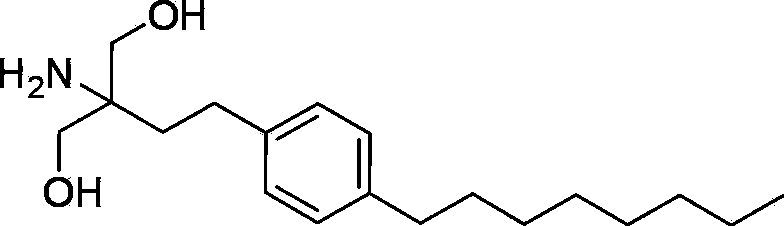
Oral solid pharmaceutical composition containing Fingolimod
A composition and drug technology, which can be used in drug combinations, antipyretic drugs, cardiovascular system diseases, etc., can solve the problems of large excipients, increased adverse reactions, and difficulty in controlling content uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Pharmaceutical compositions using only microcrystalline cellulose as excipients:
[0070]
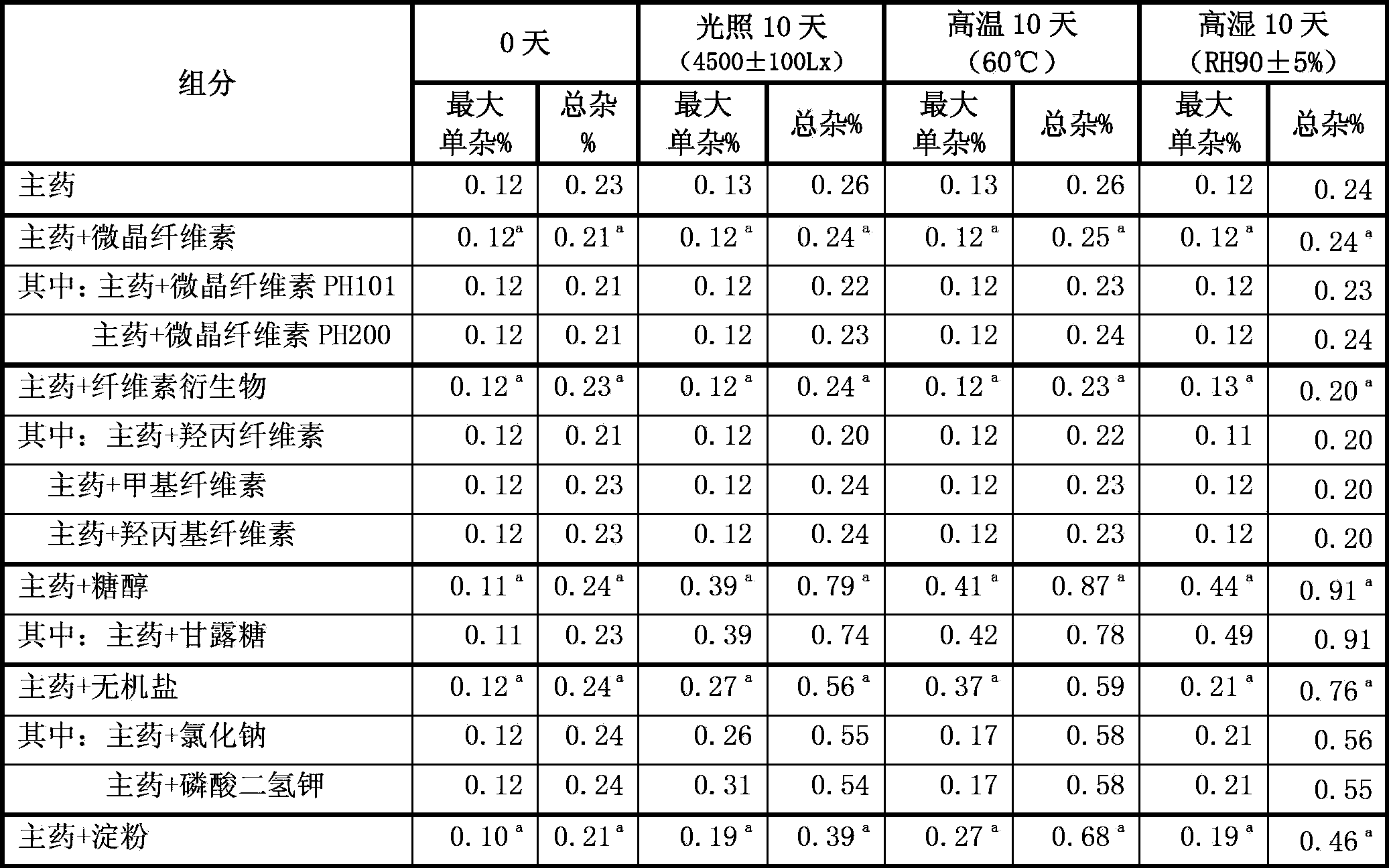
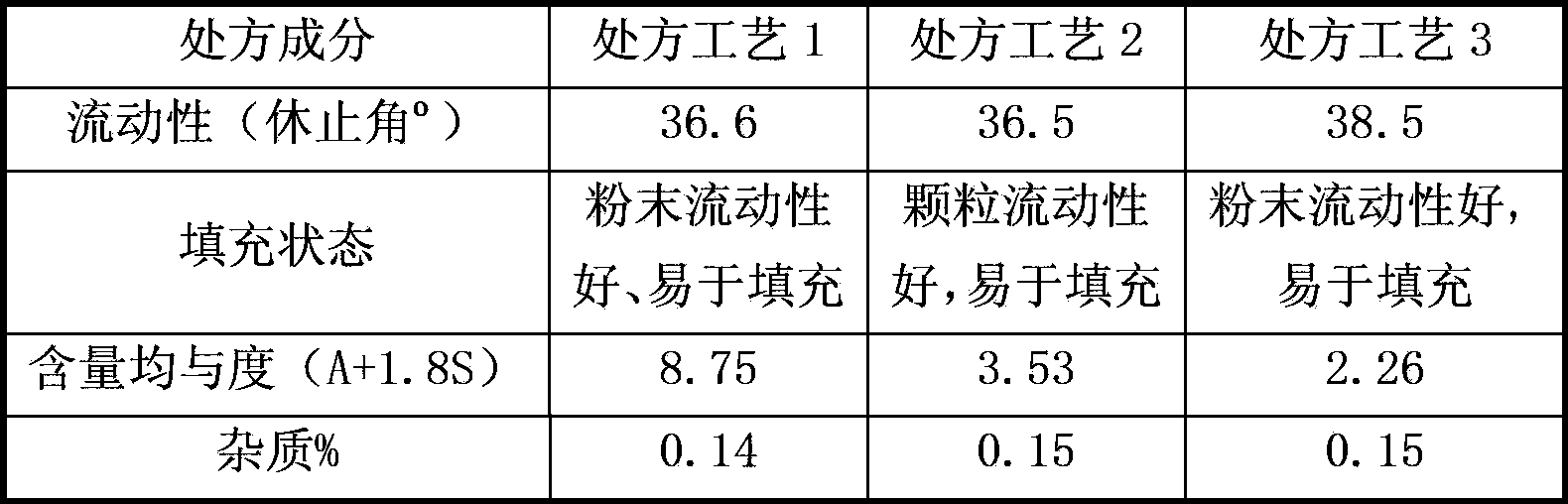
[0071] Preparation method: Pass the micronized fingolimod hydrochloride through a 200-mesh sieve. The prescribed amount of fingolimod hydrochloride and microcrystalline cellulose are mixed uniformly in equal amounts to obtain the pharmaceutical composition of the present invention. After heating at 60° C. for 40 hours, inspect and check the content changes of impurities and fingolimod hydrochloride. The inspection results show that the impurity content of the composition is 0.14, and the main drug content is 99.9%.
[0072] The composition is compressed into tablets or filled into capsules according to the required dosage.
Embodiment 2
[0074] Pharmaceutical compositions using only microcrystalline cellulose as excipients:
[0075]
[0076]Preparation method: Pass the micronized fingolimod hydrochloride through a 200-mesh sieve. The prescribed amount of fingolimod hydrochloride and microcrystalline cellulose are mixed uniformly in equal amounts to obtain the pharmaceutical composition of the present invention. After heating at 60° C. for 40 hours, inspect and check the content changes of impurities and fingolimod hydrochloride. The inspection results show that the impurity content of the composition is 0.13%, and the main drug content is 101.9%.
[0077] The composition is compressed into tablets or filled into capsules according to the required dosage.
Embodiment 3
[0079] Pharmaceutical compositions using only microcrystalline cellulose as excipients:
[0080]
[0081] Preparation method: Pass the micronized fingolimod hydrochloride through a 300-mesh sieve. The prescribed amount of fingolimod hydrochloride and microcrystalline cellulose are mixed uniformly in equal amounts to obtain the pharmaceutical composition of the present invention. After heating at 60° C. for 40 hours, inspect and check the content changes of impurities and fingolimod hydrochloride. The inspection results show that the impurity content of the composition is 0.15%, and the main ingredient content is 102.2%.
[0082] The composition is compressed into tablets or filled into capsules according to the required dosage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com