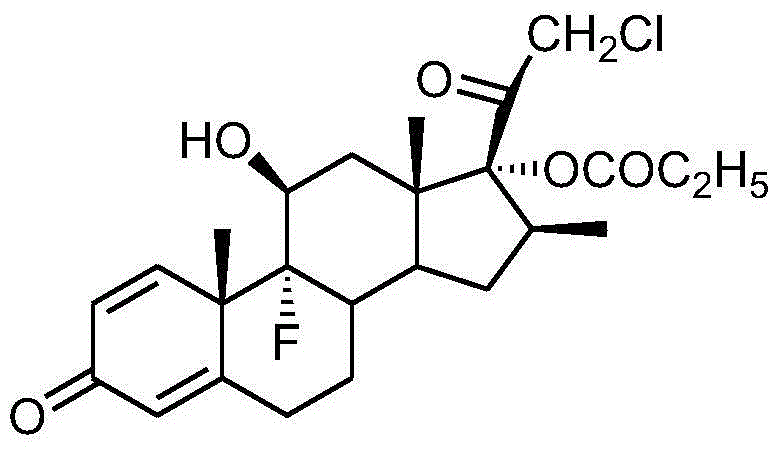
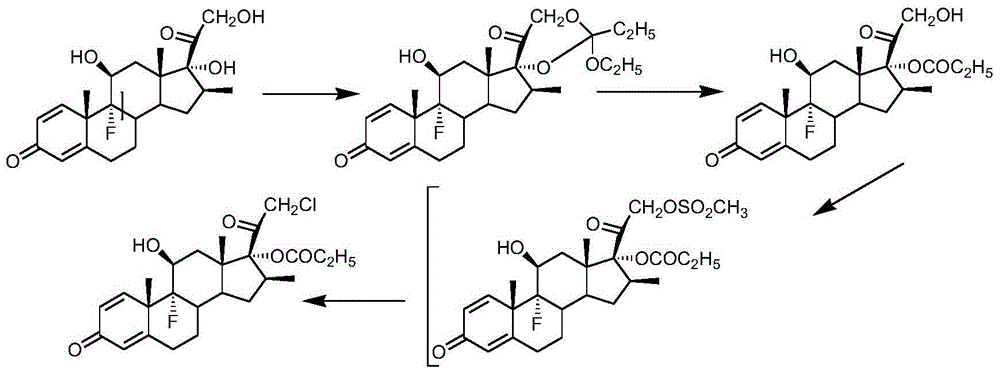
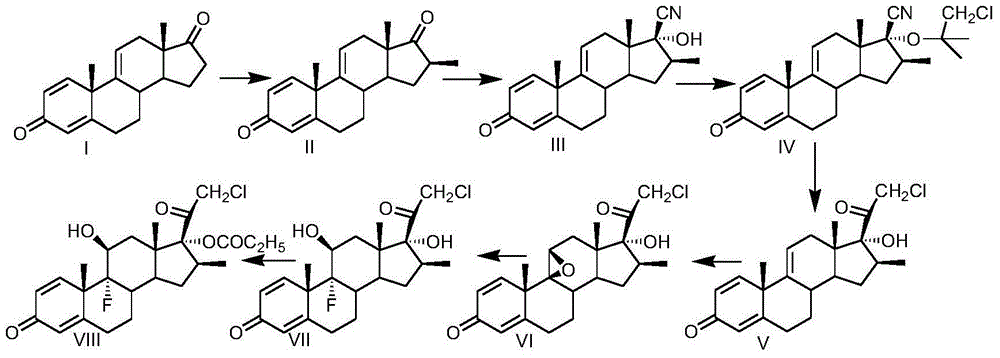
Preparation method of clobetasol and preparation method of clobetasol propionate
A technology of clobetasol propionate, clobetasol propionate, applied in the directions of steroids, organic chemistry, etc., can solve the problems of large solvent pollution, many side reactions, difficult to recover, etc., to shorten the synthesis route and reduce the production cost , the effect of production economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take a three-necked reaction flask, pass through nitrogen protection, add 100g of compound I, namely 1,4,9(11)-trienandrost-3,17-dione, add 400ml THF, cool the system to 0°C, and then add 60ml oxalic acid Diethyl ester and 30g of sodium methoxide were stirred at a temperature of 0-5°C for 1 h, then 20 g of anhydrous potassium carbonate and 28 ml of methyl iodide were added, and the temperature was raised to reflux for 12 h. After the reaction was complete, the mixture was concentrated under reduced pressure to nearly dryness. Add 400ml of anhydrous methanol, cool down to 0-5°C; then add 12g of sodium methoxide, stir at 0-5°C for 2 hours, and take a sample to check that there is no intermediate. Adjust the pH to 7-8 with glacial acetic acid, add 2000ml of water, control the temperature of the system at 0-5°C and stir for 2 hours, filter with suction, wash the filter cake with water until neutral, and dry to obtain 95g of compound II, namely 16β-methyl-1,4 , 9(11)-triene ...
Embodiment 2
[0040] Take a three-necked reaction flask, pass through nitrogen protection, add 100g of compound I, namely 1,4,9(11)-triene androst-3,17-dione, add 600ml of dioxane, cool the system to 0°C, and then Add 60ml of diethyl oxalate and 40g of sodium ethoxide, control the temperature at 0-5°C and stir for 1 hour, then add 20g of anhydrous potassium carbonate, pass in 60g of methyl bromide, raise the temperature to 60-70°C and react for 10 hours, after the reaction is complete, concentrate under reduced pressure to near dryness . Add 400ml of anhydrous methanol, cool down to 0-5°C; then add 14g of sodium ethoxide, stir at 0-5°C for 2 hours, and take a sample to check that there is no intermediate. Adjust the pH to 7-8 with glacial acetic acid, add 2000ml of water, control the temperature of the system at 0-5°C and stir for 2 hours, filter with suction, wash the filter cake with water until neutral, and dry to obtain 93g of compound II, namely 16β-methyl-1,4 , 9(11)-triene androst-3...
Embodiment 3
[0048] Take a three-necked reaction flask, pass through nitrogen protection, add 100g of compound I, namely 1,4,9(11)-triene androst-3,17-dione, add 700ml of dichloromethane, cool the system to 0°C, and then add 60ml of diethyl oxalate and 50g of potassium tert-butoxide, stirred at 0-5°C for 1 hour, then added 20g of anhydrous potassium carbonate, introduced 50g of methyl chloride, raised the temperature to 40-45°C for 30 hours, after the reaction was completed, concentrated under reduced pressure to nearly dry. Add 400ml of anhydrous methanol, cool down to 0-5°C; then add 16g of potassium tert-butoxide, stir at 0-5°C for 2 hours, and take a sample to check that there is no intermediate. Adjust the pH to 7-8 with glacial acetic acid, add 2000ml of water, control the temperature of the system at 0-5°C and stir for 2 hours, filter with suction, wash the filter cake with water until neutral, and dry to obtain 90g of compound II, namely 16β-methyl-1,4 , 9(11)-triene androst-3,17-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com