Method for combining Fingolimod intermediate
A technology of intermediates and compounds, applied in the field of compound preparation, can solve the problems of high cost of synthetic routes, expensive raw materials, difficult industrialization, etc., and achieve the effect of simple operation, short synthetic route and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1.1 Compounds 3 preparation of
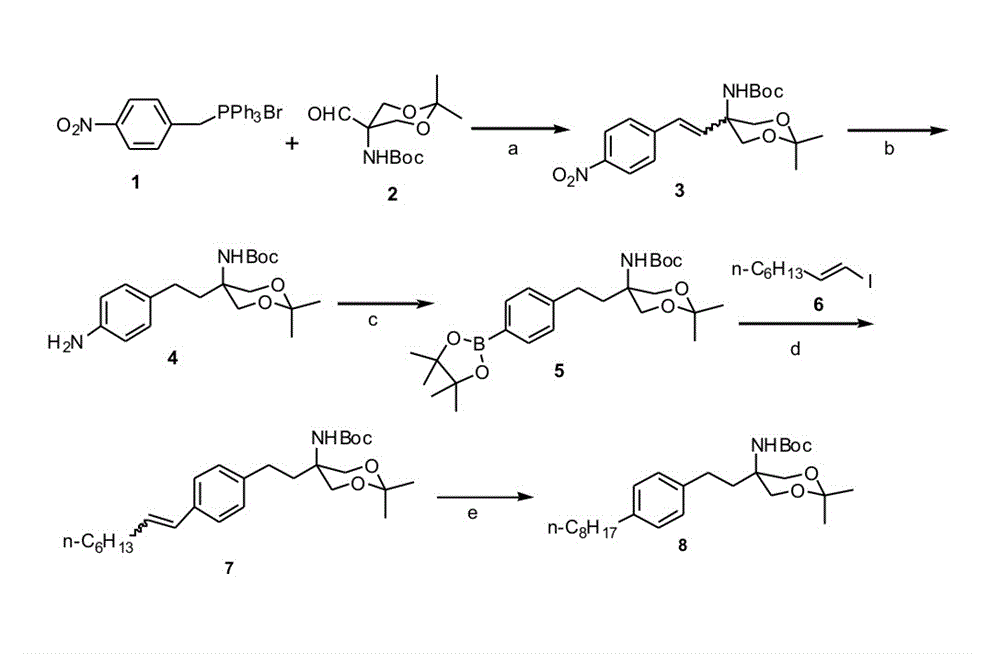
[0036] Under nitrogen protection, the compound 2 (9.9g, 38.18mmol), compound 1 (21.9g, 45.82mmol) and anhydrous K 2 CO 3 (14.8g, 106.9mmol) was added to a 500mL three-necked flask, and then a mixed solvent composed of 150mL anhydrous THF and 50mL anhydrous DMF was added, slowly heated to 80°C, and reacted for 5h. After cooling, add water, extract with ethyl acetate (100 mL×3), combine the organic phases, wash with water successively, wash with saturated brine, anhydrous Na 2 SO 4 Drying, column chromatography obtains 12.3g corresponding alkene compound 3 ; compound 3 It is a mixture with a cis-trans structure, and the yield is 85.1%.
[0037] 1.2 Compounds 3 preparation of
[0038] Under nitrogen protection, the compound 2 (9.9g, 38.18mmol), compound 1 (21.9g, 45.82mmol) and anhydrous Na 2 CO 3(11.3g, 106.9mmol) was added to a 500mL three-necked flask, and then a mixed solvent composed of 150mL anhydrous THF and 50mL an...
Embodiment 2
[0040] 2.1 Compounds 4 preparation of
[0041] compound 3 (12.0 g, 31.71 mmol) was dissolved in 150 mL of methanol, 1.2 g of Pd / C ((10 wt%) was added, and reacted at room temperature under hydrogen for 12 h. Suction filtration, evaporation of the solvent under reduced pressure gave 10.3 g of white solid product 4 , and the yield was 92.7%.
[0042] Mp: 160-163 oC; 1 H NMR (500MHz, CDCl 3 ): δ 1.41 (s, 3 H), 1.43 (s, 3 H), 1.47 (s, 9 H), 1.92 (t, 2 H), 2.44-2.48 (m, 2 H), 3.55 (s, 2 H), 3.66 (d, J = 12 Hz, 2 H), 3.88 (d, J = 11 Hz, 2 H), 4.98 (s, 1 H), 6.61 (d, J = 8 Hz, 2 H), 6.96 (d, J = 8 Hz, 2 H); HRMS (EI): m / z calcd for C 19 h 30 N 2 o 4 [M] + : 350.2206, found: 350.2203.
[0043] 2.2 Compounds 4 preparation of
[0044] compound 3 (12.0 g, 31.71 mmol) was dissolved in 150 mL of ethanol, 1.2 g of Pd / C ((10 wt%) was added, and the reaction was carried out at room temperature under hydrogen for 12 h. Suction filtration, and the solvent was evapora...
Embodiment 3
[0050] 3.1 Compounds 5 preparation of
[0051] Dissolve pinacol diboronate (7.25g, 28.53mmol) and benzoyl peroxide (0.14g, 0.57mmol) in 150mL CH 3 CN, followed by adding compounds 4 (10.0g, 28.53mmol) and tert-butyl nitrite (4.42g, 42.80mmol), react at room temperature for 6h after addition. Add water to terminate the reaction, extract with ethyl acetate (100 mL×3), combine the organic phases, wash with water successively, wash with saturated brine, anhydrous Na 2 SO 4 dry. Suction filtration, evaporation of the solvent under reduced pressure, and column chromatography yielded 7.76 g of a white solid product with a yield of 60%.
[0052] Mp: 145-148 oC; 1 H NMR (500MHz, CDCl 3 ): δ 1.33 (s, 12 H), 1.41 (s, 3 H), 1.43 (s, 3 H), 1.47 (s, 9 H), 1.98 (t, 2 H), 2.56-2.60 (m, 2 H), 3.66 (d, J = 12 Hz, 2 H), 3.88 (d, J = 11 Hz, 2 H), 4.98 (s, 1 H), 7.19 (d, J = 8 Hz, 2 H), 7.71 (d, J = 8 Hz, 2 H); HRMS (EI): m / z calcd for C 25 h 40 BNO 6 [M] + : 461.2949, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com