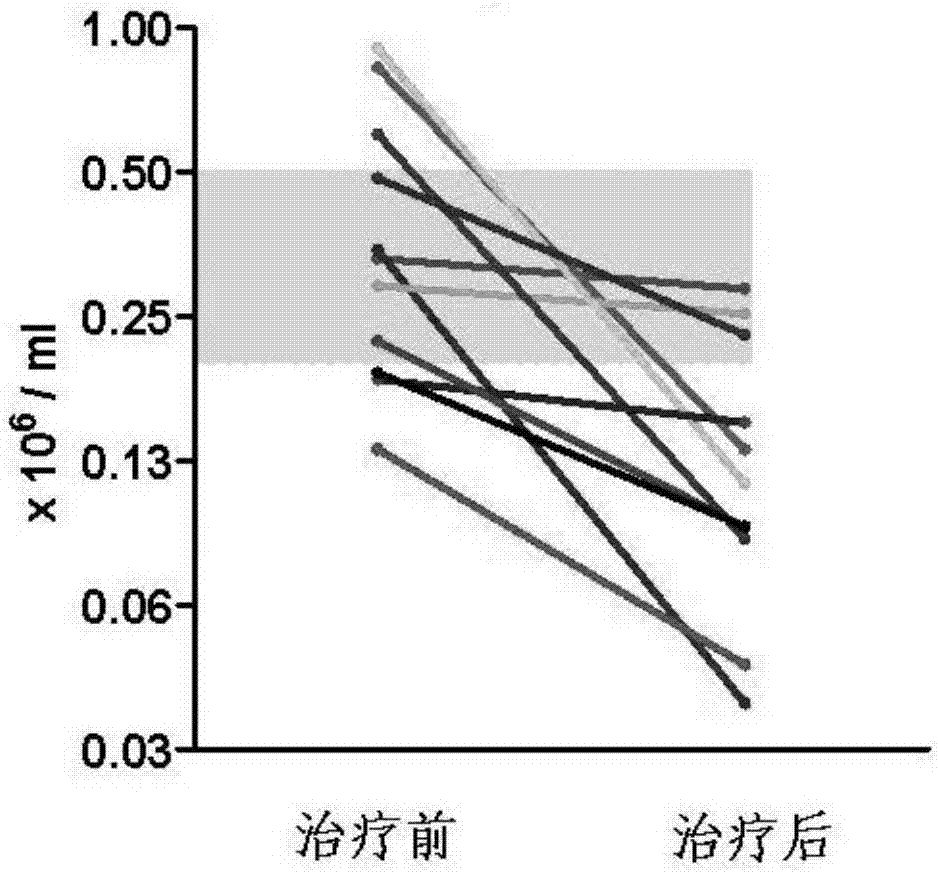
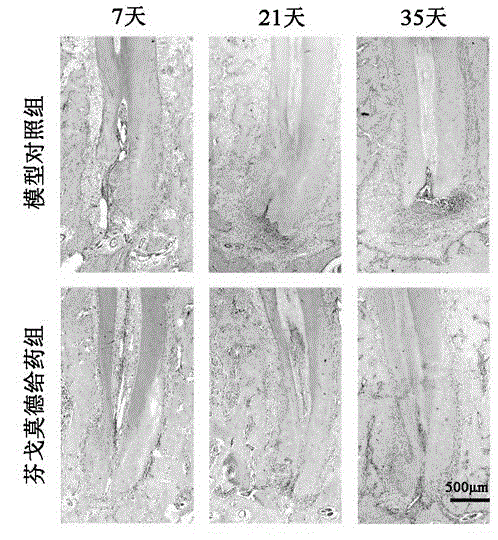
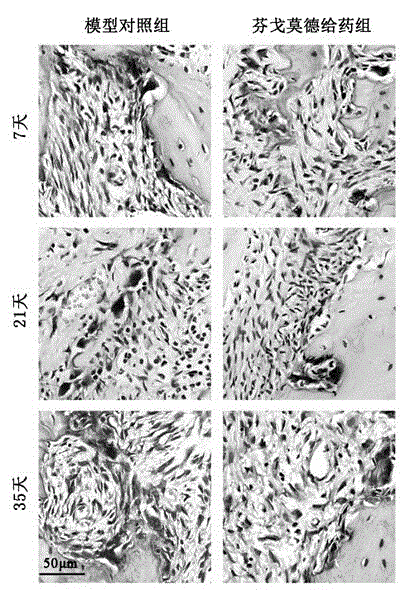
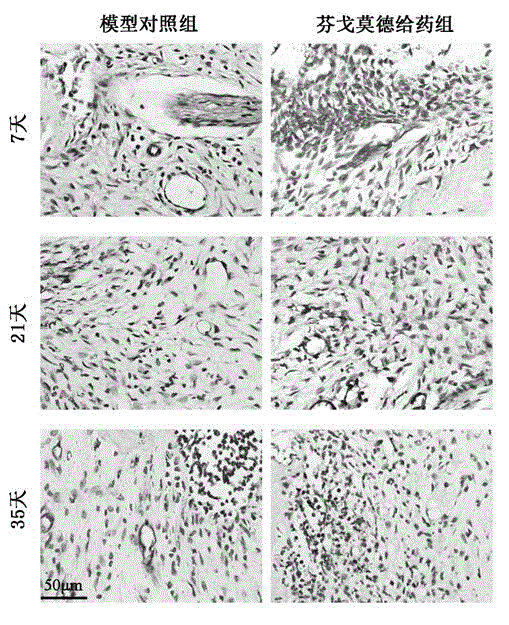
Patents
Literature
49 results about "Fingolimod" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a certain type of multiple sclerosis (relapsing multiple sclerosis-MS).
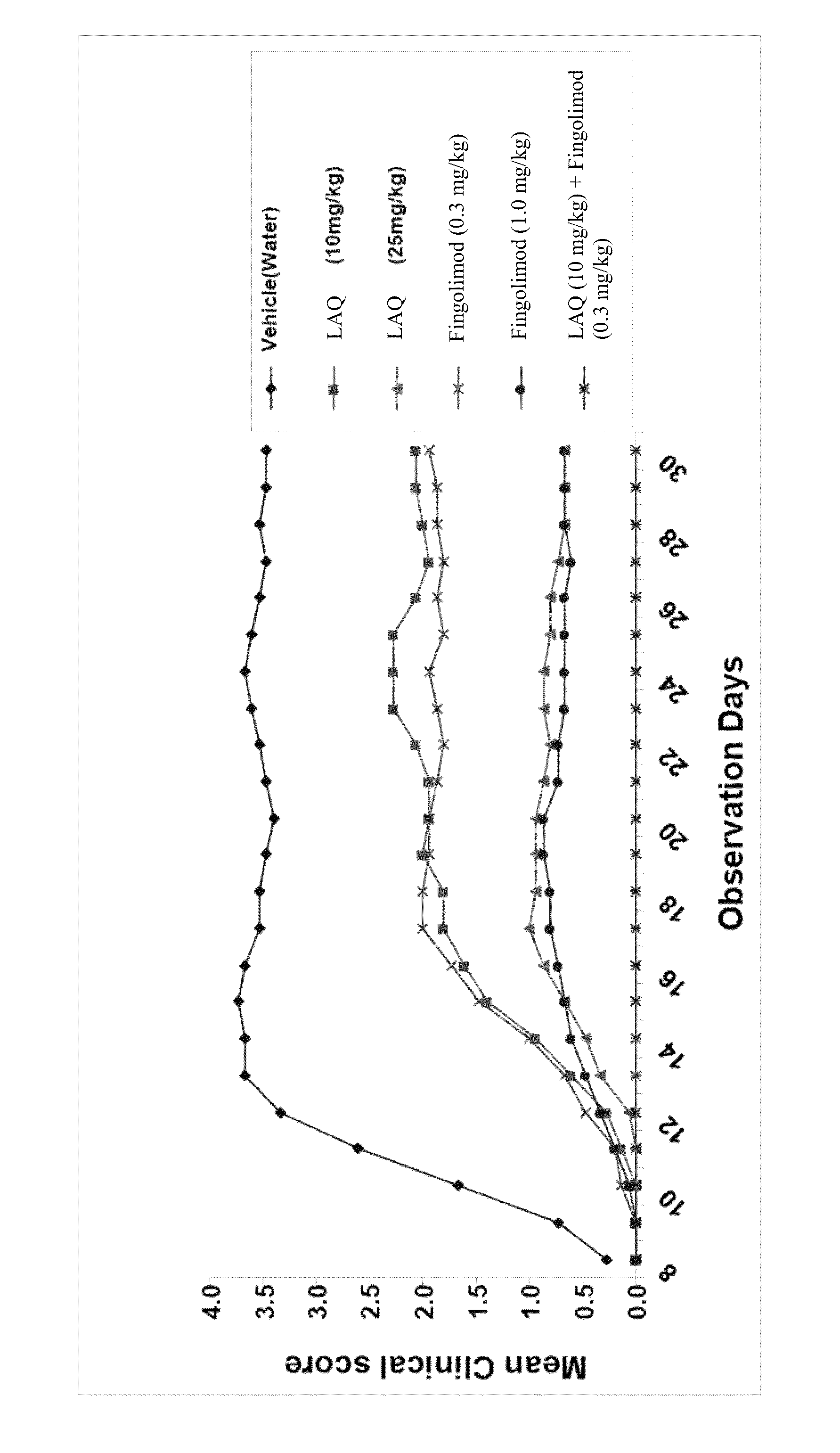
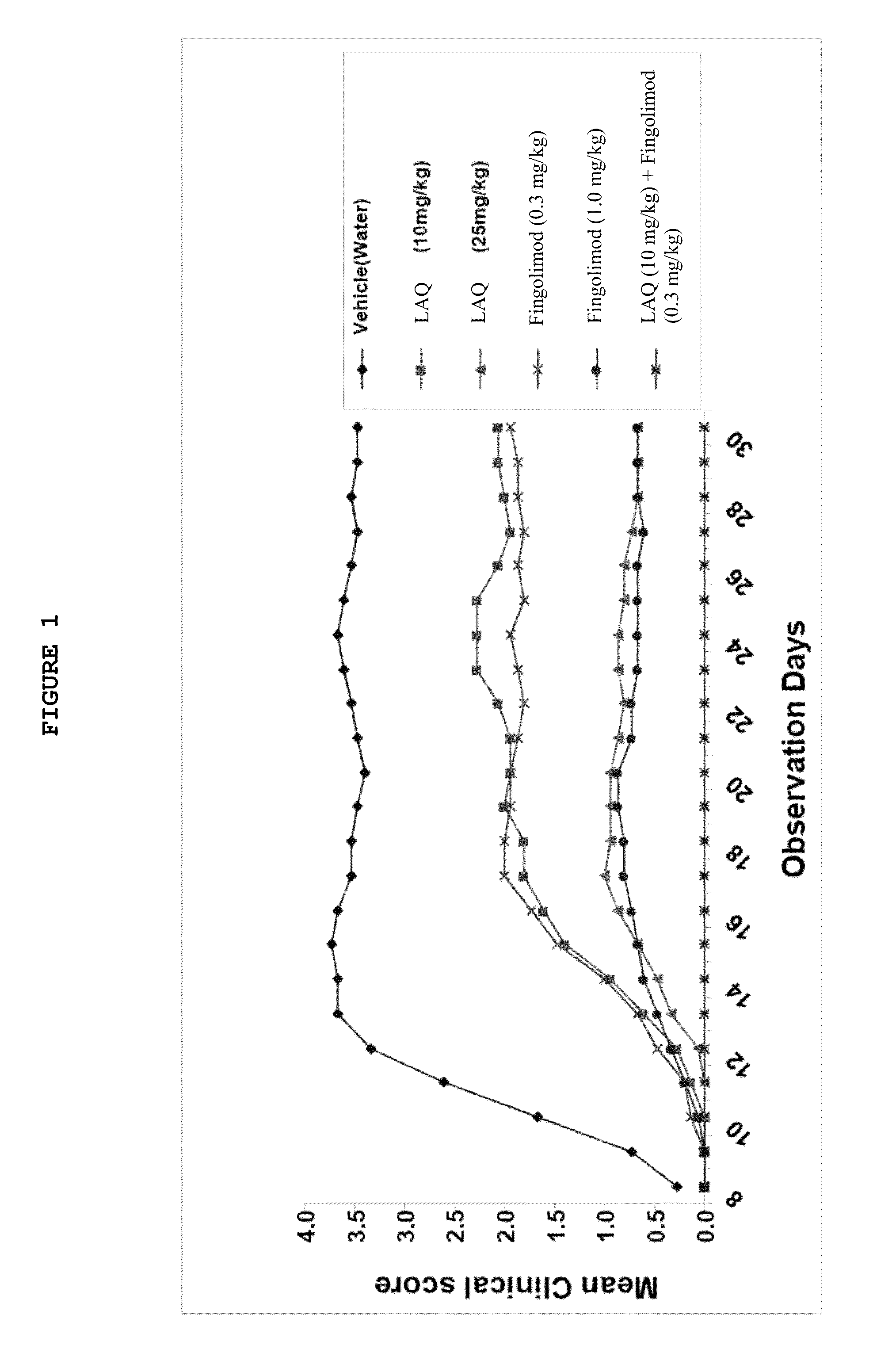
Treatment Of Multiple Sclerosis With Combination Of Laquinimod And Fingolimod
This invention provides a method of treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the subject laquinimod as an add-on therapy to or in combination with fingolimod. This invention also provides a package and a pharmaceutical composition comprising laquinimod and fingolimod for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with fingolimod in treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and fingolimod in the preparation of a combination for treating a subject afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Process for preparing pharmaceutical compositions of fingolimod
The present invention provides a process for preparing a pharmaceutical composition of fingolimod comprising: (i) obtaining a intimate admixture comprising fingolimod or a pharmaceutically acceptable salt thereof, and at least one surfactant (wetting agent), e.g., an intimate admixture of the fingolimod and the at least one surfactant, and (ii) optionally combining the intimate admixture from step (i) with one or more excipients. Also provided are pharmaceutical compositions and dosage forms obtainable by the process, uses of the pharmaceutical compositions and dosage forms, and methods of treating appropriate diseases with the pharmaceutical compositions or dosage forms.
Owner:TEVA PHARMA IND LTD
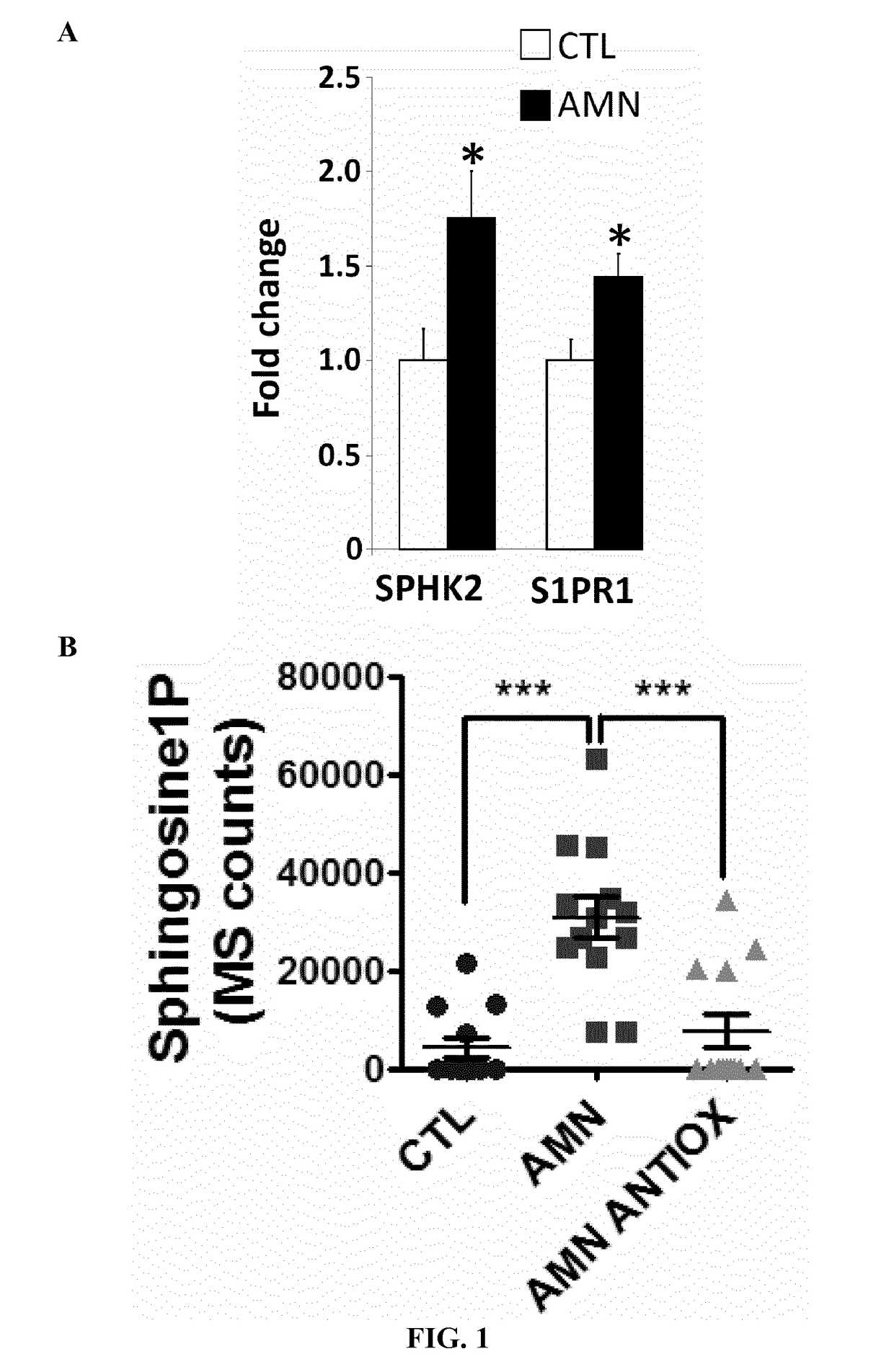
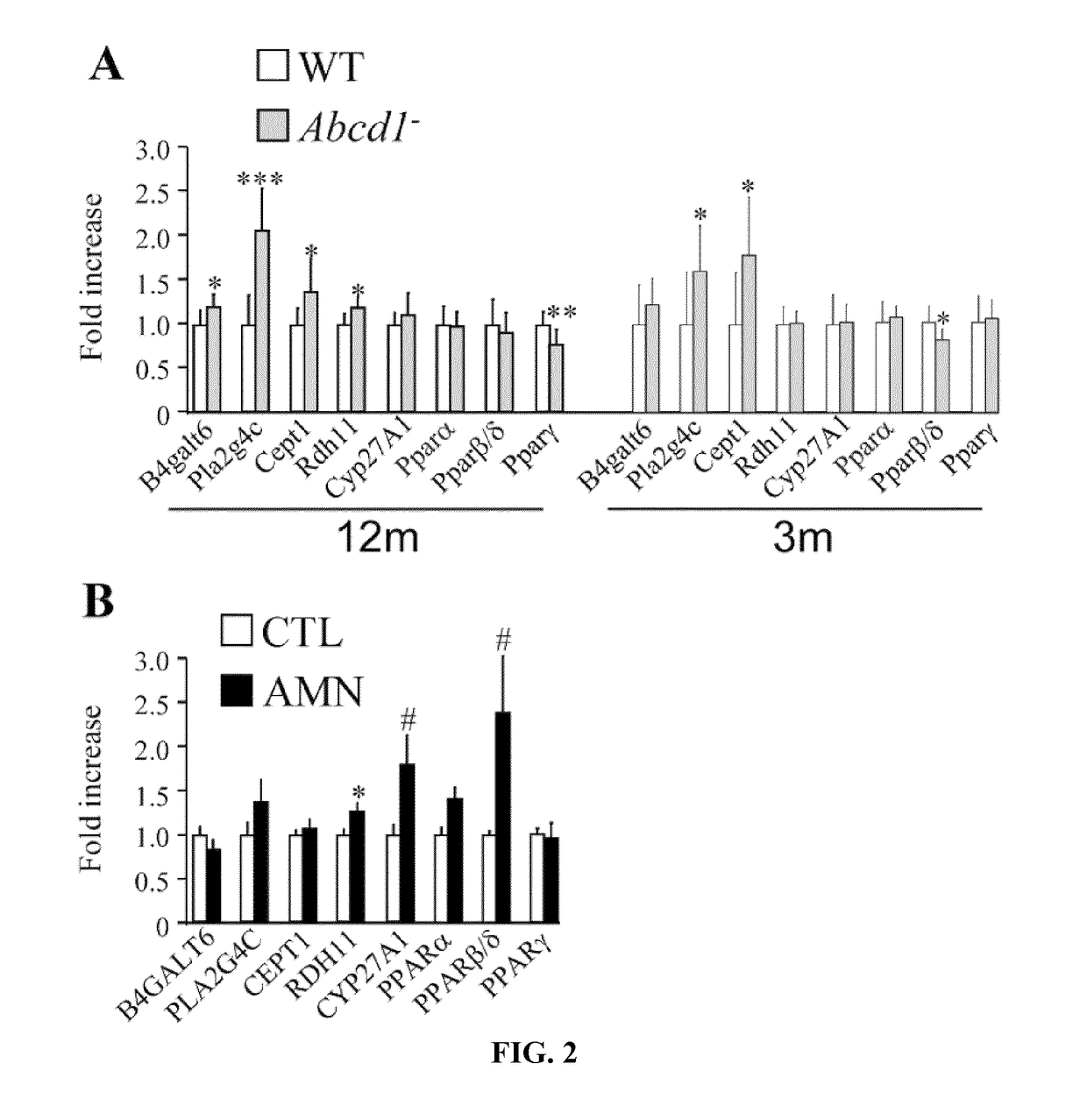
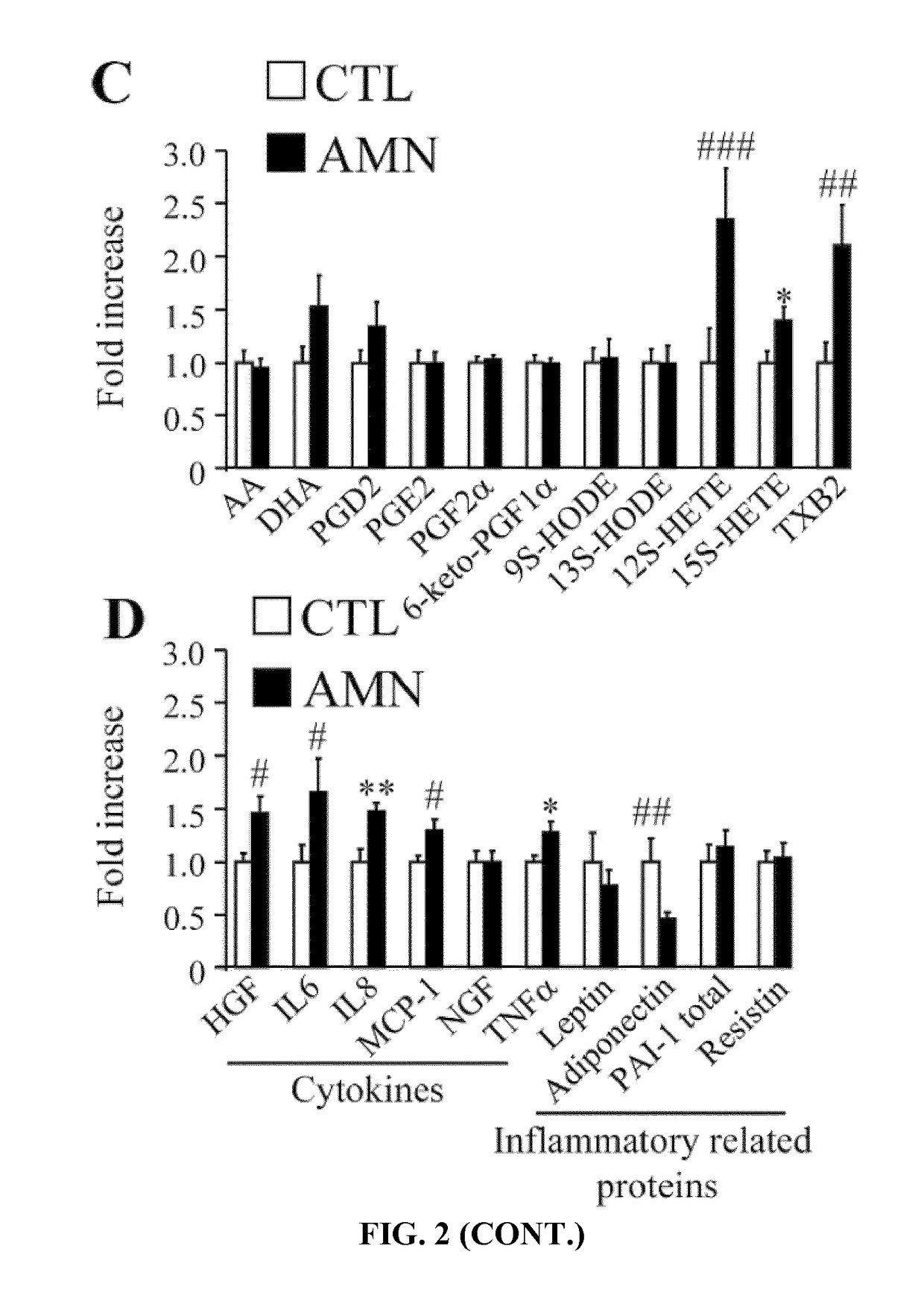
Methods and compositions for the diagnosis and for the treatment of adrenoleukodystrophy
InactiveUS20190010535A1Improve the level ofAltered levelOrganic active ingredientsMetabolism disorderX-linked adrenoleukodystrophyDiagnostic methods
The present invention is directed to a diagnostic method for adrenoleukodystrophy in a subject based on the determination of the levels of different markers. The invention also provides a method for monitoring the progression of an adrenoleukodystrophy, a method for monitoring the effect of an adrenoleukodystrophy therapy and fingolimod, an analogue, metabolite or derivative thereof, or a pharmaceutically acceptable salt thereof, for use in the treatment and / or prevention of anadrenoleukodystrophy.
Owner:FUNDACIO INST DINVESTIGACIO BIOMEDICA DE BELLVITGE IDIBELL +4
Stabilized pharmaceutical compositions of fingolimod and process for preparation thereof
InactiveUS20150141520A1Reduce frequencyAvoid rapid accumulationOrganic active ingredientsBiocideActive agentS1P Receptor Modulators
Stabilized pharmaceutical compositions comprising a S1P receptor modulator as an active agent(s), process of preparation and method of using the same are provided. The present invention also relates to stabilized pharmaceutical compositions comprising fingolimod, or pharmaceutically acceptable salts, esters, hydrates and solvates thereof, process of preparation and method of using the same.
Owner:AUROBINDO PHARMA LTD
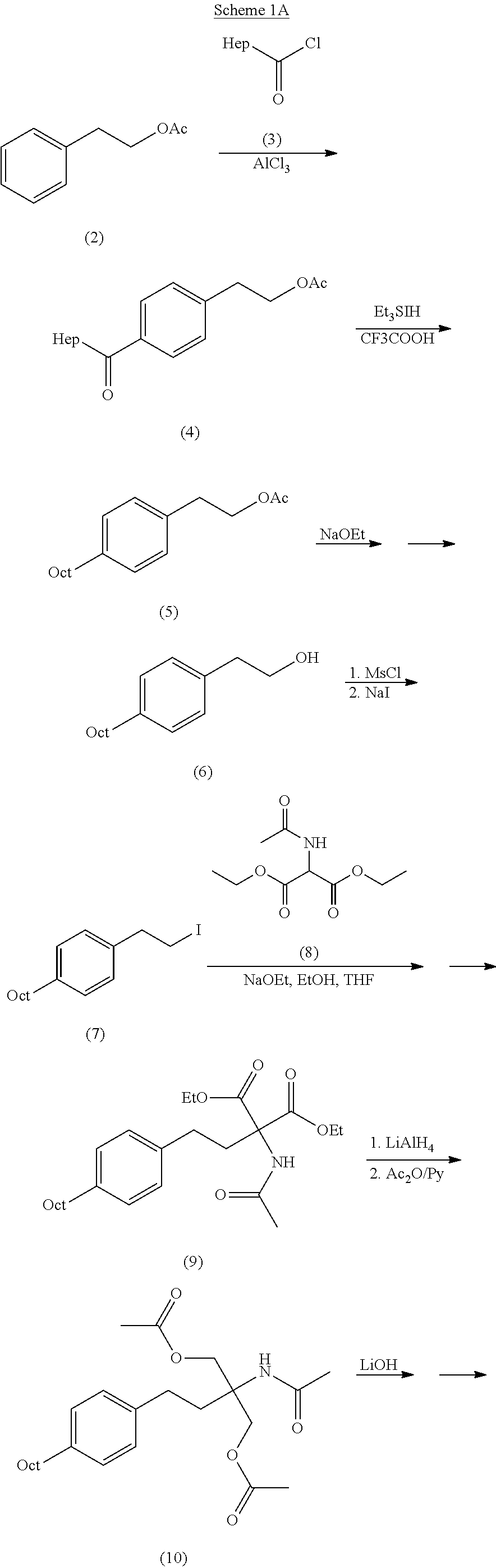
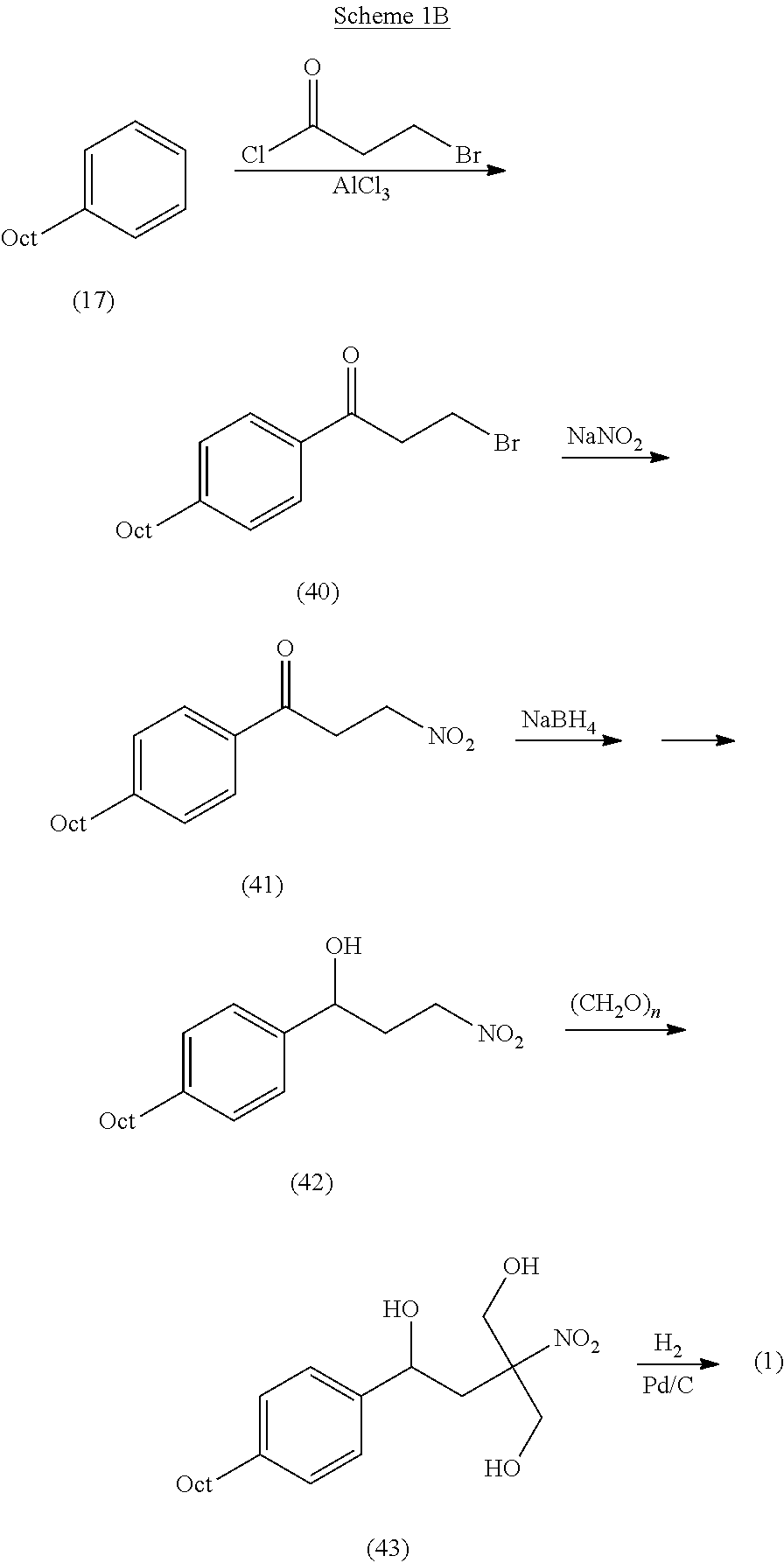
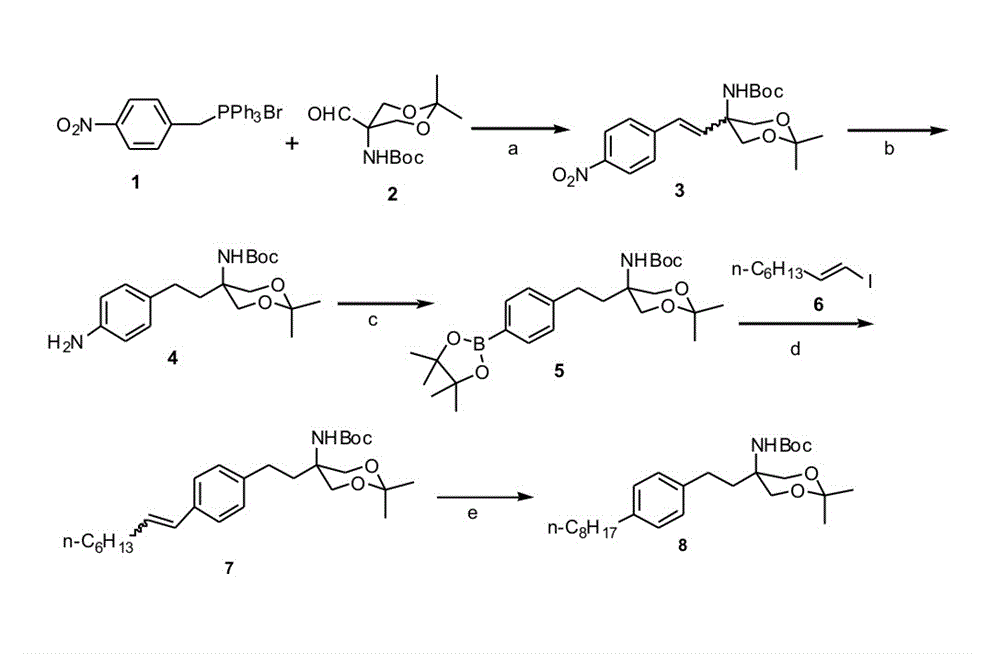
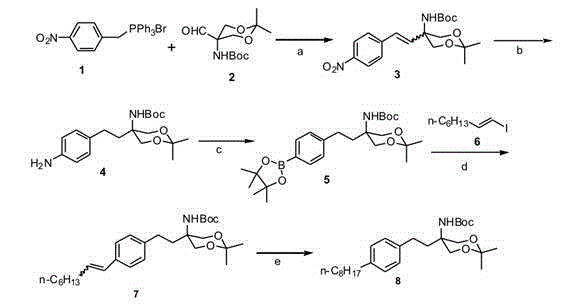
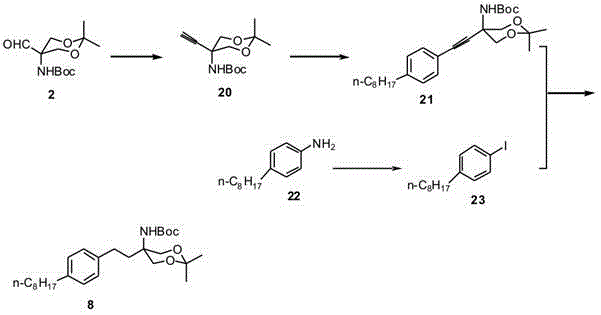
Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester
InactiveCN102850319AEasy to manufactureEasy post-processingOrganic chemistryGrignard reagentTert butyl
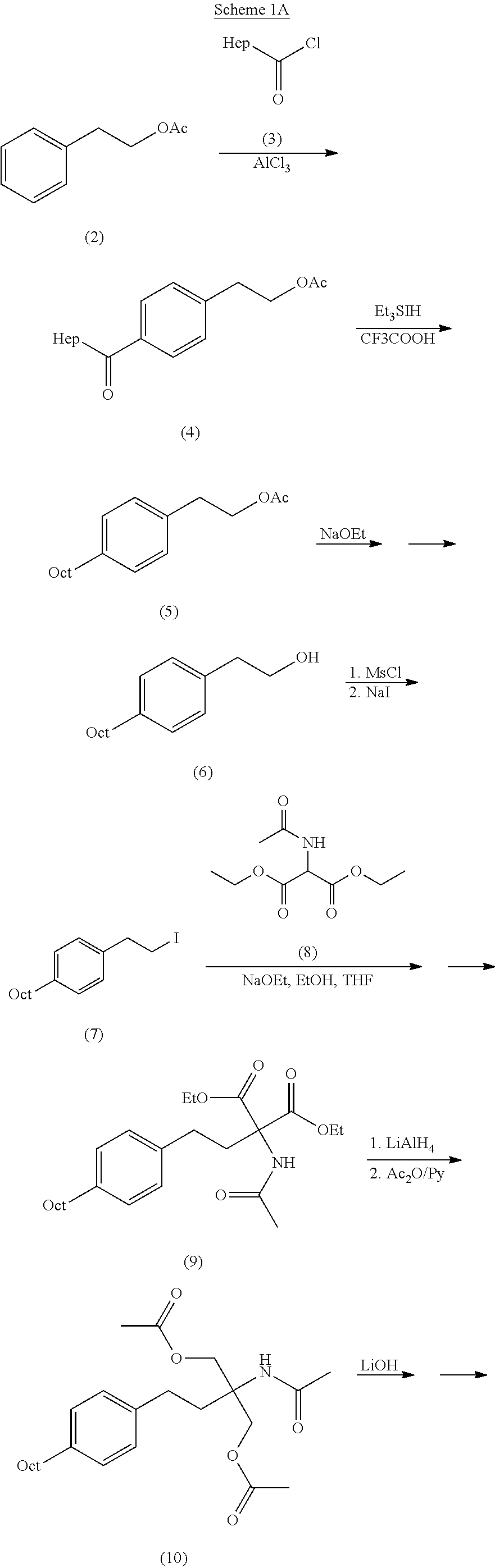
The present invention relates to a preparation method of fingolimod intermediate, specifically relates to a preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester. The method includes performing cross-coupling reaction of a Grignard reagent prepared from n-octyl alkylogen with 4-halogen-benzoate, reducing the generated 4-n-octyl benzoate to 4-n-octyl benzyl alcohol, performing halogenation reaction to give 4-n-octyl benzyl halide, reacting the halide with triphenylphosphine to generate (4-n-octyl benzyl) triphenylphosphonium halide, performing Wittig reaction of the compound with (5-formyl-2,2-dimethyl-1,3-dioxane-5-yl) carbamic acid tert-butyl ester, generating {5-[2-(4-n-octyl-phenyl)-vinyl]-2,2-dimethyl-1,3-dioxane-5-yl}-carbamic acid tert-butyl ester, and preparing the compound by hydrogenation reduction.
Owner:CHINA PHARM UNIV
Stable solid fingolimod dosage forms
The present invention relates to a solid pharmaceutical dosage forms and methods for preparing the solid pharmaceutical dosage form that contains fingolimod or its pharmaceutically acceptable salts, conjugates or complexes thereof. The solid pharmaceutical dosage forms may rapidly disintegrates in a patient's oral cavity.
Owner:HANDA NEUROSCIENCE LLC
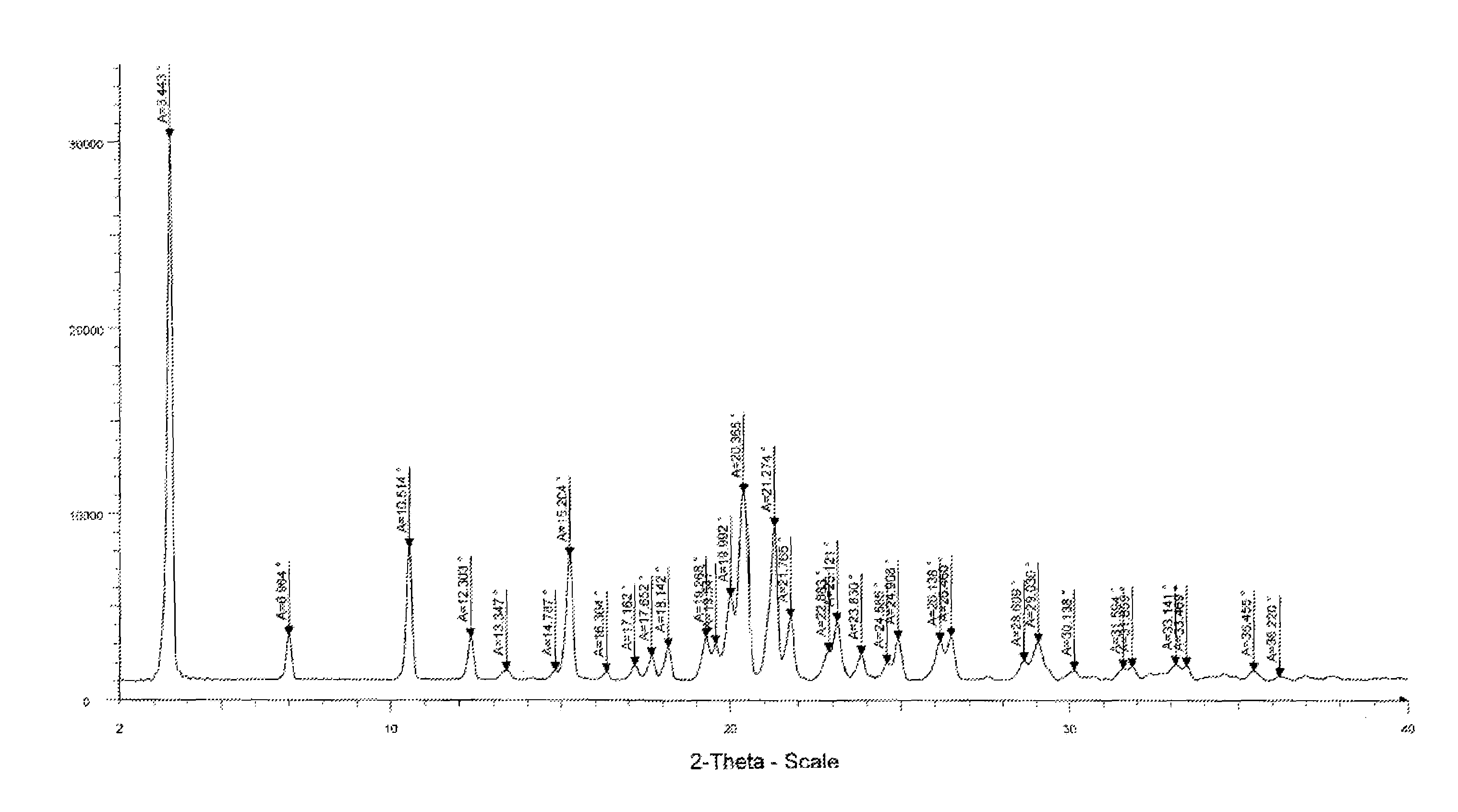
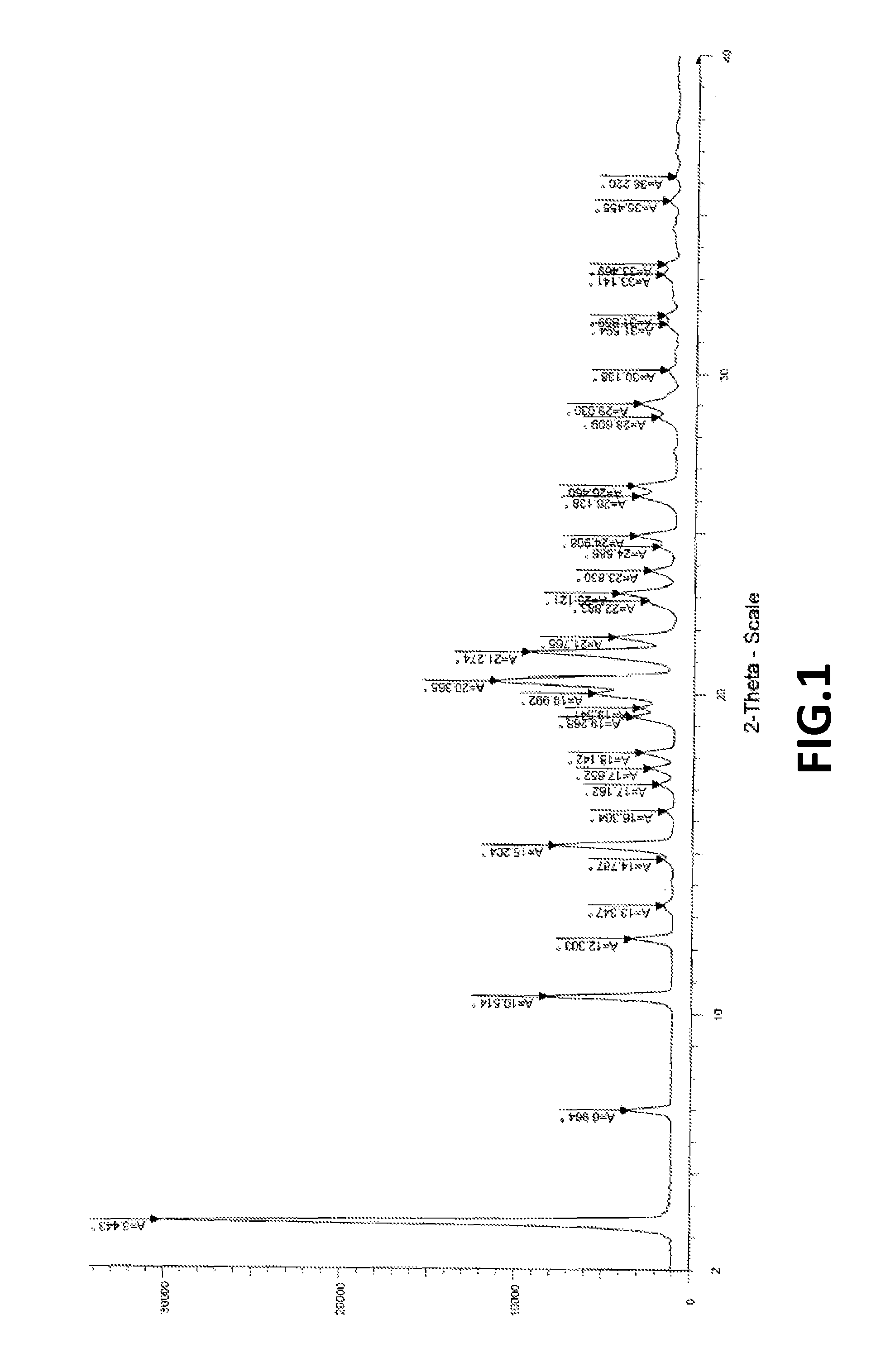
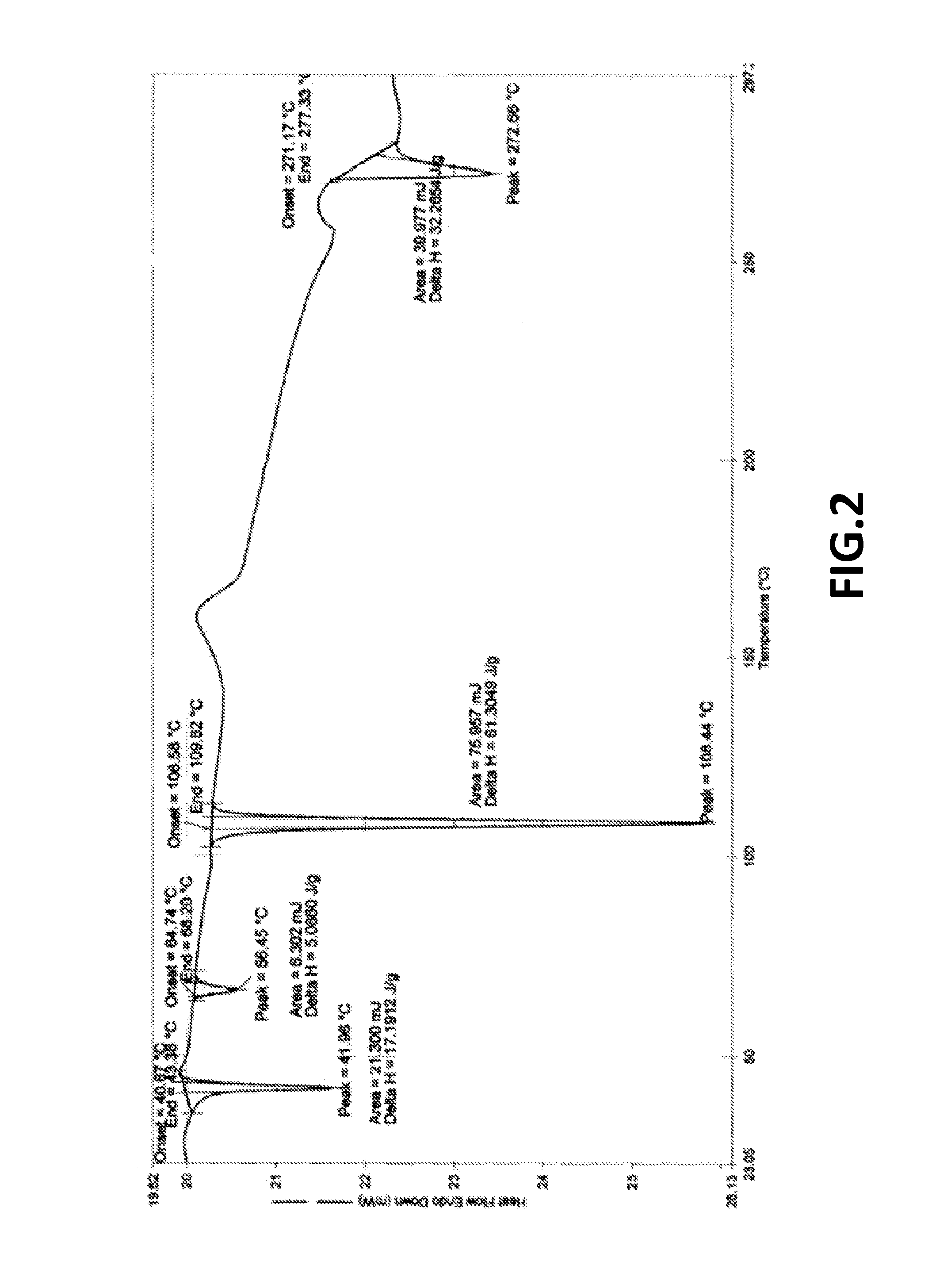
Fingolimod polymorphs and their processes
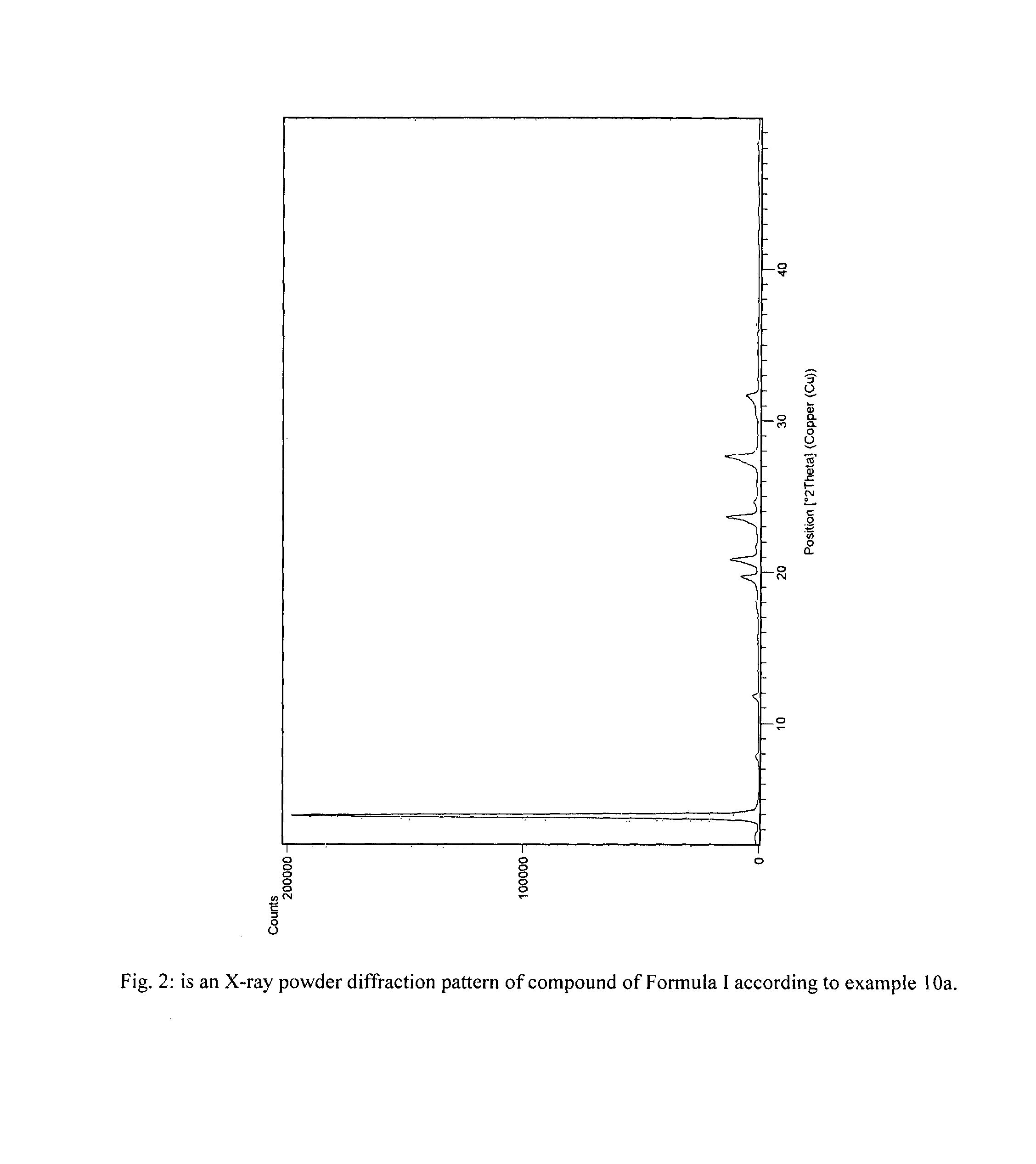
ActiveUS20130281739A1Organic compound preparationOrganic chemistry methodsRelated impuritiesMedicinal chemistry
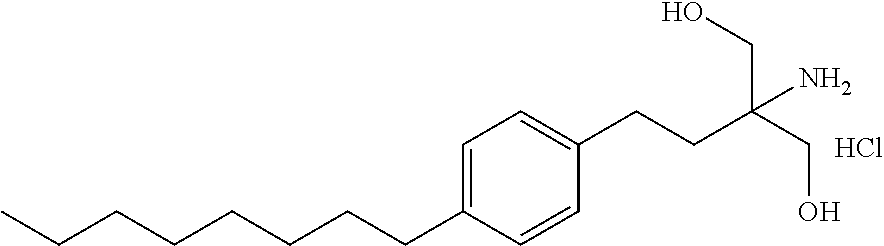
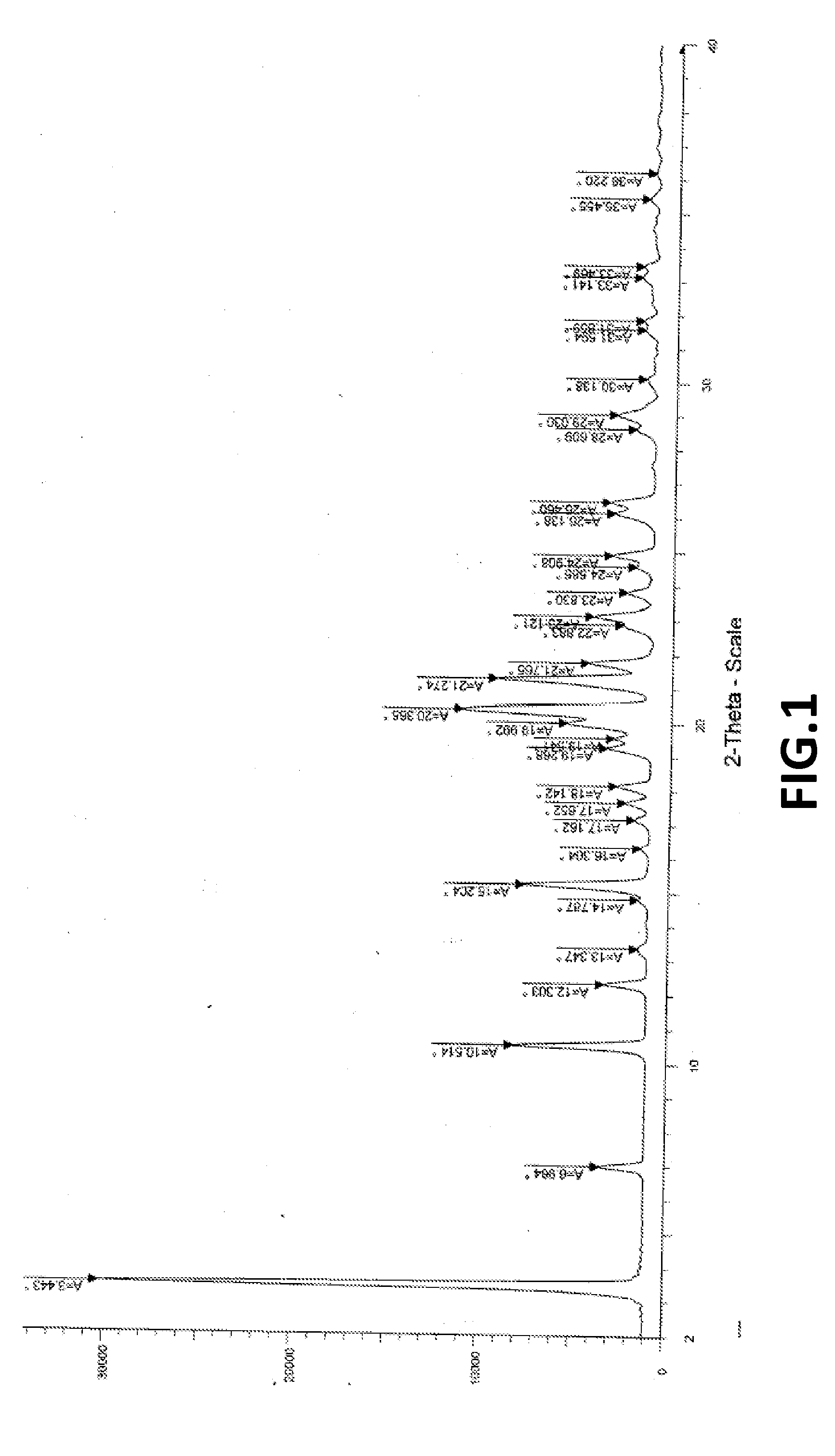
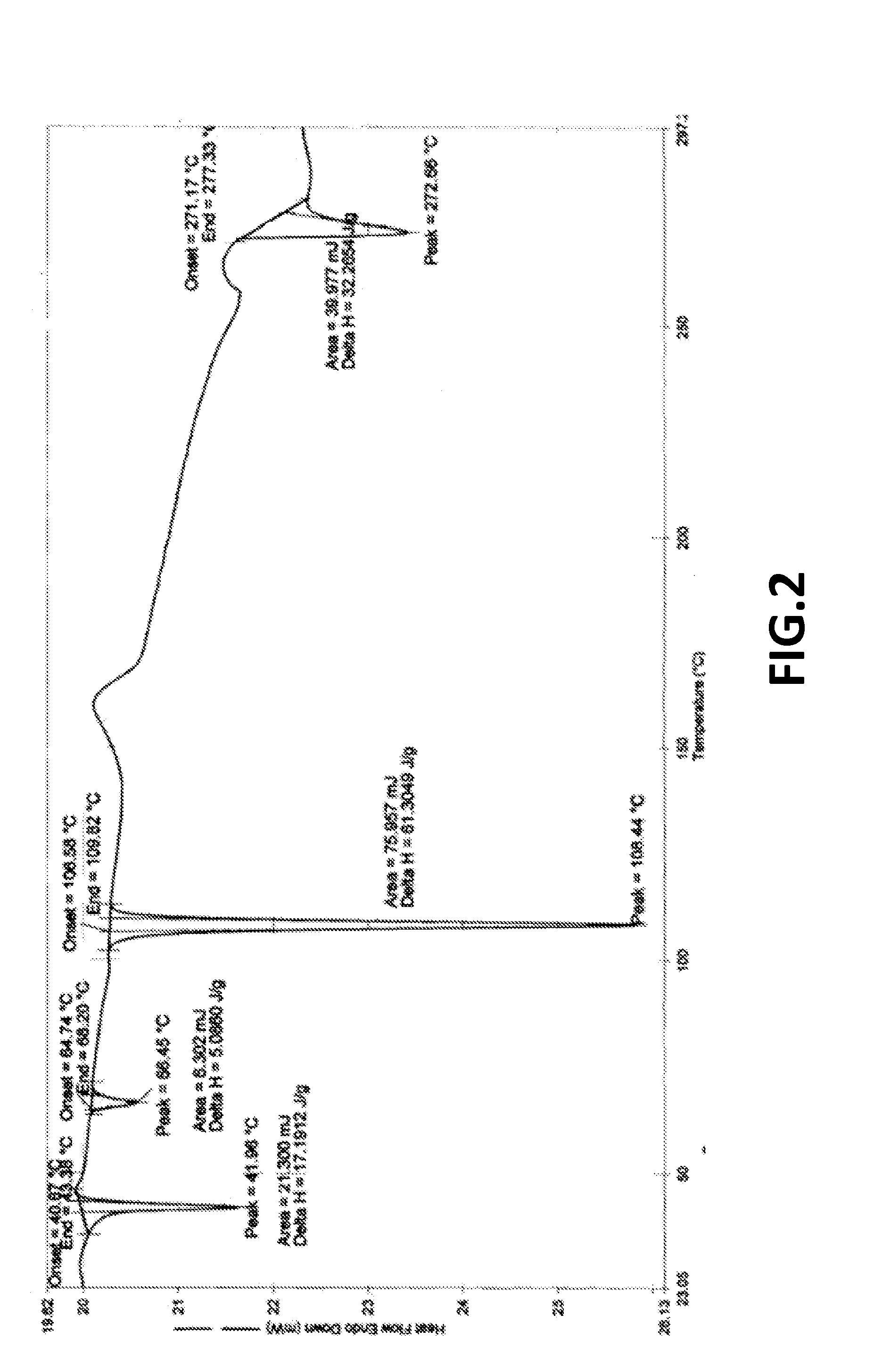
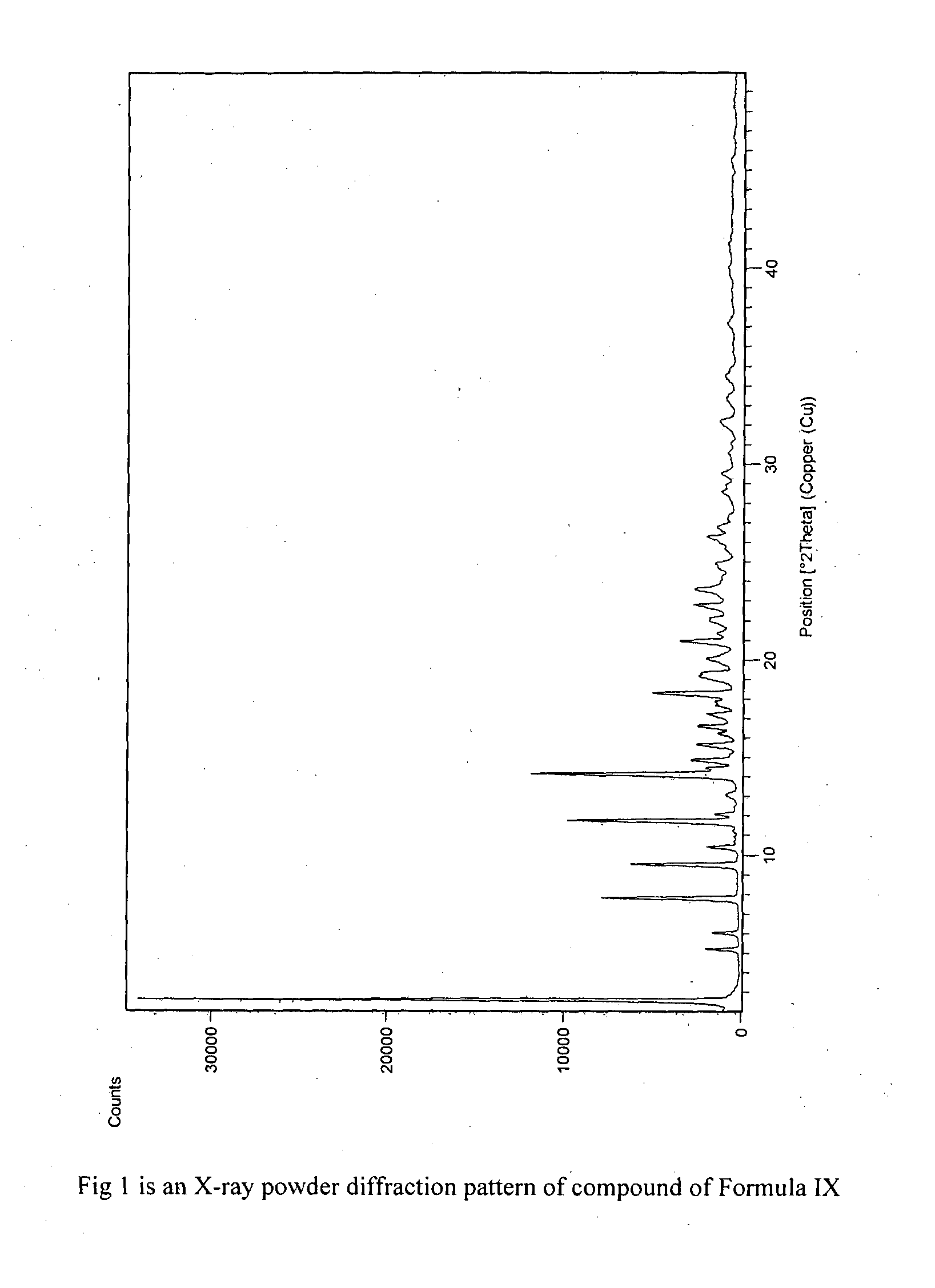
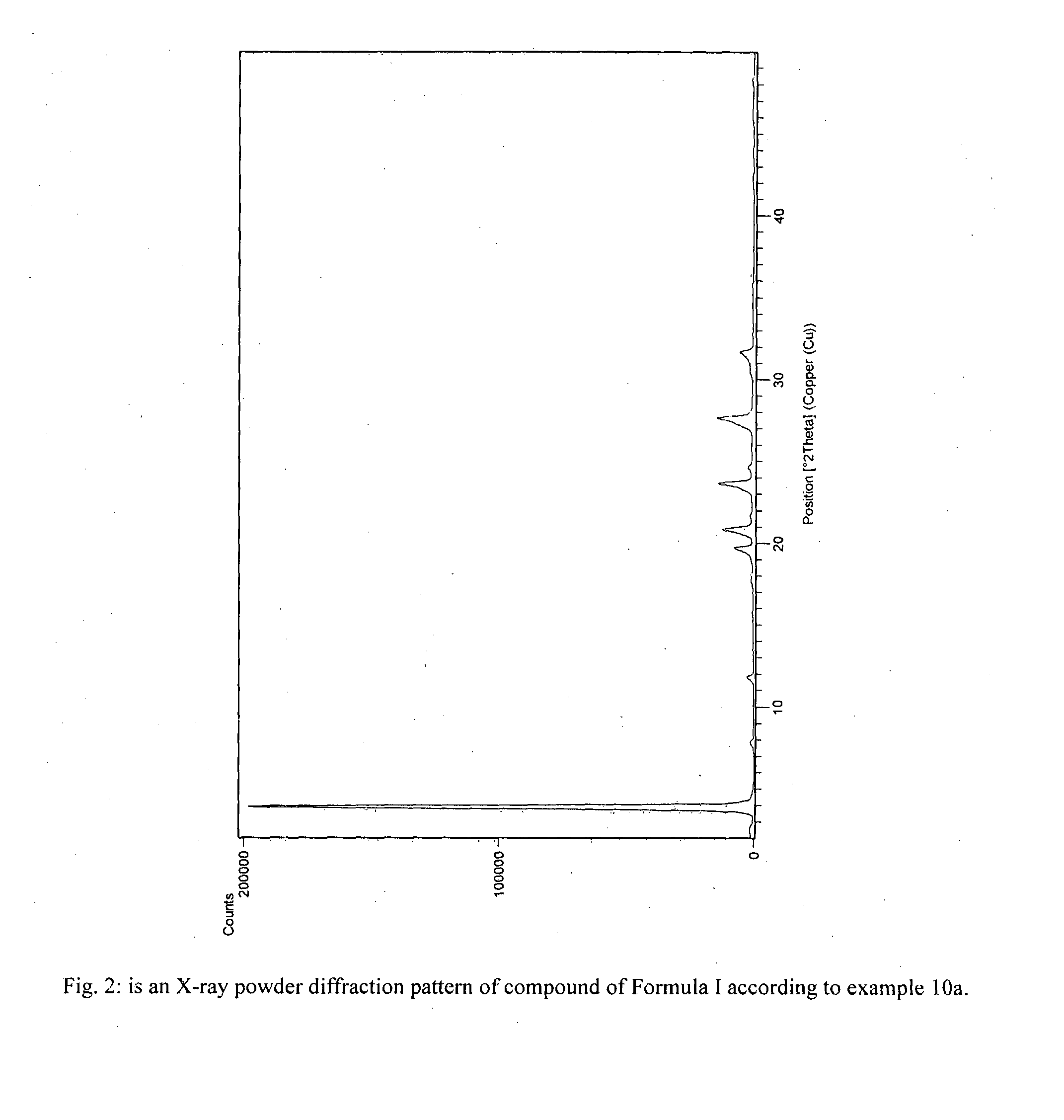
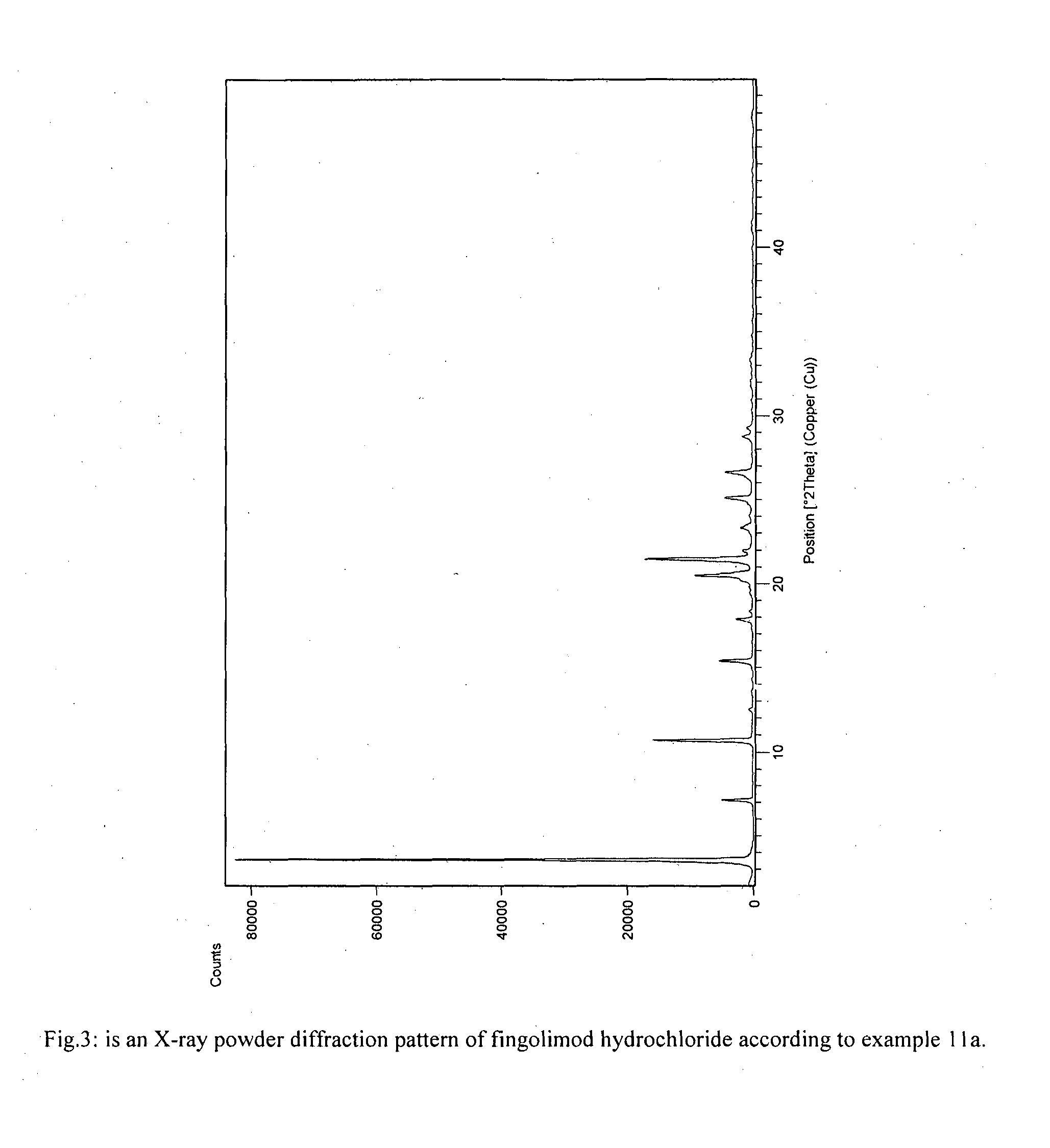
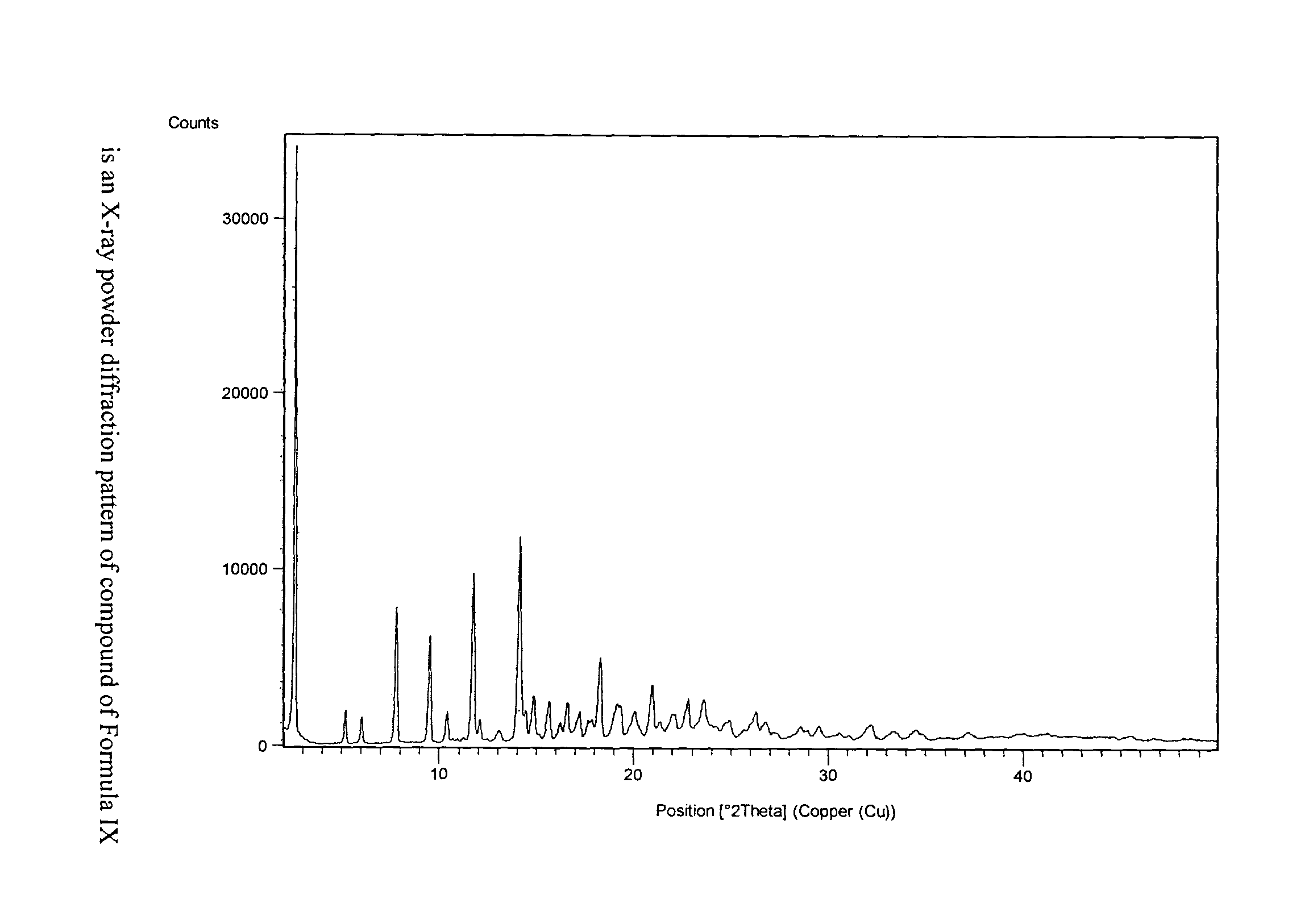
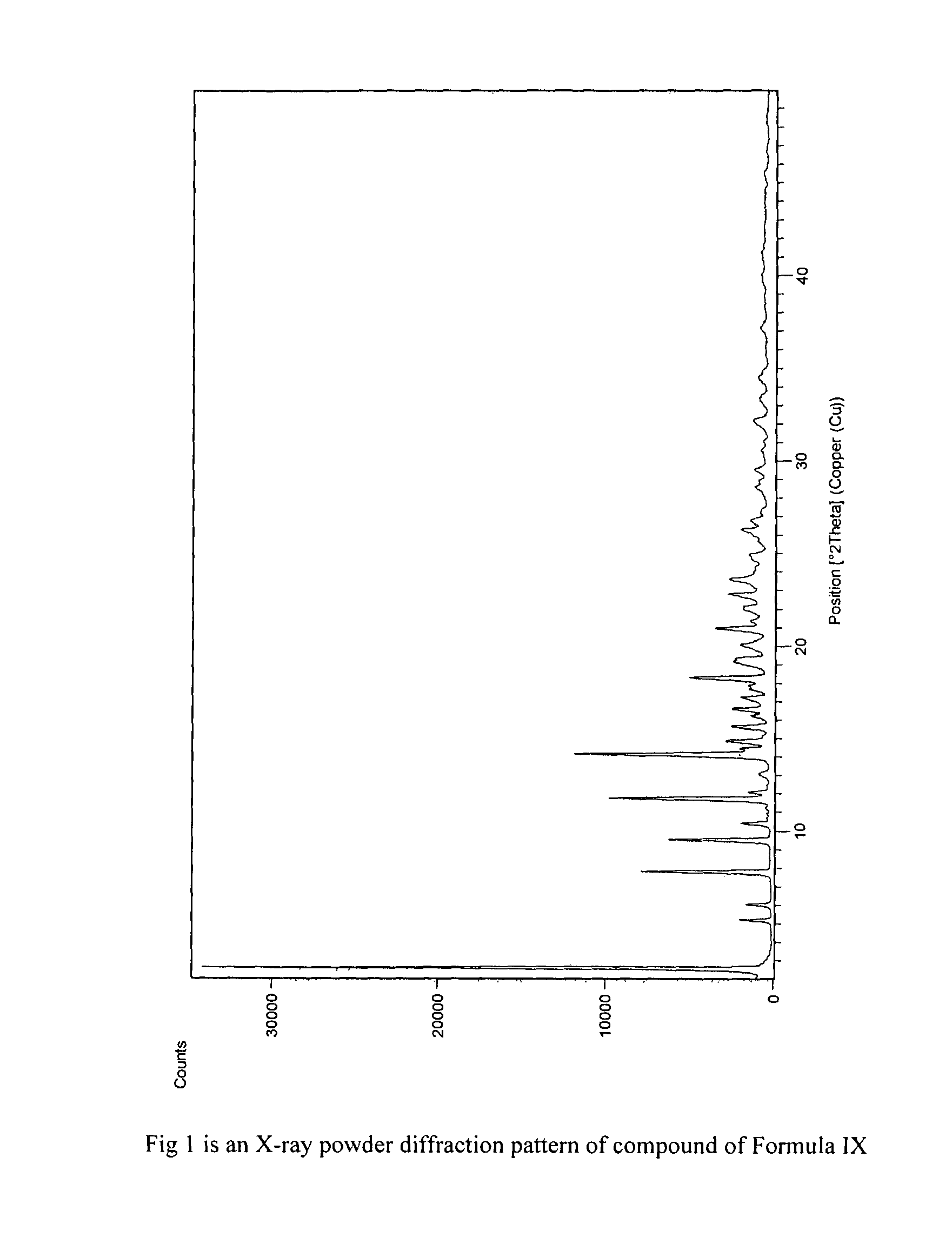
The present invention provides crystalline polymorphic forms of Fingolimod HCl (I) and processes for preparation thereof.The application provides processes for preparation of crystalline polymorphic forms-α, β and μ substantially free from process related impurities. The crystalline polymorphic forms of Fingolimod HCl (I) obtained by the processes according to the present invention having an XRDP pattern as per FIGS. 1, 3 and 5, which are useful as active pharmaceutical ingredient in pharmaceutical compositions for the treatment or prevention of autoimmune related disorder including multiple sclerosis.
Owner:SHILPA PHARM INC
Treatment of multiple sclerosis with combination of laquinimod and fingolimod
Owner:TEVA PHARMA IND LTD
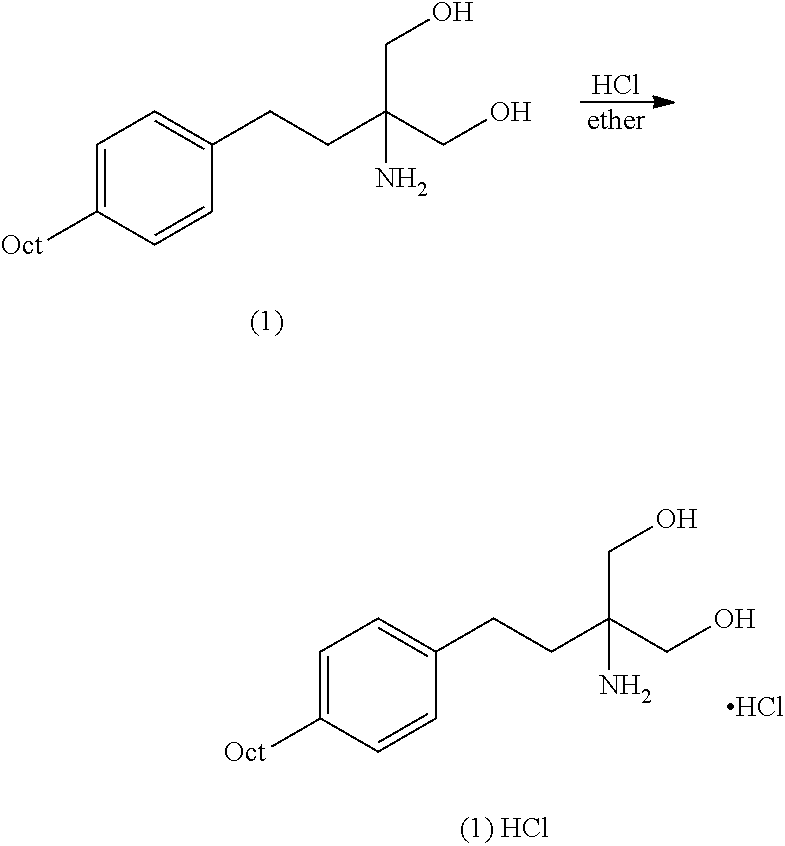
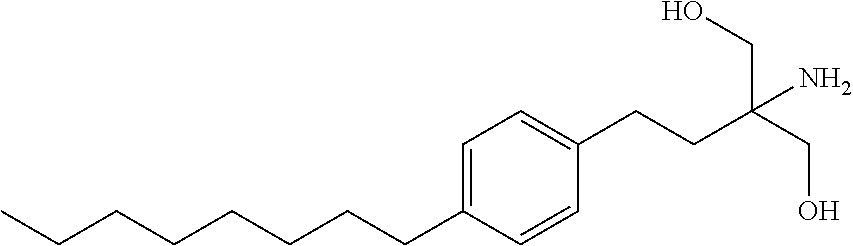
Intermediate compounds and process for the preparation of fingolimod
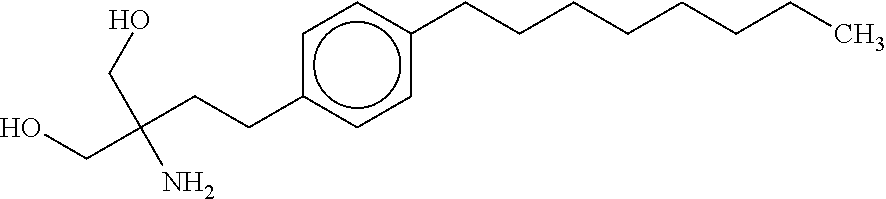
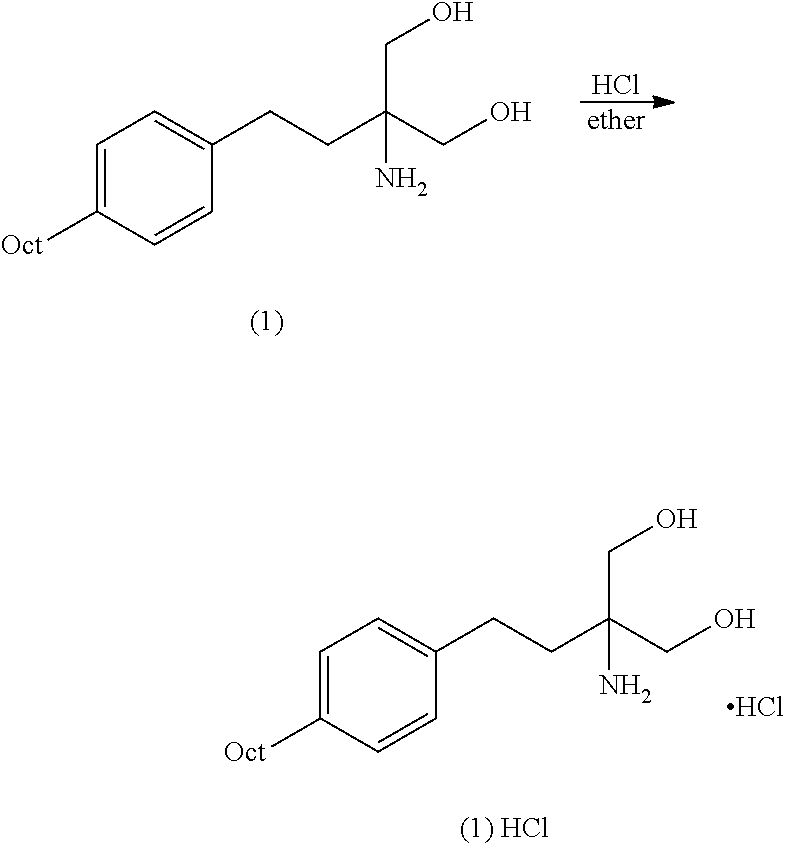
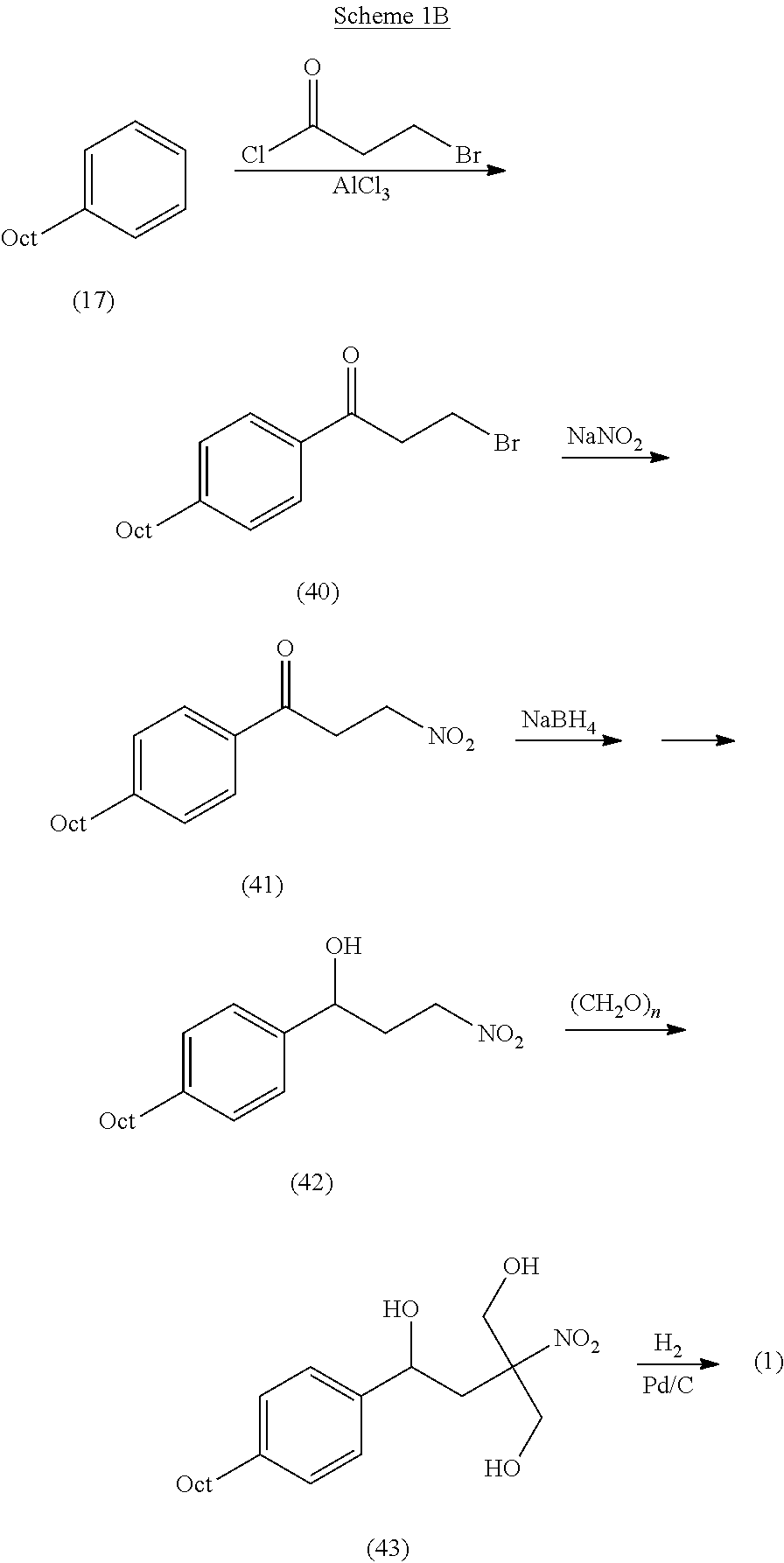
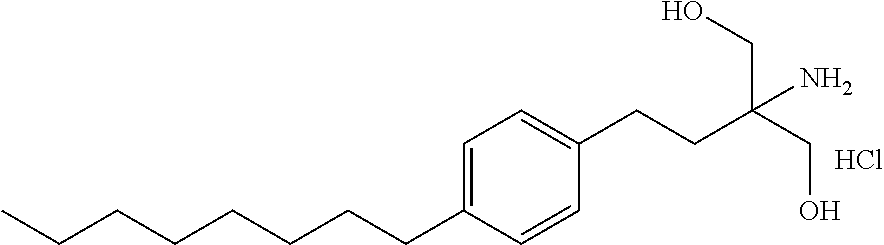
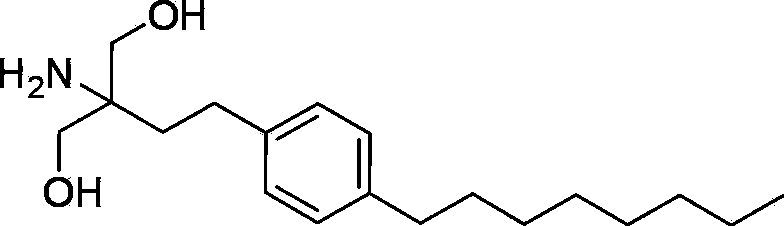
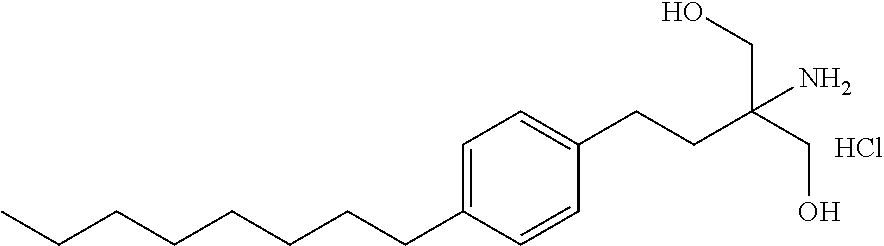
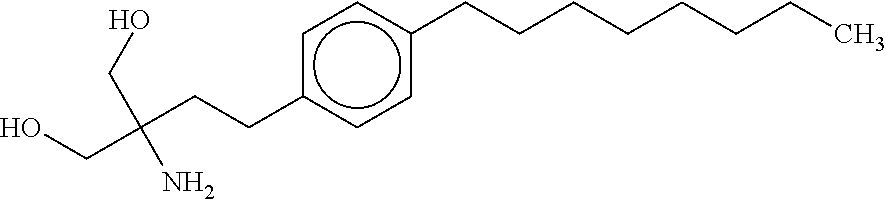
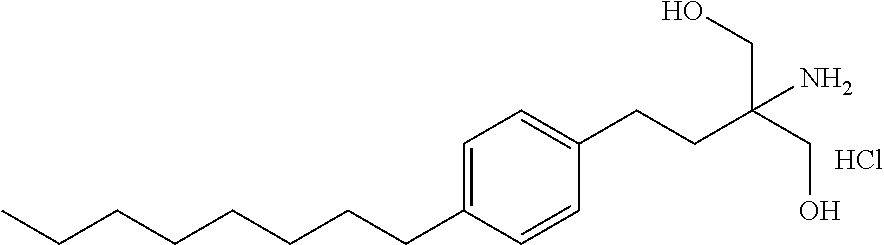
The present invention relates to processes for the preparation of (2-Amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride (Fingolimod) and pharmaceutically acceptable salts thereof, and intermediates formed in such processes.
Owner:MAPI PHARMA
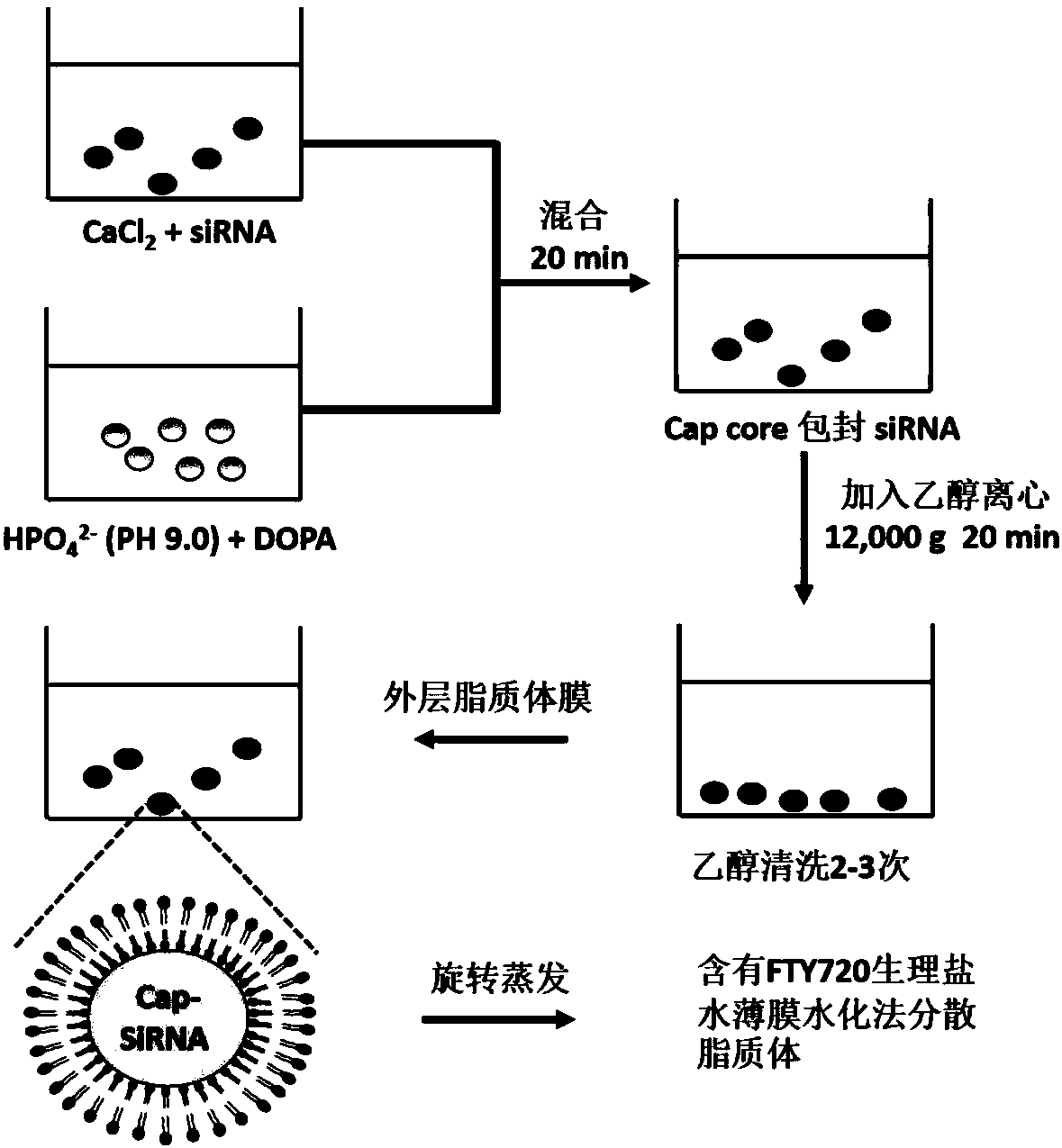
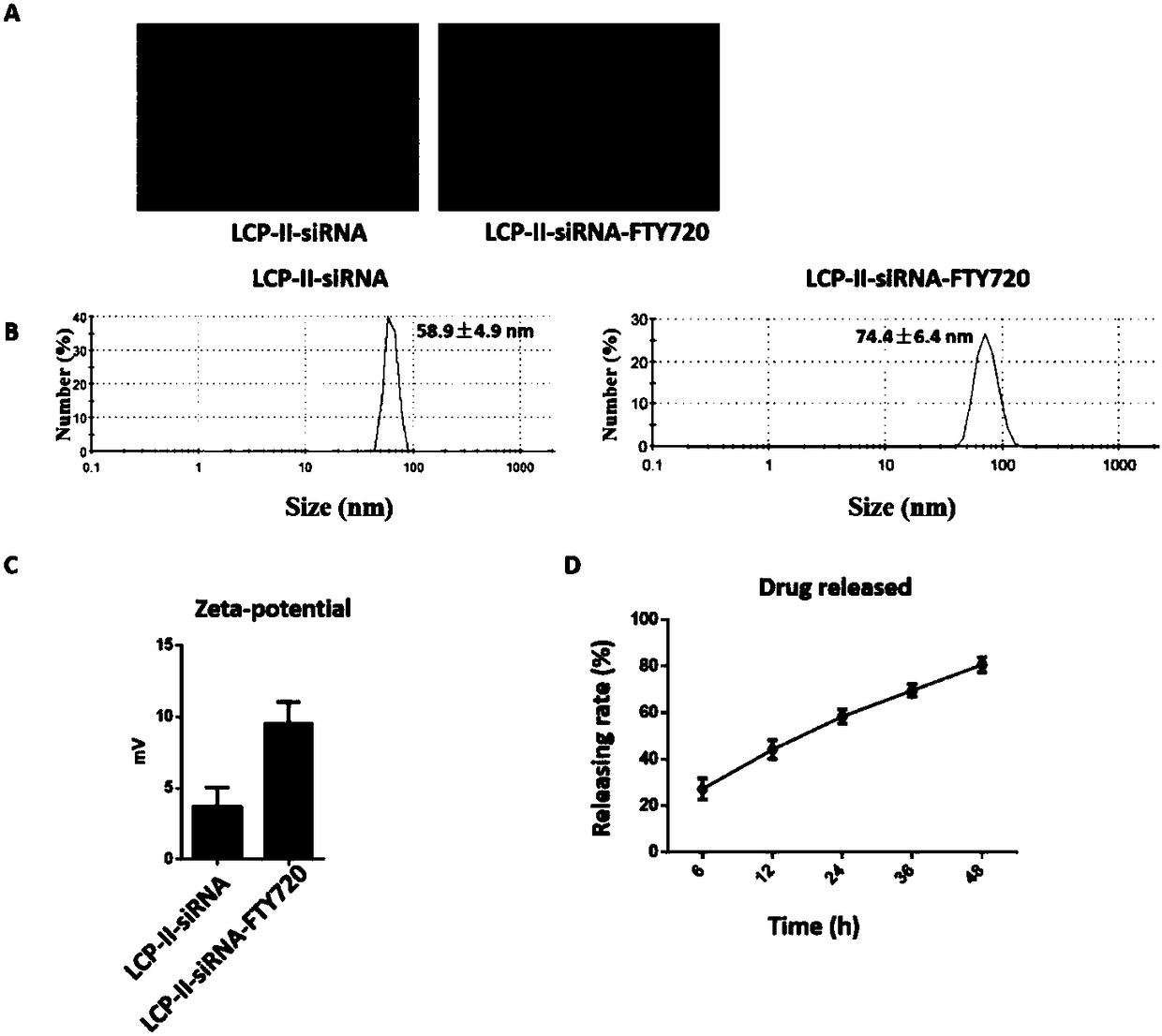
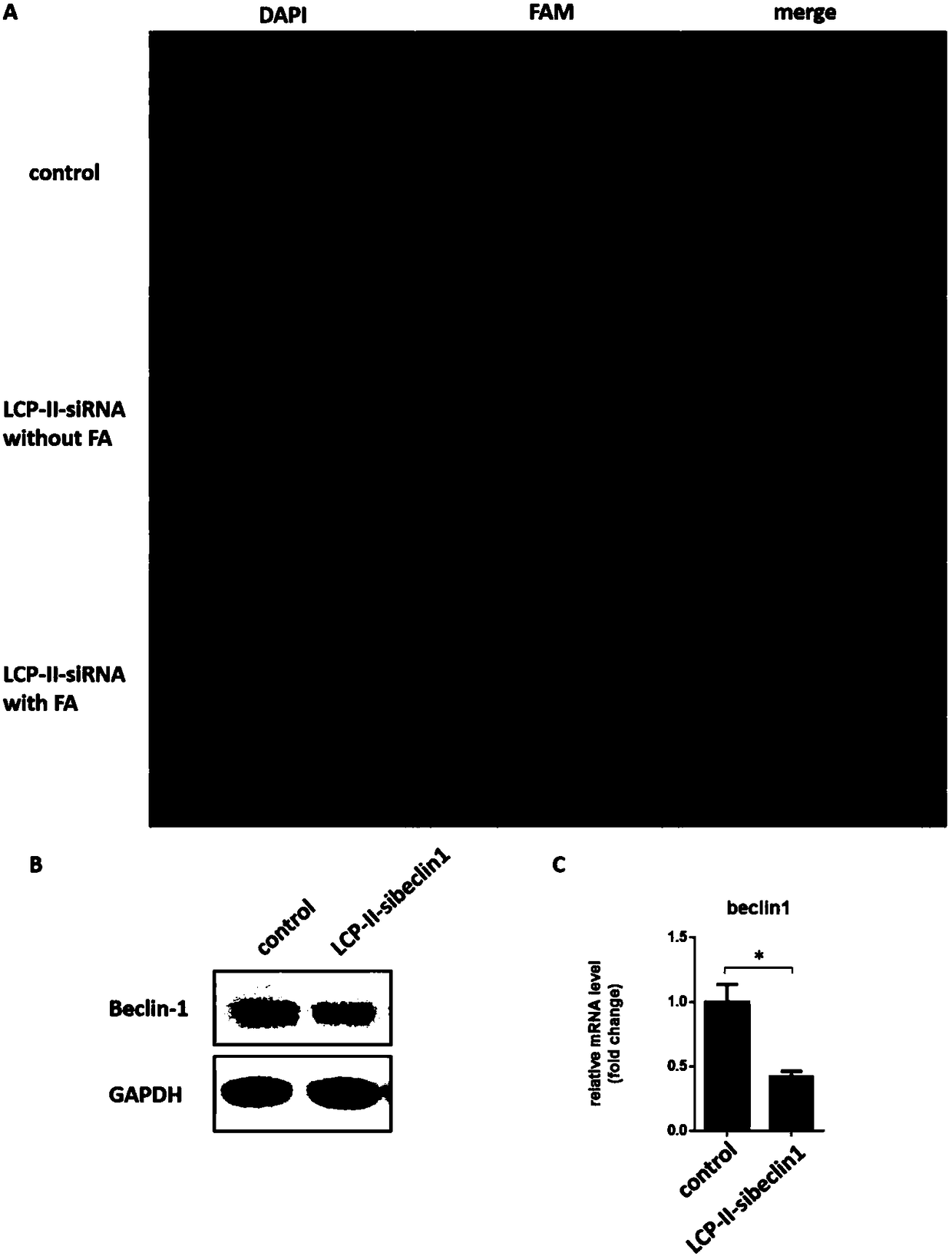
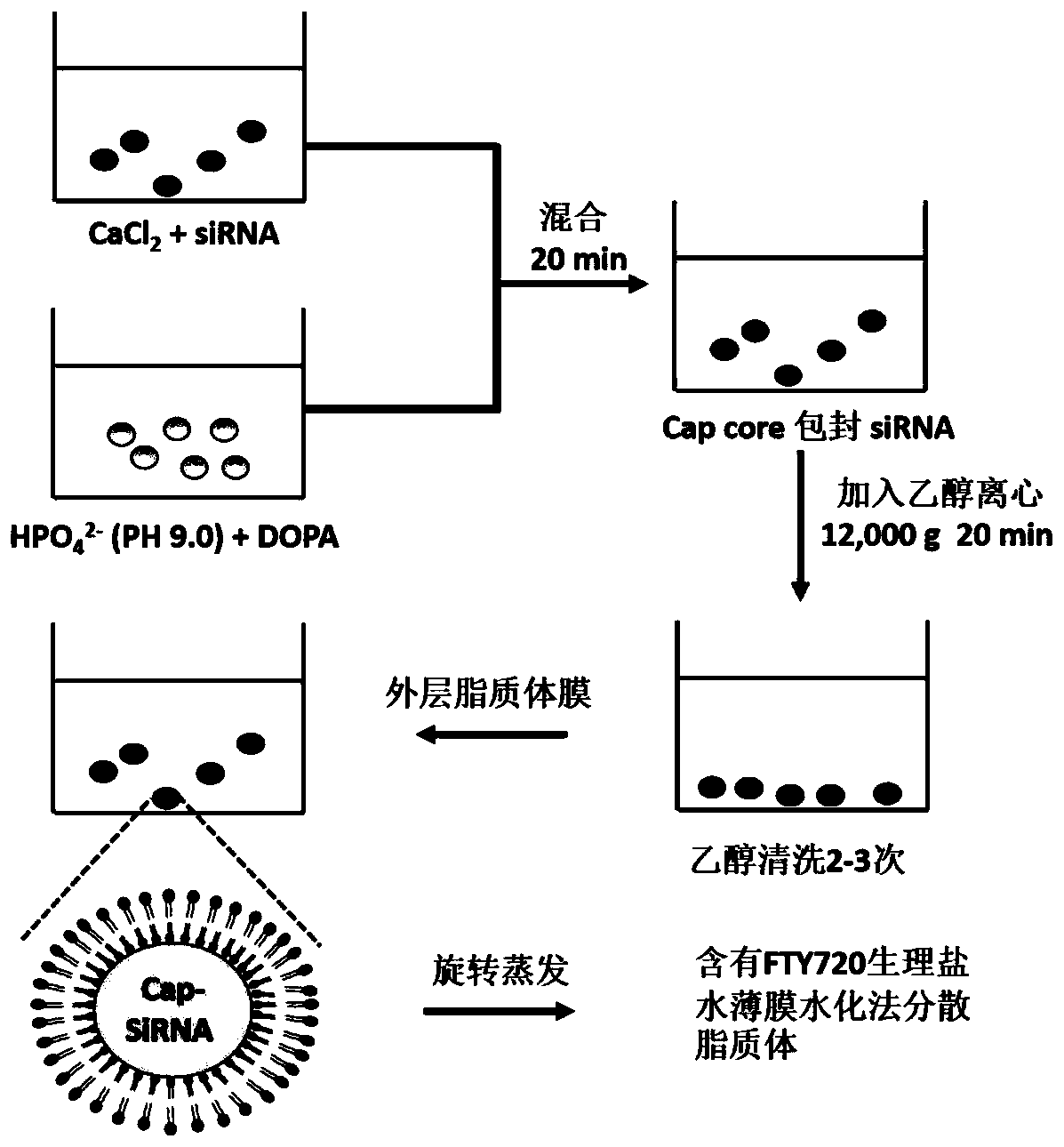
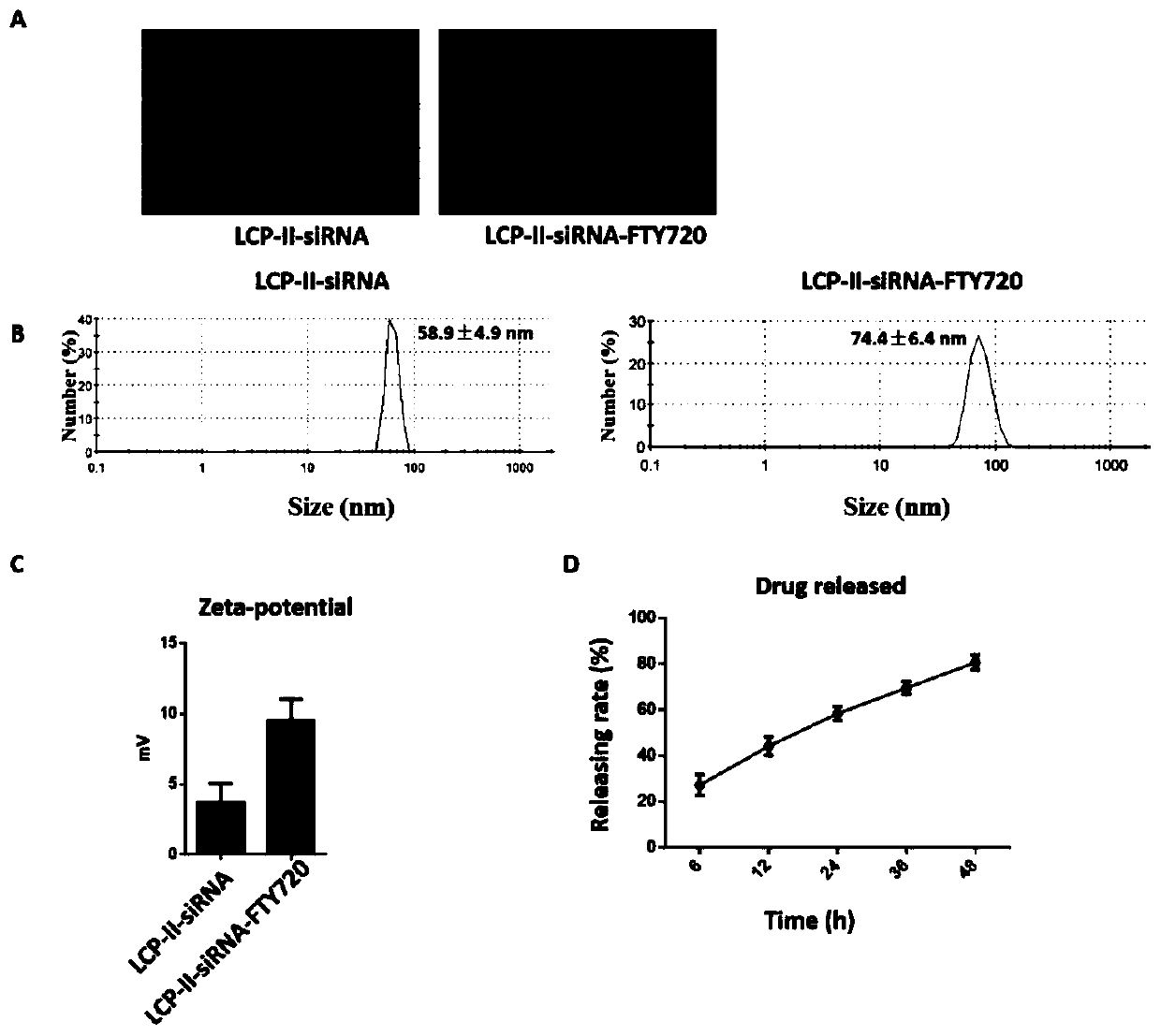
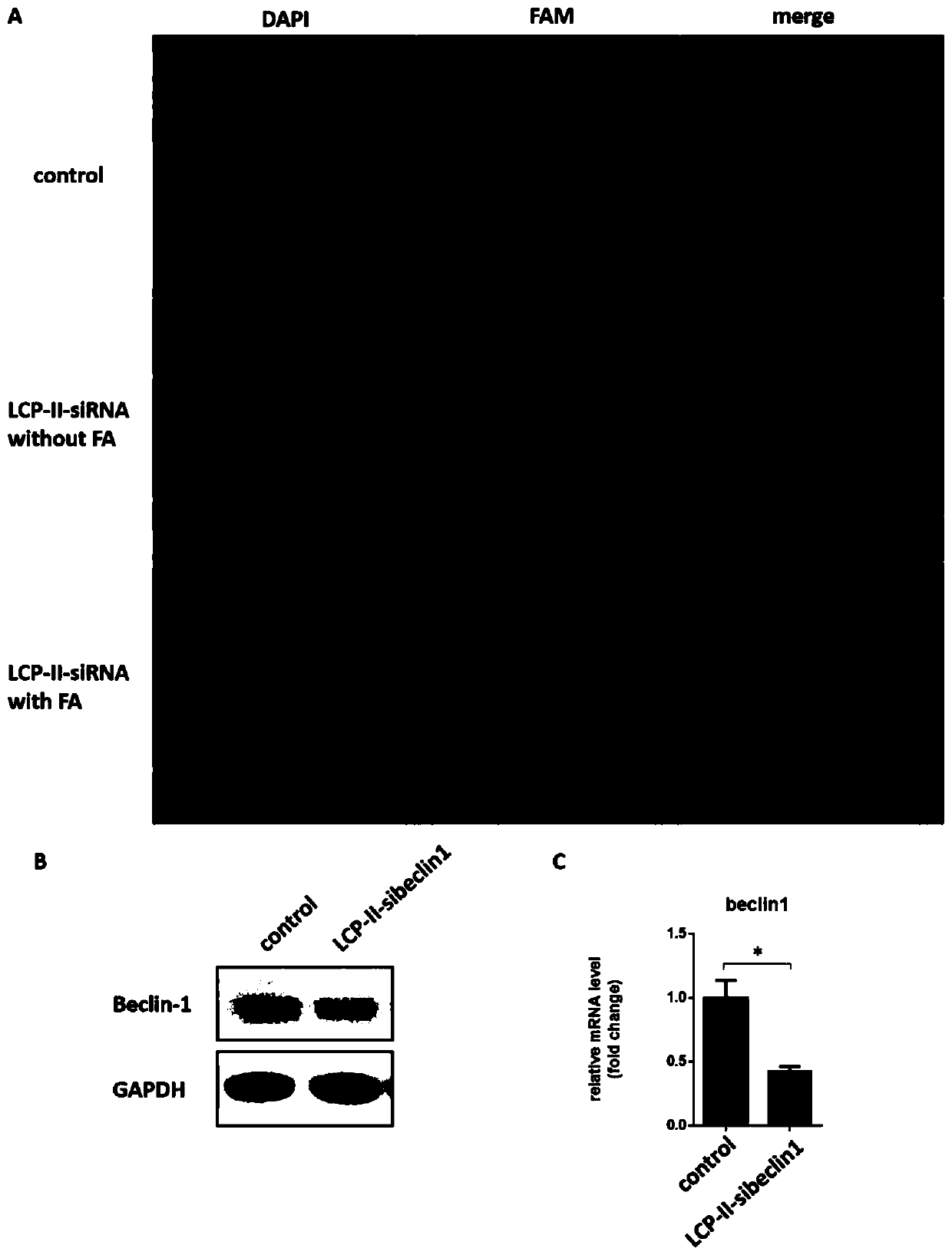
Autophagy siRNA-Fingolimod co-delivered nano drug delivery system for targeting liver cancer
ActiveCN109125306ATo achieve the purpose of actively targeting liver cancerFor the purpose of releasingOrganic active ingredientsGenetic material ingredientsMedicineAutophagic death
The invention belongs to the field of pharmaceutical technology, and in particular relates to the field of targeted delivery pharmaceutical preparation, in particular to autophagy siRNA-Fingolimod co-delivered nano drug delivery system for targeting liver cancer, and a preparation method and use. The autophagy siRNA-Fingolimod co-delivered nano drug delivery system is disclosed. The preparation method of a siRNA co-carrying autophagy protein beclin1 and a nano drug-carrying system LCP-II-sibeclin1-FTY720 for actively targeting liver cancer are disclosed. Pharmacological experiments show that the nano drug-carrying system simultaneously carries the siRNA of beclin 1 and the FTY720, actively targets the liver cancer and delivers the siRNA into the liver cancer cell, and is released under theacidic environment in the liver cancer cell, has the function of resisting liver cancer, and has the value of developing the anti-liver cancer medicament.
Owner:NANJING UNIV
Method for combining Fingolimod intermediate
ActiveCN103145689AShort synthetic routeDoes not involve the use ofOrganic chemistryBenzoyl peroxidePtru catalyst
The invention provides a method for combining a Fingolimod intermediate. The method includes that: a. compound 1 and a compound 2 conduct Wittig reaction to produce a compound 3; b. the compound 3 conducts hydrogenation reaction under a condition that catalyst Pd / C exists to produce a compound 4; c. the compound 4 reacts with bisdiboron, benzoyl peroxide and t-BuONO to produce a compound 5; d. the compound 5 and a iodo-object 6 conduct Suzuki reaction to produce a compound 7; and e. the compound 7 conducts hydrogenation reaction under the catalysis of Pd / C to produce a compound 8. The method for combining the Fingolimod intermediate is simple and short in synthetic route, easy and convenient to operate, low in cost, high in rate of production and easy for industrialized production.
Owner:NANJING JIEYUN PHARMA TECH CO LTD
Application of fingolimod and analogue thereof in preparing medicines for treating cerebral infarction
InactiveCN104146991AConvenient for clinical operationOptimize volumeOrganic active ingredientsNervous disorderMedicineCurative effect
The invention provides a new application of fingolimod and an analogue thereof in preparing medicines for treating cerebral infarction and further shows the mechanism of treatment, effective dose, suitable dosage form and the specific compound of the analogue. Experiments confirm that the fingolimod and the analogue thereof can relieve lymphocyte infiltration caused by cerebral infarction, the relieving effect can be taken as a new target for treating cerebral infraction, furthermore, based on the new properties of the fingolimod and the analogue thereof, the new application of the fingolimod and the analogue thereof in preparing medicines for treating cerebral infraction is confirmed. The clinical applicability of the fingolimod and the analogue thereof is enlarged, the fact that the immune cells involve in cerebral infarction pathological lesion is disclosed, and the medicine prepared by the fingolilmod and the analogue thereof has a remarkable curative effect on cerebral infraction injure, and has prosperous generalization prospect.
Owner:施福东 +10
Intermediate compounds and process for the preparation of fingolimod
The present invention relates to processes for the preparation of (2-Amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride (Fingolimod) and pharmaceutically acceptable salts thereof, and intermediates formed in such processes.
Owner:MAPI PHARMA
Process for preparing pharmaceutical compositions of fingolimod
The present invention provides a process for preparing a pharmaceutical composition of fingolimod comprising: (i) obtaining a intimate admixture comprising fingolimod or a pharmaceutically acceptable salt thereof, and at least one surfactant (wetting agent), e.g., an intimate admixture of the fingolimod and the at least one surfactant, and (ii) optionally combining the intimate admixture from step (i) with one or more excipients. Also provided are pharmaceutical compositions and dosage forms obtainable by the process, uses of the pharmaceutical compositions and dosage forms, and methods of treating appropriate diseases with the pharmaceutical compositions or dosage forms.
Owner:TEVA PHARMA IND LTD
Process for preparation of fingolimod
InactiveUS20150018578A1Organic compound preparationCarboxylic acid esters preparationMedicinal chemistryImpurity
The present invention provides a process for preparation of fingolimod, a compound of Formula I or a pharmaceutically acceptable salt thereof, free of regioisomeric impurity compound of Formula IA
Owner:GLENMARK LIFE SCI LTD
Process for preparation of fingolimod
InactiveUS9056813B2Organic compound preparationCarboxylic acid esters preparationMedicinal chemistryImpurity
The present invention provides a process for preparation of fingolimod, a compound of Formula I or a pharmaceutically acceptable salt thereof, free of regioisomeric impurity compound of Formula IA.
Owner:GLENMARK LIFE SCI LTD
Application of fingolimod or salts thereof in treatment of cystic diseases
InactiveCN105560219AThe effect is accurateCyst size shrinksOrganic active ingredientsDigestive systemCystic diseaseCytotoxicity
The invention provides application of fingolimod (2-amino-2-[2-(4-octylphenyl) ethyl]-1,3-propanediol) or salts thereof in preparation of medicinal compositions for treating cystic diseases. Particularly, the inventor finds that fingolimod or salts thereof have the effects of inducing the apoptosis and / or inhibiting the proliferation of a cystic cell by inhibiting HDACs and arresting the cell cycle of the cystic cell at G2 / M so as to reduce the cystic volume and restore the renal function. In addition, cytotoxicity experiments also confirm that during the treatment of the cystic diseases, fingolimod, as a known marketed medicine, has no obvious toxicity under the therapeutic dose. Fingolimod, as a medicine for treating the cystic diseases, especially an autosomal dominant polycystic kidney disease, has a wide application prospect.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +2
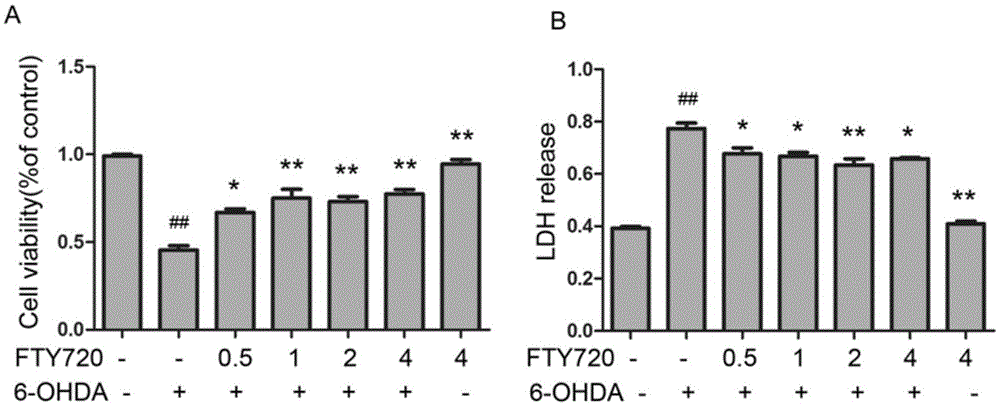
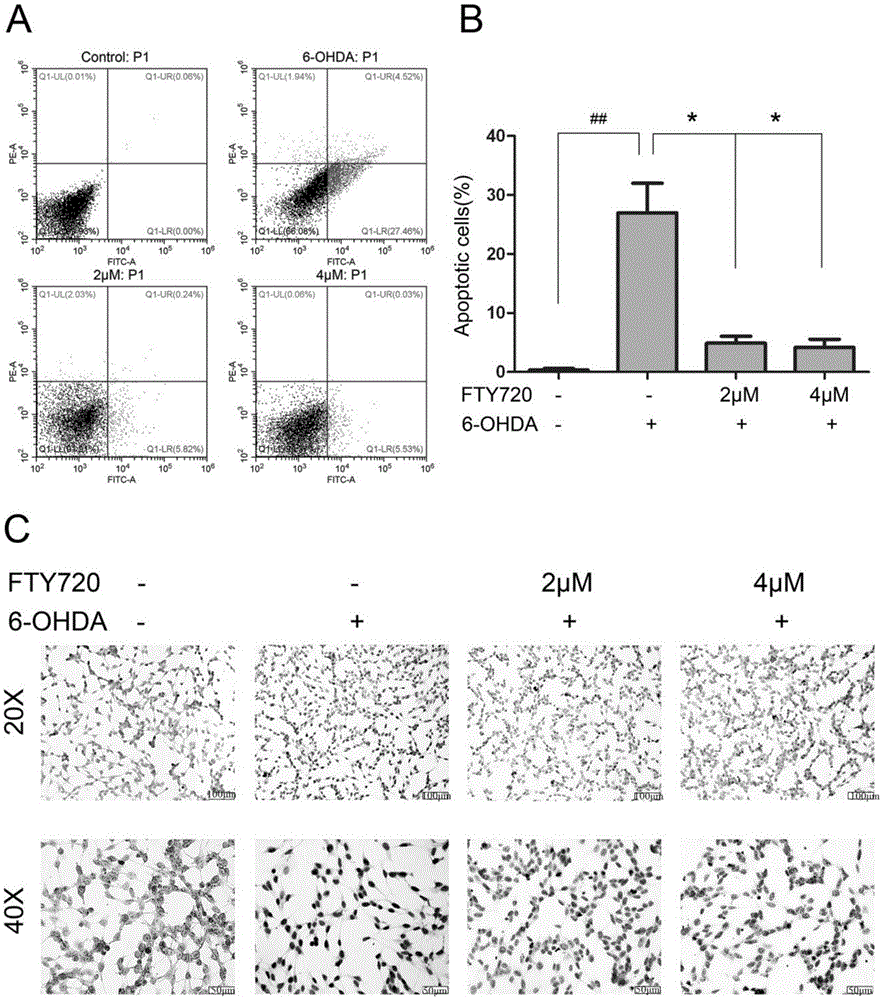
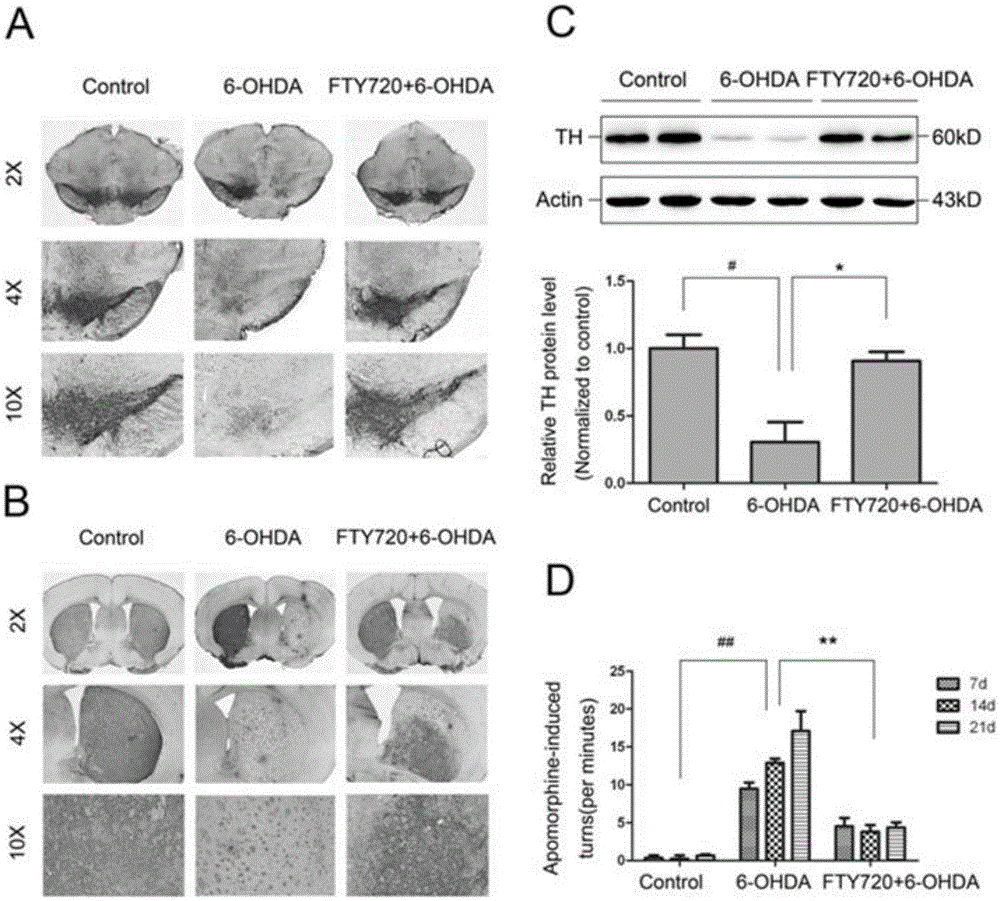
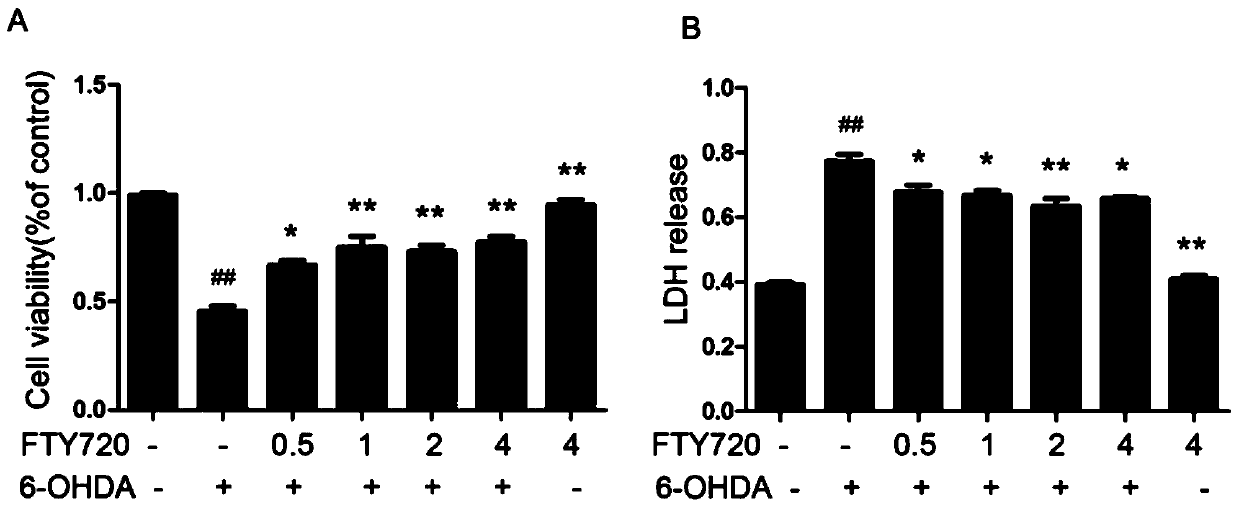
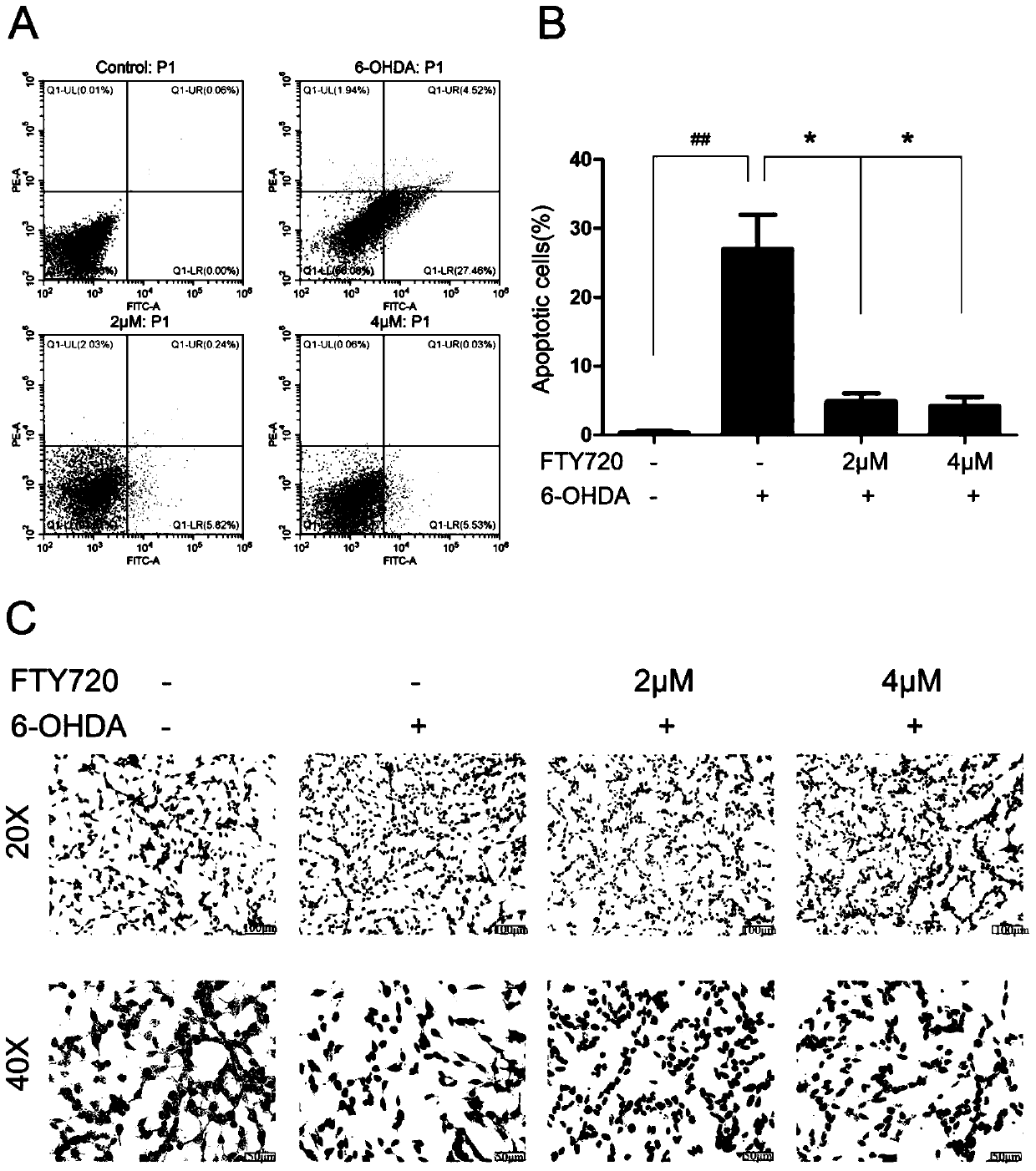
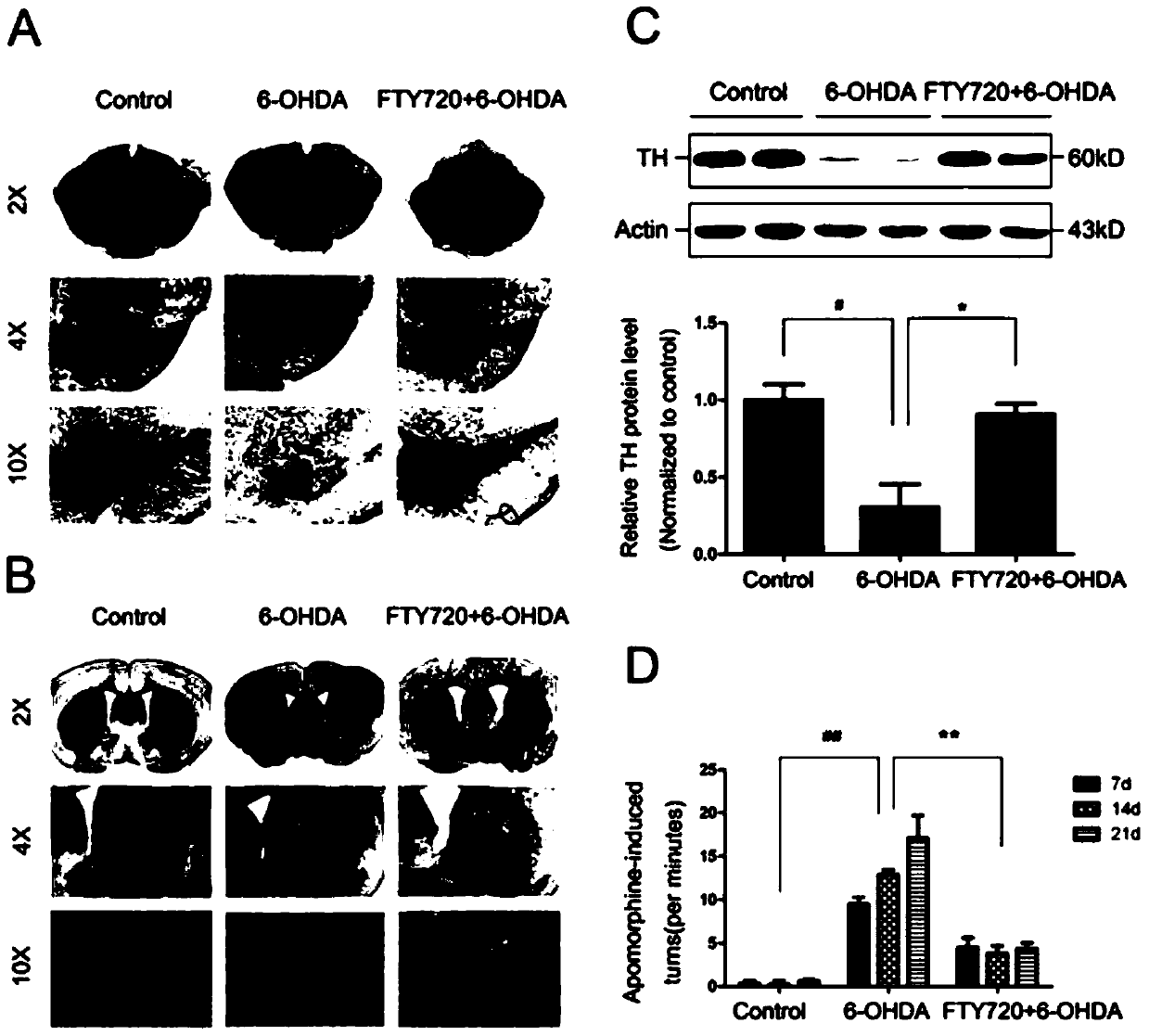
Novel applications of medicament Fingolimod
InactiveCN105663100AGood anti-Parkinson's effectReduce processOrganic active ingredientsNervous disorderDiseaseNatural product
The invention discloses new applications of a medicament Fingolimod. The applications comprise applications of Fingolimod or pharmaceutically acceptable salts, esters, and solvates thereof to preparation of a medicament for protecting a dopaminergic nerve cell strain SH-SY5Y damaged by 6-OHDA, and applications of Fingolimod or pharmaceutically acceptable salts, esters, and solvates thereof to prevention and / or treatment of a neurodegenerative disease, wherein the neurodegenerative disease is Parkinson's disease. Researches show that Fingolimod can delay progression of the Parkinson's disease with substantial effects; mechanisms of action of Fingolimod which is used as a novel medicament derived from natural products are different from mechanisms of action of clinical existed medicaments for resisting Parkinson's disease, and mainly have special emphasis on protection effects of dopaminergic neurons, so that a cross resistance phenomenon does not happen.
Owner:SHANDONG UNIV
Fingolimod polymorphs and their processes
ActiveUS9266816B2Organic compound preparationOrganic chemistry methodsRelated impuritiesMedicinal chemistry
The present invention provides crystalline polymorphic forms of Fingolimod HCl (I) and processes for preparation thereof.The application provides processes for preparation of crystalline polymorphic forms-α, β and μ substantially free from process related impurities. The crystalline polymorphic forms of Fingolimod HCl (I) obtained by the processes according to the present invention having an XRDP pattern as per FIGS. 1, 3 and 5, which are useful as active pharmaceutical ingredient in pharmaceutical compositions for the treatment or prevention of autoimmune related disorder including multiple sclerosis.
Owner:SHILPA PHARM INC
Oral solid pharmaceutical composition containing Fingolimod
InactiveCN103908446AStable storageGood content uniformityOrganic active ingredientsNervous disorderPharmaceutical formulationPharmaceutical Excipient
The invention relates to the field of chemical pharmaceutical preparations, especially to an oral solid pharmaceutical composition containing Fingolimod or its pharmaceutically acceptable salt. Specifically, the invention relates to a pharmaceutical composition containing Fingolimod or its pharmaceutically acceptable salt, microcrystalline cellulose and (or) other pharmaceutical excipients or carriers. The pharmaceutical composition provided by the invention has the characteristics of stable preservation performance, high content uniformity, and excellent dissolution performance.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Intermediate compounds and process for the preparation of fingolimod
The present invention relates to processes for the preparation of (2-Amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride (Fingolimod) and pharmaceutically acceptable salts thereof, and intermediates formed in such processes.
Owner:MAPI PHARMA
Application of fingolimod and its structural analog in preparation of cerebral hemorrhage treatment medicines
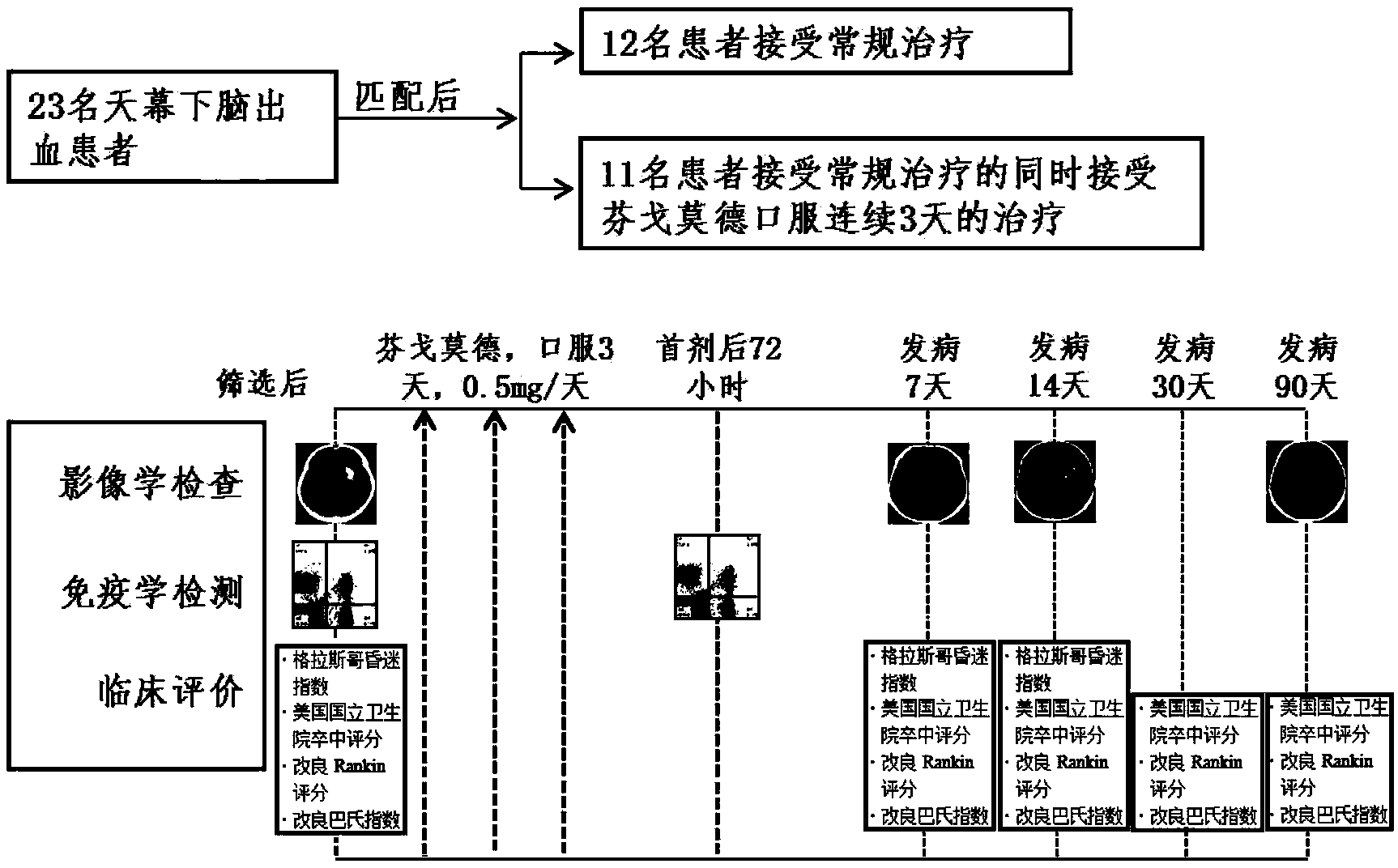
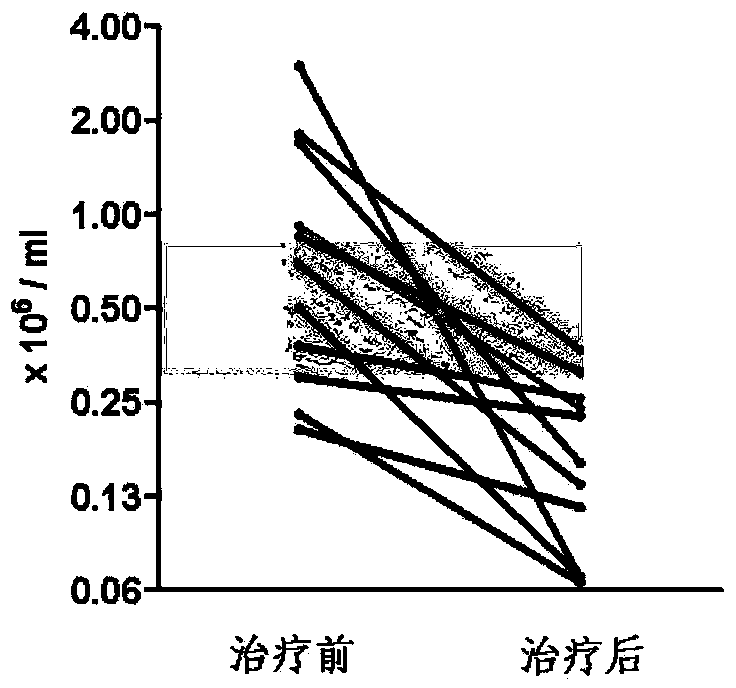
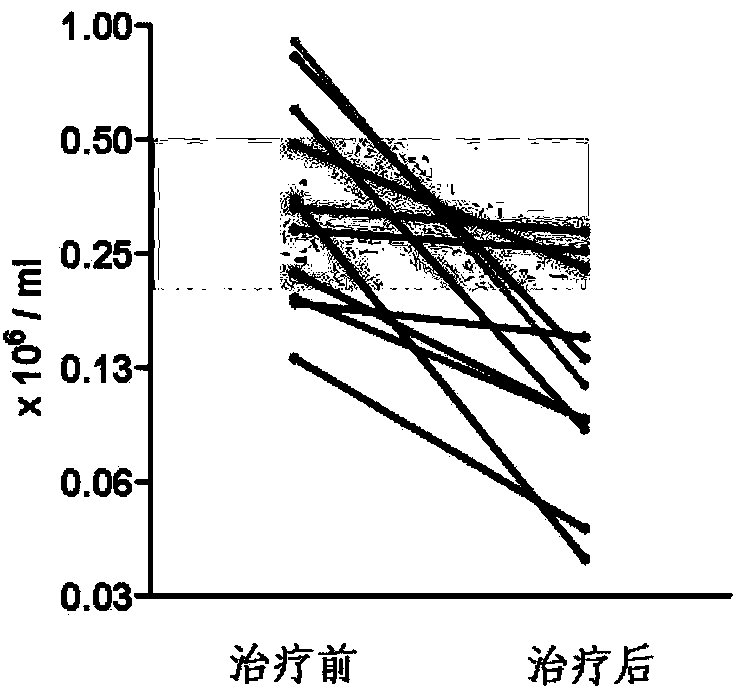
InactiveCN104161742AIncrease mass effectPlay a therapeutic effectOrganic active ingredientsNervous disorderDiseaseCurative effect
The invention provides a new use of fingolimod and its structural analog in the preparation of cerebral hemorrhage treatment medicines, and further clarifies a treatment mechanism, an effective dosage, a suitable dosage form, and a concrete compound referred to the structural analog. Experiments prove that fingolimod and its structural analog can alleviate lymphocyte infiltration caused by cerebral hemorrhage, and the above alleviation effect can be used as a new cerebral hemorrhage target in order to determine the new use of the fingolimod and its structural analog in the preparation of cerebral hemorrhage treatment medicines based on the above property of the fingolimod and its structural analog. The clinic adaptation disease of the fingolimod and its structural analog is enlarged, and the participation of immune cells in the pathological lesion of the cerebral hemorrhage is disclosed. The medicines prepared in the invention have a substantial curative effect on the cerebral hemorrhage, and have a protruding popularization prospect.
Owner:施福东 +10
Stable solid fingolimod dosage forms
The present invention relates to a solid pharmaceutical dosage forms and methods for preparing the solid pharmaceutical dosage form that contains fingolimod or its pharmaceutically acceptable salts, conjugates or complexes thereof. The solid pharmaceutical dosage forms may rapidly disintegrates in a patient's oral cavity.
Owner:HANDA NEUROSCIENCE LLC
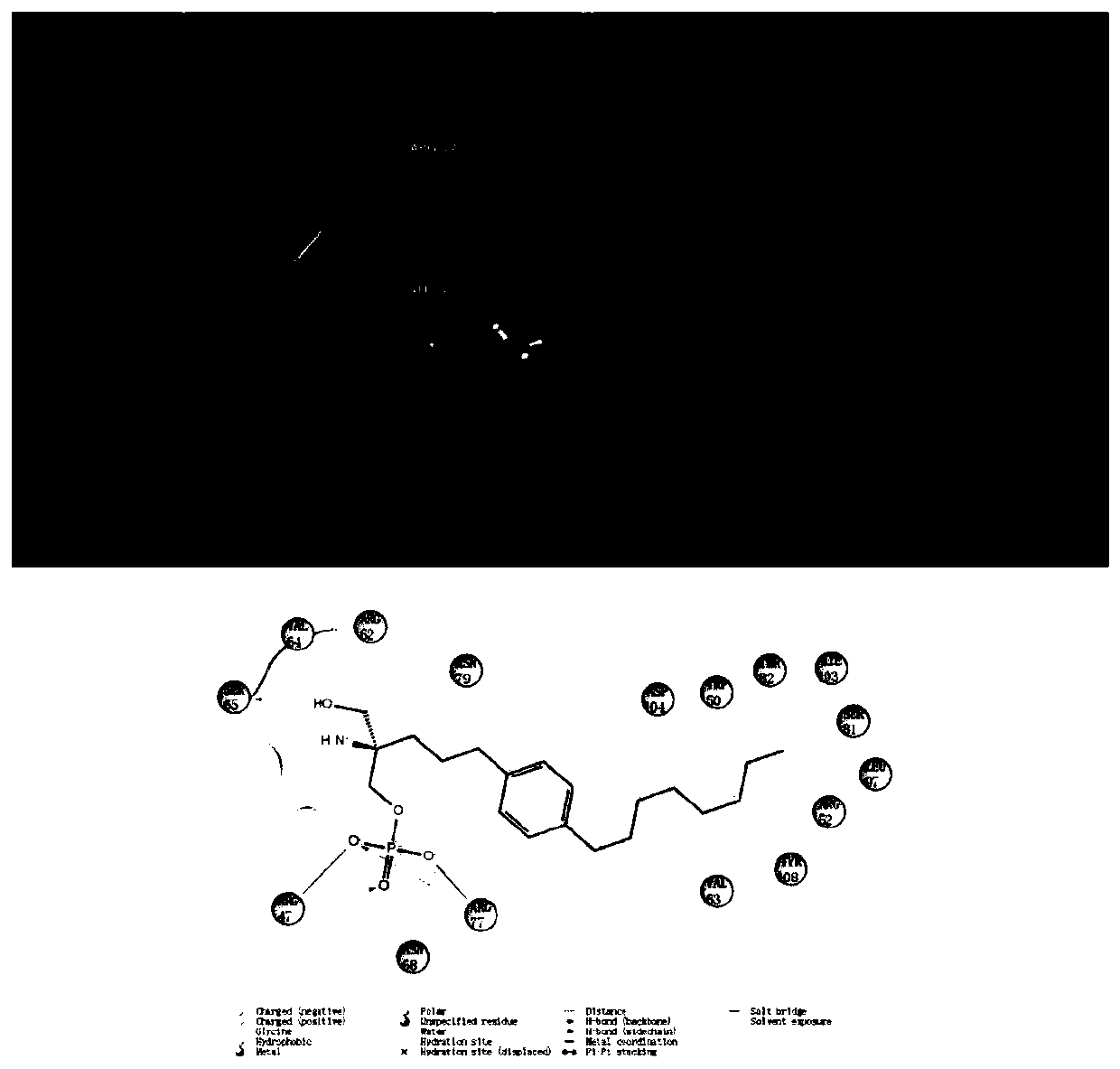
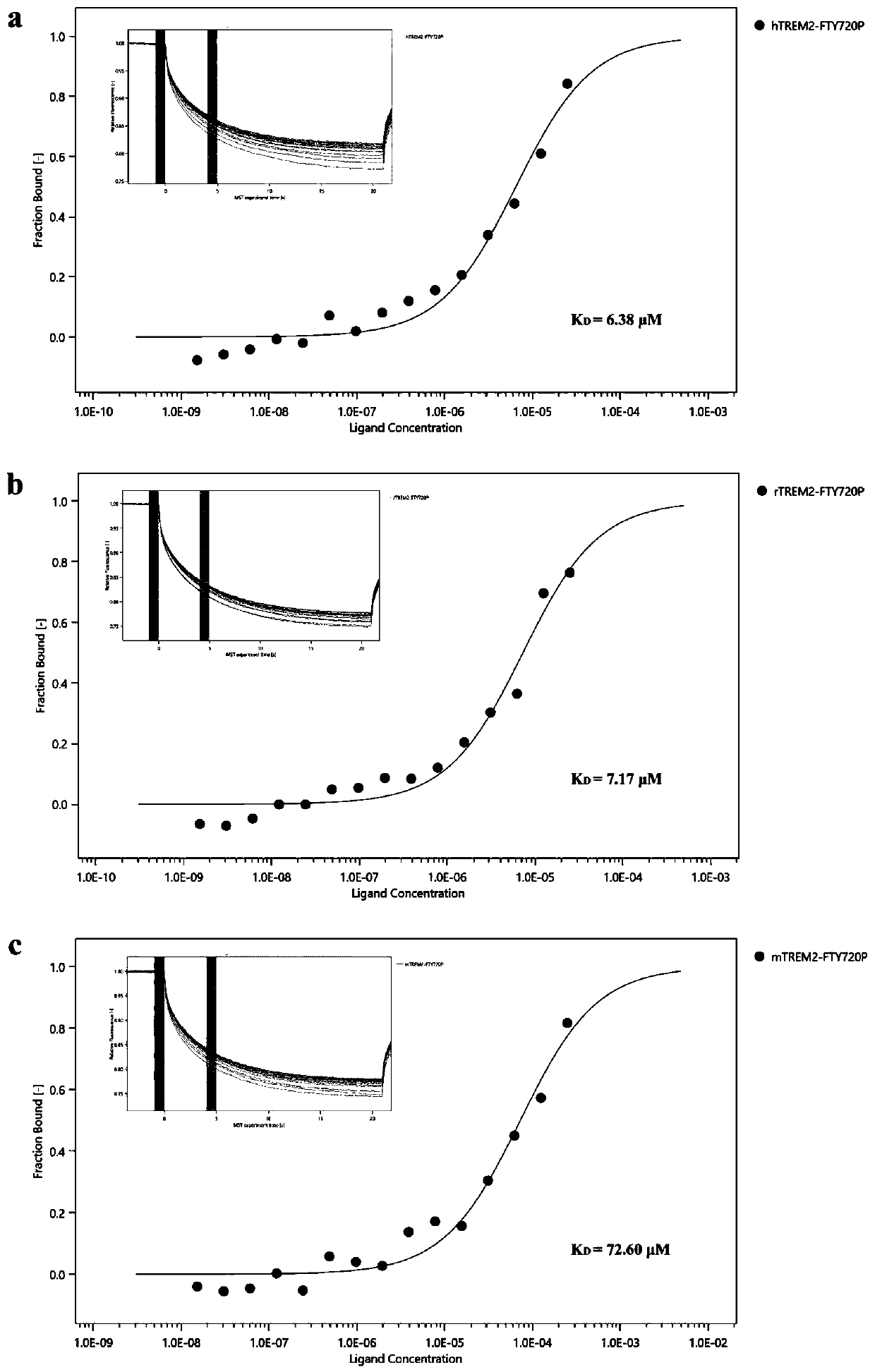
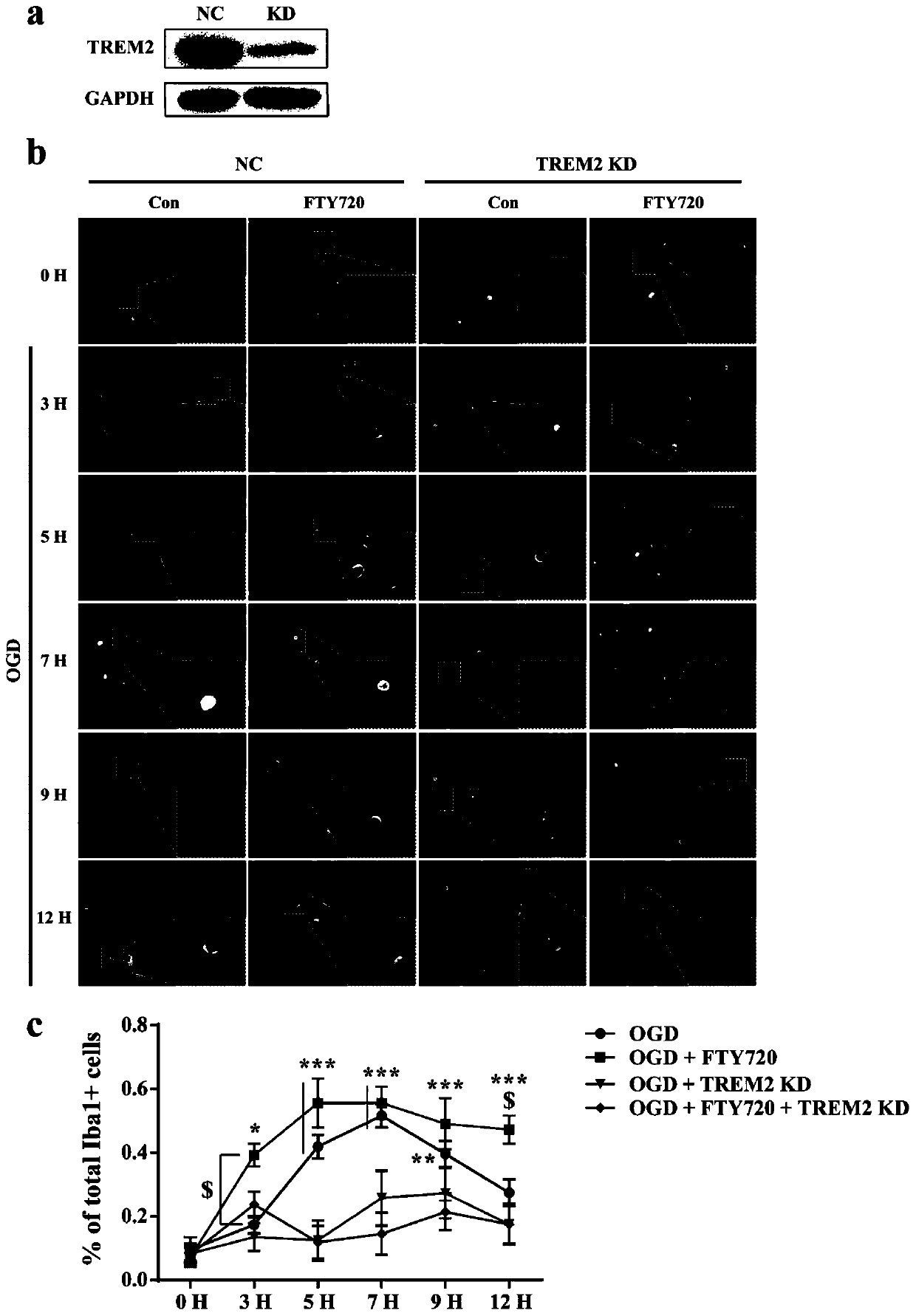
Application of FTY720-Phosphate in preparation of activating pharmacy of TREM2
PendingCN110882262AEnhance phagocytosisPromote phagocytosisOrganic active ingredientsAntipyreticBiotechnologyPhosphorylation
The invention discloses application of FTY720-Phosphate in preparation of activating pharmacy of a TREM2, thereby realizing application of regulation of cell phagocytosis. The invention discloses thatFTY720-Phosphate is an agonist of TREM2 for the first time, discloses a fact that phosphorylated fingolimod enhances the phagocytic function of microglial cells by activating the TREM2, and illustrates a new mechanism of FTY720-Phosphate for regulating the phagocytic function of microglial cells.
Owner:NANJING MEDICAL UNIV
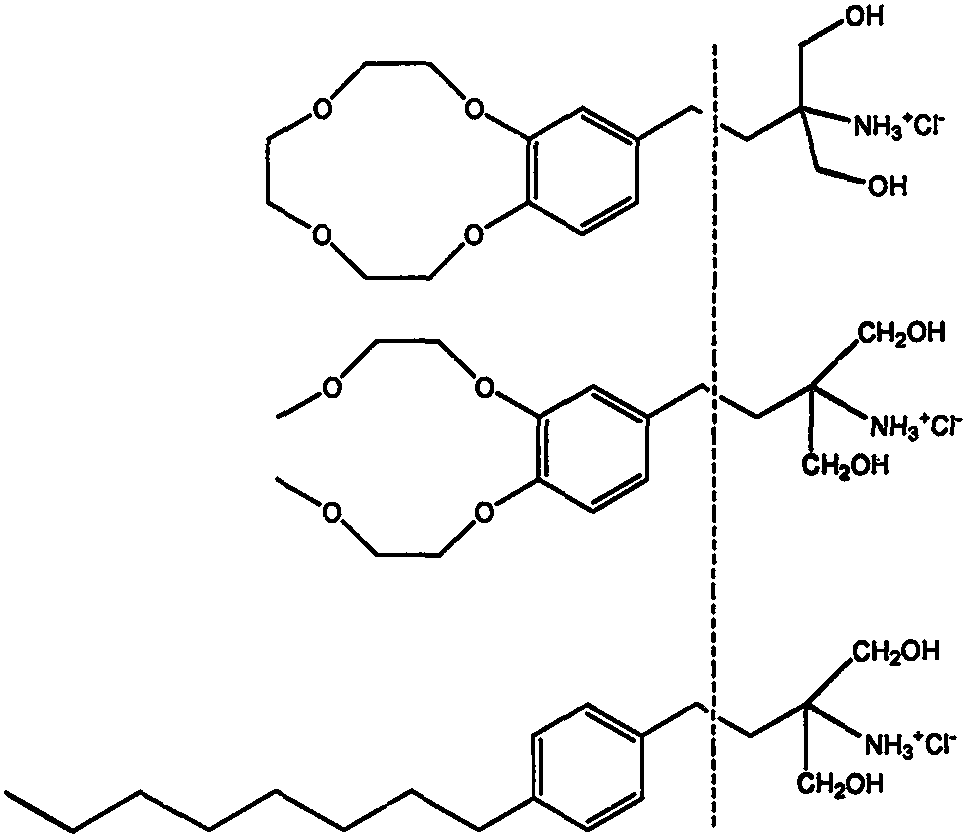
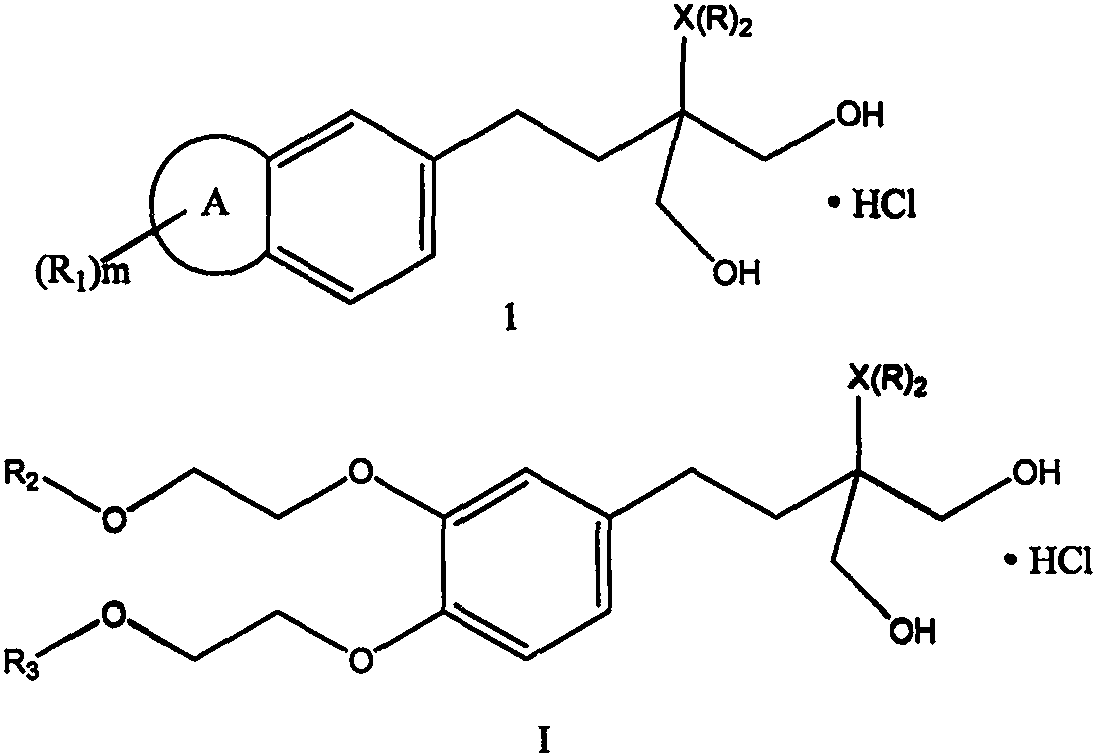
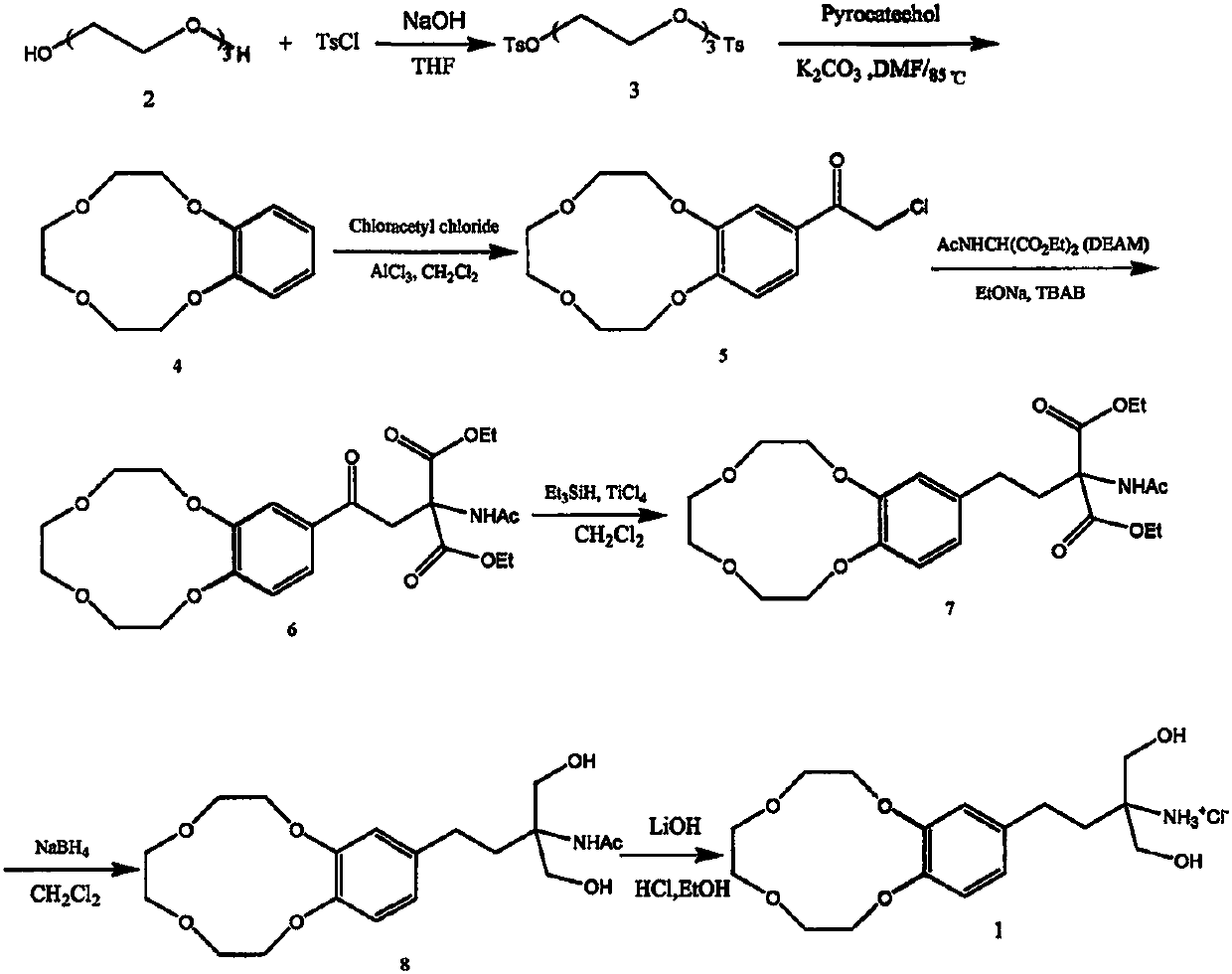
Fingolimod derivative containing crown ether and di (2-methoxyethoxy) structure
A series of fingolimod derivatives containing crown ether or di (2-methoxyethoxy) structures are designed and synthesized by modifying the structure of an immunosuppressant fingolimod for treating multiple sclerosis on the market. The hypoglycemic effect of the target compound is evaluated through in-vivo animal experiments. Results show that the compounds including 1c-1e and Ic-Ie have a certaineffect of reducing blood sugar, and have application prospects in preparation of drugs for treating diabetes.
Owner:REVIVALLON BIOPHARMACEUTICAL CO LTD
Application of fingolimod and structural analogue of fingolimod in preparing drug for treating cerebral hemorrhage
InactiveCN107126434AIncrease mass effectPlay a therapeutic effectOrganic active ingredientsNervous disorderCurative effectStructural analog
The invention provides a novel application of fingolimod and structural analogue of fingolimod in preparing a drug for treating cerebral hemorrhage, and further determines a treatment mechanism, an active dose and an appropriate dosage form and a specific compound pointed by the structural analogue. The nature of the fingoliimod and the structural analogue of the fingolimod for alleviating lymphocytes infiltration caused by cerebral hemorrhage is made clear by virtue of experimental means, and the alleviation effect is ensured to be used as a novel target spot for treating the cerebral hemorrhage, so that the novel application of the fingolimod and the structural analogue of the fingolimod in preparing the drug for treating the cerebral hemorrhage is determined on the basis of the novel nature f the fingolimod and the structural analogue of the fingolimod. The novel application of the invention enlarges the clinical applicability of the fingolimod and the structural analogue of the fingolimod, and discloses the fact that the immune cells anticipate in the cerebral hemorrhage pathological lesion, and the drug prepared by the invention is significant in curative effect for the cerebral hemorrhage and has remarkable popularization prospect.
Owner:施福东 +10
Application of Fingolimod in treatment of periapical periodontitis
The invention discloses an application of Fingolimod in treatment of periapical periodontitis. According to the invention, an in vivo experiment shows that Fingolimod can be used for obviously inhibiting infiltration of local inflammatory cells and bone destruction due to pulp exposing at a lesion of the periapical periodontitis; and S1P1 and RANKL expression level is reduced, local RANKL / OPG ratio is dynamically regulated, and the effect of treating the periapical periodontitis is achieved. The results show that Fingolimod is a medicine capable of effectively treating the periapical periodontitis, and Fingolimod can be used for preparing a medicine used for treating the periapical periodontitis.
Owner:WUHAN UNIV
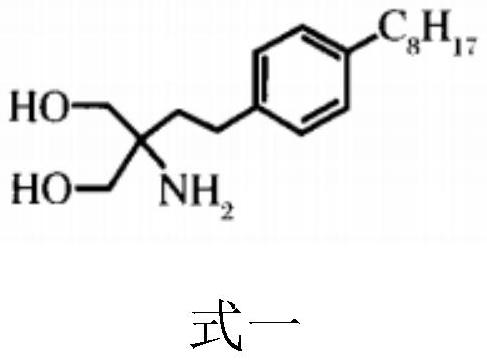
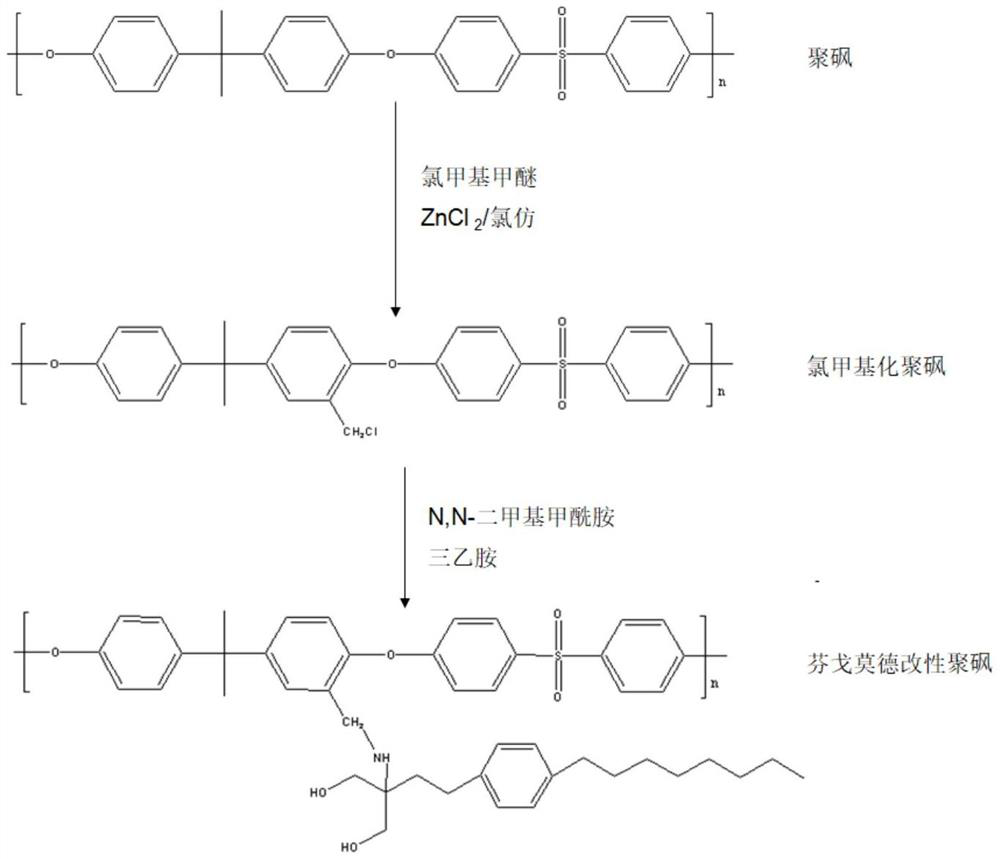
Modified blood purification membrane and preparation method thereof
ActiveCN114177778AGood blood compatibilityInhibitory activityMembranesSemi-permeable membranesThrombusLymphocyte
The invention discloses a modified blood purification membrane and a preparation method thereof, and belongs to the technical field of medicine and pharmacology. The preparation method comprises the following steps: (1) preparing a chloromethylated polymer; (2) preparing a fingolimod modified polymer; and (3) preparation of the fingolimod modified polymer film. The invention also provides the modified blood purification membrane obtained by the preparation method. The fingolimod is grafted to polysulfone or polyethersulfone, so that the prepared modified blood purification membrane has better blood compatibility, can directly inhibit lymphocyte activity to achieve an anti-inflammatory effect, and can improve hydrophilic performance through hydroxyl of the fingolimod while effectively resisting inflammation, thereby reducing formation of thrombus and improving the blood purification effect. The medicine is suitable for treatment of patients with various severe infections and multi-organ function failure. The preparation method disclosed by the invention is safe and simple, can be realized through a two-step synthesis method, is relatively short in preparation period and can be suitable for industrial batch production.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV +1
Co-delivery of lipid nanoparticles with autophagy siRNA-fingolimod targeting liver cancer
ActiveCN109125306BTo achieve the purpose of actively targeting liver cancerPlay a releaseOrganic active ingredientsGenetic material ingredientsPharmacometricsPharmaceutical drug
The invention belongs to the field of pharmaceutical technology, and in particular relates to the field of targeted delivery pharmaceutical preparation, in particular to autophagy siRNA-Fingolimod co-delivered nano drug delivery system for targeting liver cancer, and a preparation method and use. The autophagy siRNA-Fingolimod co-delivered nano drug delivery system is disclosed. The preparation method of a siRNA co-carrying autophagy protein beclin1 and a nano drug-carrying system LCP-II-sibeclin1-FTY720 for actively targeting liver cancer are disclosed. Pharmacological experiments show that the nano drug-carrying system simultaneously carries the siRNA of beclin 1 and the FTY720, actively targets the liver cancer and delivers the siRNA into the liver cancer cell, and is released under theacidic environment in the liver cancer cell, has the function of resisting liver cancer, and has the value of developing the anti-liver cancer medicament.
Owner:NANJING UNIV
Uses of the drug fingolimod
InactiveCN105663100BGood anti-Parkinson's effectReduce processOrganic active ingredientsNervous disorderDiseaseNatural product
The invention discloses new applications of a medicament Fingolimod. The applications comprise applications of Fingolimod or pharmaceutically acceptable salts, esters, and solvates thereof to preparation of a medicament for protecting a dopaminergic nerve cell strain SH-SY5Y damaged by 6-OHDA, and applications of Fingolimod or pharmaceutically acceptable salts, esters, and solvates thereof to prevention and / or treatment of a neurodegenerative disease, wherein the neurodegenerative disease is Parkinson's disease. Researches show that Fingolimod can delay progression of the Parkinson's disease with substantial effects; mechanisms of action of Fingolimod which is used as a novel medicament derived from natural products are different from mechanisms of action of clinical existed medicaments for resisting Parkinson's disease, and mainly have special emphasis on protection effects of dopaminergic neurons, so that a cross resistance phenomenon does not happen.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester](https://images-eureka.patsnap.com/patent_img/43792b04-02a7-458c-a359-674a1d2ebc14/BSA00000525402200011.PNG)
![Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester](https://images-eureka.patsnap.com/patent_img/43792b04-02a7-458c-a359-674a1d2ebc14/BSA00000525402200021.PNG)
![Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester Preparation method of {5-[2-(4-n-octyl-phenyl)ethyl]-2,2-dimethyl-1,3-dioxane-5-yl} carbamic acid tert-butyl ester](https://images-eureka.patsnap.com/patent_img/43792b04-02a7-458c-a359-674a1d2ebc14/BSA00000525402200022.PNG)