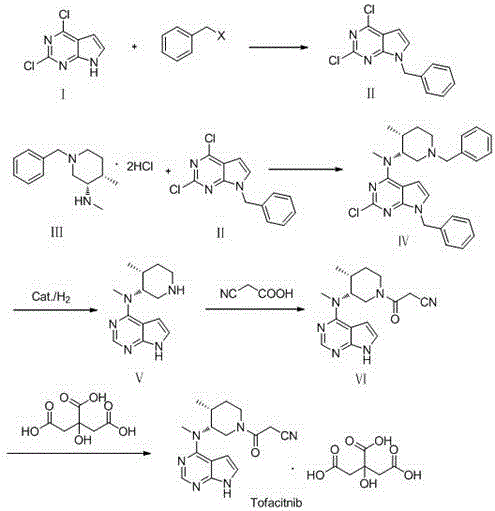
Preparation method of JAKs inhibitor drug tofacitinib
A technology of tofacitinib and inhibitors, which is applied in the field of preparation of JAKs inhibitor drug tofacitinib, can solve the problems of long synthetic route, not suitable for large-scale production, high production cost, etc., and achieve simple operation and easy control of purity , the effect of little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Preparation of Compound II:
[0022] Add 190g of 2,4-dichloro-7H pyrrole[2,3-D]pyrimidine, 280g of potassium carbonate, 1000mL of acetonitrile into the reaction flask, stir, heat to 45°C, then dropwise add 250g of benzyl bromide; °C reaction, TLC monitoring reaction. After the reaction is complete, cool to room temperature, evaporate most of the solvent, add 1000mL water and 2000mL ethyl acetate, stir, let stand for liquid separation, extract the aqueous layer with 800mL ethyl acetate, combine organic phases, dry over anhydrous sodium sulfate, and reduce pressure Concentrate to dryness, add 800mL of methyl tert-butyl ether to make a slurry, and obtain 255g of solid product Compound II.
[0023] (2) Preparation of Compound IV:
[0024] Add 200g (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride, 210g 7-benzyl-2,4-dichloro- 7H pyrrole[2,3-D]pyrimidine (Compound II), 160g of potassium carbonate, 500mL of ethanol and 1000mL of water, stirred and rai...
Embodiment 2
[0032] (1) Preparation of Compound II:
[0033] Add 150g of 2,4-dichloro-7H pyrrole[2,3-D]pyrimidine, 205g of N,N-diisopropylethylamine, 600mL of N,N-dimethylformamide into the reaction flask, stir and heat to 45°C , and then dropwise added 152g of benzyl chloride; after dropping, the temperature was raised to 90°C for reaction, and the reaction was monitored by TLC. After completion of the reaction, cool to room temperature, add 1000mL of water and 1500mL of ethyl acetate, stir, stand still for liquid separation, extract the water layer twice with 1000mL of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure. Add 600mL of isopropyl ether and make a slurry to obtain 204g of solid product Compound II.
[0034](2) Preparation of Compound IV:
[0035] Add 160g (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride, 168g 7-benzyl-2,4-dichloro- 7H pyrrole[2,3-D]pyrimidine, 93g piperidine, a...
Embodiment 3
[0043] (1) Preparation of Compound II:
[0044] Add 190g of 2,4-dichloro-7H pyrrole[2,3-D]pyrimidine, 153g of triethylamine, 1000mL of DMSO into the reaction flask, stir, heat to 45°C, then dropwise add 207g of benzyl bromide; °C reaction, TLC monitoring reaction. After the reaction is complete, cool to room temperature, evaporate most of the solvent, add 1000mL water and 2000mL ethyl acetate, stir, let stand for liquid separation, extract the aqueous layer with 800mL ethyl acetate, combine organic phases, dry over anhydrous sodium sulfate, and reduce pressure Concentrate to dryness, add 800mL of methyl tert-butyl ether to make a slurry, and obtain 250g of solid product Compound II.
[0045] (2) Preparation of Compound IV:
[0046] Add 200g (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride, 230g 7-benzyl-2,4-dichloro- 7H pyrrole[2,3-D]pyrimidine (Compound II), 133g of N,N-diisopropylethylamine, 500mL of ethanol and 1000mL of water, stirred and raised to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com