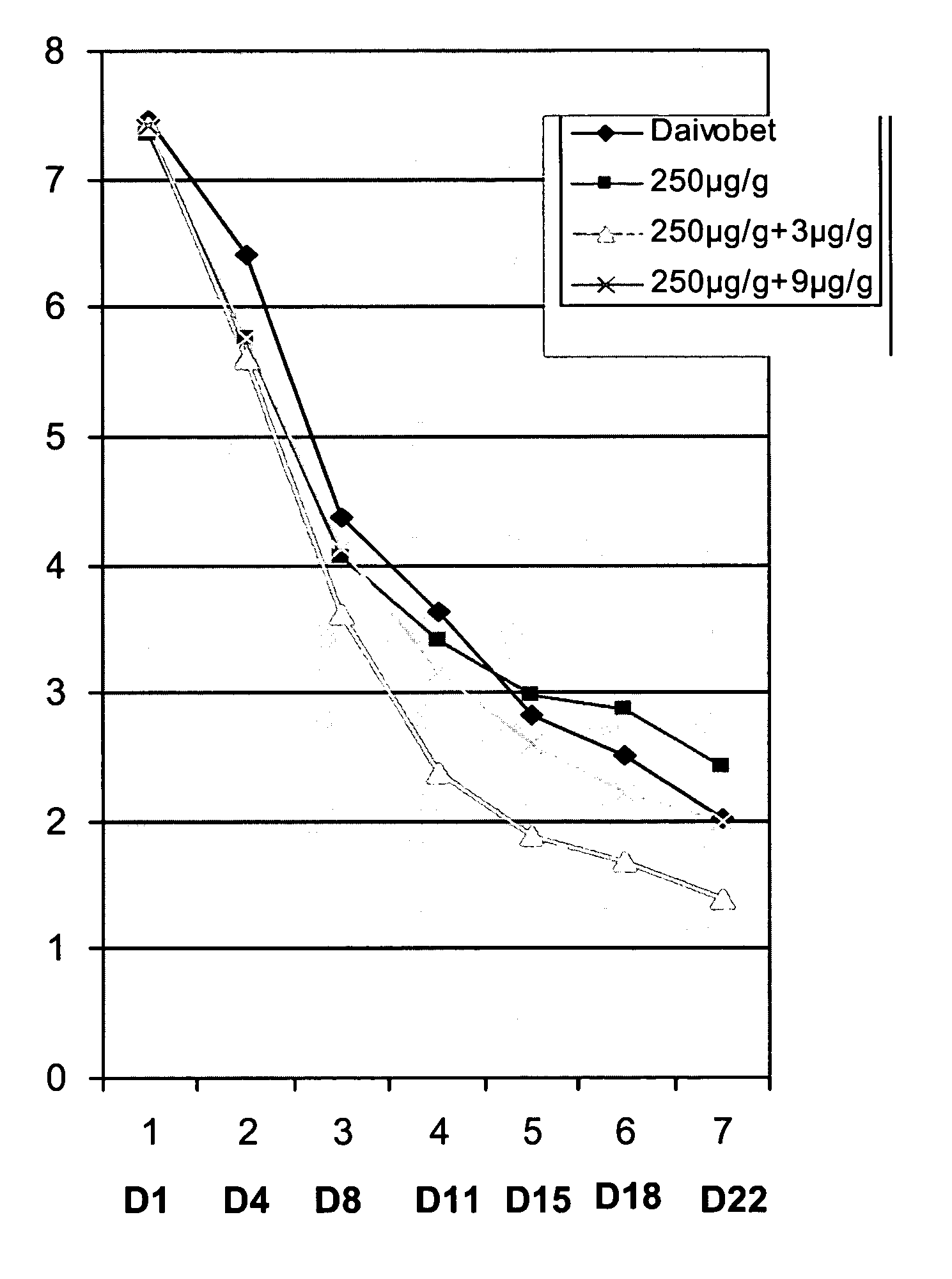
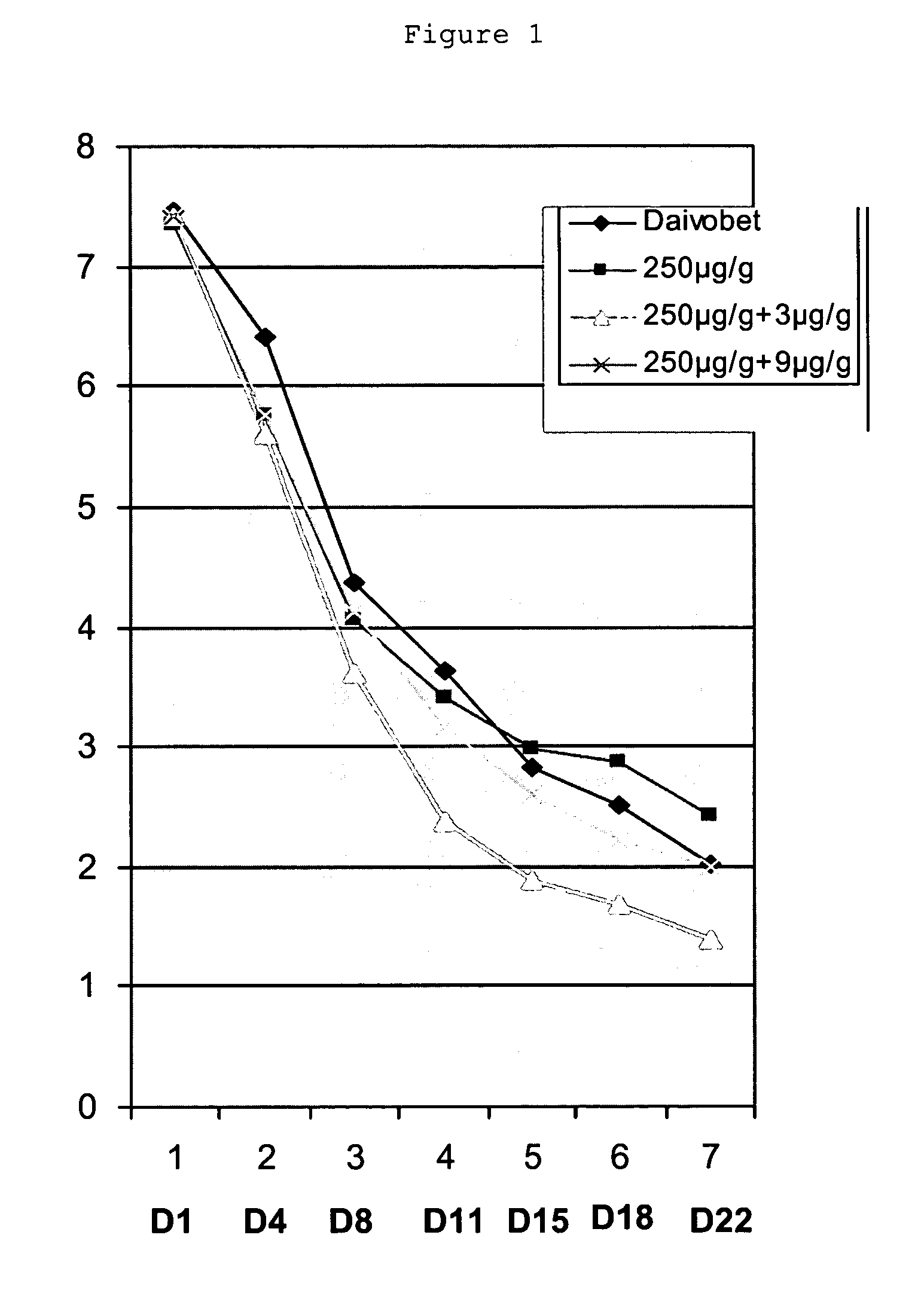
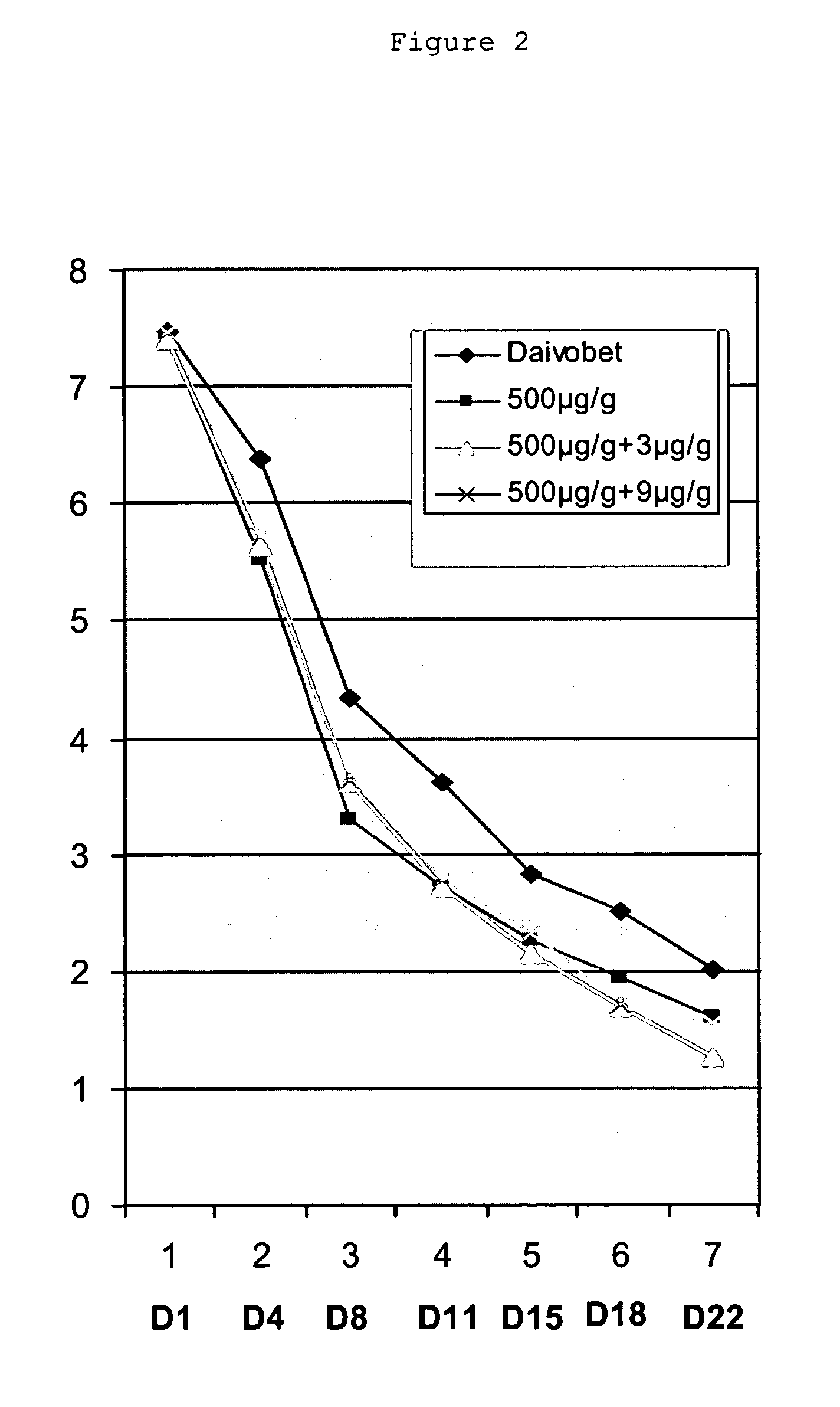
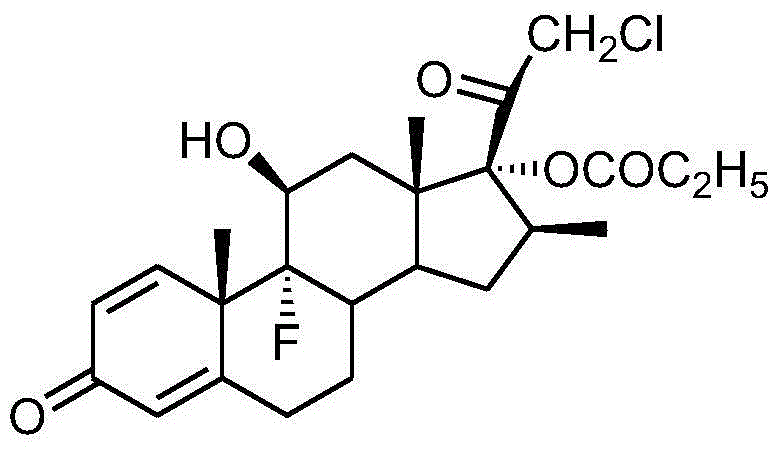
Patents
Literature
55 results about "Clobetasol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clobetasol (brand name Dermovat) is a synthetic glucocorticoid corticosteroid which is marketed in Denmark. A propionate ester of clobetasol, clobetasol propionate, has also been marketed, and is far more widely used in comparison.
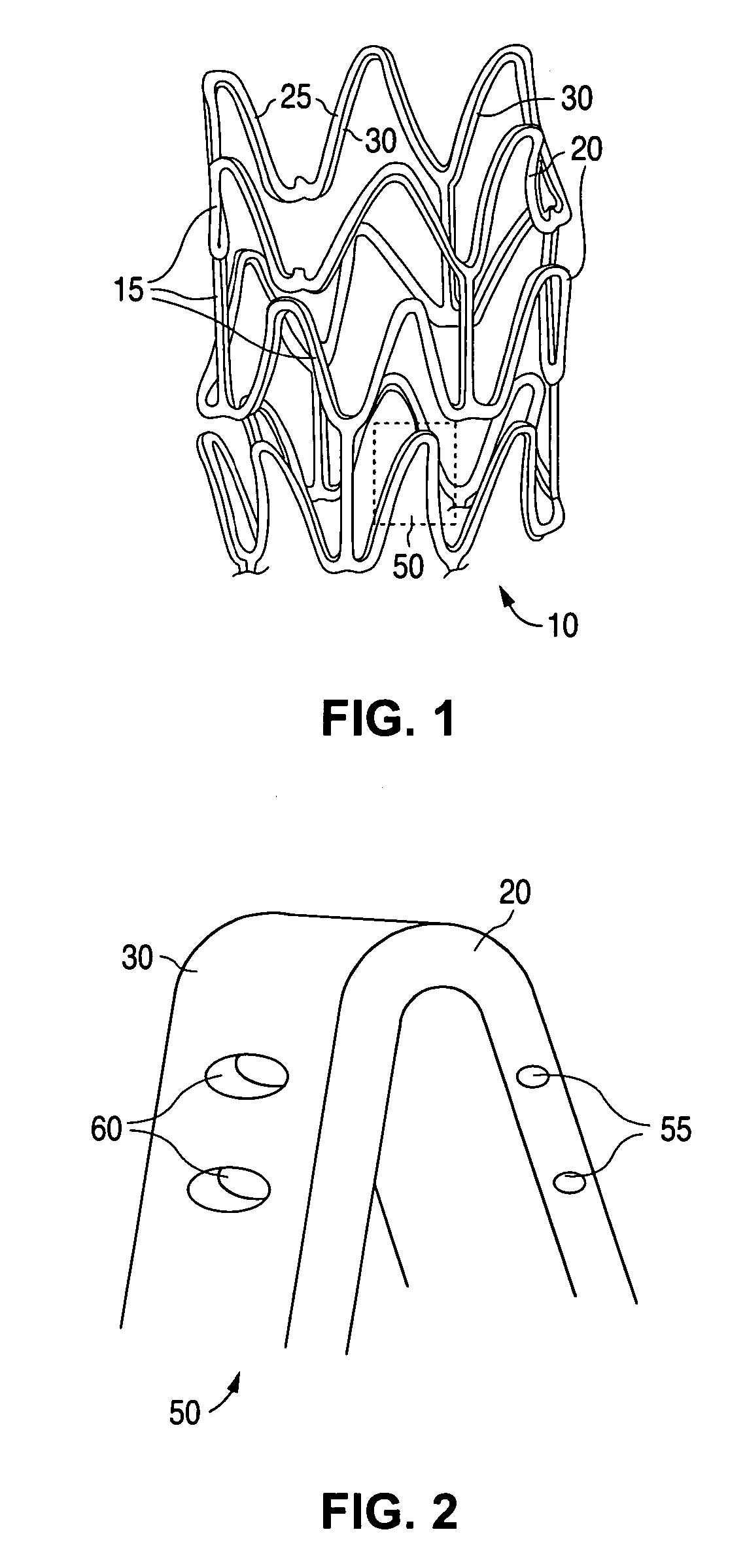
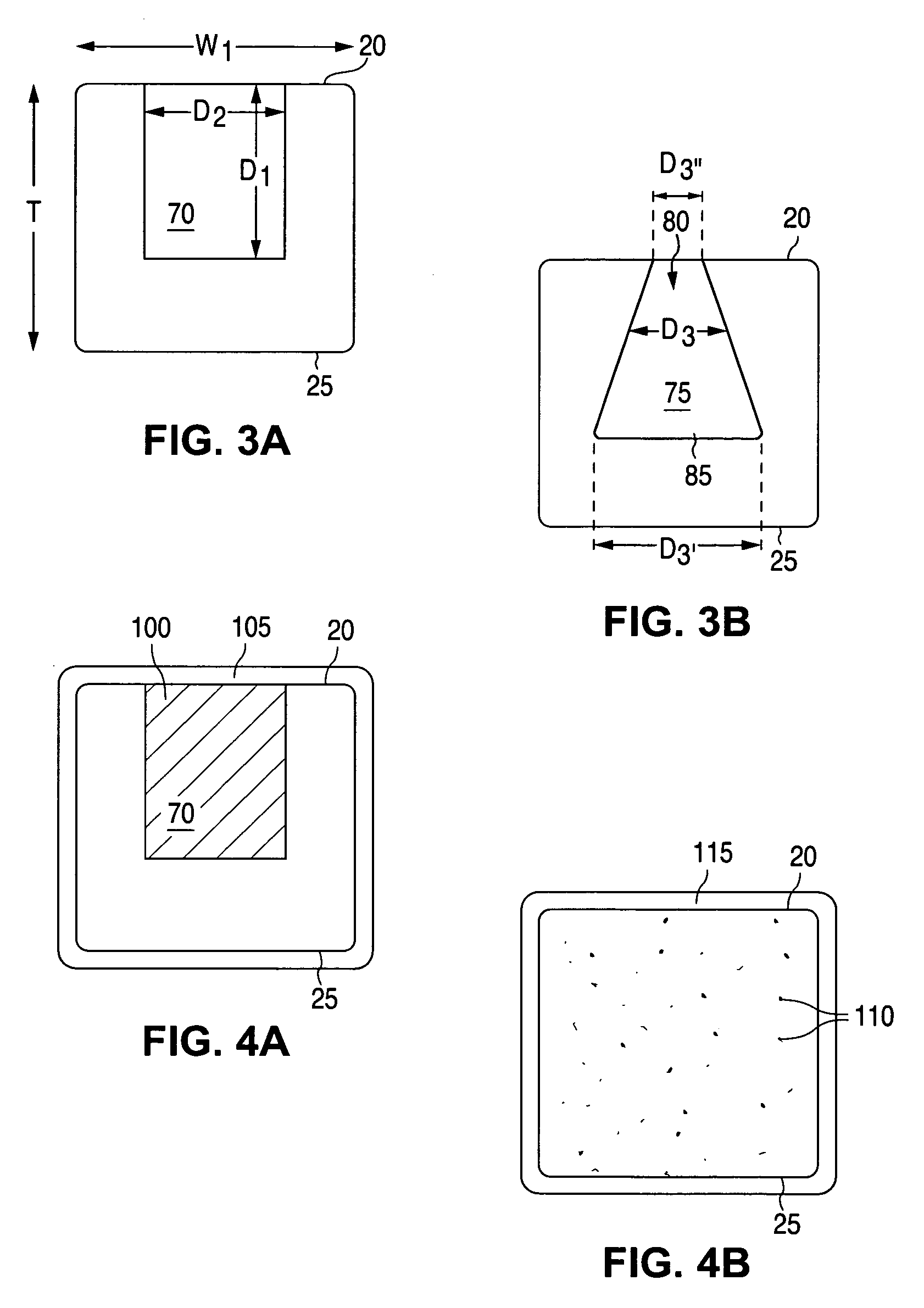
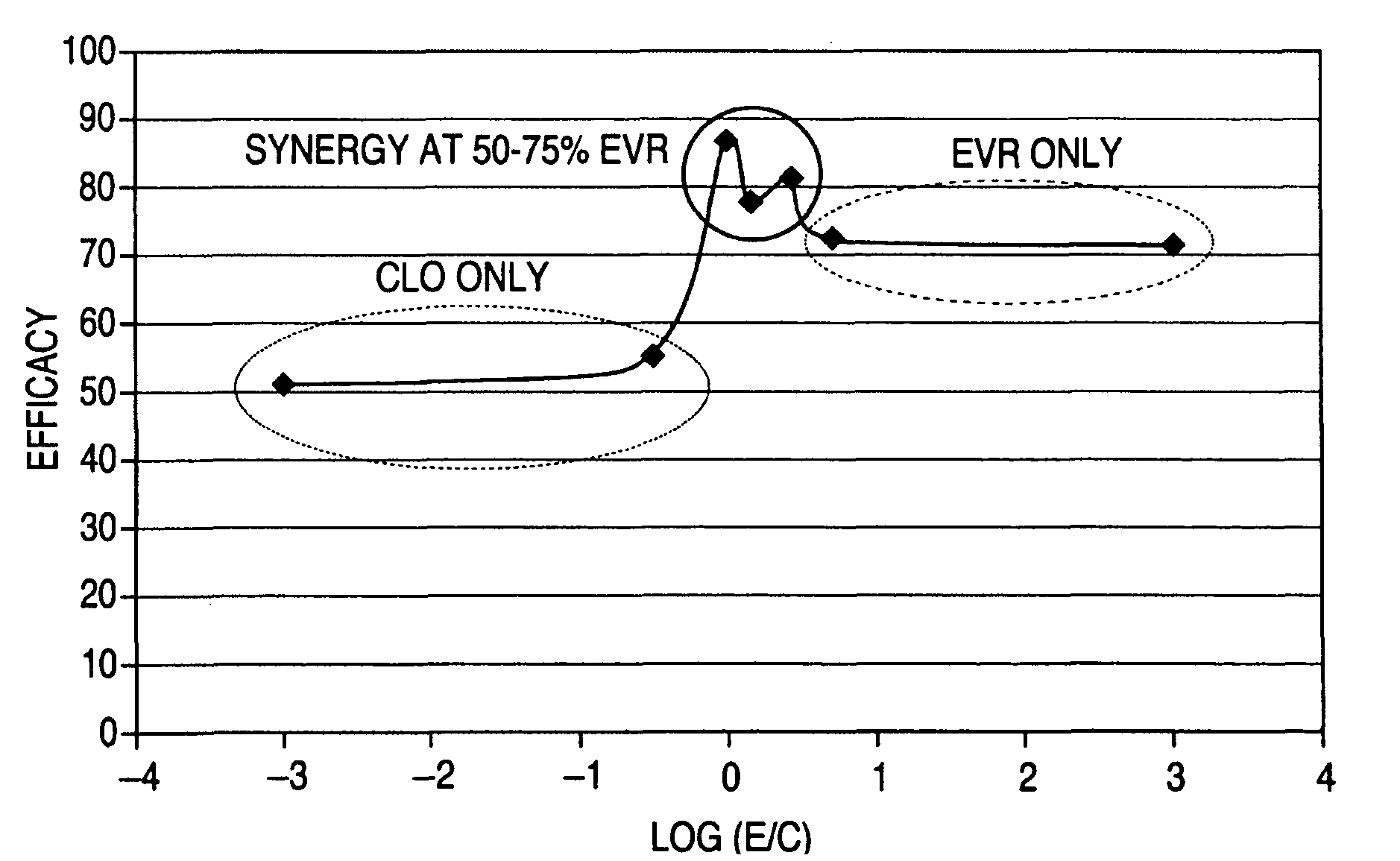
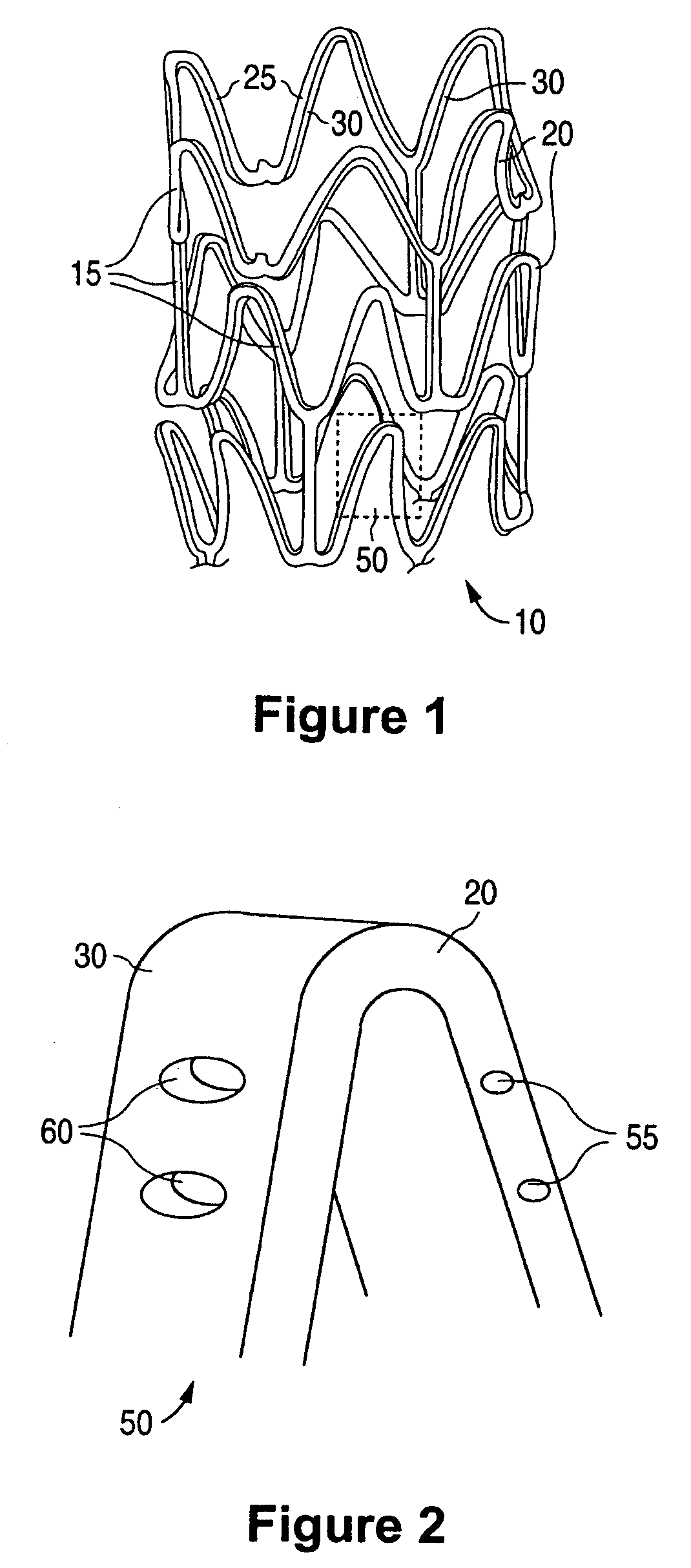
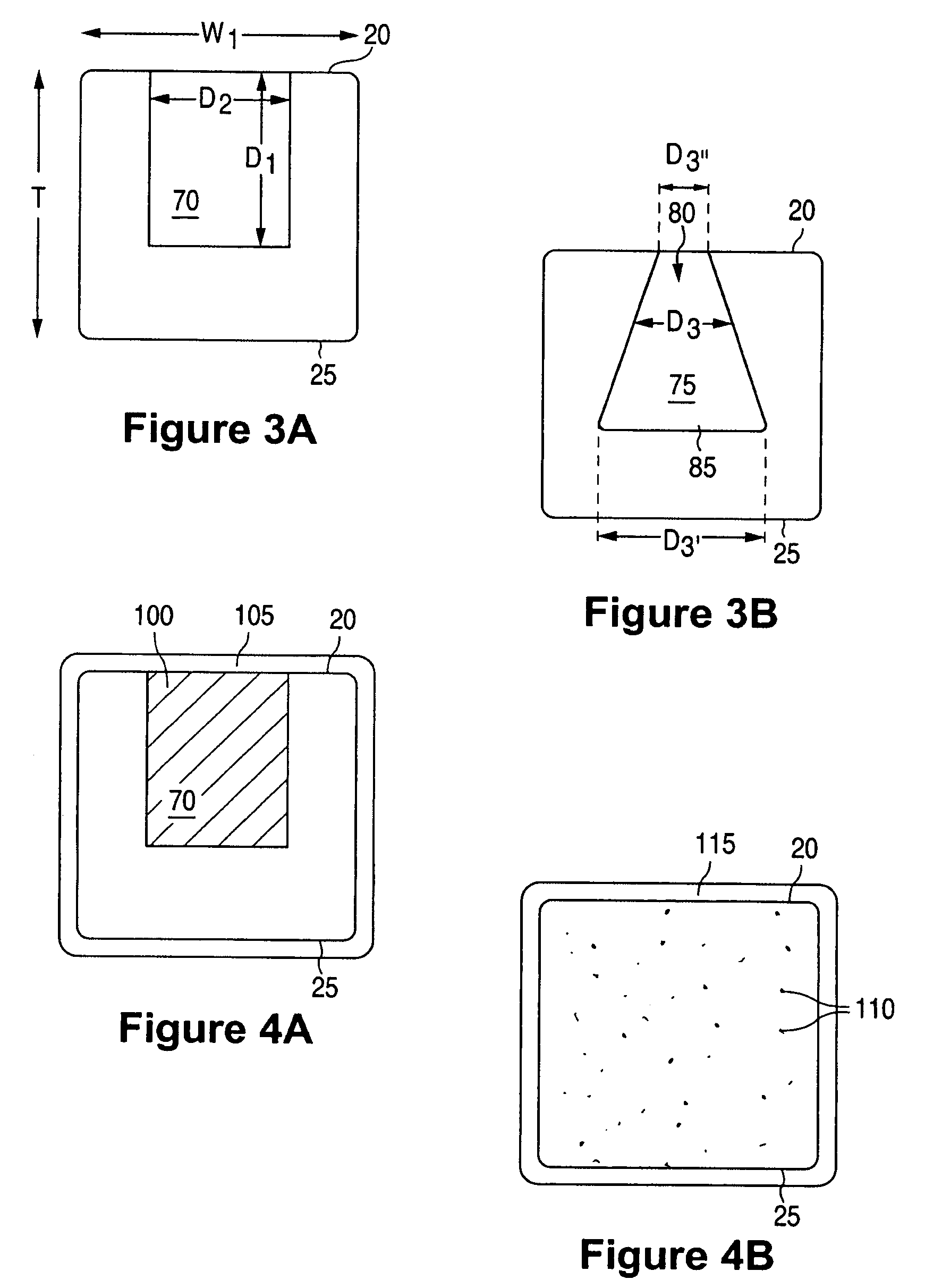
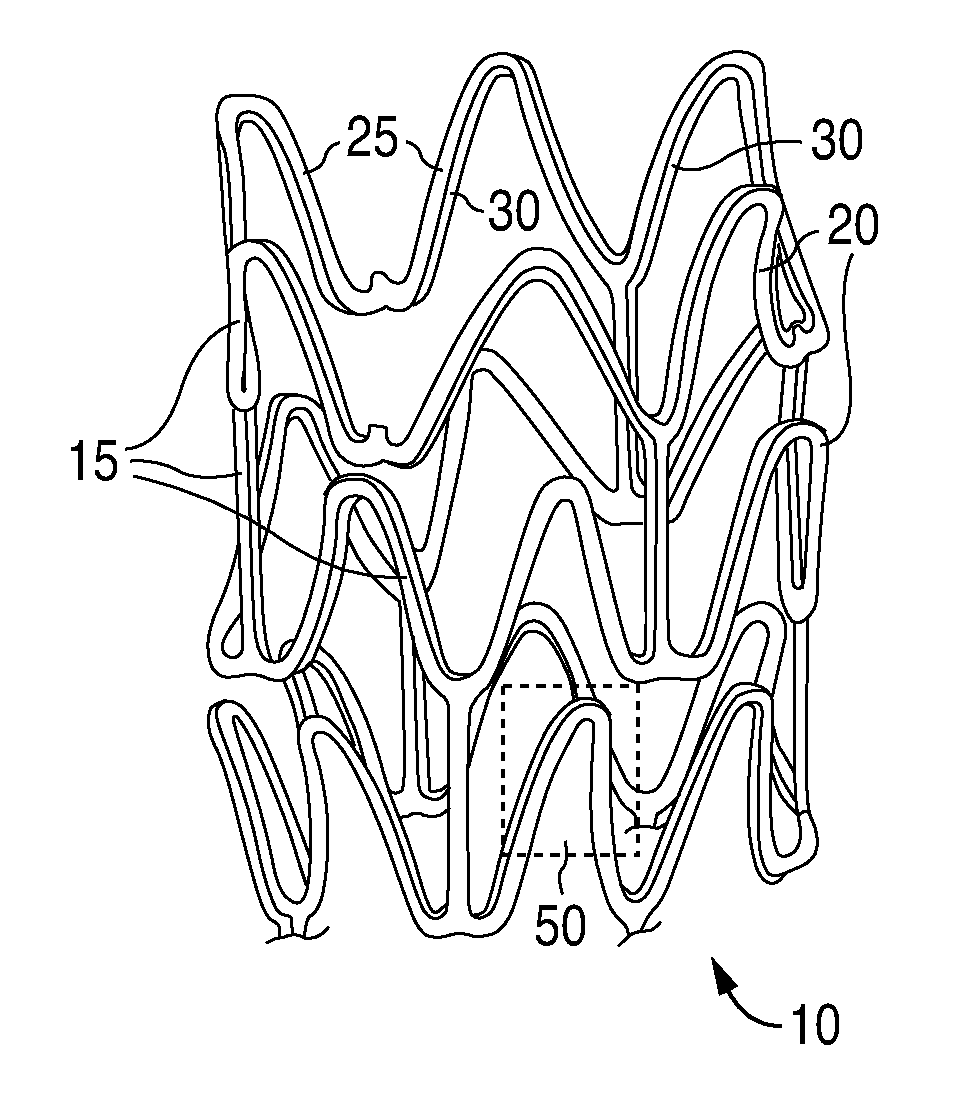
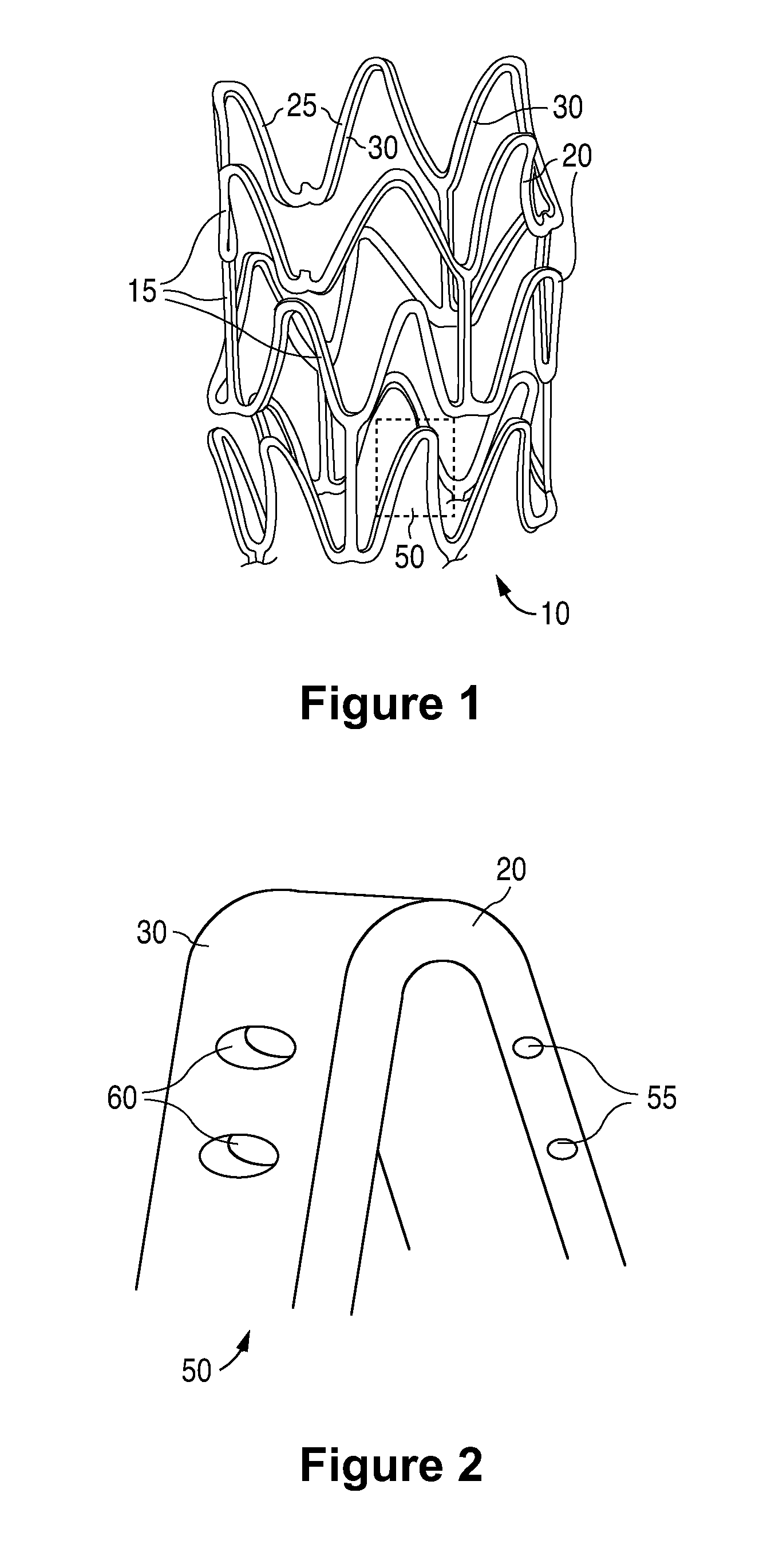
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders
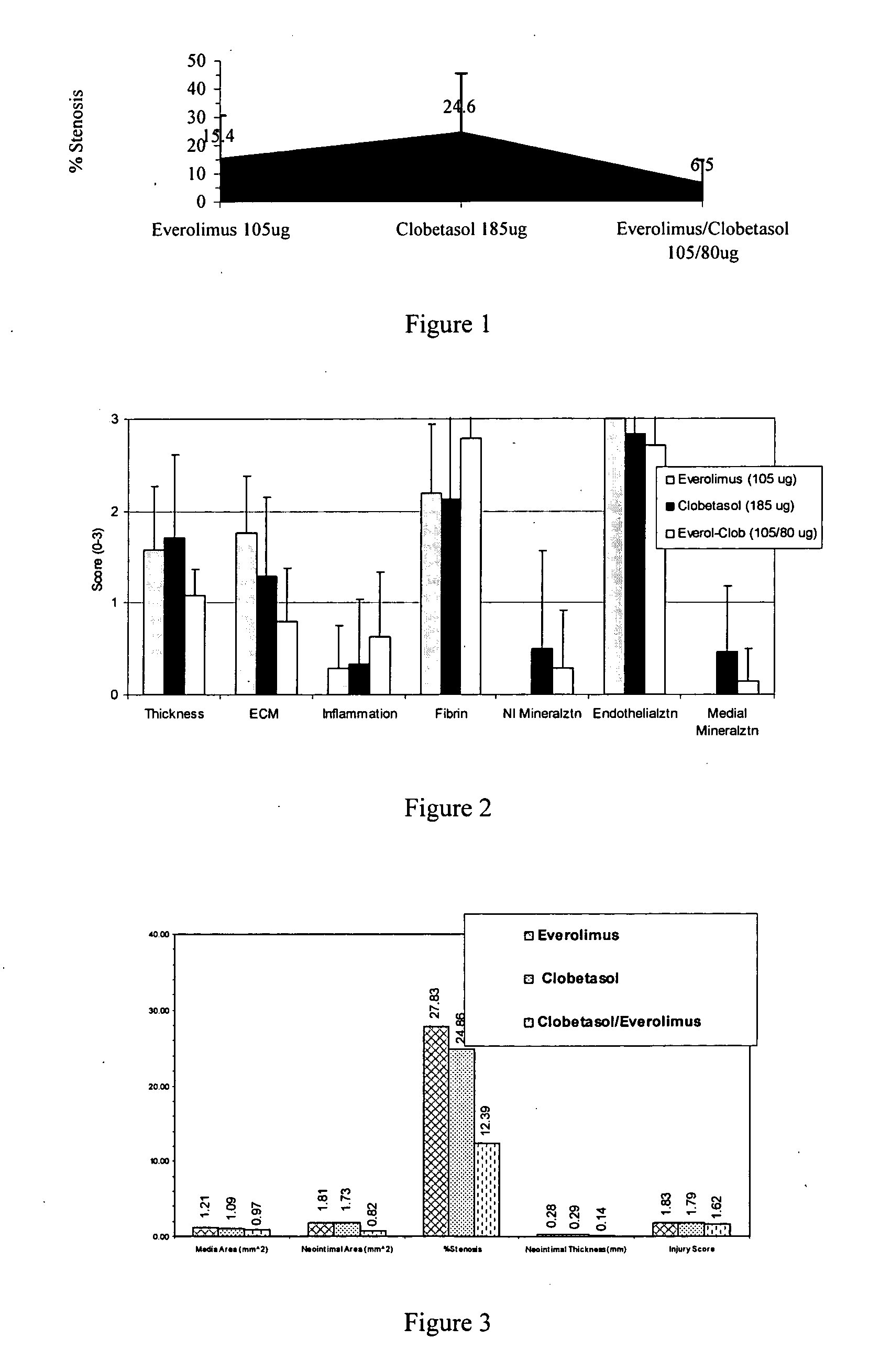
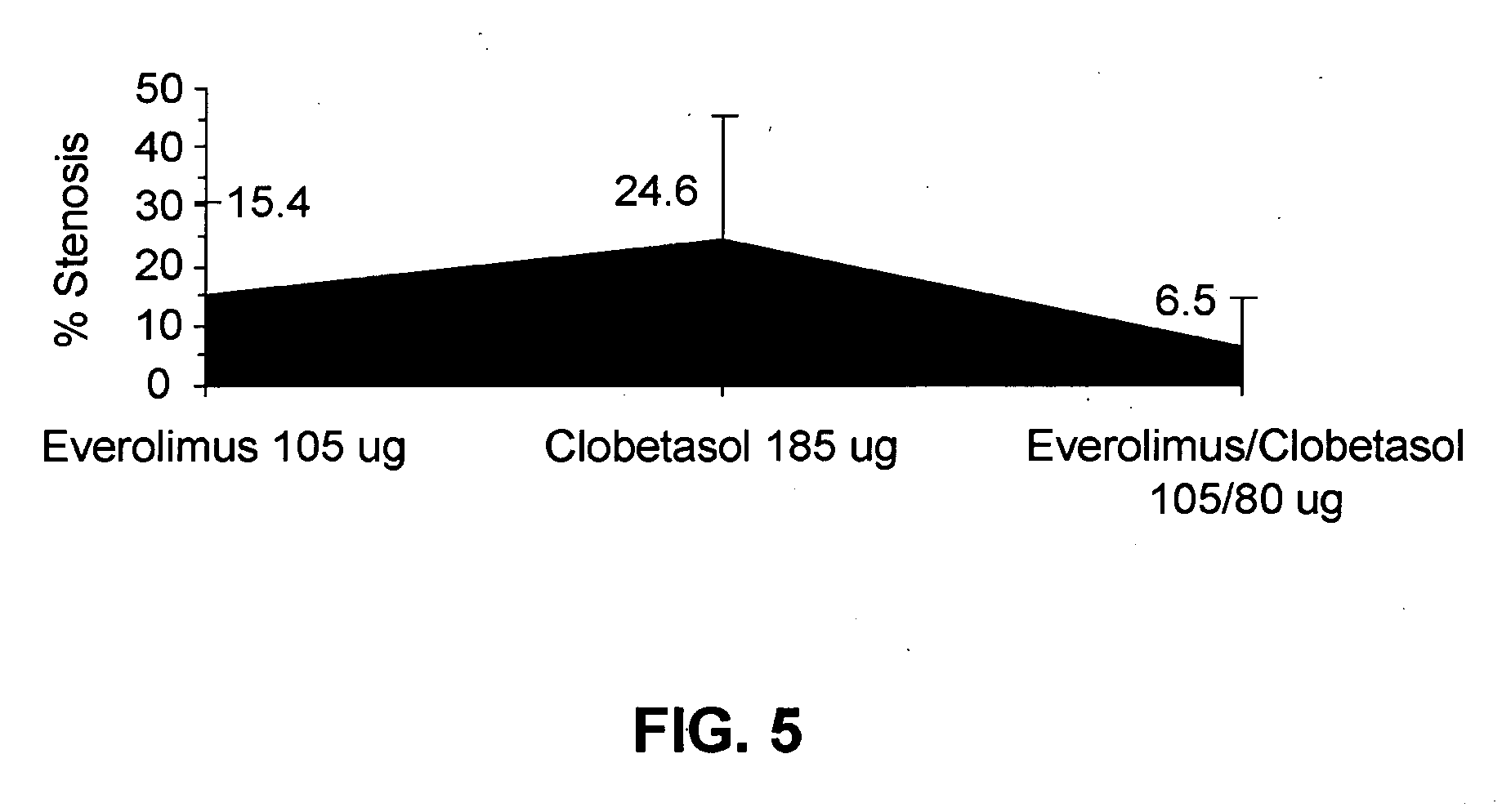
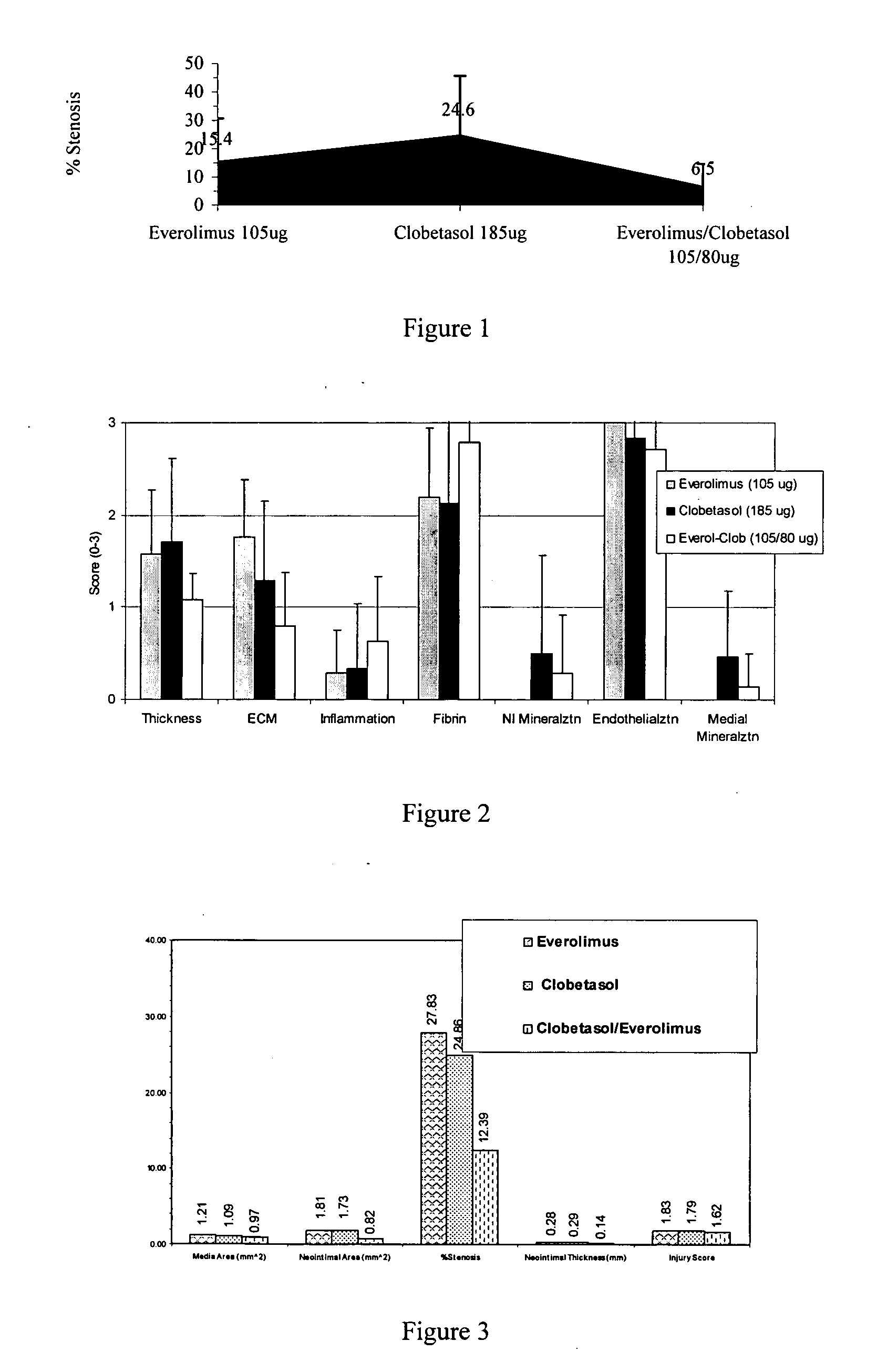
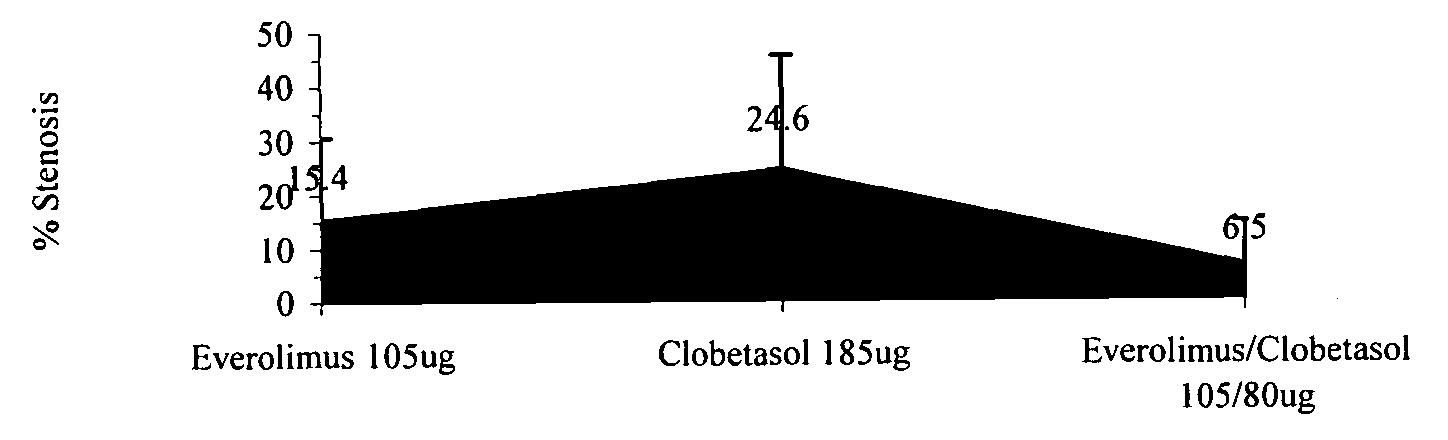
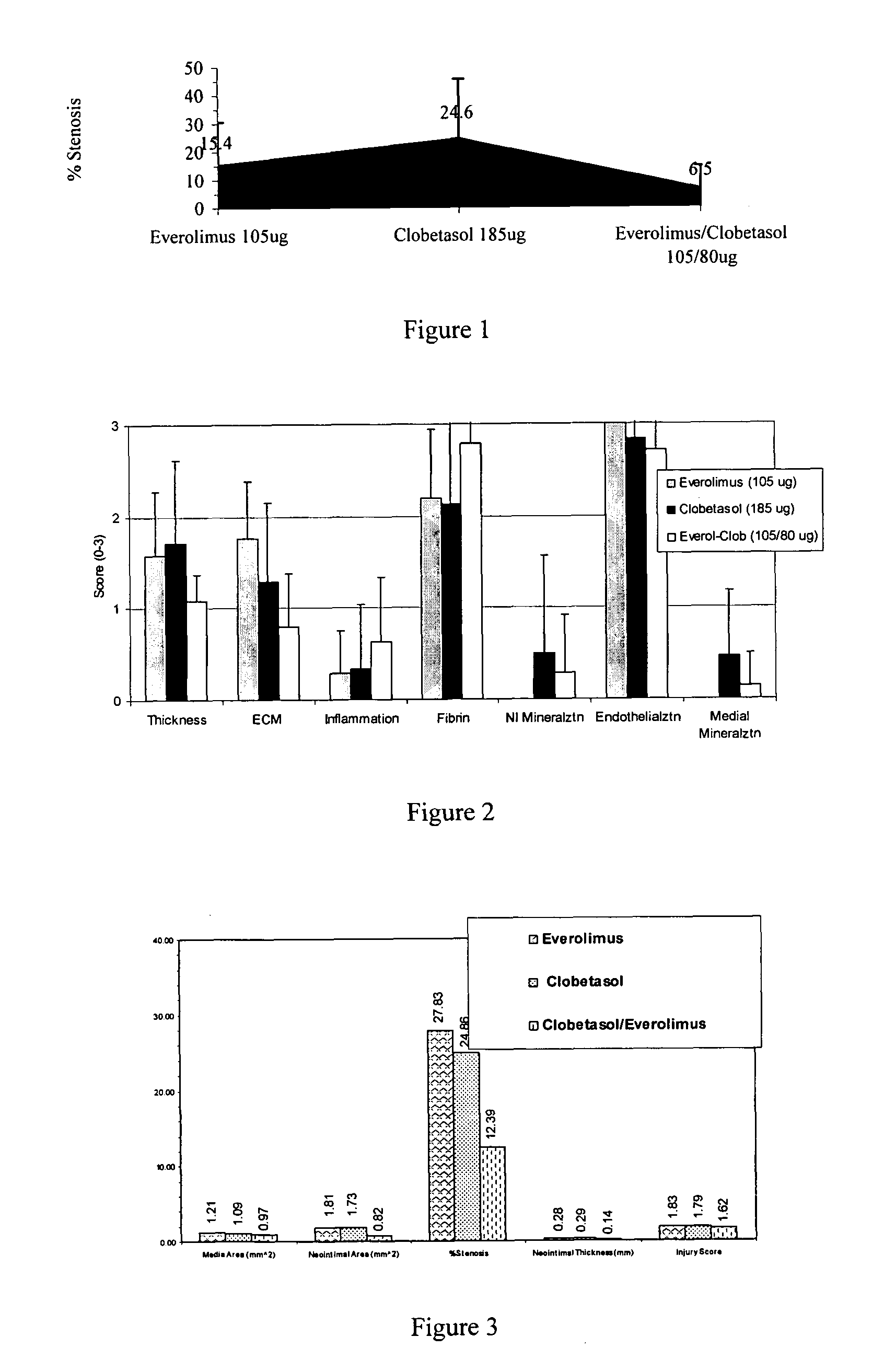
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Biosoluble coating comprising Anti-proliferative and Anti-inflammatory agent combination for treatment of vascular disorders
InactiveUS20090297578A1Less neointima thicknessPromote healingBiocideSurgeryEverolimusPercent Diameter Stenosis
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque
Owner:ABBOTT CARDIOVASCULAR
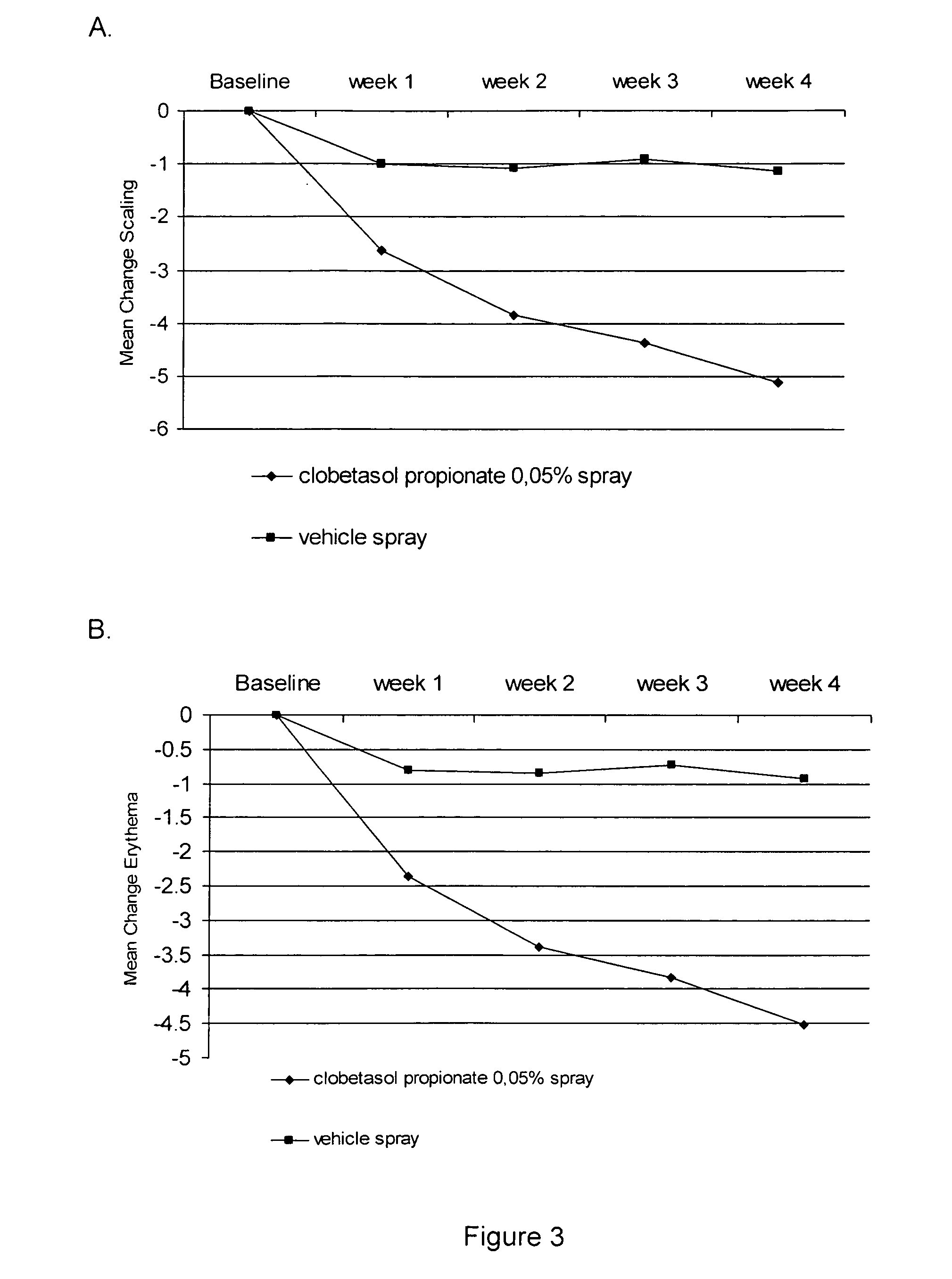
Use of a clobetasol spray formulation to treat psoriasis
InactiveUS20060239929A1Increased clinical benefitBeneficial therapeutic resultHydroxy compound active ingredientsAerosol deliveryAlcoholSURFACTANT BLEND
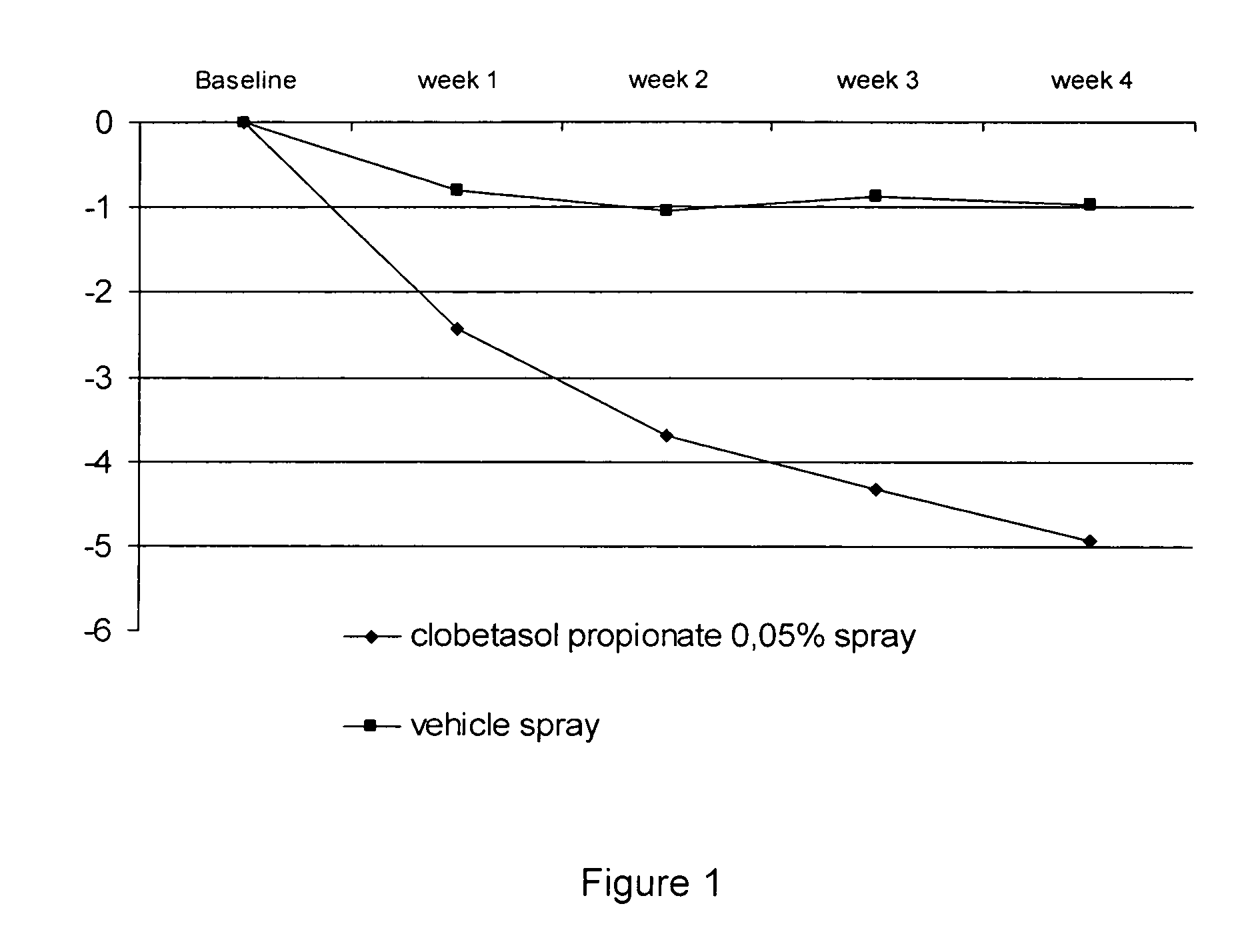
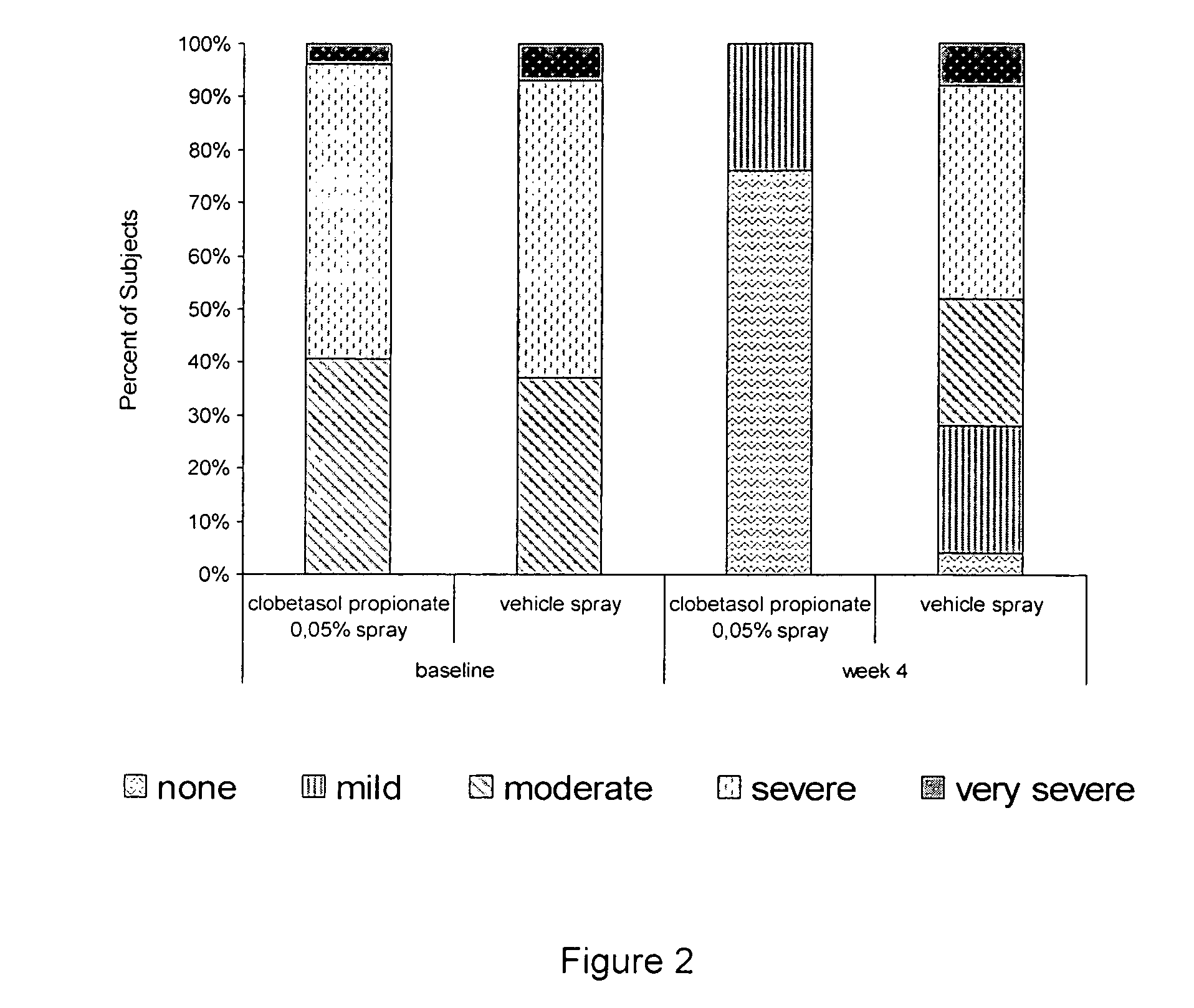
The present invention provides a method for treating psoriasis, by spraying onto the skin with psoriasis daily for at least 4 weeks a pharmaceutical composition containing an effective amount of clobetasol propionate. A preferred pharmaceutical composition containing clobetasol propionate, ethyl alcohol, isopropyl myristate, and anionic surfactant.
Owner:DOW PHARMA SCI INC +1
Anti-Proliferative and Anti-Inflammatory Agent Combination for Treatment of Vascular Disorders with an Implantable Medical Device
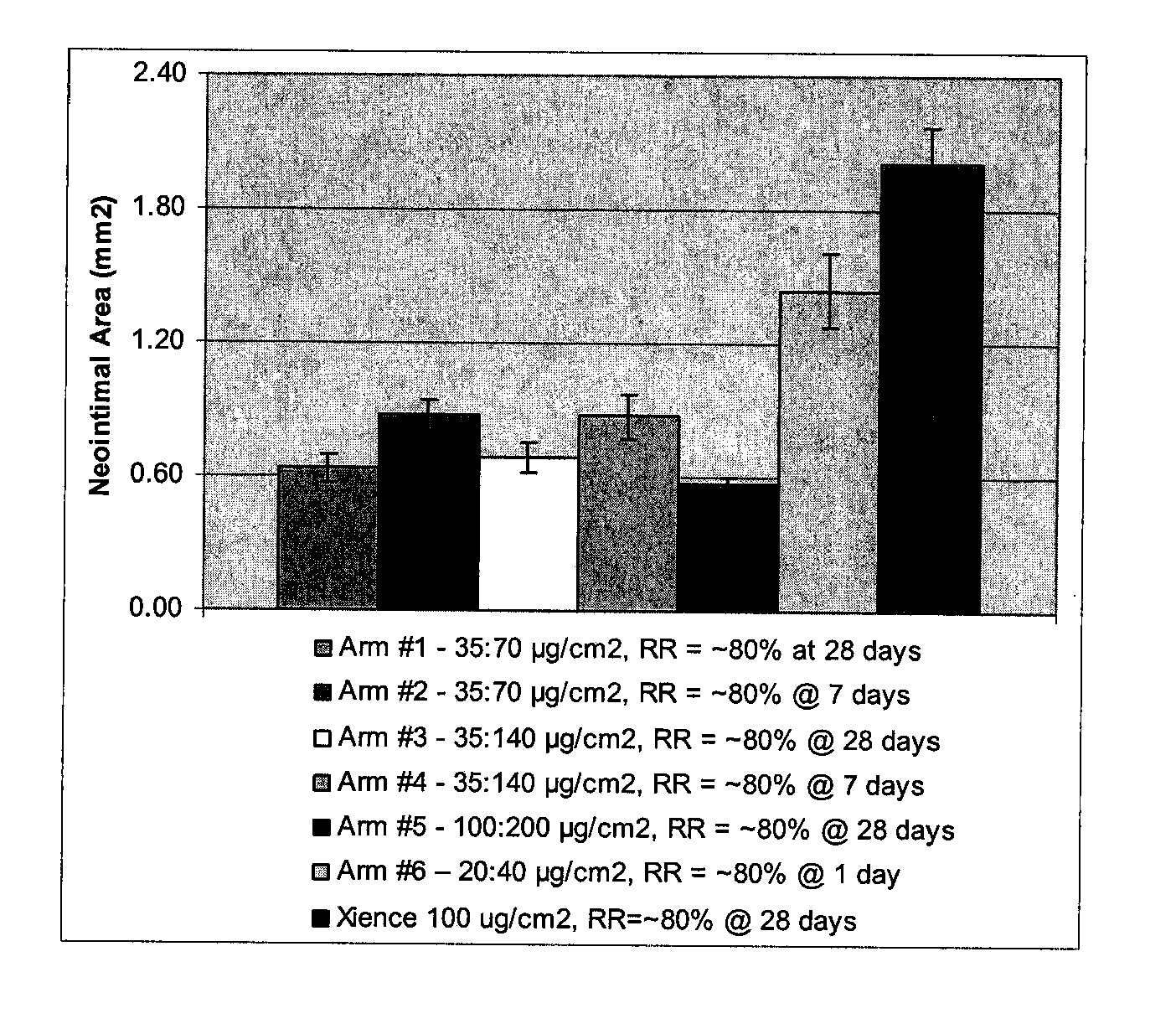
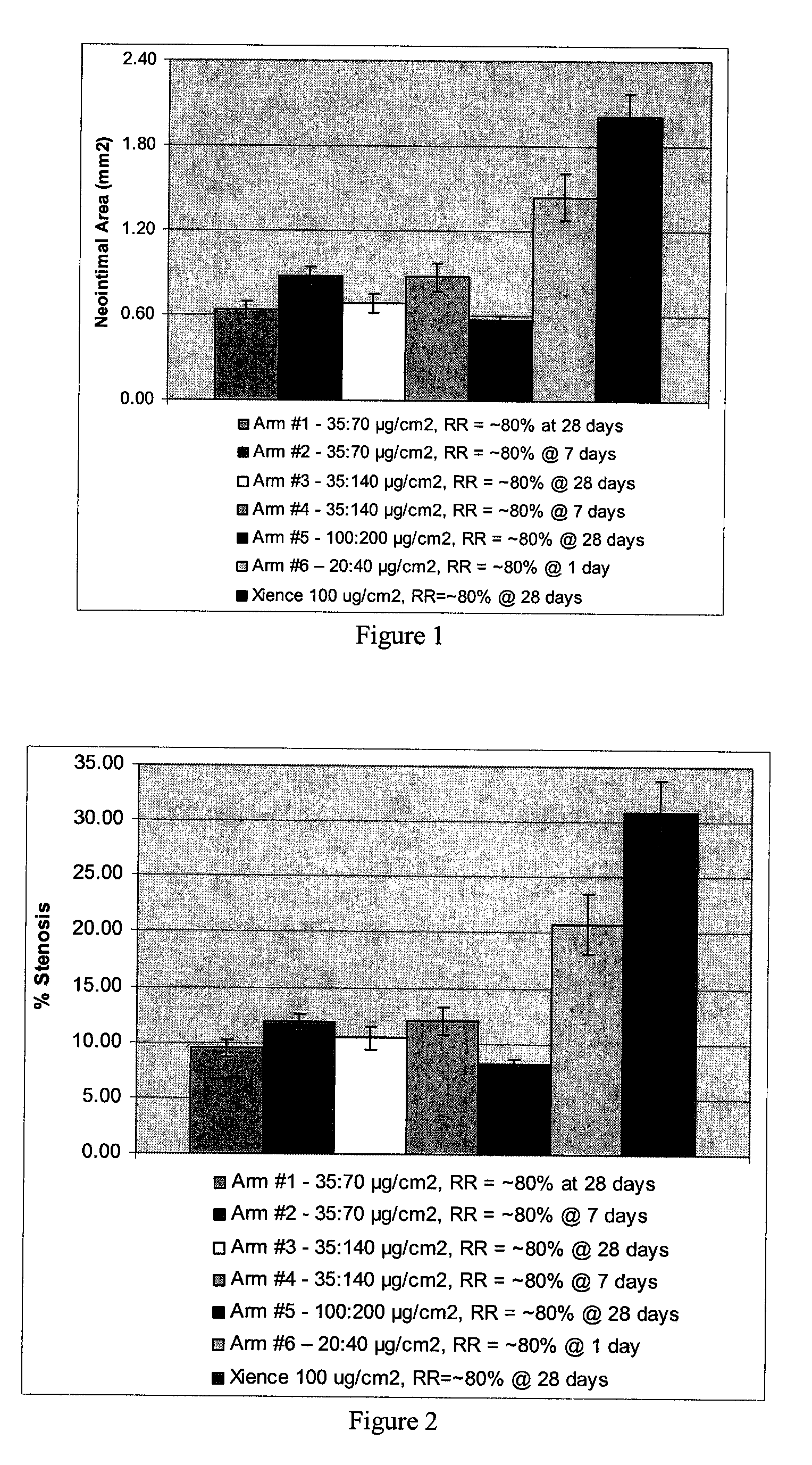
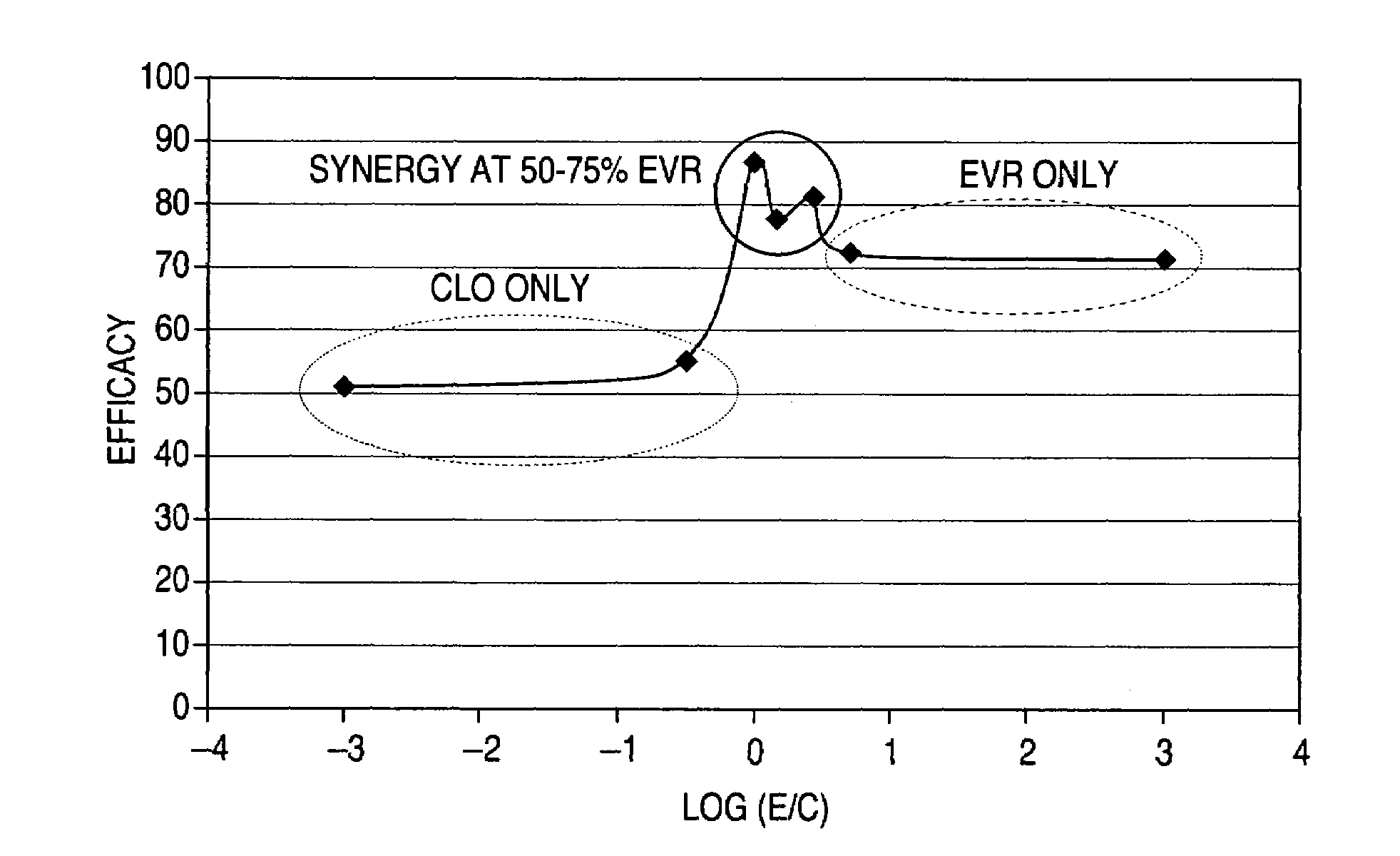
A drug-delivery system is provided including at least 100 μg of everolimus and clobetasol, such that the ratio of everolimus to clobetasol is at least 10:1 (w / w) or the amount of everolimus by weight is at least 10 times more than clobetasol. The system can be a stent. Also provided a method of treating restenosis or vulnerable plaque of a blood vessel, the method includes locally administering to a patient a first drug selected from a group consisting of rapamycin (sirolimus), Biolimus A9, deforolimus, AP23572, tacrolimus, temsirolimus, pimecrolimus, zotarolimus (ABT-578), 40-O-(2-hydroxy)ethylrapamycin (everolimus), 40-O-(3-hydroxy)propylrapamycin, 40-O-[2-(2-hydroxy)ethoxy]ethylrapamycin, 40-O-tetrazolylrapamycin and 40-epi-(N1-tetrazolyl)rapamycin, and locally administering to a patient a second drug consisting of clobetasol, wherein the minimum amount of the first drug that is locally administered is 100 μg, and wherein the ratio of the first drug to the second drug is, for example, 10:1 to 100:1 (w / w).
Owner:ABBOTT CARDIOVASCULAR
Oleaginous ointments comprising two solubilized bioactive agents for the treatment of psoriasis
Topically applicable, anhydrous, hydrophobic and physically / chemically stable dermatological / pharmaceutical oleaginous ointments having effective release / penetration capacity and also having therapeutically effective amounts of calcitriol and clobetasol 17-propionate solubilized therein, are well suited for the treatment of psoriasis.
Owner:GALDERMA SA
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-inflammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque
Owner:ABBOTT CARDIOVASCULAR
Calcitriol/clobetasol propionate compositions for the treatment of psoriasis
InactiveUS20050282792A1Good synergyOrganic active ingredientsDermatological disorderRegimenCalcitriol
Topically applicable pharmaceutical compositions useful for the treatment of psoriasis contain respective amounts of calcitriol and clobetasol propionate permitting a once-per-day effective regimen of topical application onto the part or parts of the skin affected by psoriasis.
Owner:GALDERMA SA
Topical formulation of low level clobetasol propionate for treating disorders of the skin and mucous membranes
InactiveUS20100249060A1Good chemical stabilityLong durabilityBiocideOrganic active ingredientsPolyethylene glycolGlycerol
A new topical formulation is provided, with a high chemical stability, of for example a low dose clobetasol propionate, suitable for the topical treatment of skin and mucous membrane conditions associated with disorders including psoriasis, eczema, and other forms of dermatitis and also topical use associated with the mouth, such as lichen planus. The formulation includes an aqueous vehicle of based on propylene glycol as a solvent and moisture-retaining agent, and macrogol-glycerol hydroxystearate as a non-ionic emulsifier, being capable of holding surprisingly low concentrations of clobetasol. The vehicle holds concentrations about 0.005% to about 0.05% by weight of 17-clobetasol propionate, more preferably about 0.02 to 0.025%, even more preferably 0.025% by weight of 17-clobetasol propionate. The formulation has a good chemical stability, resulting in a long durability.
Owner:SMITH JAN G
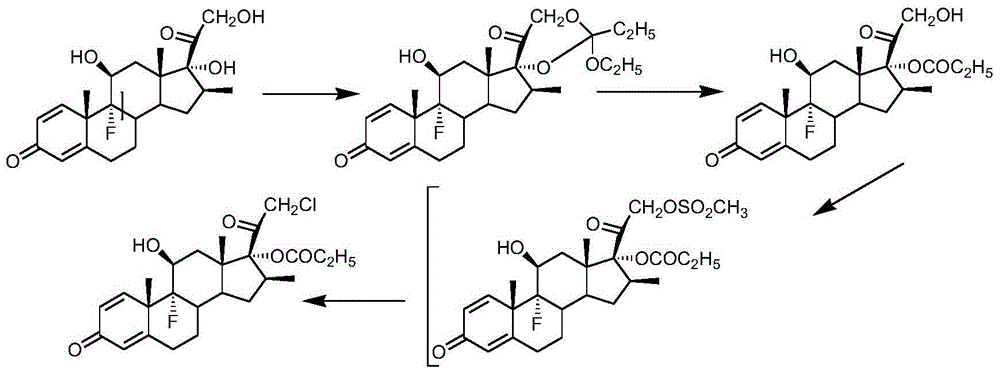
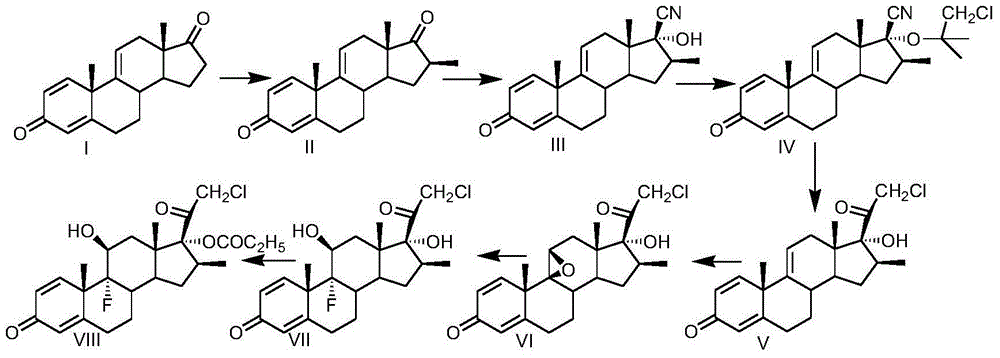
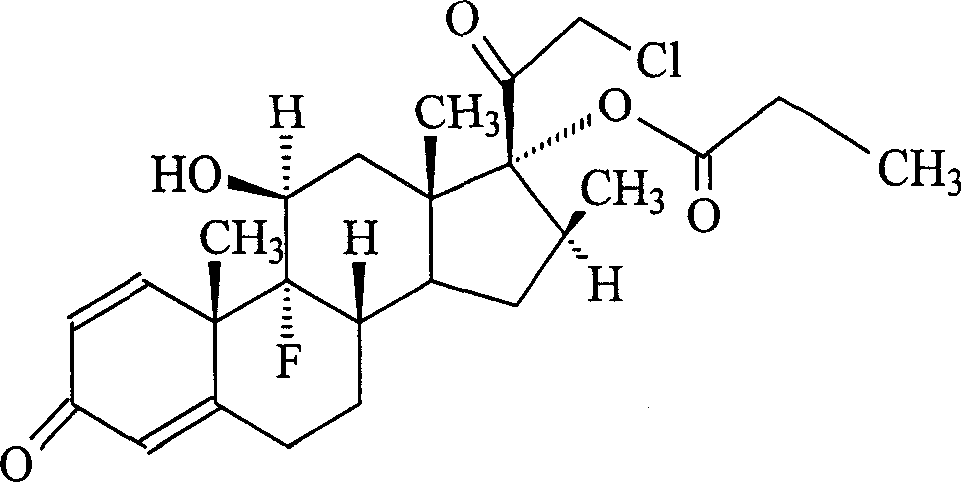
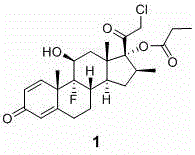
Preparation method of clobetasol and preparation method of clobetasol propionate
The invention discloses a preparation method of clobetasol and the preparation method of clobetasol propionate. The preparation method of the clobetasol comprises the following steps: by taking a compound I, namely 1,4,9(11)-triene androstane-3,17-diketone as an initial raw material, performing a methylation reaction, a cyan substitution reaction, a siloxy protection reaction, an intramolecular nucleophilic substitution reaction, a bromoepoxy reaction and a fluorination reaction to prepare a compound VII which is clobetasol. The compound VII is subjected to a propyl esterification reaction to prepare a compound VIII which is clobetasol propionate. According to the preparation method disclosed by the invention, since relatively basic initial raw materials which are cheap are used, each step of reaction is relatively easy to implement and high yield is achieved; the operation of multi-step protection and deprotection is simplified; moreover, 21 sites of fluorine are directly arranged in one step during arrangement of a side chain, and multiple steps of reaction for arranging the 21 sites of fluorine in the prior art are directly avoided, so that the synthetic route is greatly shortened, the total yield is increased, the product quality is improved and the production cost is greatly lowered.
Owner:江西赣亮医药原料有限公司
Topical pharmaceutical compositions containing nanodroplets for the treatment psoriasis
InactiveUS20130273172A1Improve bioavailabilityExcellent characteristicsSalicyclic acid active ingredientsPowder deliveryNano sizeSalicylic acid
The present invention relates to stable topical pharmaceutical compositions for the treatment of psoriasis. Disclosed are in particular nonoemulsions comprising nano size droplets of one or more anti-psoriasis agents, e.g. clobetasol and / or salicylic acid. These compositions exhibit greater permeability, and improved bioavailability. The invention also relates to processes for the preparation of such compositions.
Owner:CADILA HEALTHCARE LTD
Preparation method of propionic acid clobetasol
The invention discloses a preparing method of chlorotestosterone betaprodine, which comprises the following steps: blending betamisong 17-propionic ester sulphonation and anhydrous lithium chloride with the rate at 1: 1-2; dissolving in the DMF to do chloridization reaction; elutriating through icy water; drying through centrifuging to obtain rough product; dissolving rough product in the carbinol or alcohol; adding activated charcoal; decoloring; filtering; recycling activated charcoal; condensing the filtrate; crystallizing; dehydrating; drying to obtain the product.
Owner:ZHEJIANG DINGTAI PHARMA
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device
A drug-delivery system is provided including at least 100 μg of everolimus and clobetasol, such that the ratio of everolimus to clobetasol is at least 10:1 (w / w) or the amount of everolimus by weight is at least 10 times more than clobetasol. The system can be a stent. Also provided a method of treating restenosis or vulnerable plaque of a blood vessel, the method includes locally administering to a patient a first drug selected from a group consisting of rapamycin (sirolimus), Biolimus A9, deforolimus, AP23572, tacrolimus, temsirolimus, pimecrolimus, zotarolimus (ABT-578), 40-O-(2-hydroxy)ethylrapamycin (everolimus), 40-O-(3-hydroxy)propylrapamycin, 40-O-[2-(2-hydroxy)ethoxy]ethylrapamycin, 40-O-tetrazolylrapamycin and 40-epi-(N1-tetrazolyl)rapamycin, and locally administering to a patient a second drug consisting of clobetasol, wherein the minimum amount of the first drug that is locally administered is 100 μg, and wherein the ratio of the first drug to the second drug is, for example, 10:1 to 100:1 (w / w).
Owner:ABBOTT CARDIOVASCULAR
A kind of oil-in-water type compound ketoconazole nano medicine and preparation method thereof
InactiveCN102283850ASpray evenlyApply evenlyAntibacterial agentsOrganic active ingredientsDiseasePropanoic acid
The invention discloses an oil-in-water compound ketoconazole nano-medicament. The grain diameter of the medicament is 1-100nm. The oil-in-water compound ketoconazole nano medicament comprises the following raw materials in percentage by weight: 0.01-8.0 percent of ketoconazole, 0.01-10 percent of eugenol, 0.001-5.0 percent of clobetasol propionate, 25.0-45.0 percent of surfactant, 0-10.0 percentof cosurfactant, 0.1-25.0 percent of oil and the balance of distilled water. The oil-in-water compound ketoconazole nano-medicament can be used for treating stubborn skin mycotic infection, mycodermatitis, chronic mucocutaneous candidiasis, dermatitis blastomycosis, coccidioidomycosis, histoplasmosis, chromoblastomycosis and paracoccidioidomycosis, and dermatomycosis and tinea versicolor caused by dermatophyte and microzyme as well as trichophyton disease, scytitis and pruritus. The oil-in-water compound ketoconazole nano-medicament has the advantages of strong osmosis, favorable stability, favorable infiltrating property, durable acting time, remarkable effect, low cost, simple preparation method and convenience in popularization and application.
Owner:NORTHWEST A & F UNIV
Topical formulation of low level clobetasol propionate for treating disorders of the skin and mucous membranes
InactiveUS20120238535A1Good chemical stabilityLong durabilityOrganic active ingredientsAerosol deliveryPolyethylene glycolGlycerol
A new topical formulation is provided, with a high chemical stability, of for example a low dose clobetasol propionate, suitable for the topical treatment of skin and mucous membrane conditions associated with disorders including psoriasis, eczema, and other forms of dermatitis and also topical use associated with he mouth, such as lichen planus. The formulation includes an aqueous vehicle of based on propylene glycol as a solvent and moisture-retaining agent, and macrogol-glycerol hydroxystearate as a non-ionic emulsifier, being capable of holding surprisingly low concentrations of clobetasol. The vehicle holds concentrations about 0.005% to about 0.05% by weight of 17-clobetasol propionate, more preferably about 0.02 to 0.025%, even more preferably 0.025% by weight of 17-clobetasol propionate. The formulation has a good chemical stability, resulting in a long durability.
Owner:SMITH JAN G
Anti-Proliferative And Anti-Inflammatory Agent Combination For Treatment Of Vascular Disorders With An Implantable Medical Device
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque
Owner:ABBOTT CARDIOVASCULAR
Clobetasol spray
InactiveUS20080102038A1Reduce stimulationReduce solubilityBiocideOrganic active ingredientsPreservativeDimethyl isosorbide
A spray foaming dosage form comprising clobetasol propionate, dimethyl isosorbide, propylene glycol, polysorbate, sodium dodecyl sulphate, buffer, optional preservative, optional further excipients, and water.
Owner:NUPHARM LAB
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
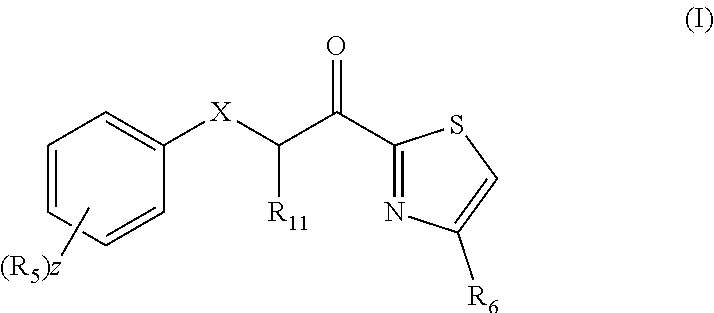
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
One-pot preparation method of clobetasol propionate intermediate
The invention discloses an improved production technology of a clobetasol propionate intermediate (betamethasone-17-propionate). Two steps namely cyclo-esterification and hydrolysis in the conventional technology are merged into one step. By merging the steps, the procedure and production period are shortened, the product purity and yield are increased, the energy consumption is reduced, the raw materials and auxiliary materials are saved; the environment pollution is reduced, and the production cost is reduced.
Owner:SHANDONG TAIHUA BIO & TECH
Topical pharmaceutical compositions containing nanodroplets for the treatment psoriasis
InactiveUS8992994B2Improve bioavailabilityExcellent characteristicsBiocidePowder deliveryNano sizeSalicylic acid
The present invention relates to stable topical pharmaceutical compositions for the treatment of psoriasis. Disclosed are in particular nonoemulsions comprising nano size droplets of one or more anti-psoriasis agents, e.g. clobetasol and / or salicylic acid. These compositions exhibit greater permeability, and improved bioavailability. The invention also relates to processes for the preparation of such compositions.
Owner:CADILA HEALTHCARE LTD
Pharmaceutical composition including anti-fungal agent and steroid
InactiveCN106170285ADo not cause mutual interferenceOrganic active ingredientsAntimycoticsDiseaseMometasone
The present invention addresses the problem of providing, in order to treat dermatomycosis accompanying severe inflammations such as flare ups, an externally applied pharmaceutical composition which includes a combination of an anti-fungal agent and a steroidal agent, and in which the anti-fungal agent and the steroidal agent do not interfere with each other. This externally applied pharmaceutical composition for treating mycosis is characterized by including: a compound represented by general formula (1) shown below (in general formula (1), R represents hydrogen or a halogen, and X represents a halogen), and / or a salt thereof; and a steroid selected from prednisolone, dexamethasone, hydrocortisone, clobetasone, clobetasol, betamethasone, and mometasone.
Owner:NIHON NOHYAKU CO LTD +1
Preparation method of intermediate of steroidal drug with 16-beta-methyl
InactiveCN101851263BEasy accessSimple processSteroidsBetamethasone Sodium PhosphateHydrogenation reaction
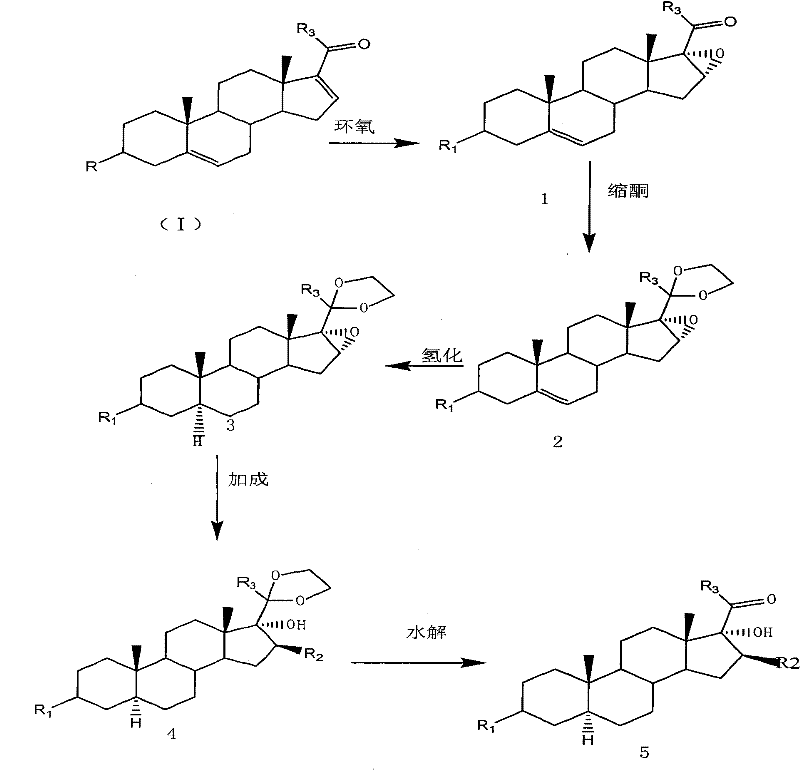
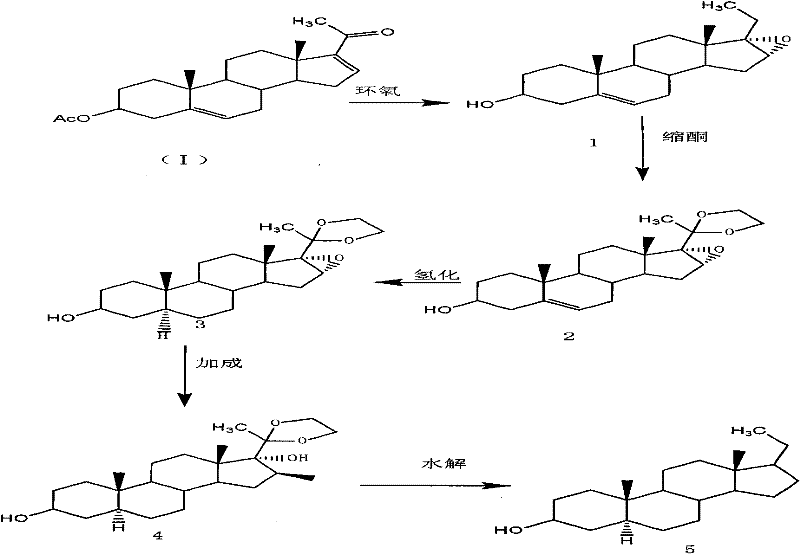
The present invention provides a preparation method of an intermediate of a steroidal drug with a 16-beta-methyl. In the method, a steroidal compound I is used as a starting material, and a steroidal compound II of the intermediate of the steroidal drug with the 16-beta-methyl can be prepared through epoxy reaction, ketal reaction, hydrogenation reaction, addition reaction and hydrolysis reaction for transformation of Position 3, Position 5, Position 6, Position 16, Position 17 and Position 20 or Position 5, Position 6, Position 16, Position 17 and Position 20. The steroidal compound II with the 16-beta-methyl is generally applied in preparation of the glucocorticoid drugs commonly used in clinical operation, such as betamethasone, betamethasone sodium phosphate, betamethasone acetate, clobetasolpropionate, beclometasone dipropionate, betamethasone valerate and the like. In the formulas of the steroidal compound I and the steroidal compound II, R represents OH or OCOCH3, R1 represents OH, R2 represents CH3, and R3 represents CH3.
Owner:GUANGXI WANDE PHARMA
Dandruff-removal composition
InactiveCN102670427AWide applicabilityGood for long termOrganic active ingredientsCosmetic preparationsGlycerolButylated hydroxytoluene
The invention discloses a dandruff-removal composition, which comprises the following components: ketoconazole, clobetasol propionate, vitamin B6, dimethyl sulfoxide, octadecanol, glycerin monostearate, glycerol distearates, cyproheptadine, 2,6-butylated hydroxytoluene and glycerol. Verified by experiments performed by the applicant, the dandruff-removal composition disclosed by the invention is obvious in effect of completely removing dandruffs when being used for dandruff-removal hair care. Moreover, the holding time is long, and dandruffs cannot relapse in at least two years.
Owner:林权飞
Compound clobetasol propionate nano-medicament and preparation method thereof
InactiveCN102327273ASystem stabilityOrganic active ingredientsAntimycoticsPropanoic acidEczematous rash
Owner:NORTHWEST A & F UNIV
Traditional Chinese medicine composition for treating psoriasis and preparation method and application thereof
InactiveCN110075199AGood anti-itch effectLittle side effectsDermatological disorderPlant ingredientsTherapeutic effectOfficinalis
The invention belongs to the technical field of medicines, and specifically relates to a traditional Chinese medicine composition for treating psoriasis and a preparation method and application thereof. The formula of the traditional Chinese medicine composition comprises the following components: 70-90 parts by weight of rhizoma atractylodis, 70-90 parts by weight of tangerine peel, 70-90 parts by weight of cortex magnoliae officinalis, 110-125 parts by weight of radix angelicae dahuricae, 110-125 parts by weight of poria cocos, 110-125 parts by weight of pericarpium arecae, 70-90 parts by weight of unprocessed rhizoma pinelliae, 5-15 parts by weight of extractum glycyrrhizae, 0.5-1.2 parts by volume of patchouli oil, and 0.2-0.6 part by volume of perilla leaf oil. The medicine of the psoriasis prepared from the traditional Chinese medicine composition can treat skin itching, dermatitis, rash and the like caused by a plurality of reasons, treatment range is wide, security coefficientis high, and a patient has no hormonal dependence after recovery; a better therapeutic effect is realized when the Chinese medicine composition for treating the psoriasis is used in combination with ointments for external use of urea ointments, tretinoin, acitretin, vitamin D, compound flumethasone ointments, compound clobetasol cream and the like.
Owner:CHONGQING TAIJI MEDICAL RES INST CO LTD +1
Patch for treatment of eyelid disease containing clobetasol
A patch for treatment of eyelid diseases that is provided with a support, a pressure-sensitive adhesive layer, and a release layer in this order, wherein (a) the pressure-sensitive adhesive layer includes the following (a-1) to (a-4): (a-1) a styrene-isoprene-styrene block copolymer, a tackifier resin, and a softening agent are contained; (a-2) a ratio (mass ratio) of the styrene-isoprene-styrene block copolymer and the tackifier resin is 1:2 to 1:4; (a-3) a content of the softening agent is 40% to 60% by mass; and (a-4) further 0.005% to 5% by mass of clobetasol or acid ester thereof is contained; and (b) the support has elastic modulus with a Young's modulus of 0.01 to 0.5 GPa: and a method for producing the patch for treatment of eyelid diseases including: forming a pressure-sensitive adhesive layer on a upper surface of a release layer.
Owner:NICHIBAN CO LTD
Anti-Proliferative and Anti-Inflammatory Agent Combination for Treatment of Vascular Disorders with an Implantable Medical Device
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Compound clobetasol propionate lipid composite ointment and preparation method thereof
ActiveCN103349663ASmall and uniform particle sizeFacilitate throughHydroxy compound active ingredientsAerosol deliveryMedicineOil phase
The present invention provides a clobetasol propionate and tretinoin ointment agent. According to the present invention, a phospholipid and a positively charged lipid are mainly adopted to wrap drugs to prepare a lipid complex, wherein the positively charged lipid complex can concurrently wrap two drugs, encapsulation efficiency is high, and leakage is not easily generated; with the lipid complex, drug absorption can be promoted, and drug retention in skin can be increased; the multi-component low co-melting system is adopted to prepare the solid dispersion, such that the composite antioxidant component in the formula is uniformly dispersed in the same system; an oil phase matrix and an emulsifying agent are added so as to further prepare into a cream agent or an ointment agent in one step; and the preparation has characteristics of good emulsifying uniformity and high stability, and can be used for treatment of psoriasis, chronic dermatitis and eczema, skin papule type amyloidopathia, lichen planus, and non-atopic dermatitis.
Owner:JIANGSU SEMPOLL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com