Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
A technology of cefoxidone sodium and cefoxidone is applied in directions such as pharmaceutical formulations, antibacterial drugs, powder delivery, etc., and can solve problems such as being unsuitable for industrialized large-scale production, low yield of cefoxidone, and increased synthesis cost, and achieves the The effect of easy control, simplified purification process and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
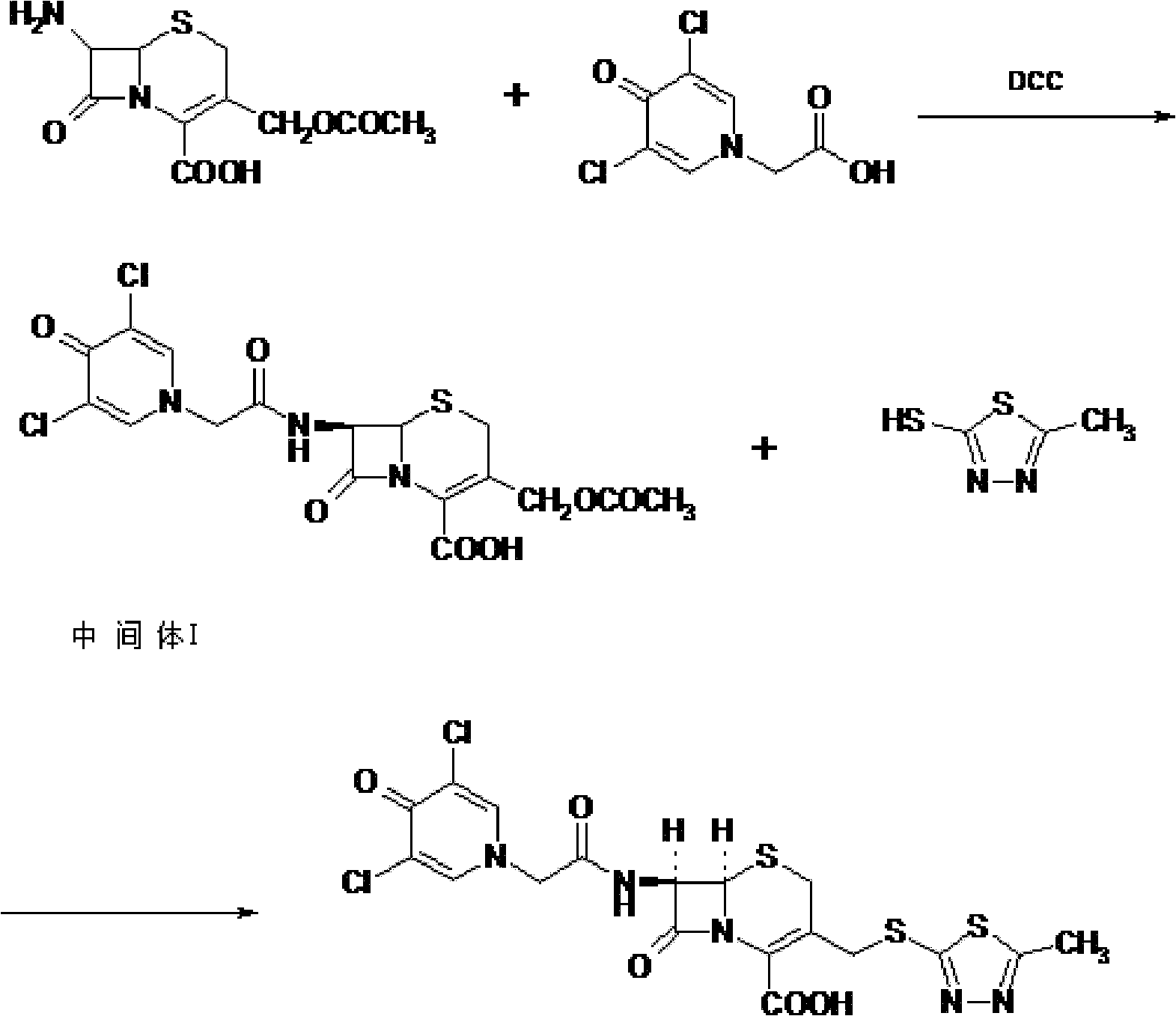
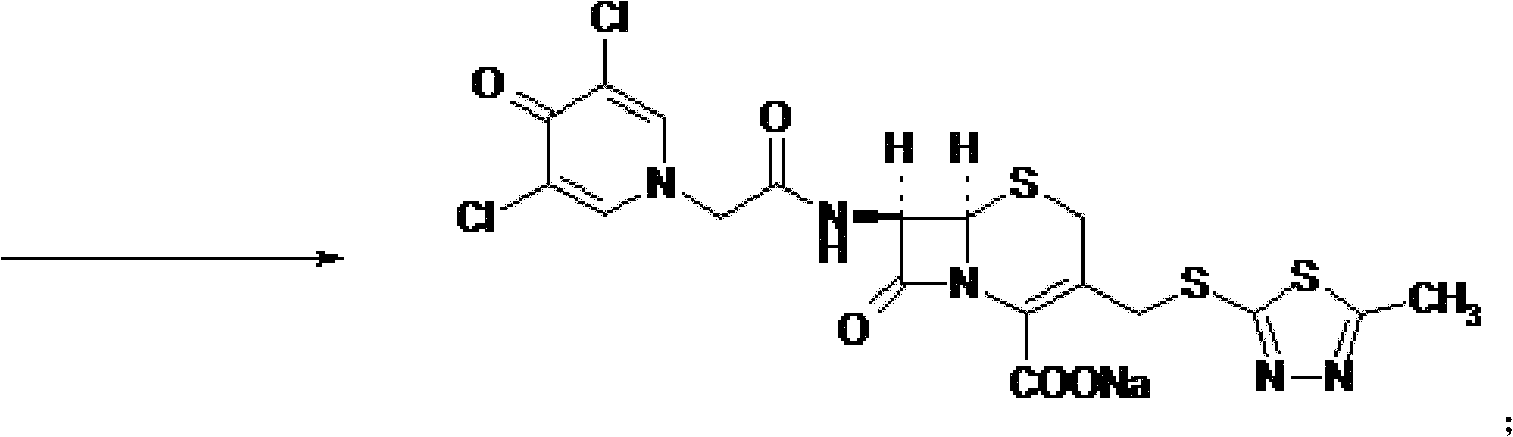
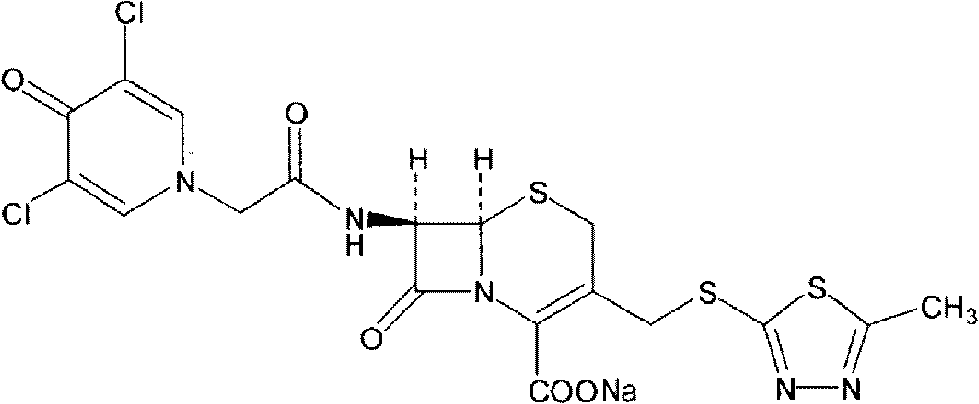
[0054] Synthesis of Intermediate I:
[0055] Put 20g (0.0735mol) of 7-ACA into the flask, add 200ml of tetrahydrofuran, 7.5g of triethylamine, add 17.2g (0.085mol) of dicyclohexylcarbodiimide (DCC), add dichloromethane / DMF= 1 / 1(v / v) solution 200ml, cooling to 0℃ in an ice bath, adding 17.6g (0.0793mol) of 3,5-dichloropyridone acetic acid, adding 3,5-dichloropyridone acetic acid every minute After 1 / 20 of the total amount was added, the reaction was stirred at 20°C for 0.5 hour. After filtration, the filtrate was evaporated to dryness under reduced pressure at 50°C, recrystallized with ether, crystallized under refrigeration for more than 8 hours, filtered, and dried under vacuum at 50°C to obtain 33.1g of intermediate I with a yield of 94.5%. The infrared characteristic absorption peak of Intermediate I is: 3400cm -1 , 1654.48cm -1 , 1600cm -1 , 1400cm -1 .
Embodiment 2
[0057] Synthesis of Intermediate I:
[0058] Put 20g (0.0735mol) of 7-ACA into the flask, add 200ml of dichloromethane, 7.5g of triethylamine, add 19.68g (0.0955mol) of dicyclohexylcarbodiimide (DCC), add dichloromethane solution 200ml, cooled to -5°C in an ice bath, add 19.6g (0.0882mol) of 3,5-dichloropyridone acetic acid at a rate of 1 / 10 of the total amount of 3,5-dichloropyridone acetic acid added per minute. , The reaction was stirred at 25°C for 0.5 hour. After filtration, the filtrate was evaporated to dryness under reduced pressure at 50°C, recrystallized with ether, crystallized under refrigeration for more than 8 hours, filtered, and dried under vacuum at 50°C to obtain 33.7g of intermediate I, with a yield of 96.4%. The infrared characteristic absorption peak of Intermediate I is: 3400cm -1 , 1654.48cm -1 , 1600cm -1 , 1400cm -1 .
Embodiment 3
[0060] Synthesis of Intermediate I:
[0061] Put 20g (0.0735mol) of 7-ACA into the flask, add 200ml of N,N-dimethylformamide, 7.5g of triethylamine, add 15.2g (0.0735mol) of dicyclohexylcarbodiimide (DCC), Add 200ml of dichloromethane / DMF=1 / 1(v / v) solution, cool to 0℃ in an ice bath, add 24.47g (0.11mol) of 3,5-dichloropyridone acetic acid, add 3 per minute After adding 1 / 15 of the total amount of 5-dichloropyridone acetic acid, the reaction was stirred at 35°C for 0.5 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure at 50°C, ether was recrystallized, crystallized under refrigeration for more than 8 hours, filtered, and dried under vacuum at 50°C to obtain 31.7g of intermediate I with a yield of 90.6%. The infrared characteristic absorption peak of Intermediate I is: 3400cm -1 , 1654.48cm -1 , 1600cm -1 , 1400cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com