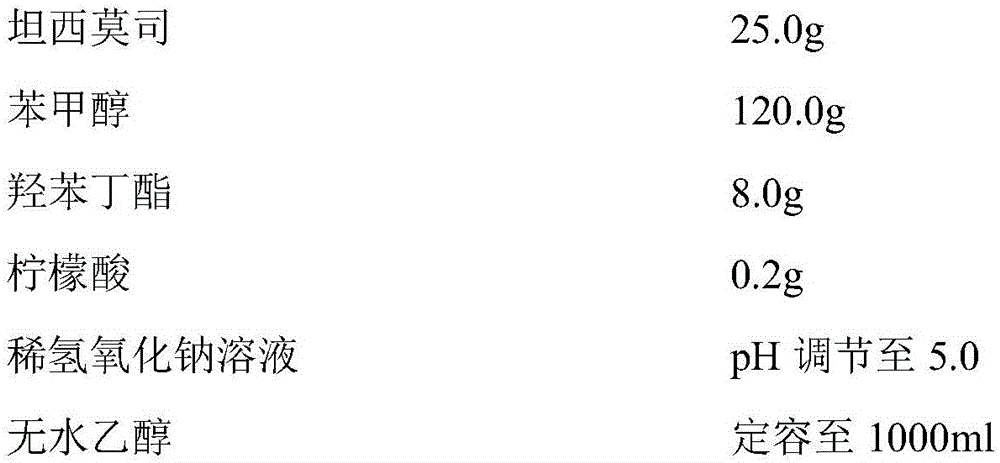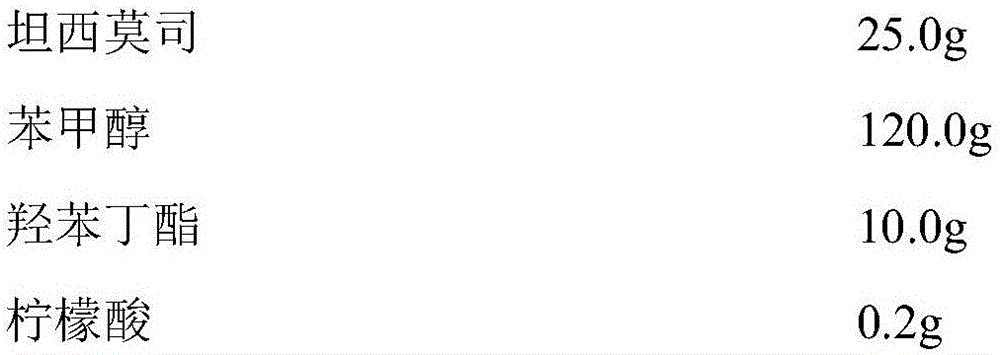Patents
Literature
62 results about "Temsirolimus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat kidney cancer.
Anti-Proliferative and Anti-Inflammatory Agent Combination for Treatment of Vascular Disorders with an Implantable Medical Device
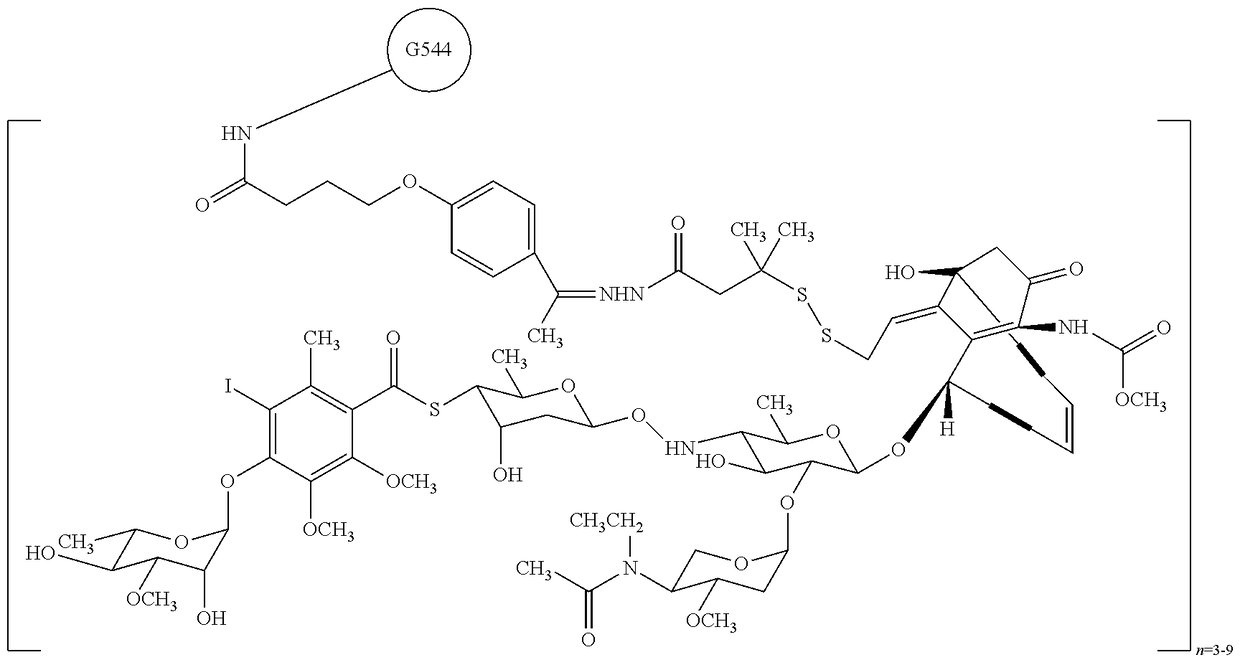
A drug-delivery system is provided including at least 100 μg of everolimus and clobetasol, such that the ratio of everolimus to clobetasol is at least 10:1 (w / w) or the amount of everolimus by weight is at least 10 times more than clobetasol. The system can be a stent. Also provided a method of treating restenosis or vulnerable plaque of a blood vessel, the method includes locally administering to a patient a first drug selected from a group consisting of rapamycin (sirolimus), Biolimus A9, deforolimus, AP23572, tacrolimus, temsirolimus, pimecrolimus, zotarolimus (ABT-578), 40-O-(2-hydroxy)ethylrapamycin (everolimus), 40-O-(3-hydroxy)propylrapamycin, 40-O-[2-(2-hydroxy)ethoxy]ethylrapamycin, 40-O-tetrazolylrapamycin and 40-epi-(N1-tetrazolyl)rapamycin, and locally administering to a patient a second drug consisting of clobetasol, wherein the minimum amount of the first drug that is locally administered is 100 μg, and wherein the ratio of the first drug to the second drug is, for example, 10:1 to 100:1 (w / w).
Owner:ABBOTT CARDIOVASCULAR
Antineoplastic combinations of temsirolimus and sunitinib malate
A combination of temsirolimus and sunitinib malate in the treatment of cancer is provided. Also provided are regimens and kits for treatment of renal cell carcinoma, containing temsirolium and sunitinib malate, optionally in combination with other anti-neoplastic or immune modulators.
Owner:WYETH
Combination of inotuzumab ozogamicin and torisel for the treatment of cancer
InactiveUS9642918B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAfter treatmentInotuzumab ozogamicin
The present invention relates to a therapeutic method for the treatment of cancer that comprises the use of a combination of inotuzumab ozogamicin (CMC-544) and temsirolimus. The enhanced antitumor of the combination therapy is particularly useful for patient population that are recalcitrant to inotuzumab ozogamicin or temsirolimus therapy, relapse after treatment with inotuzumab ozogamicin or temsirolimus or where enhanced antitumor effect reduces toxicities associated with treatment using inotuzumab ozogamicin or temsirolimus.
Owner:PFIZER INC +1
Therapeutic methods
InactiveUS20070185150A1Great cumulative exposureImprove compromiseBiocideAnimal repellantsEverolimusMedicine
Disclosed are methods for treating a patient with an mTOR inhibitor such as AP23573, sirolimus, temsirolimus, everolimus, etc.
Owner:ARIAD PHARMA INC
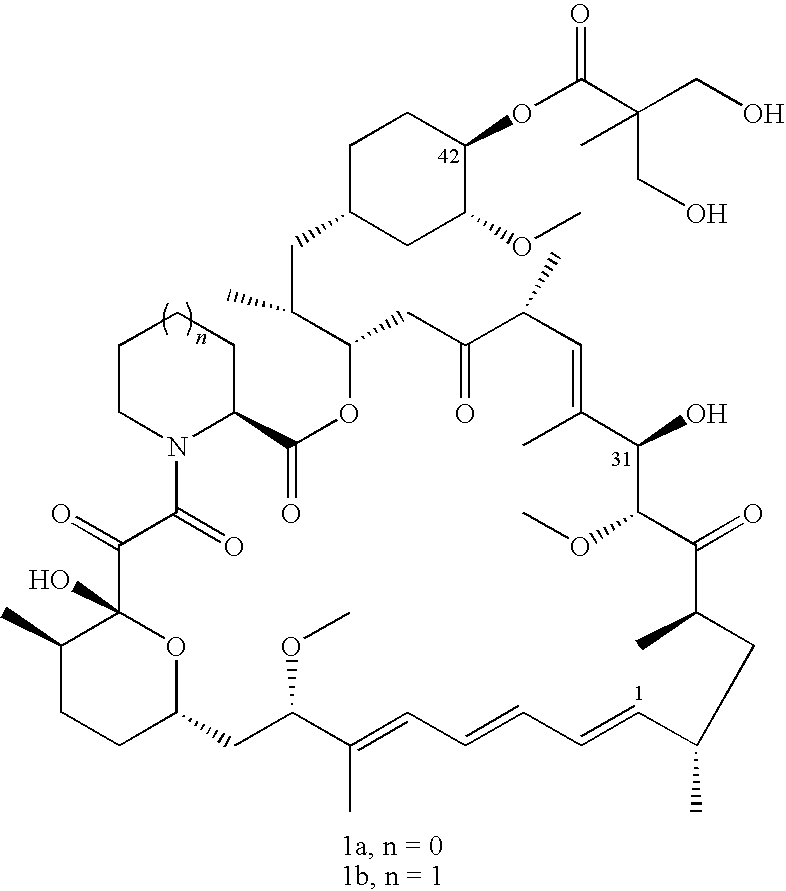
Process for preparation of temsirolimus
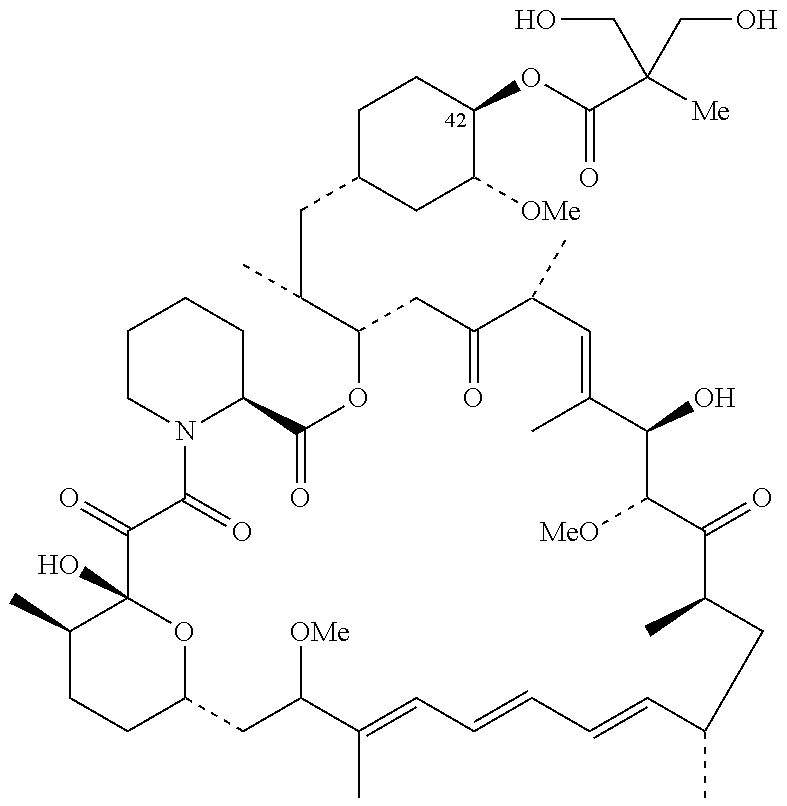
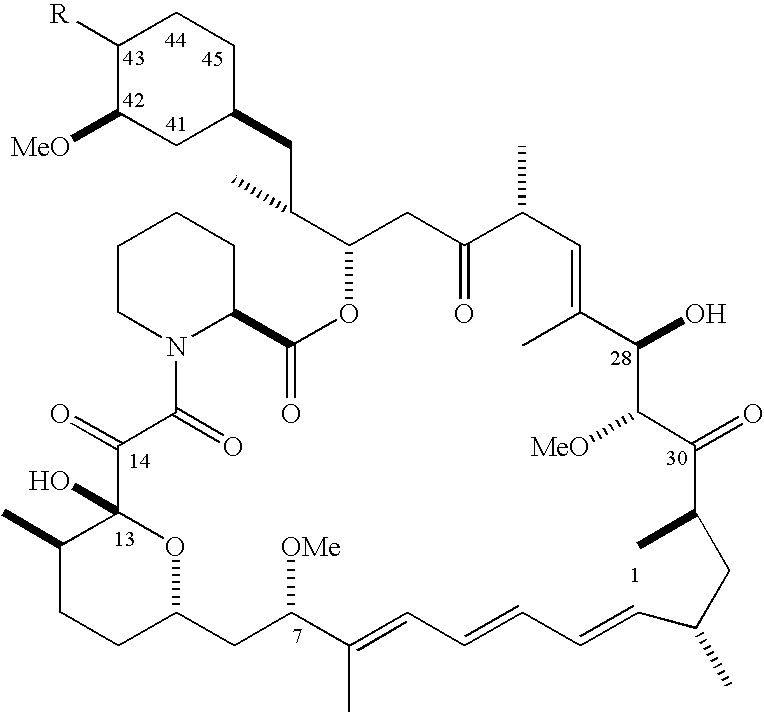
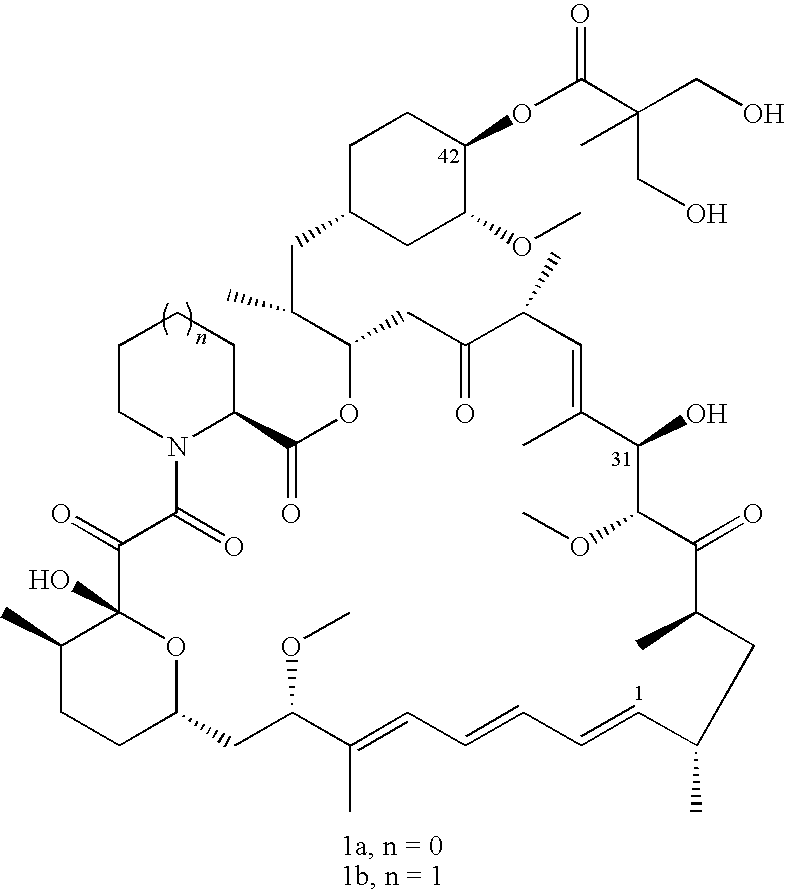
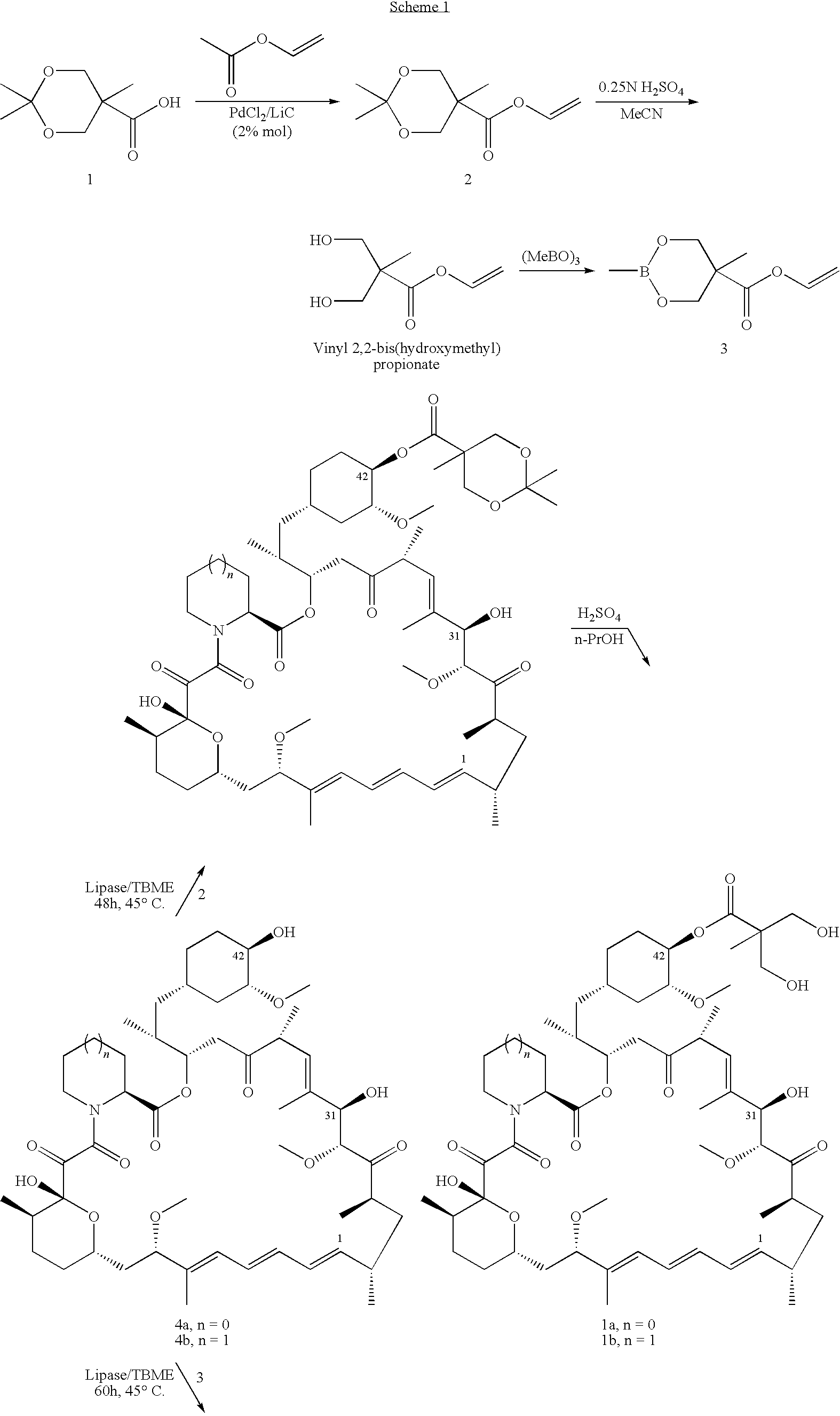
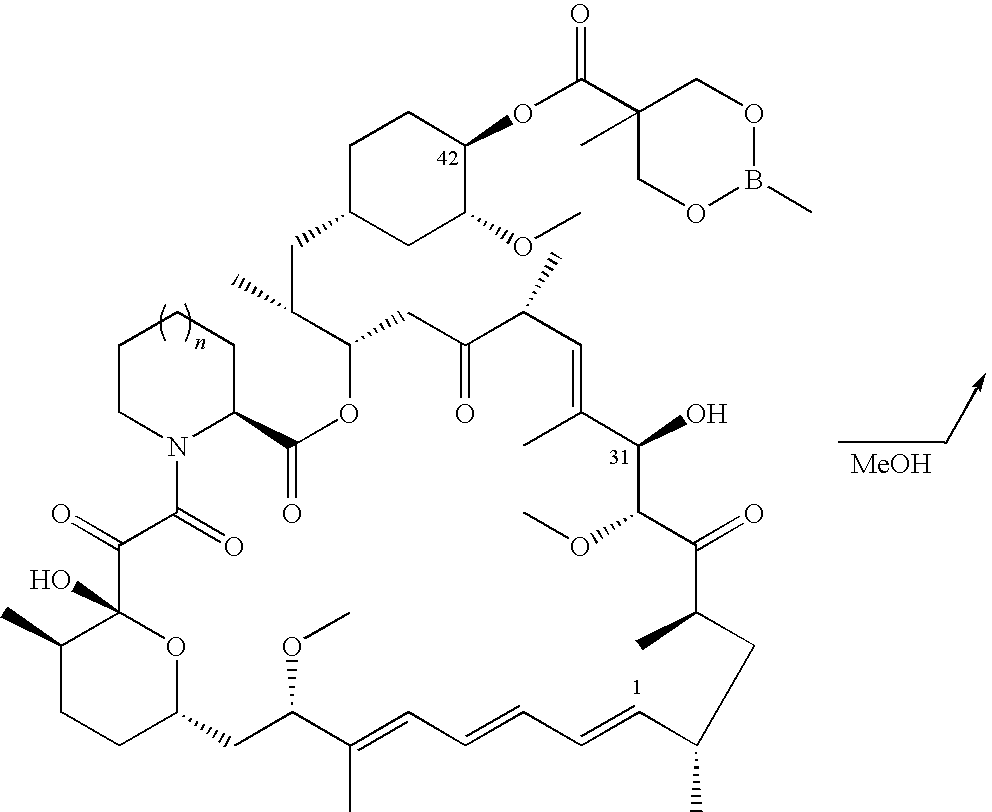
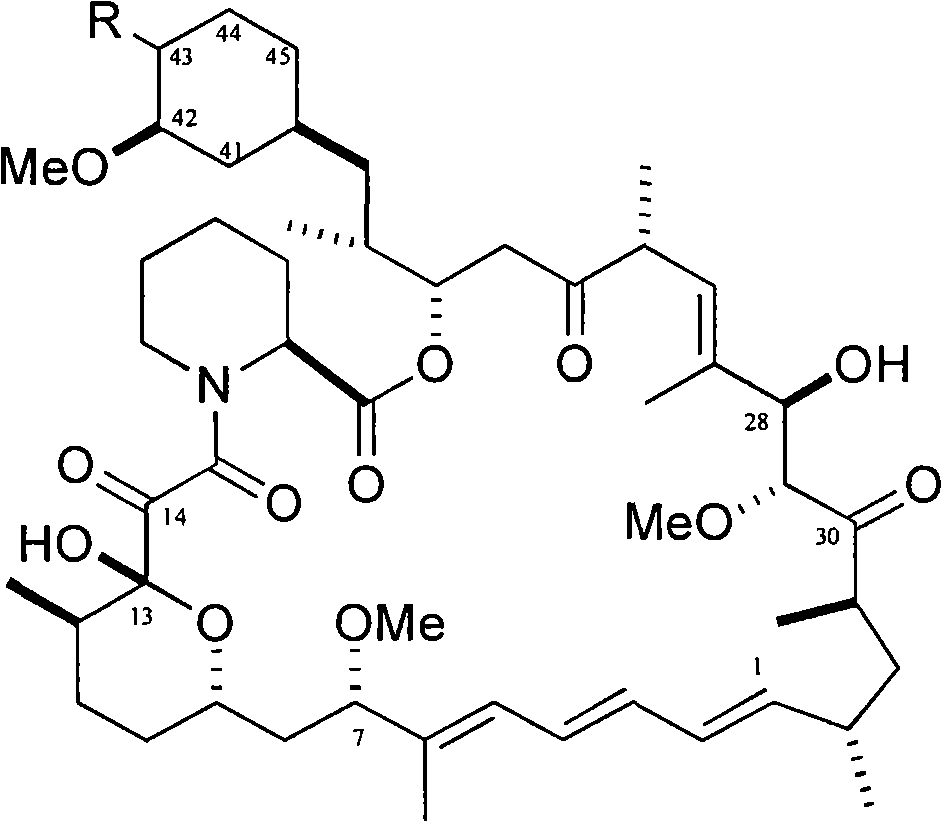
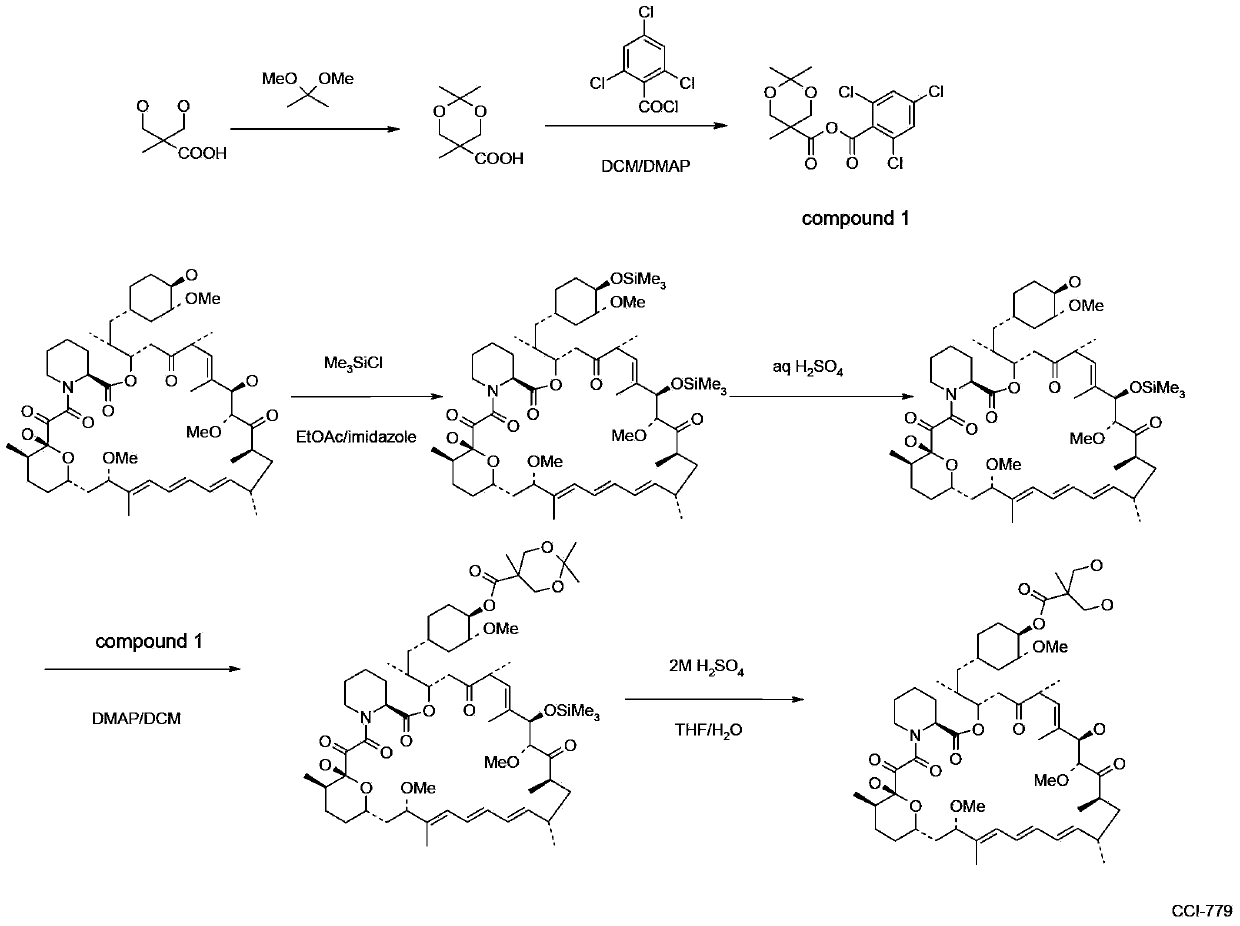
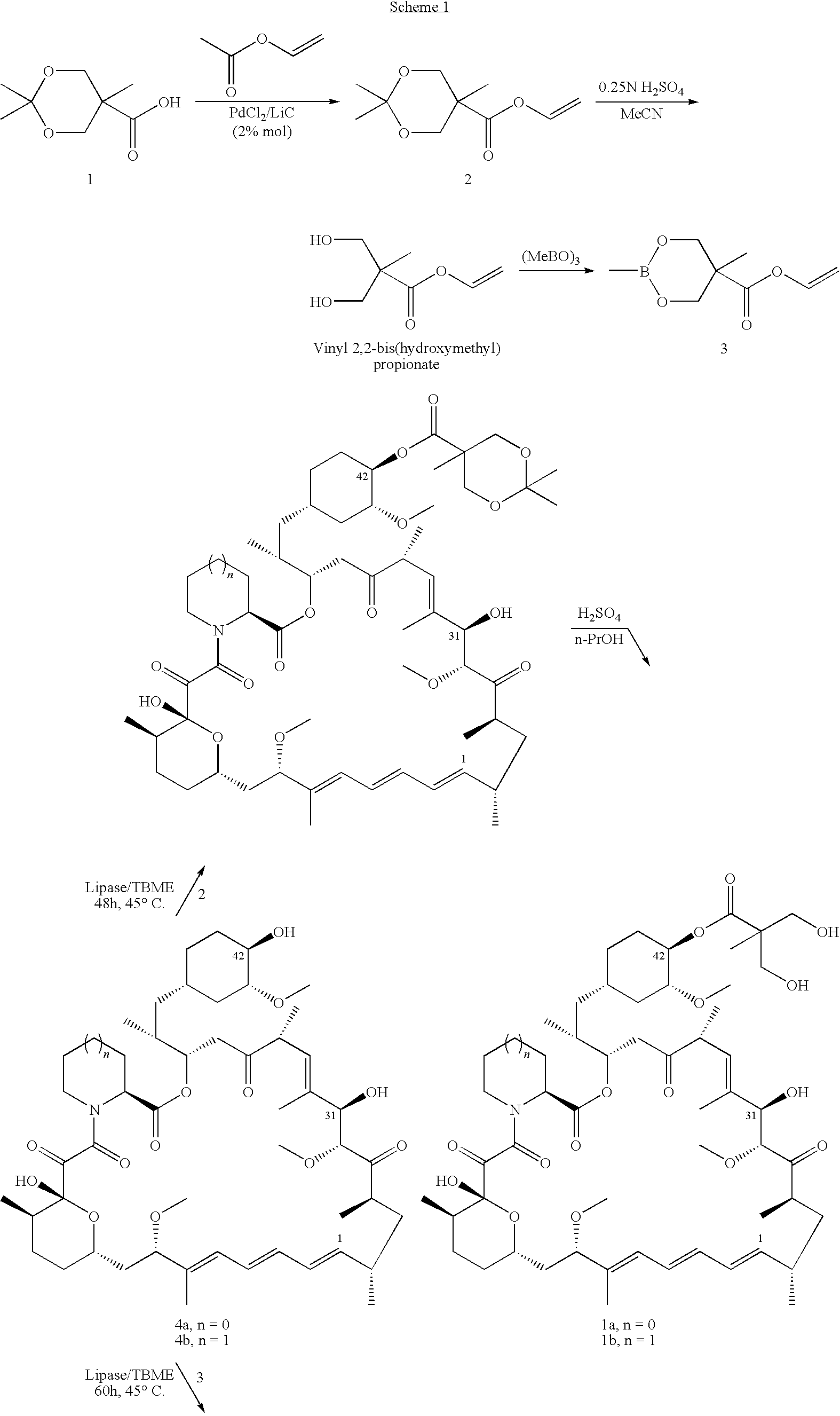
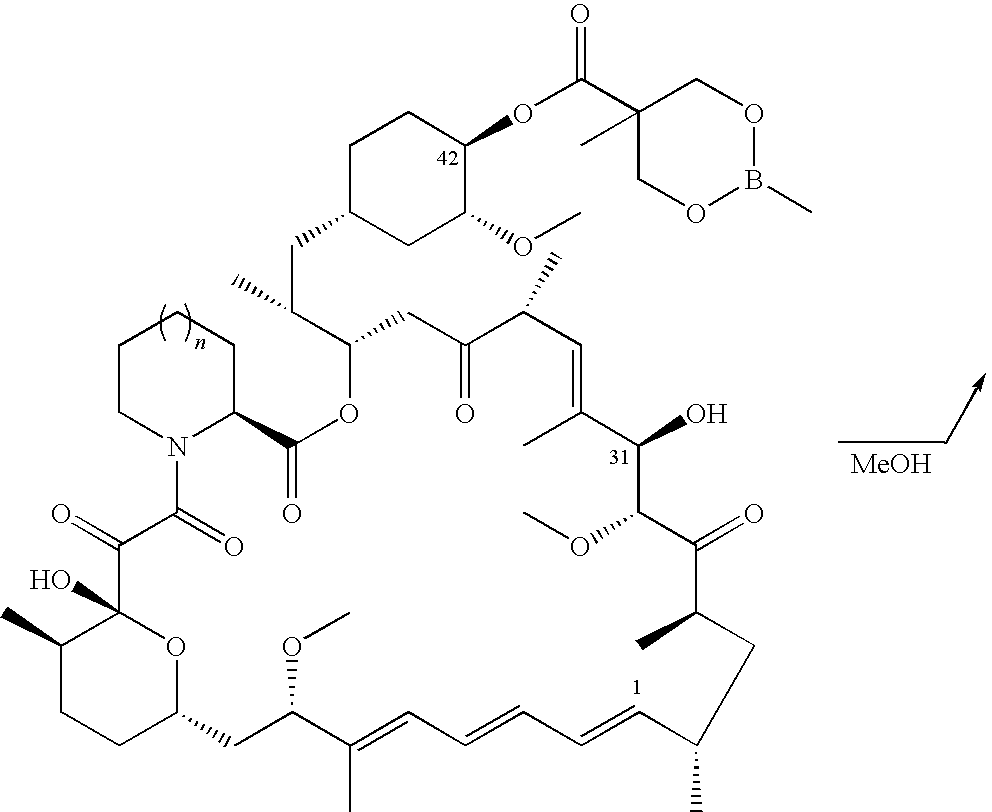
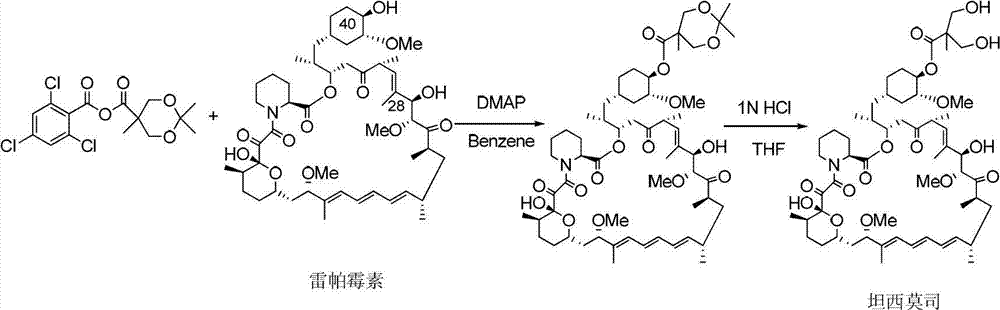
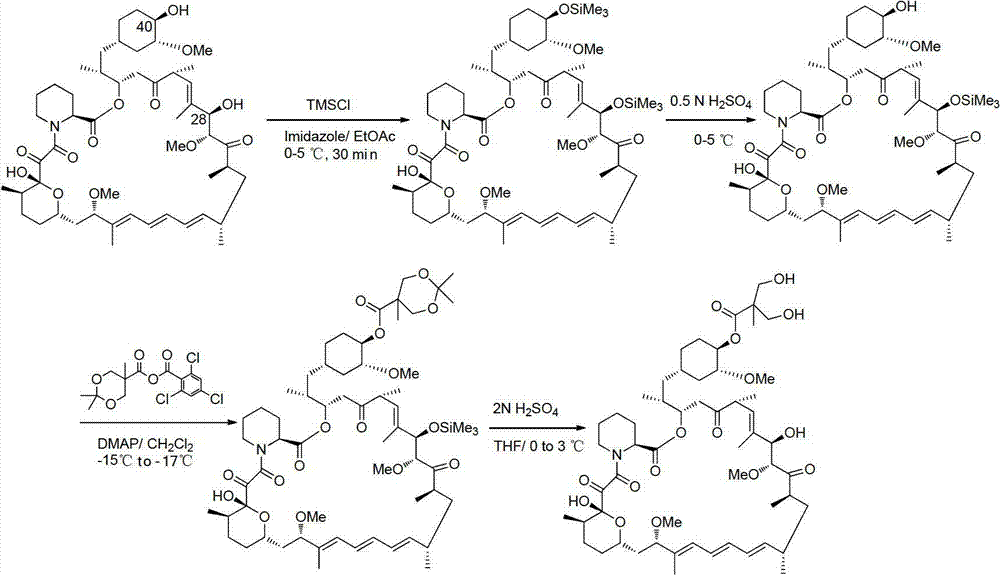
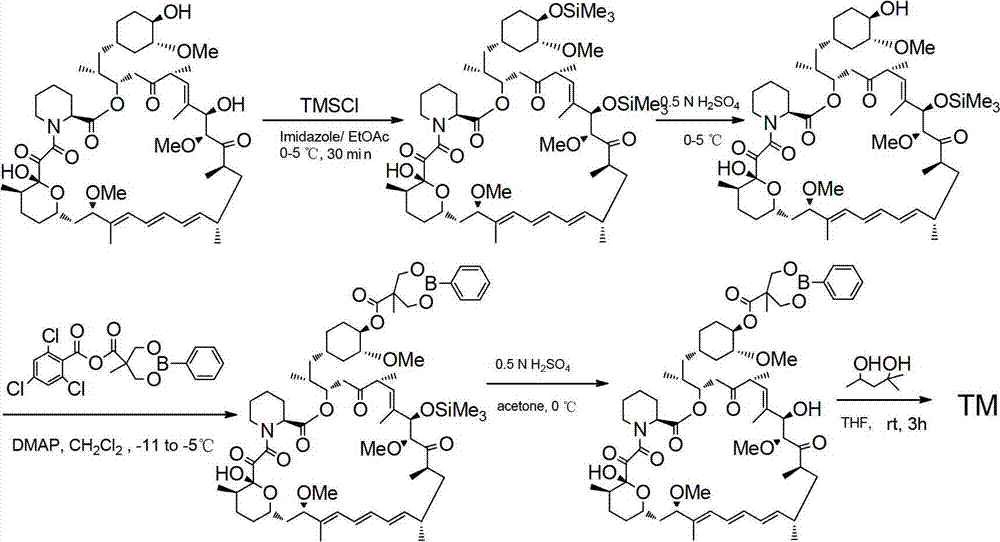
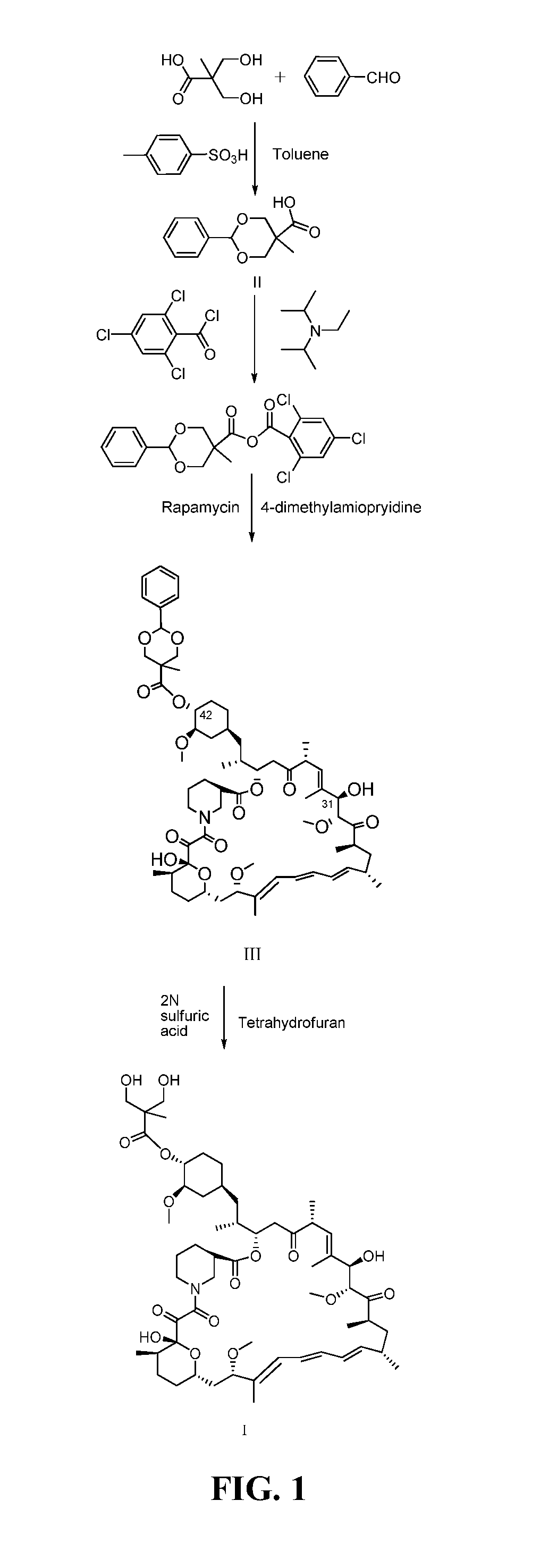
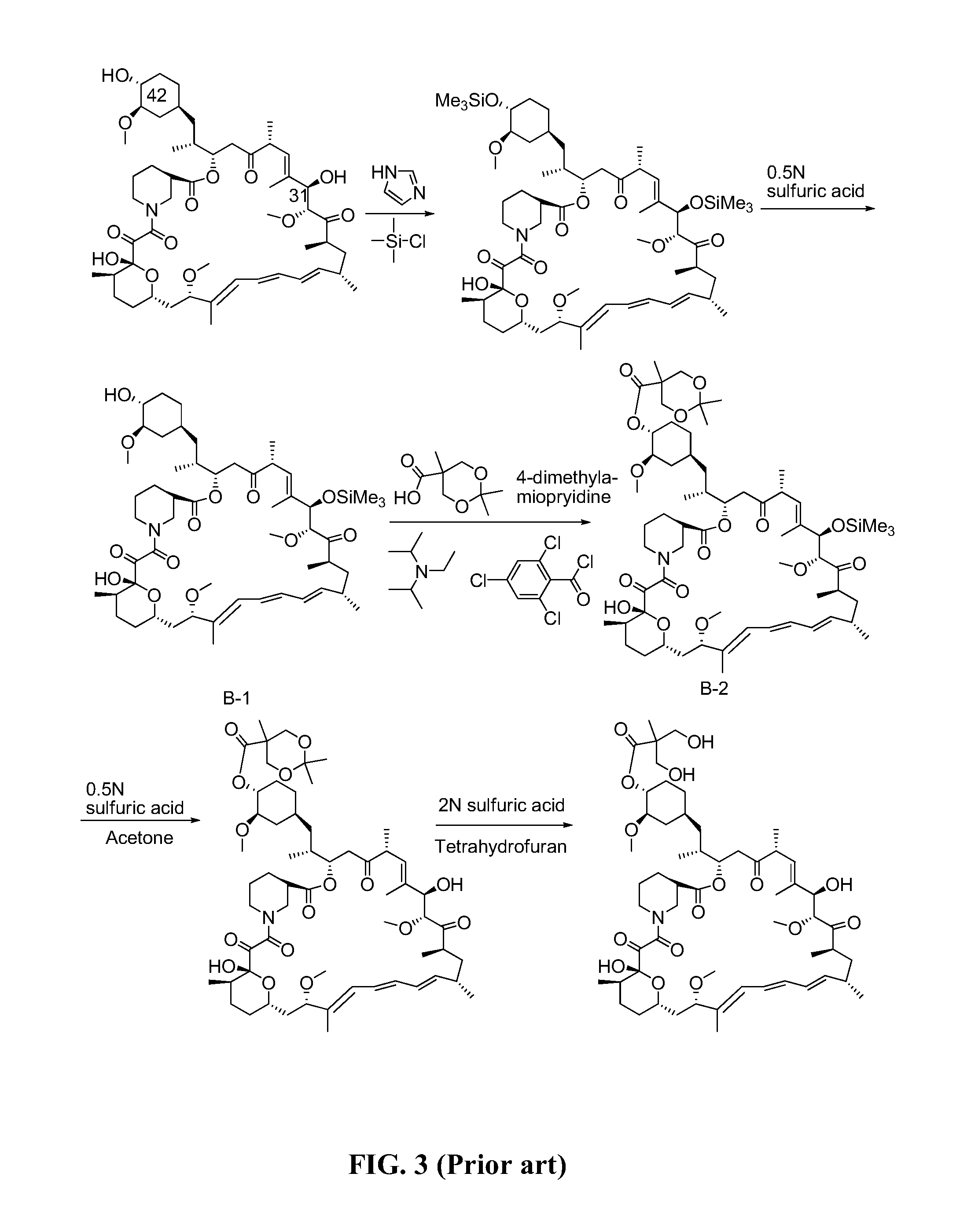
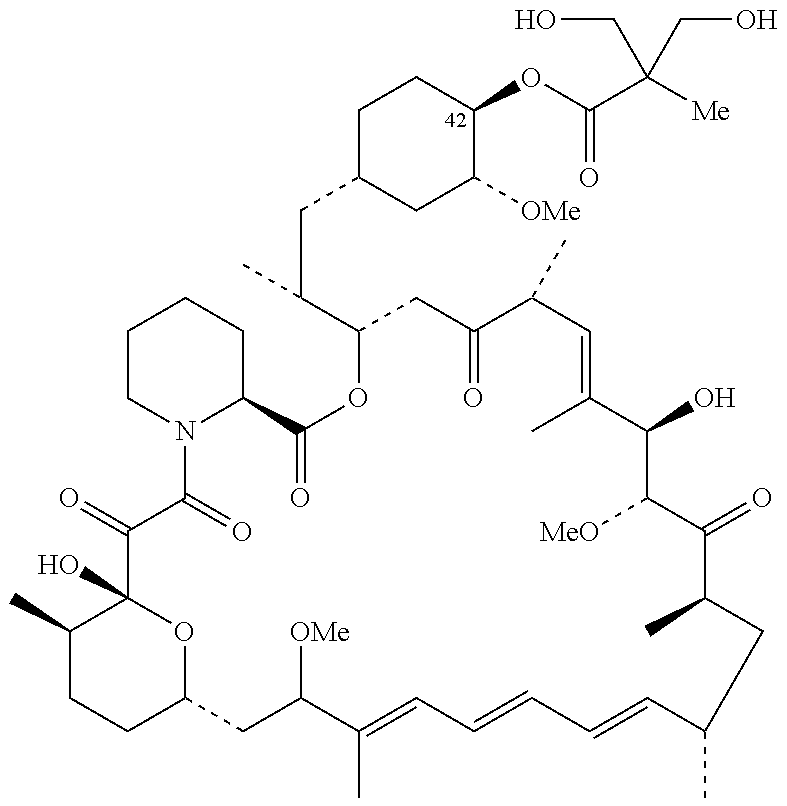
The present invention provides two synthetic routes for the preparation of Temsirolimus (compound 1b and analog of Temsirolimus 1a). The first route includes the synthesis of CCI-779 by directly reacting rapamycin (4b) or Prolyl-rapamycin (4a) with substituent-2,2-bis(methoxy) propionic acid anhydride(11) in the presence of an organic base, followed by deprotection to give CCI-779 or Proline CCI-779. The second route includes a process involving a reaction of rapamycin-OH-31-sily ether (4d) or Prolyl-rapamycin-OH-31-sily ether (4c) with substituent-2,2-bis(methoxy) propionic acid anhydride(11) in the presence of an organic base and followed by subsequent hydrolysis step to obtain the desired CCI-779 or Proline CCI-779.Compound 11, as described in this invention, is stable at room temperature, cost effective and ease of processing.
Owner:CHUNGHWA CHEM SYNTHESIS & BIOTECH
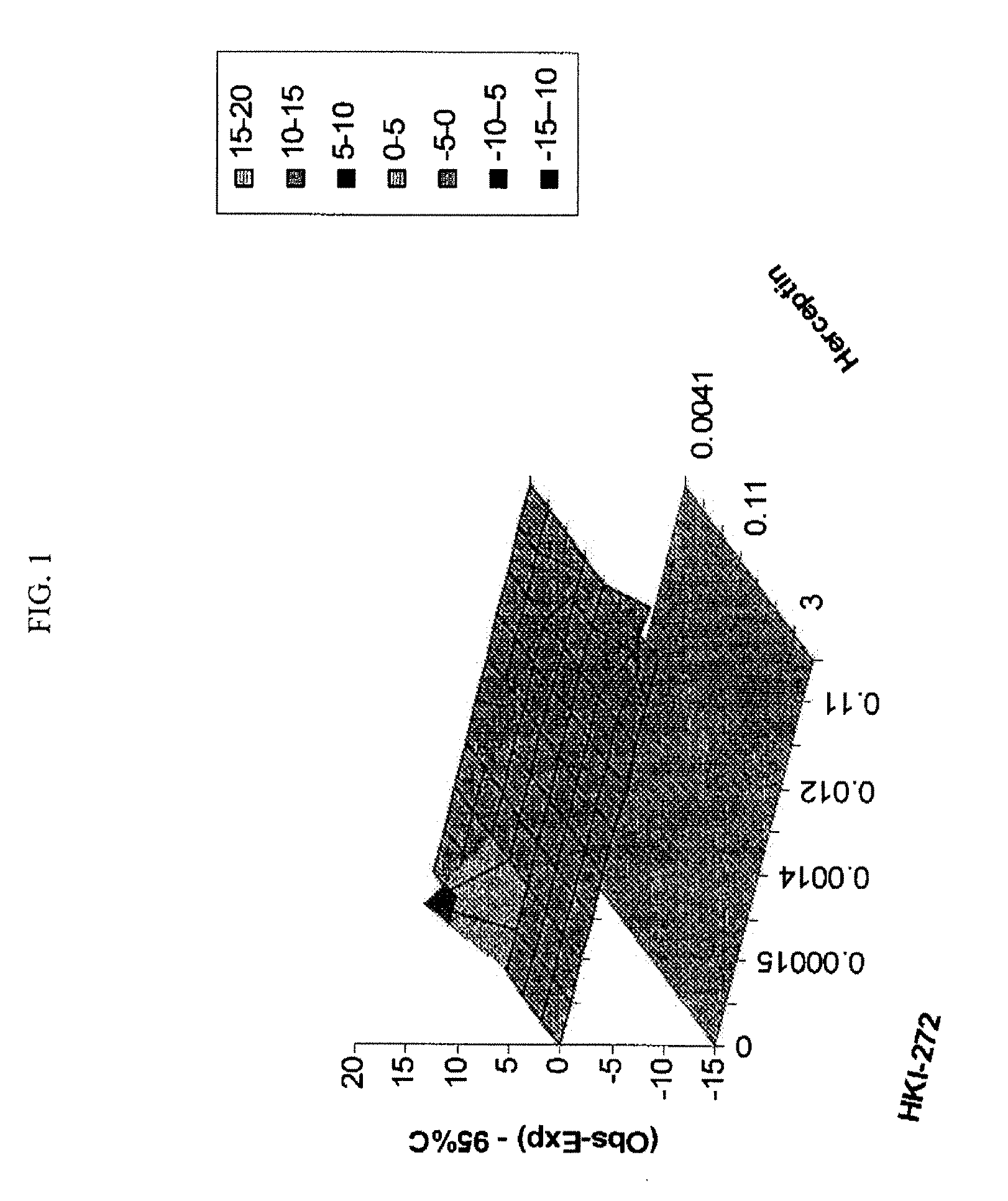
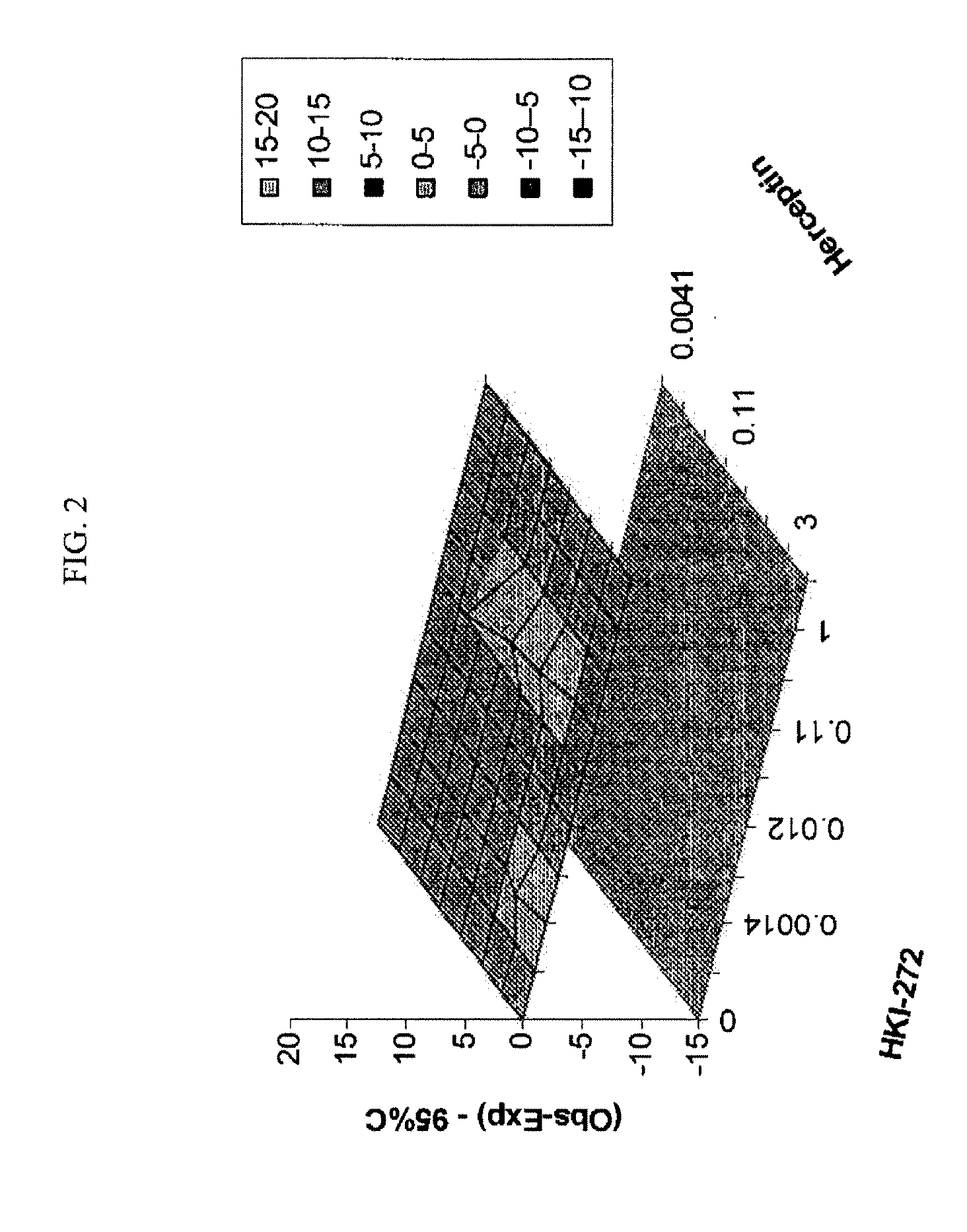
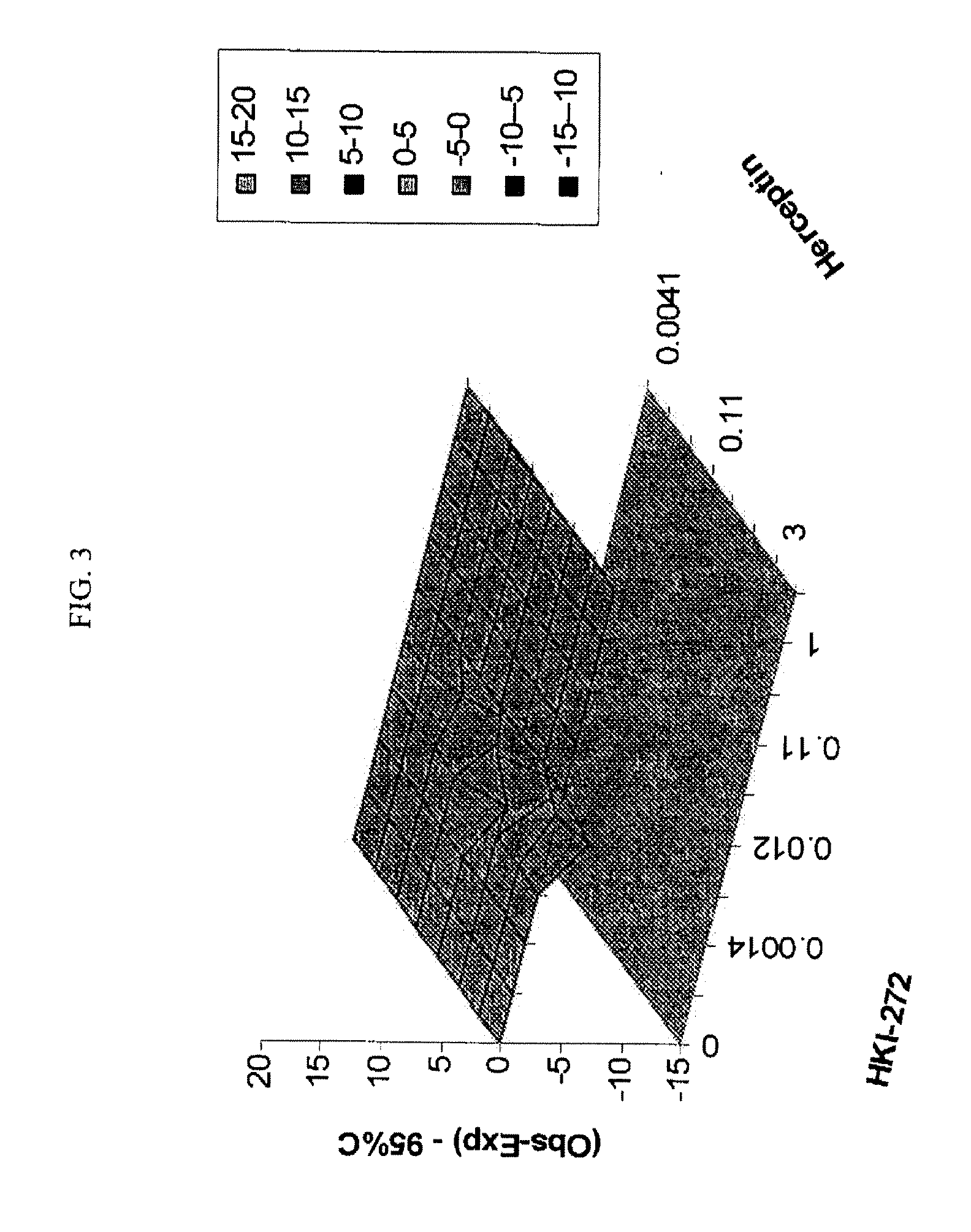
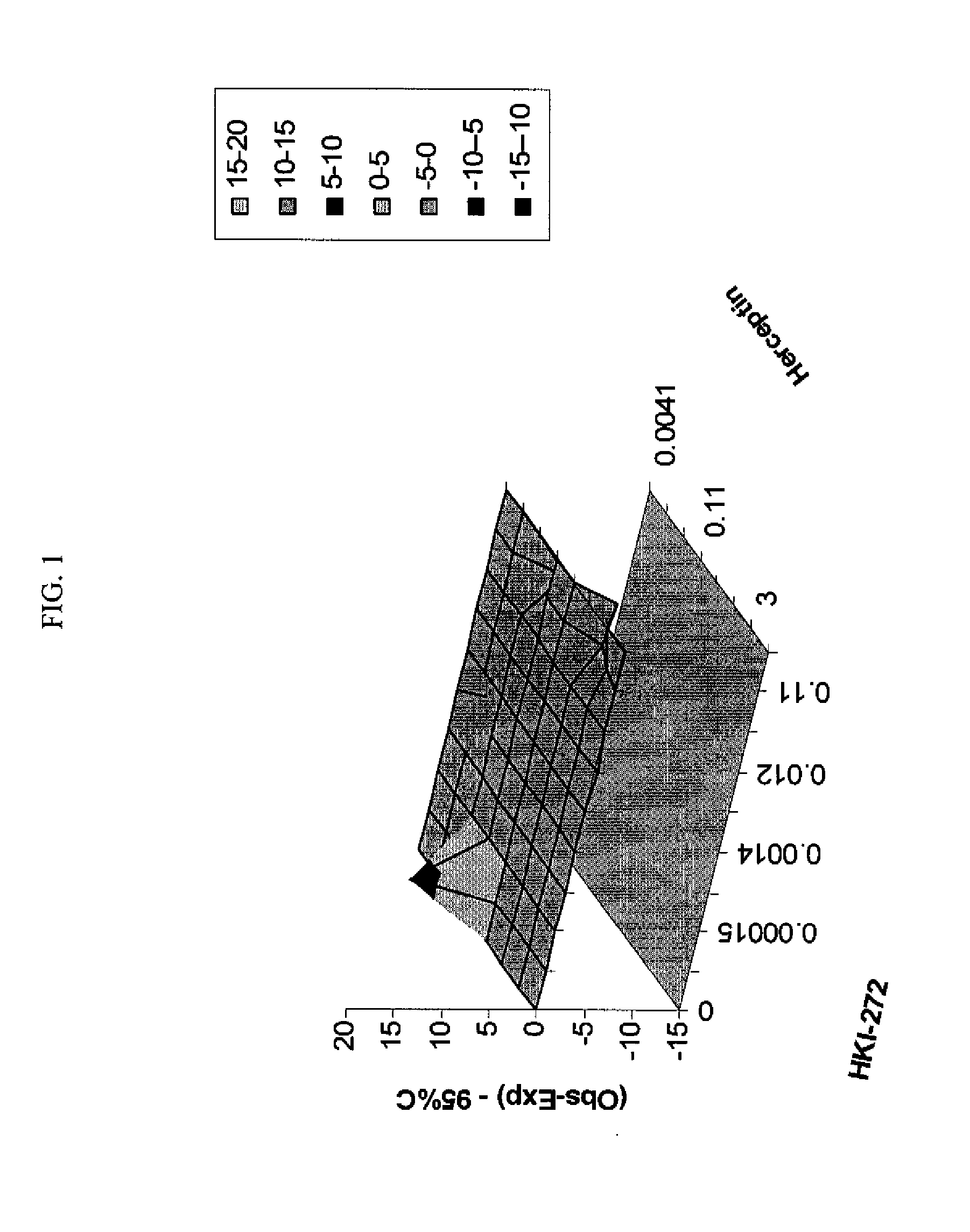
Antineoplastic Combinations with mTOR Inhibitor, Trastuzumab and/or HKI-272
A combination of temsirolimus and trastuzumab in the treatment of cancer is provided. A combination of temsirolimus and HKI-272 is provided. A combination of a trastuzumab and a HKI-272 is also provided. Regimens and kits for treatment of metastatic breast cancer, containing trastuzumab, temsirolimus and / or HKI-272, optionally in combination with other anti-neoplastic agents, or immune modulators are described.
Owner:WYETH LLC
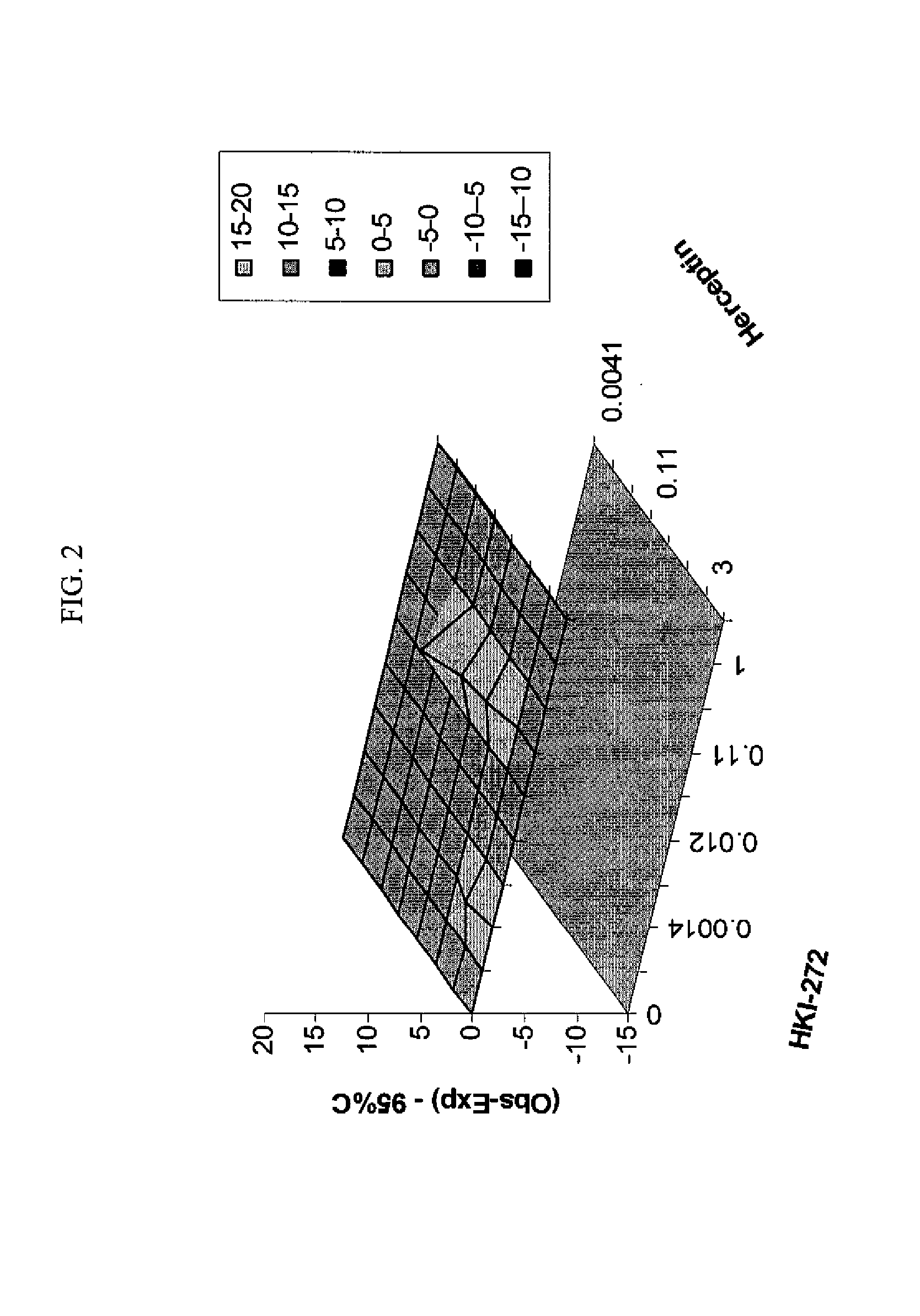
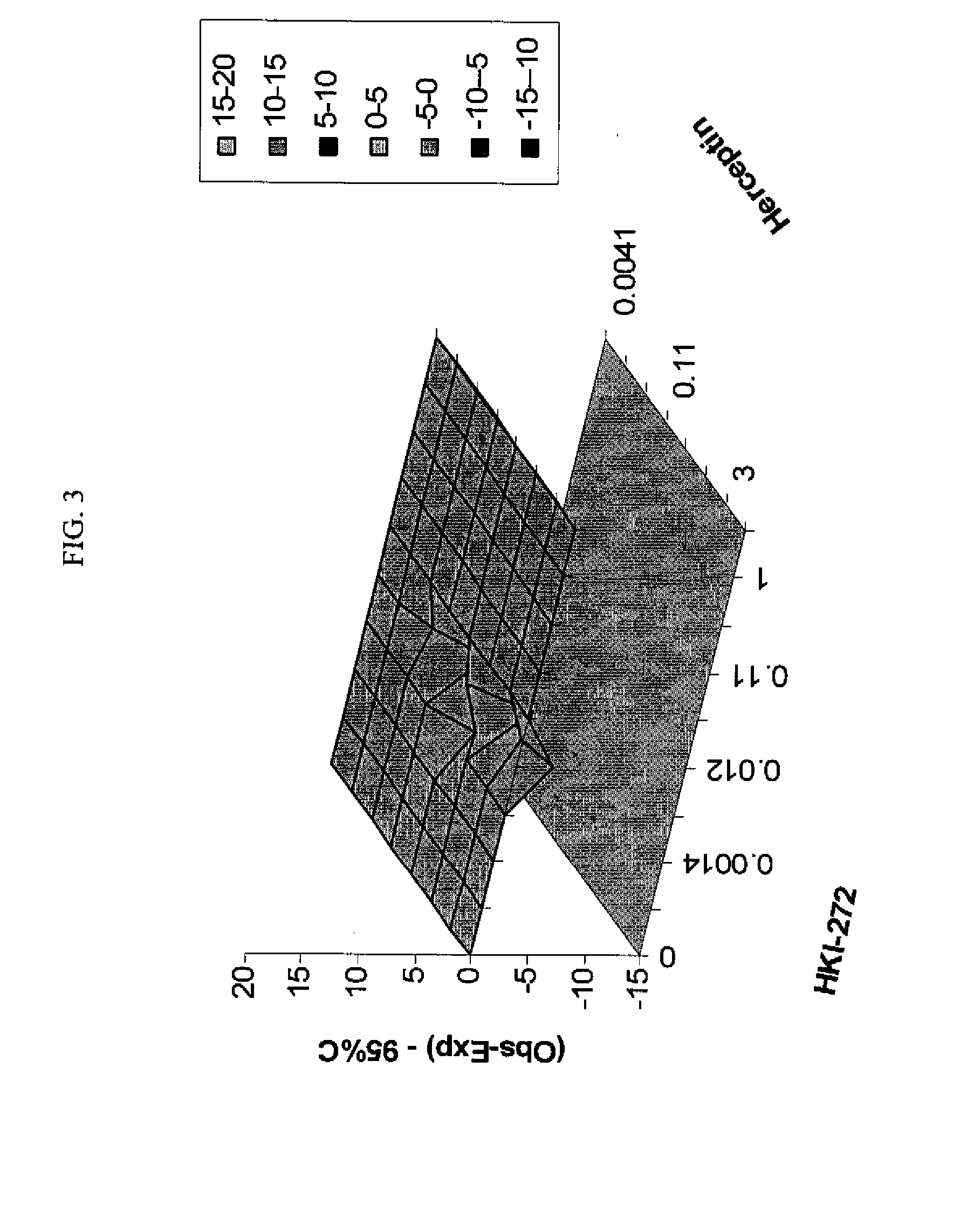
ANTINEOPLASTIC COMBINATIONS WITH mTOR INHIBITOR, TRASTUZUMAB, AND/OR HKI-272
A combination of temsirolimus and trastuzumab in the treatment of cancer is provided. A combination of temsirolimus and HKI-272 is provided. A combination of a trastuzumab and a HKI-272 is also provided. Regimens and kits for treatment of metastatic breast cancer, containing trastuzumab, temsirolimus and / or HKI-272, optionally in combination with other anti-neoplastic agents, or immune modulators are described.
Owner:WYETH LLC
Administration of mntor inhibitor to treat patients with cancer
Owner:ARIAD PHARMA INC
Combination therapy
InactiveUS20100266590A1High dose levelReduced incidence and severityOrganic active ingredientsPeptide/protein ingredientsEverolimusDose level
Disclosed are methods for treating various cancers. Methods encompass the administration of a first drug such as AP23573, temsirolimus or everolimus in combination with a second drug selected from Remicade, Humira, Enbrel, Raptiva, Abatacept, Actermra, Cimzia or anakinra.The methods are aimed at providing a desirable therapeutic window while maintaining prior, if not higher, dose levels of the first drug.
Owner:DEMETRI GEORGE D +2
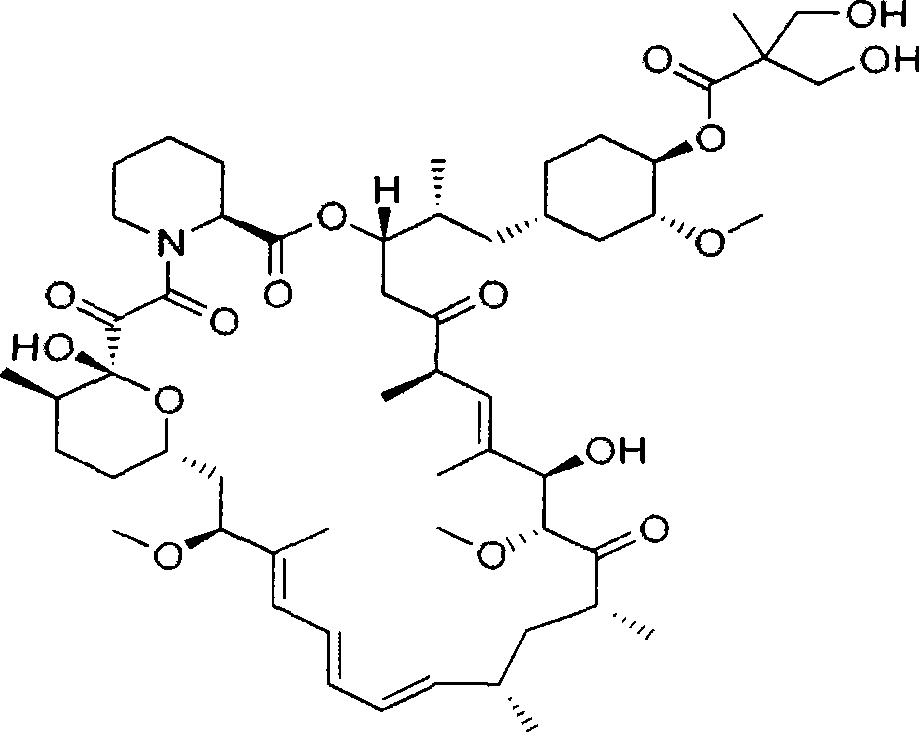
Temsirolimus for injection and preparation method thereof
ActiveCN103099806AAdvantages and Notable ImprovementsImprove stabilityOrganic active ingredientsAntineoplastic agentsAlcoholFreeze-drying
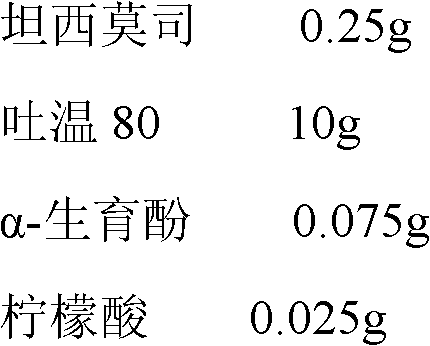
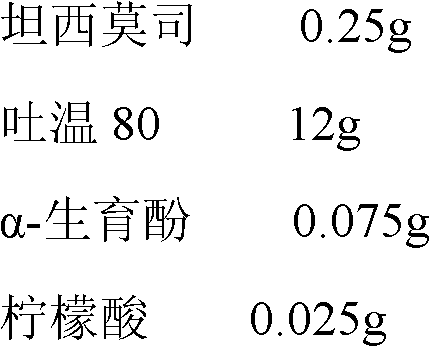
The invention belongs to the technical field of pharmaceutical preparations, and concretely relates to temsirolimus for injection and a preparation method thereof. The preparation method comprises dissolving a prescribed amount of temsirolimus and an anti-oxidant in anhydrous alcohol, mixing uniformly, adding a dispersant, re-mixing uniformly, freeze drying and removing the ethanol to obtain the temsirolimus. The preparation provided by the invention is few in prescription component; and the preparation method is convenient and easy, improves disadvantages of complex technology in present prescription, and greatly minimizes components such as alcohols and the like that may cause injection pain, thereby being relatively safe and reliable.
Owner:SHANDONG NEWTIME PHARMA
Combination therapy for treatment of restenosis
Described herein are methods for distributing a combination of a cell division inhibitor (e.g., temsirolimus or paclitaxel) and dexamethasone to a tissue surrounding a blood vessel for treating vascular diseases. Also disclosed are injectable compositions of a cell division inhibitor (e.g., temsirolimus or paclitaxel) and dexamethasone for delivery into the tissue surrounding a blood vessel for treating vascular diseases.
Owner:MERCATOR MEDSYST
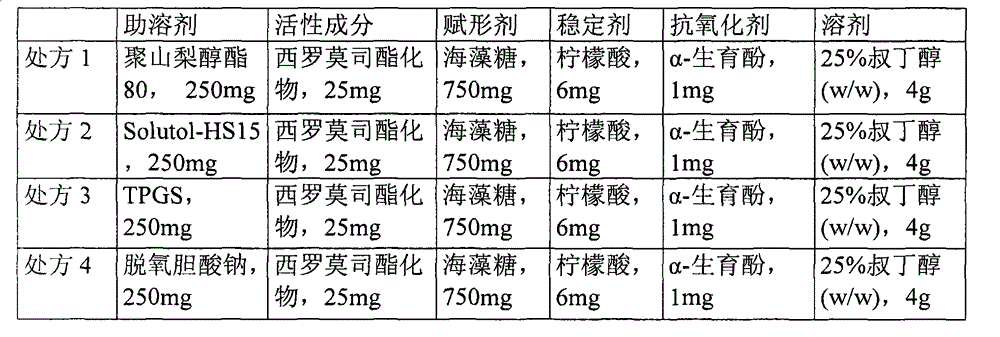
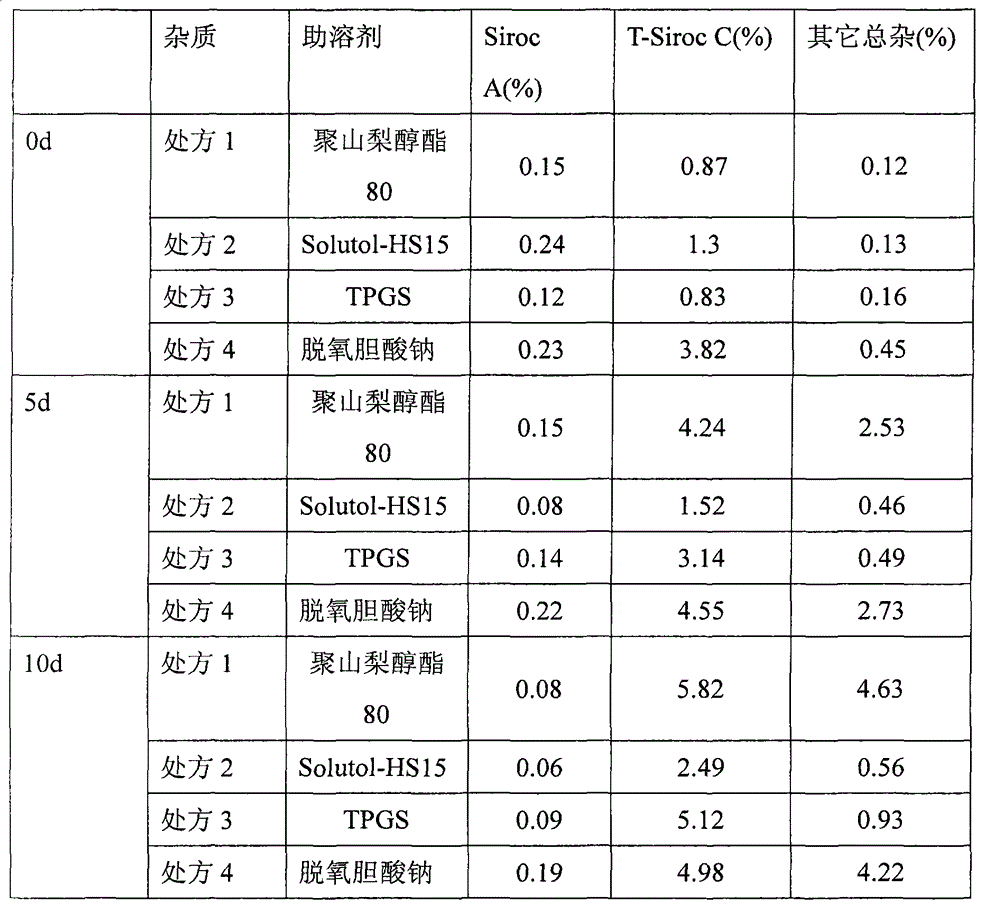
Medicinal composition containing temsirolimus and preparation method of medicinal composition
InactiveCN102940630AAvoid irritationAvoid toxicityOrganic active ingredientsAntimycoticsFreeze-dryingPolyethylene glycol
The invention relates to a medicinal composition containing temsirolimus and a preparation method of the medicinal composition. Polyethylene glycol stearate 15 as latent solvent is added in a formula, so that the problem of settlement generated by clinically using physiological saline to dilute a temsirolimus freeze-dried powder injection is effectively solved, further the content of an isomer of the temsirolimus and the content of oxidative degradation impurities are reduced and the stability and the safety of the medicament are improved. The freeze-dried medicinal composition disclosed by the invention is simple in preparation process and low in production cost and is suitable for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
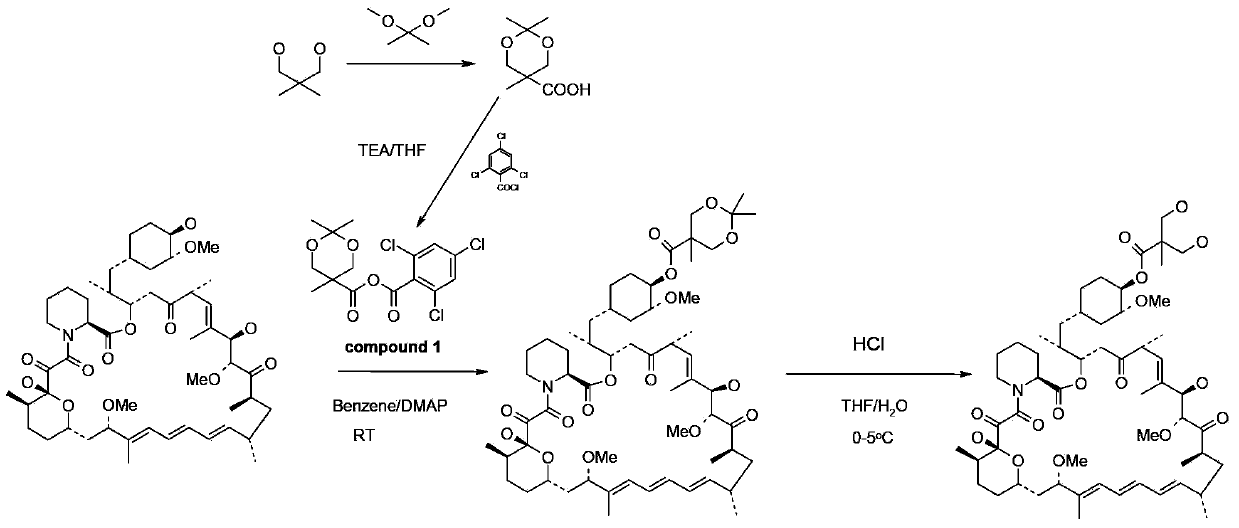
Synthesis process for temsirolimus
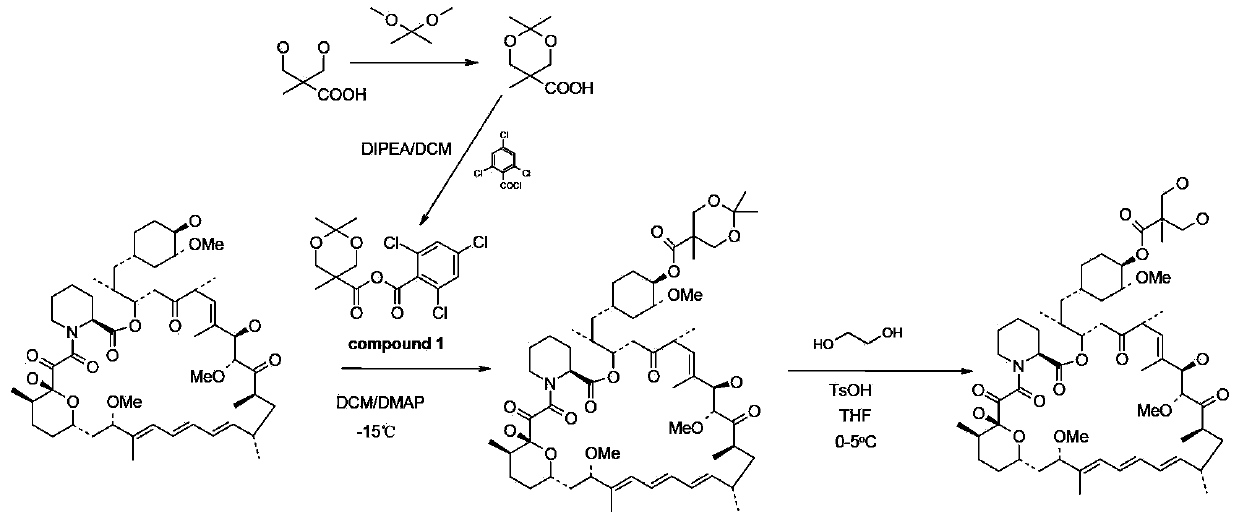
The invention provides a synthesis process for temsirolimus. The synthesis process comprises the following steps: step 1, preparing 2,2,5-trimethyl-5-carboxyl-1, 3-dioxane; step 2, preparing anhydride; step 3, carrying out esterification reaction; step 4, carrying out hydrolysis reaction and finally obtaining the target product, temsirolimus. According to the invention, in the reaction of the step 2, DIPEA (diisopropanolamine) is selected as alkali and methylene chloride is selected as solvent so that anhydride reaction liquid obtained directly can be directly used in the reaction in the step 3, and technological operation is reduced; the selectivity of the esterification reaction is directly achieved by lowering the temperature and controlling the usage amount of DMAP (dimethylaminopyridine) and the usage amount of anhydride, and the by-products of 31-esterification are reduced; esterification selectivity is improved greatly, and the reaction route is simplified; by selecting an ethylene-glycol, para-toluenesulfonic acid and tetrahydrofuran deprotection system, the reaction time is reduced greatly, and the productivity is improved.
Owner:FUJIAN INST OF MICROBIOLOGY
Method for preparing high-purity temsirolimus
InactiveCN104086564AMeet quality requirementsReduce the impactOrganic chemistryChromatographic separationGradient elution
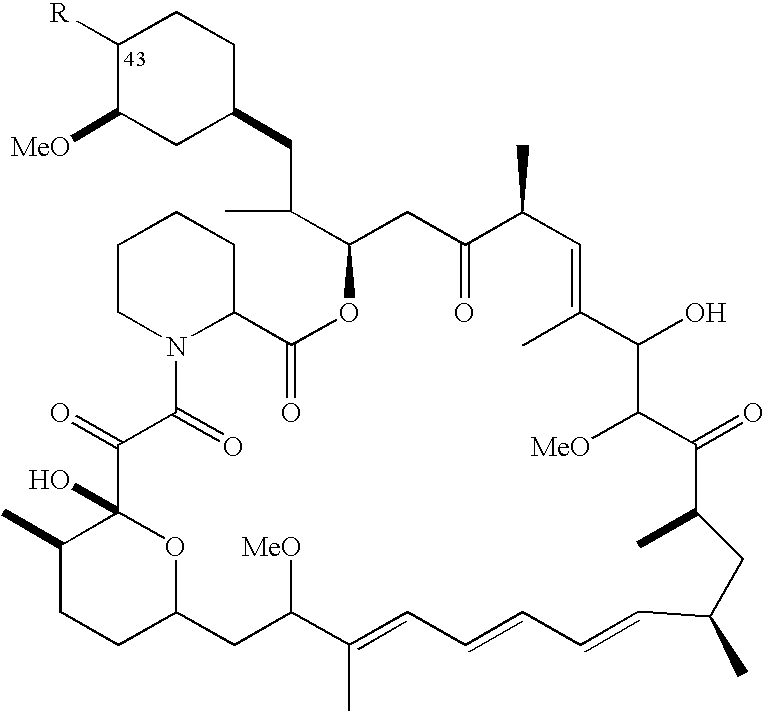
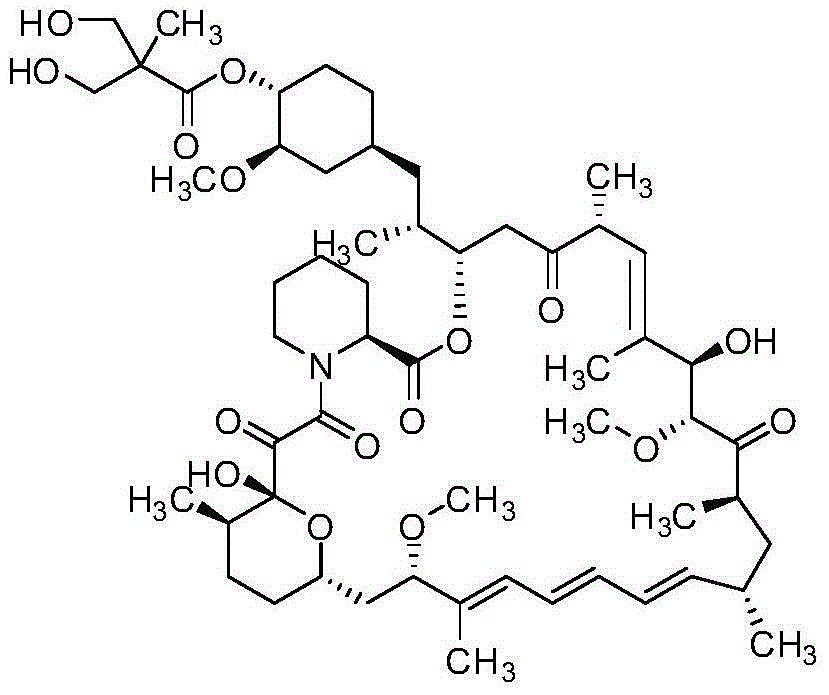
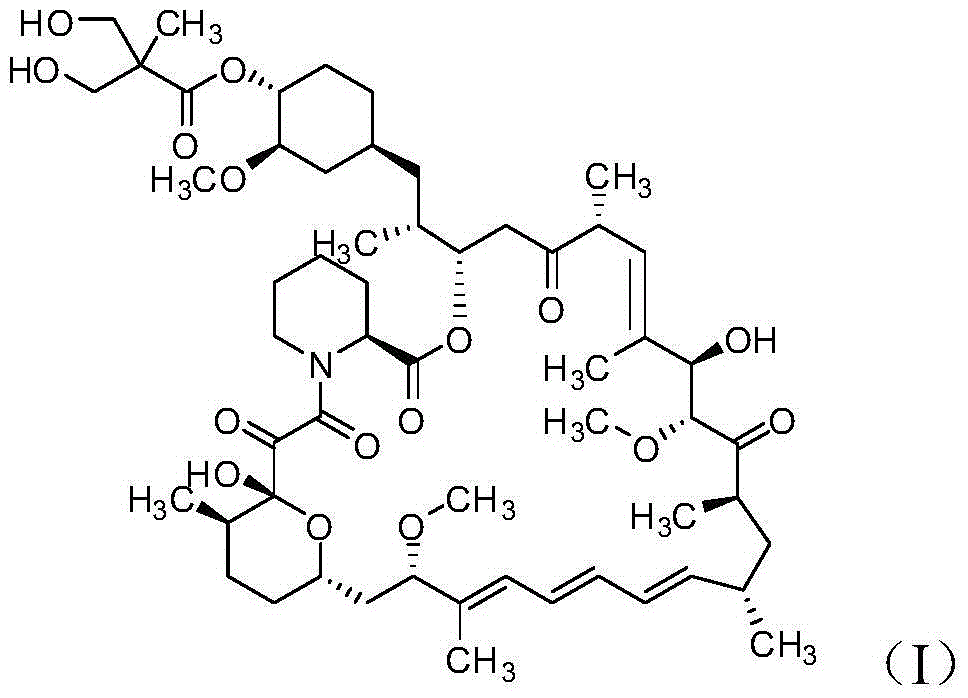
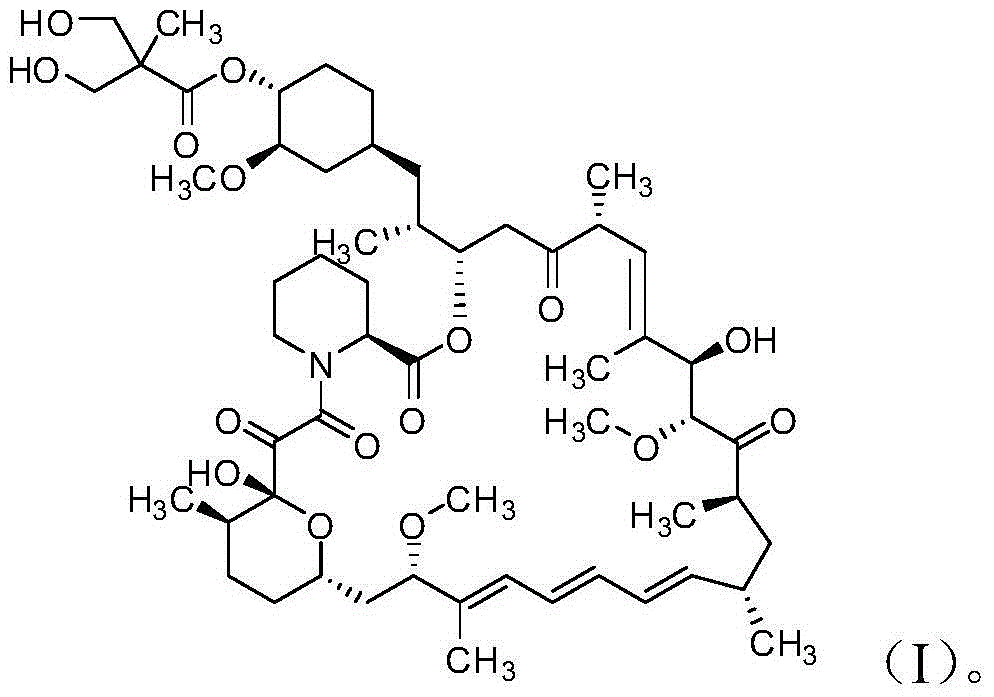
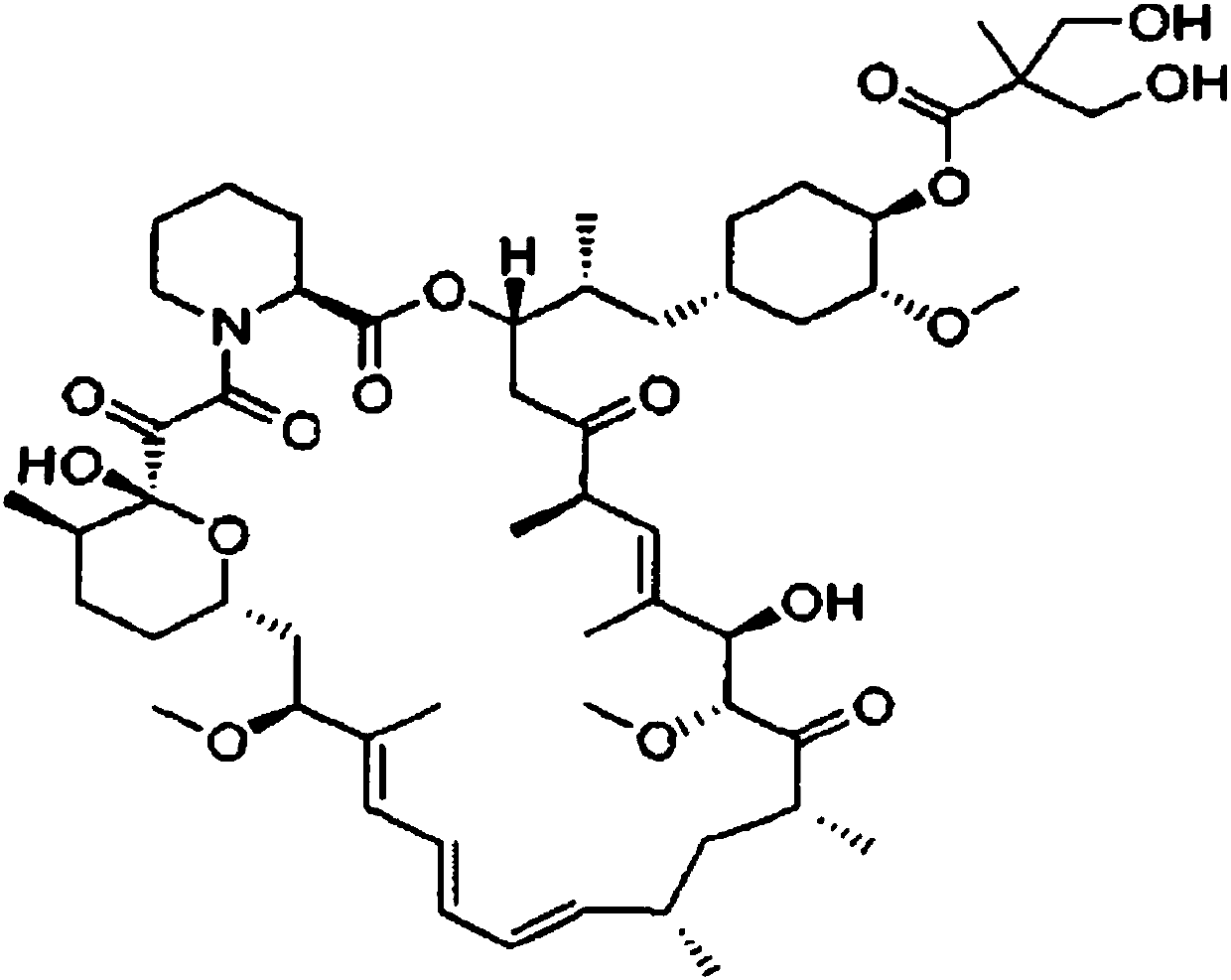
The invention discloses a high-purity temsirolimus represented by the formula (I), the HPLC purity of the temsirolimus can be above 99.5%. The invention also provides a method for preparing high-purity temsirolimus by virtue of a preparative high performance liquid chromatographic separation system. The method comprises, but not limited to the sequence of the steps, the following steps of A) normal-phase high performance liquid chromatography and B) reverse phase high performance liquid chromatographic separation, wherein in the step A), gradient elution is carried out by taking a silicon ball filler as a stationary phase, ethyl acetate of which the concentration is 60-100v / v% and n-hexane or n-heptane of which the concentration is 40-0v / v% as mobile phases; in step B), gradient elution is carried out by taking C4, C6, C8 or C18 fillers as stationary phases and acetonitrile and aqueous solution of which the pH value is 3.5-6.0 as mobile phases; and the temperature of the system is 10-30 DEG C. The process is simple and environmentally friendly and high-purity and high-quality temsirolimus can be obtained by virtue of the process.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Senolytic compounds
PendingCN110678187AHalogenated hydrocarbon active ingredientsCyclic peptide ingredientsDiseaseNitrofurazone
The present invention relates to an agent for use in selectively killing one or more senescent cells, wherein the agent is selected from the following: a cardiac glycoside or alglycone, a focal adhesion kinase (FAK) inhibitor, an HMG-CoA reductase inhibitor, JFD00244, Cyclosporine, Tyrphostin AG879, Cantharidin, Diphenyleneiodonium chloride, Rottlerin, 2,3-Dimethoxy-1,4-naphthoquinone, LY-367,265,Rotenone, Idarubicin, Dequalintum chloride, Vincristine, Nitazoxanide, Nitrofurazone, Temsirolimus, Eltrombopag, Adapalene, Azacyclonol, Enoxacin and Raltegravir, and pharmaceutically acceptable salts thereof. Another aspect relates to compounds for use in treating or preventing a senescence- associated disease or disorder, and methods relating thereto.
Owner:英国研究与创新公司
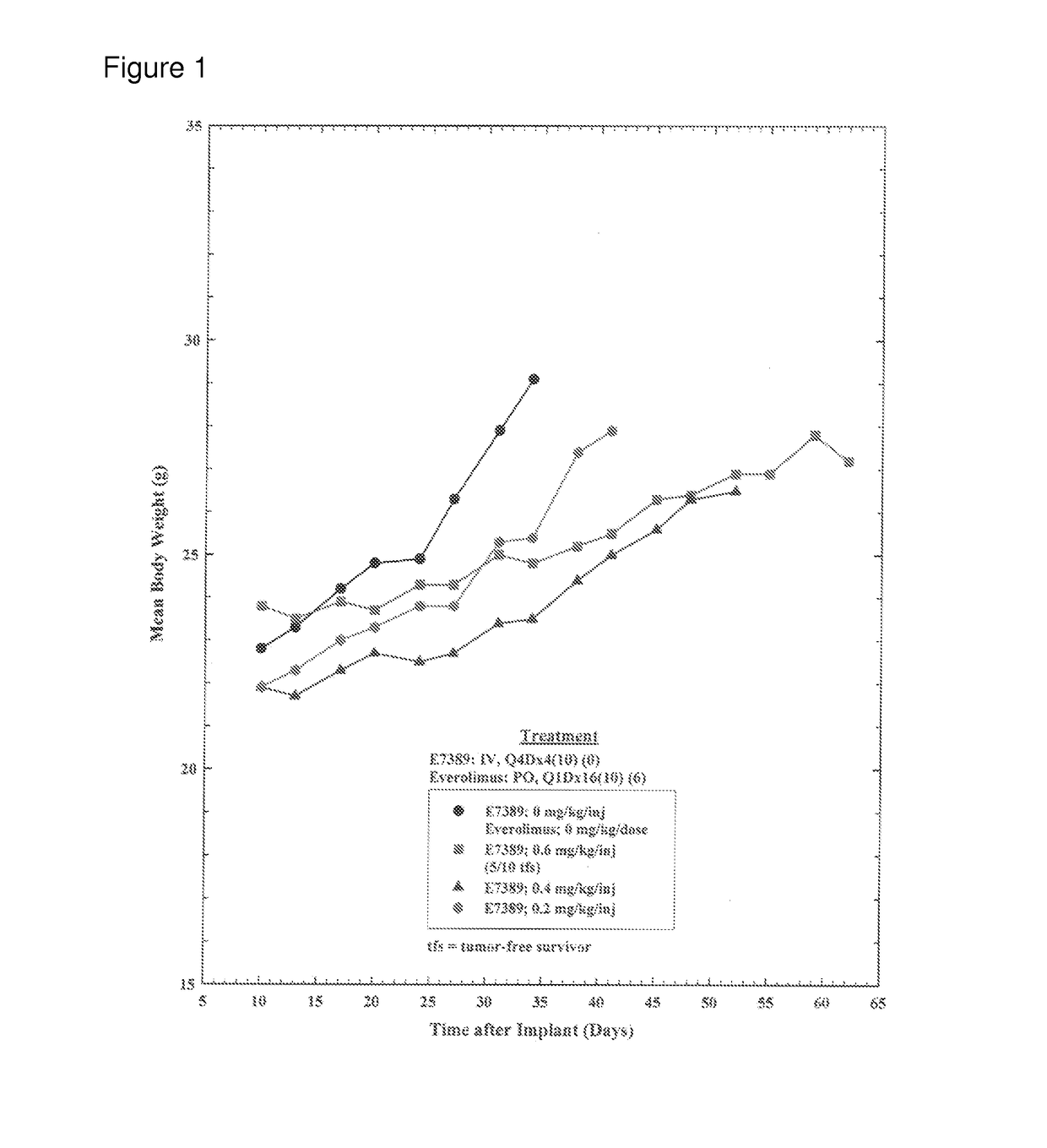
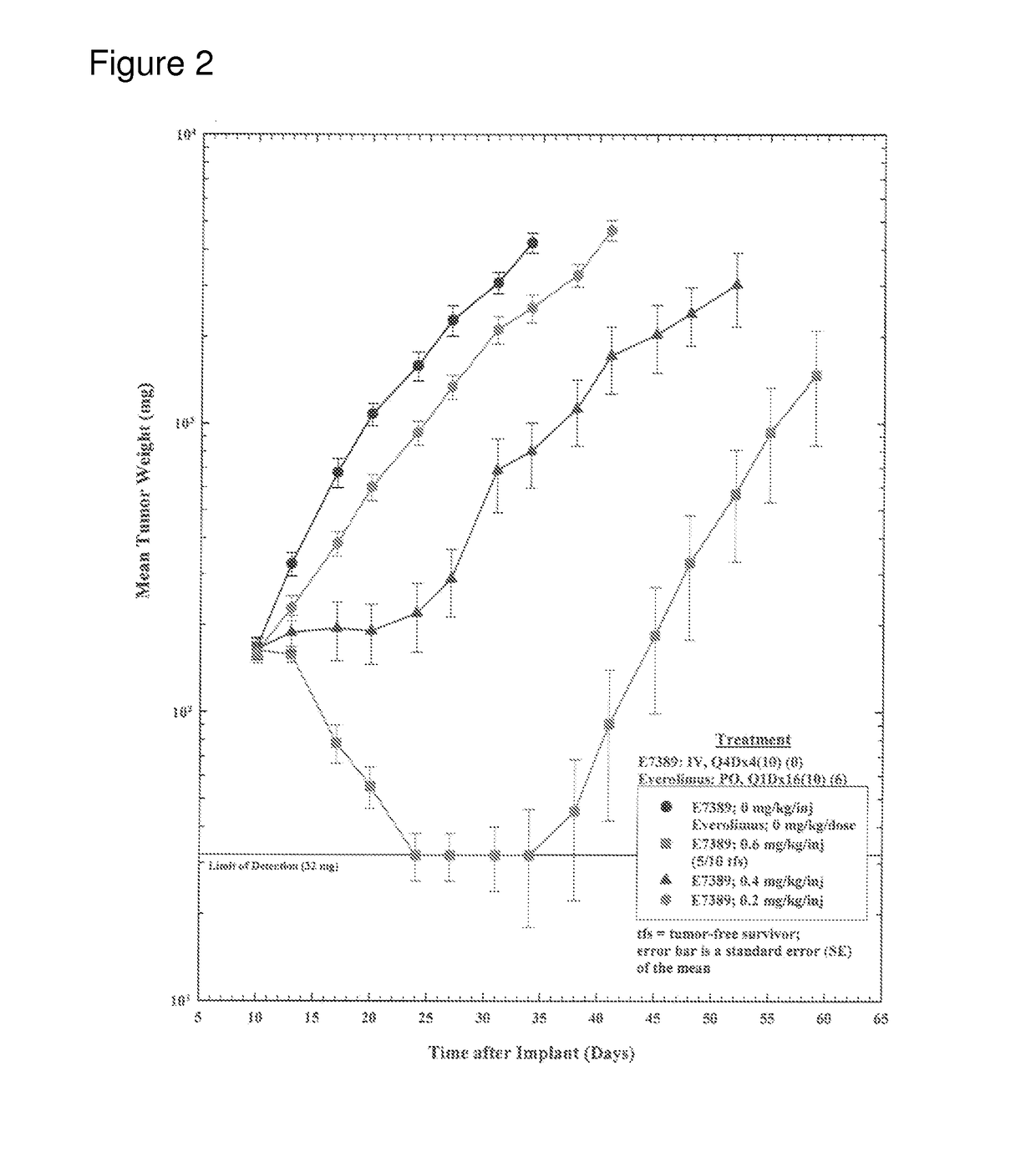
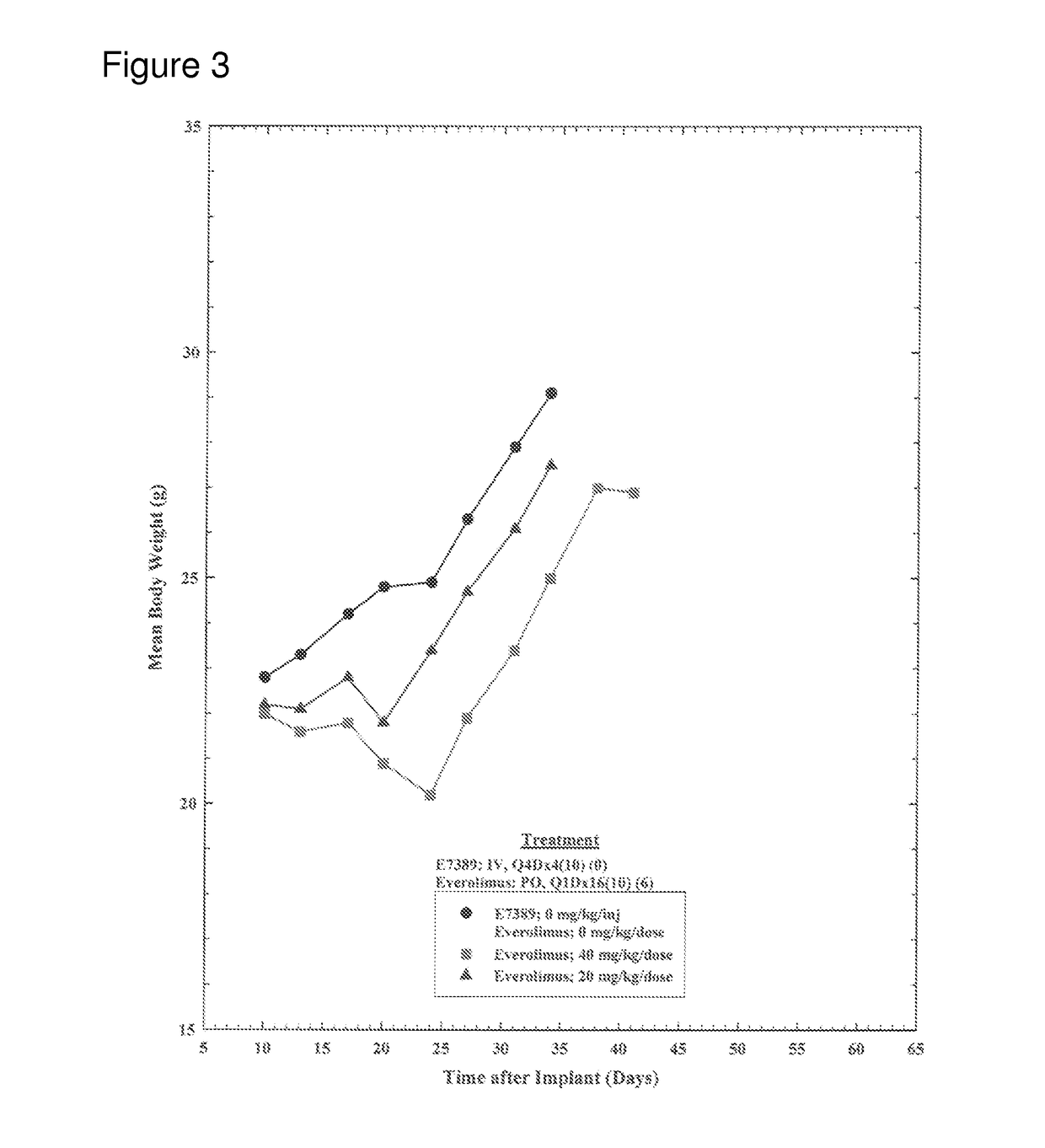
Use of eribulin and mtor inhibitors as combination therapy for the treatment of cancer
InactiveUS20170071903A1Reduce in quantityReduce tumor volumeOrganic active ingredientsPharmaceutical delivery mechanismEribulinPharyngeal cancer
Methods for treating cancer (e.g., breast cancer, lung cancer, pancreatic cancer, primitive neuroectodermal tumors, lung cancer, ovarian cancer, endometrial cancer, pharyngeal cancer, esophageal cancer, and sarcoma) in a subject (such as an human patient) in need thereof by administering eribulin (e.g., eribulin mesylate, i.e., E7389, Halaven) in combination with one or more mammalian target of rapamycin (mTOR) inhibitors (e.g., everolimus, ridaforolimus, and temsirolimus), and kits therefor are provided.
Owner:EISIA R&D MANAGEMENT CO LTD
Treatment of restenosis using temsirolimus
ActiveUS20180169075A1Reduce vessel stiffeningReduce thickeningOrganic active ingredientsSolution deliveryRestenosisVascular disease
Described herein are methods for distributing temsirolimus to a tissue surrounding a blood vessel for treating vascular diseases. Also disclosed are injectable compositions of temsirolimus for delivery into the tissue surrounding a blood vessel for treating vascular diseases.
Owner:MERCATOR MEDSYST
Anticancer Treatments
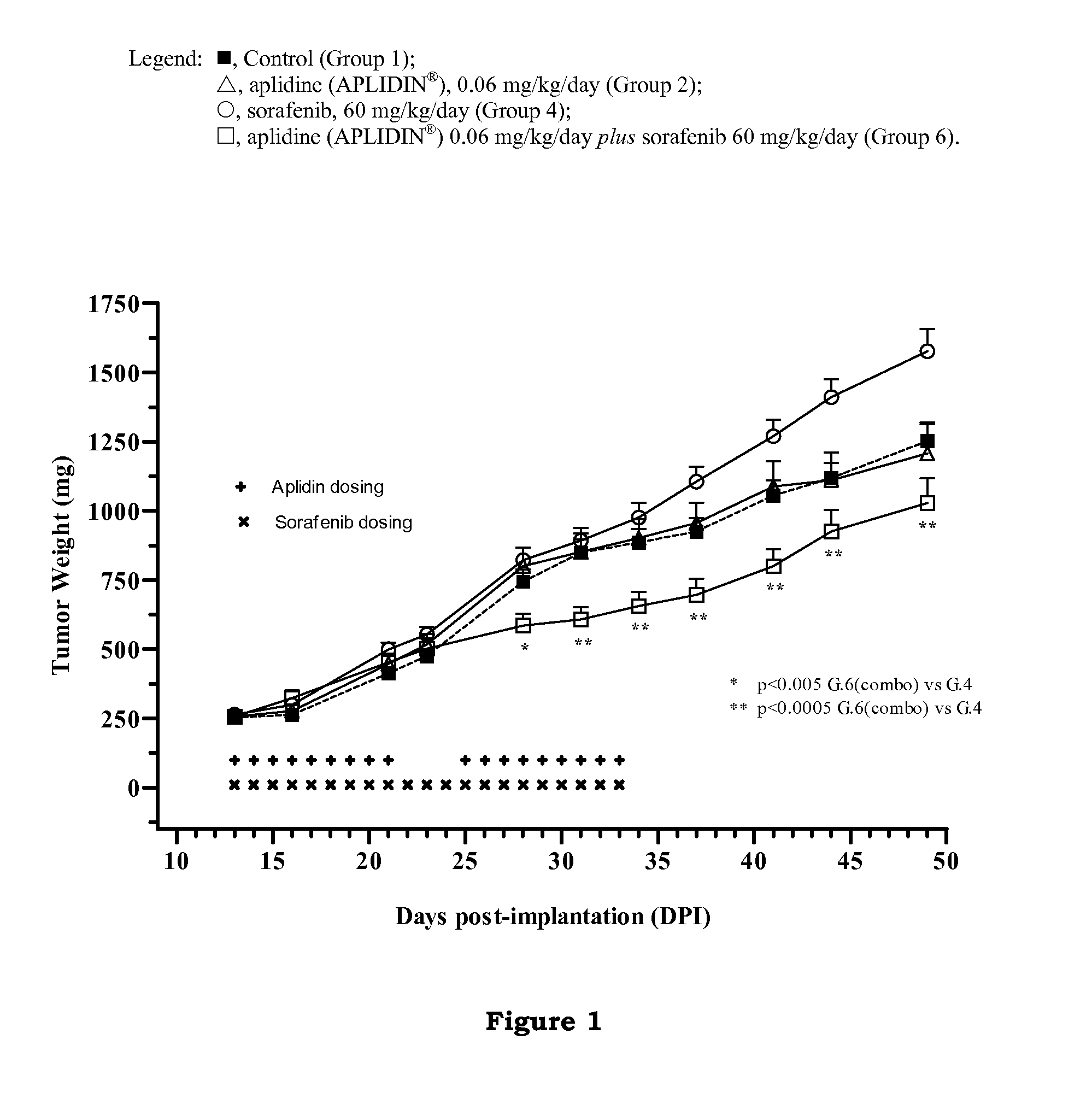
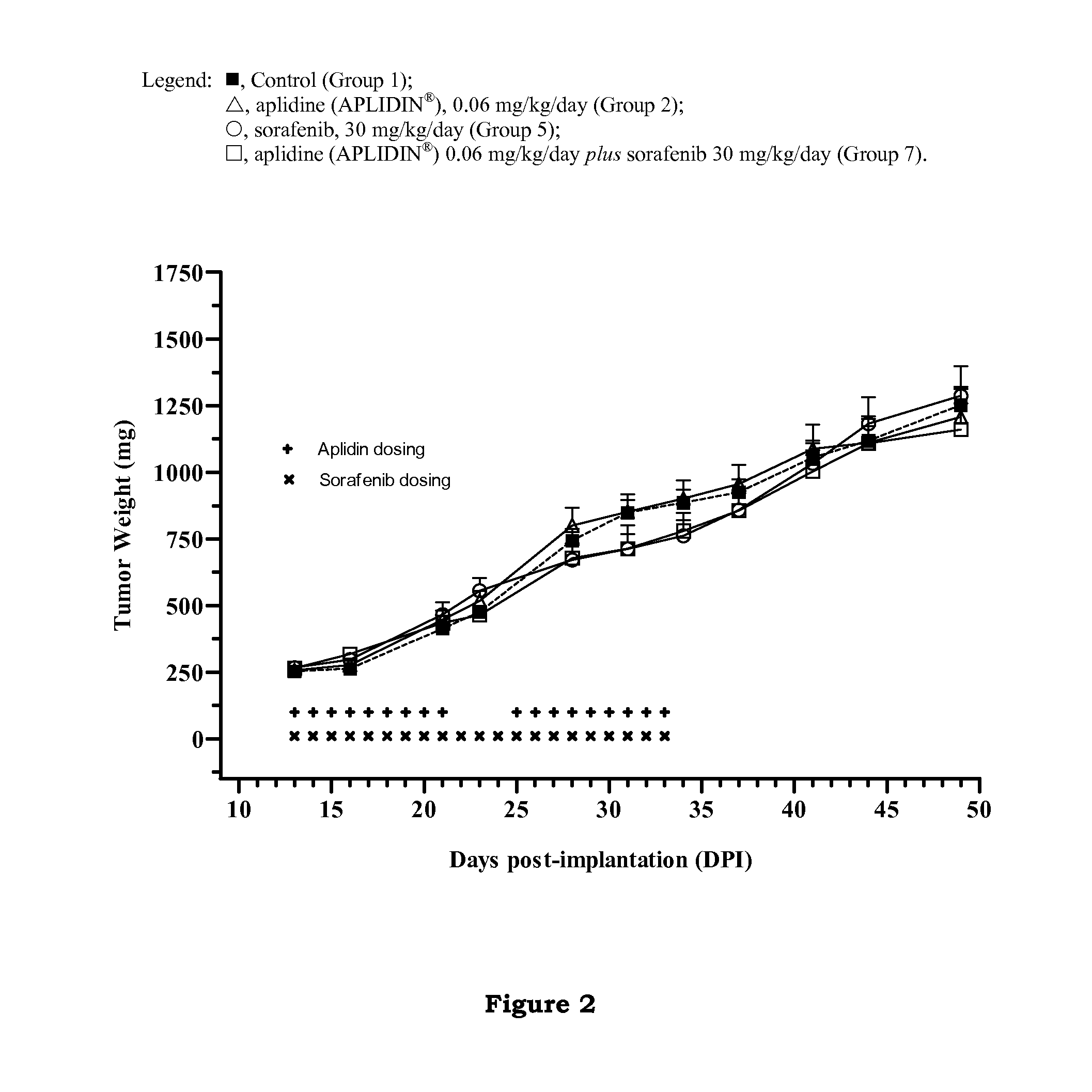
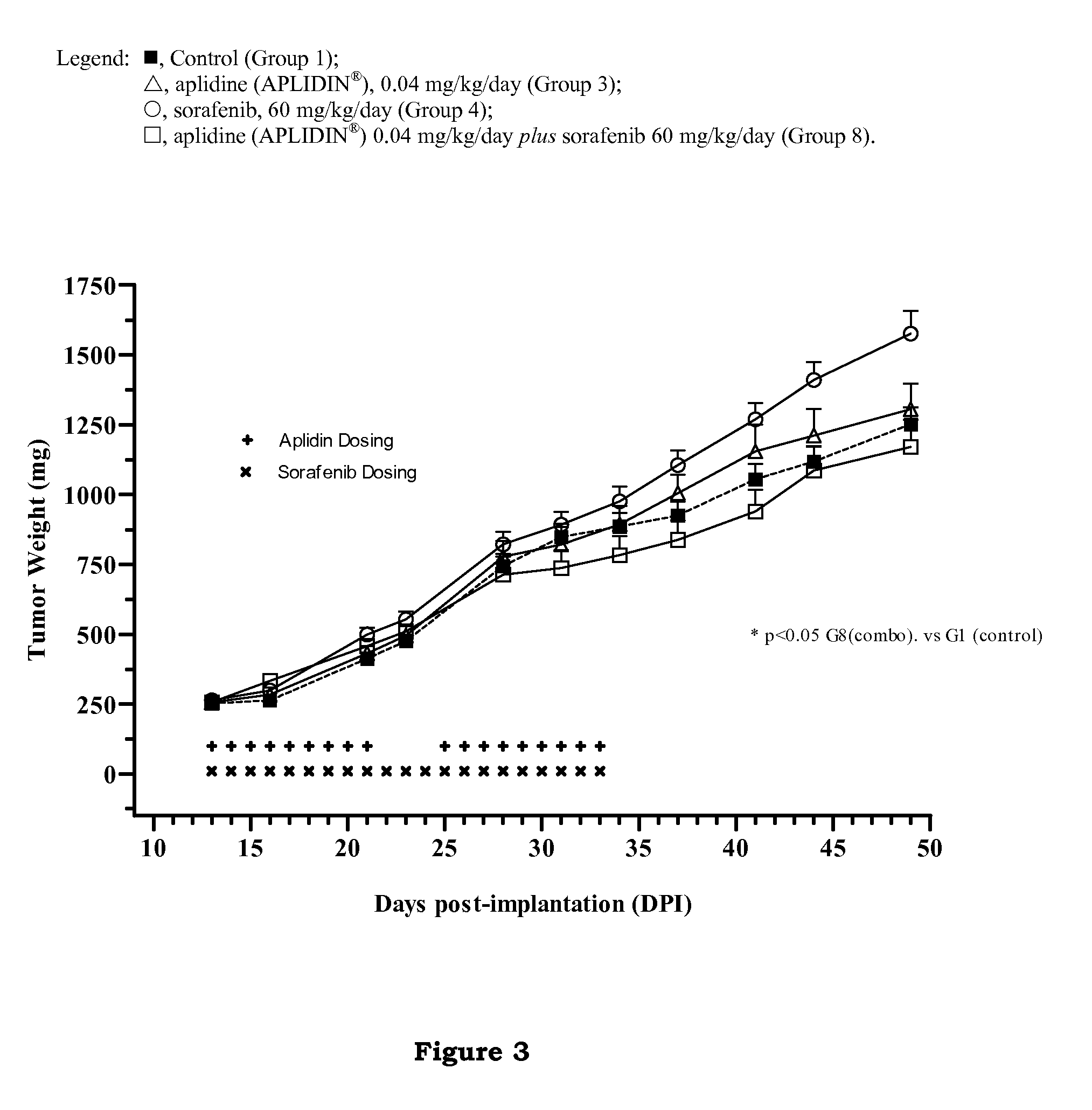
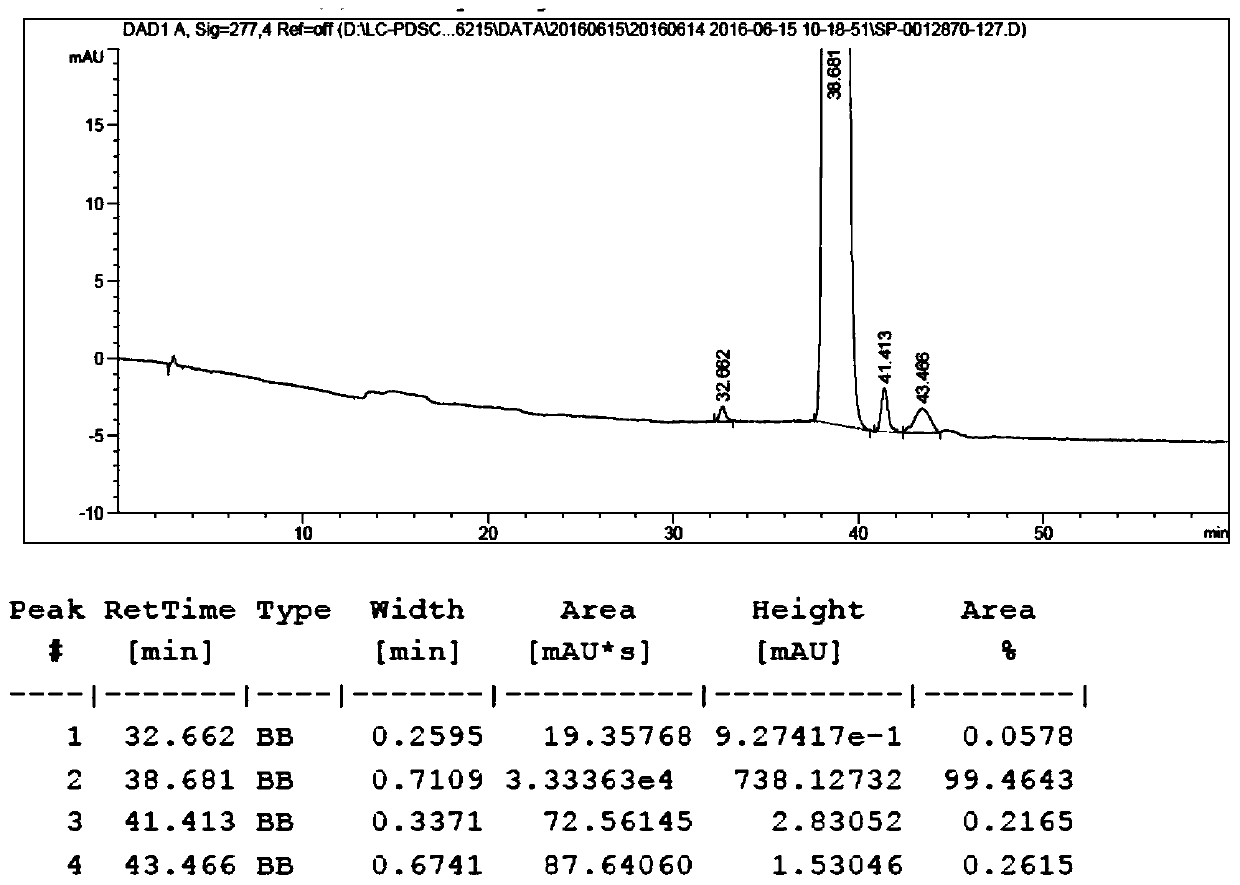
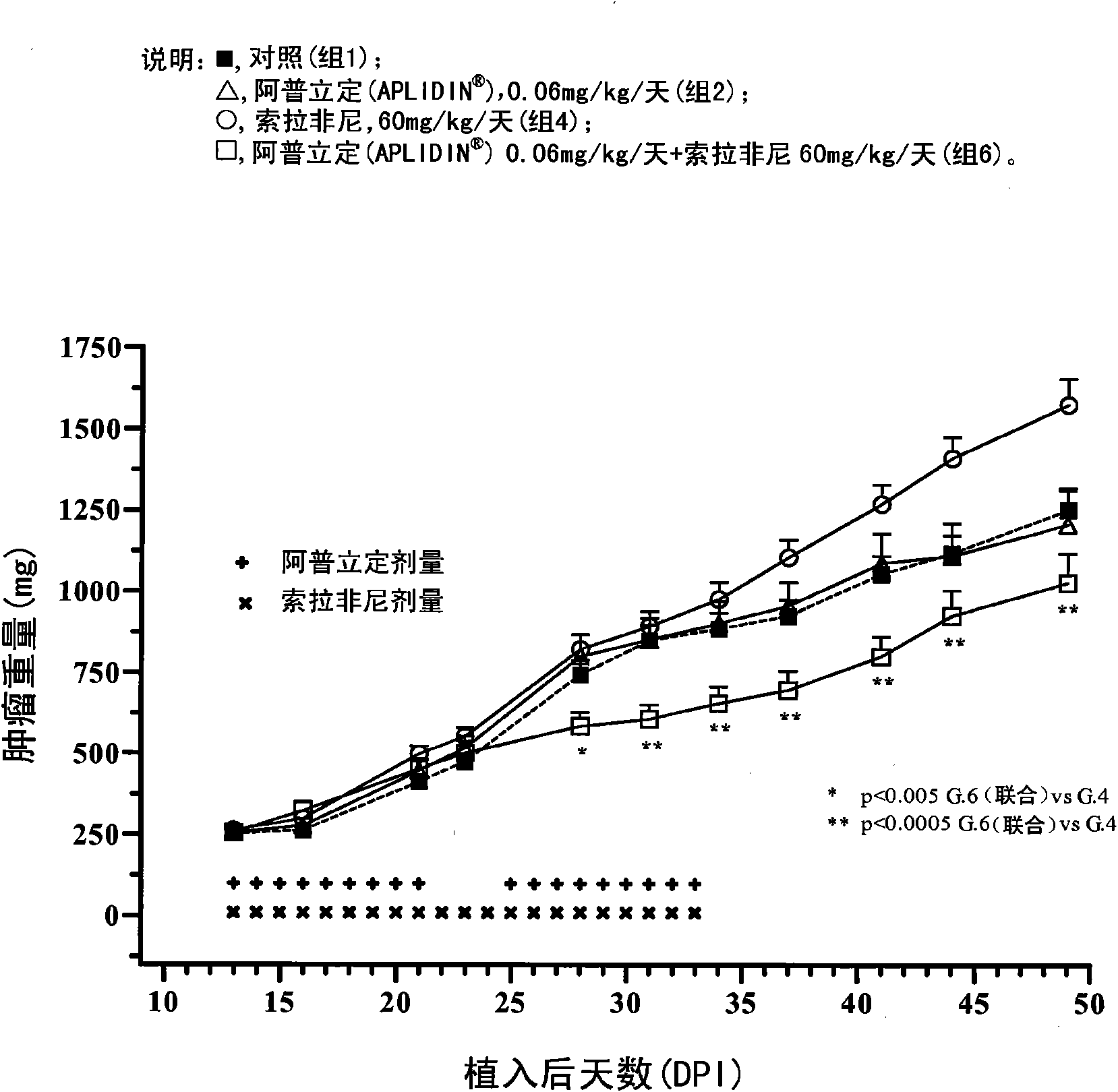
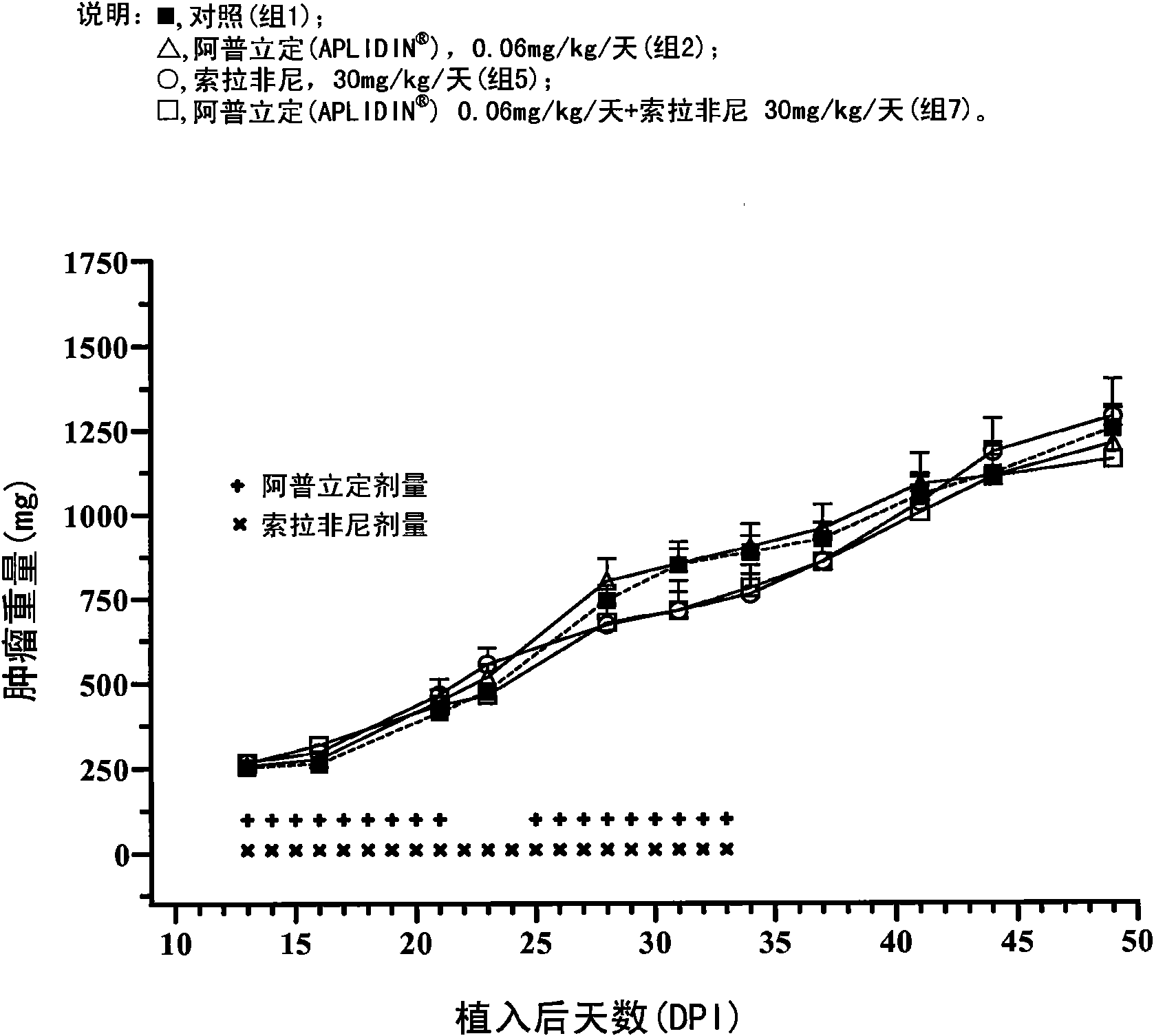
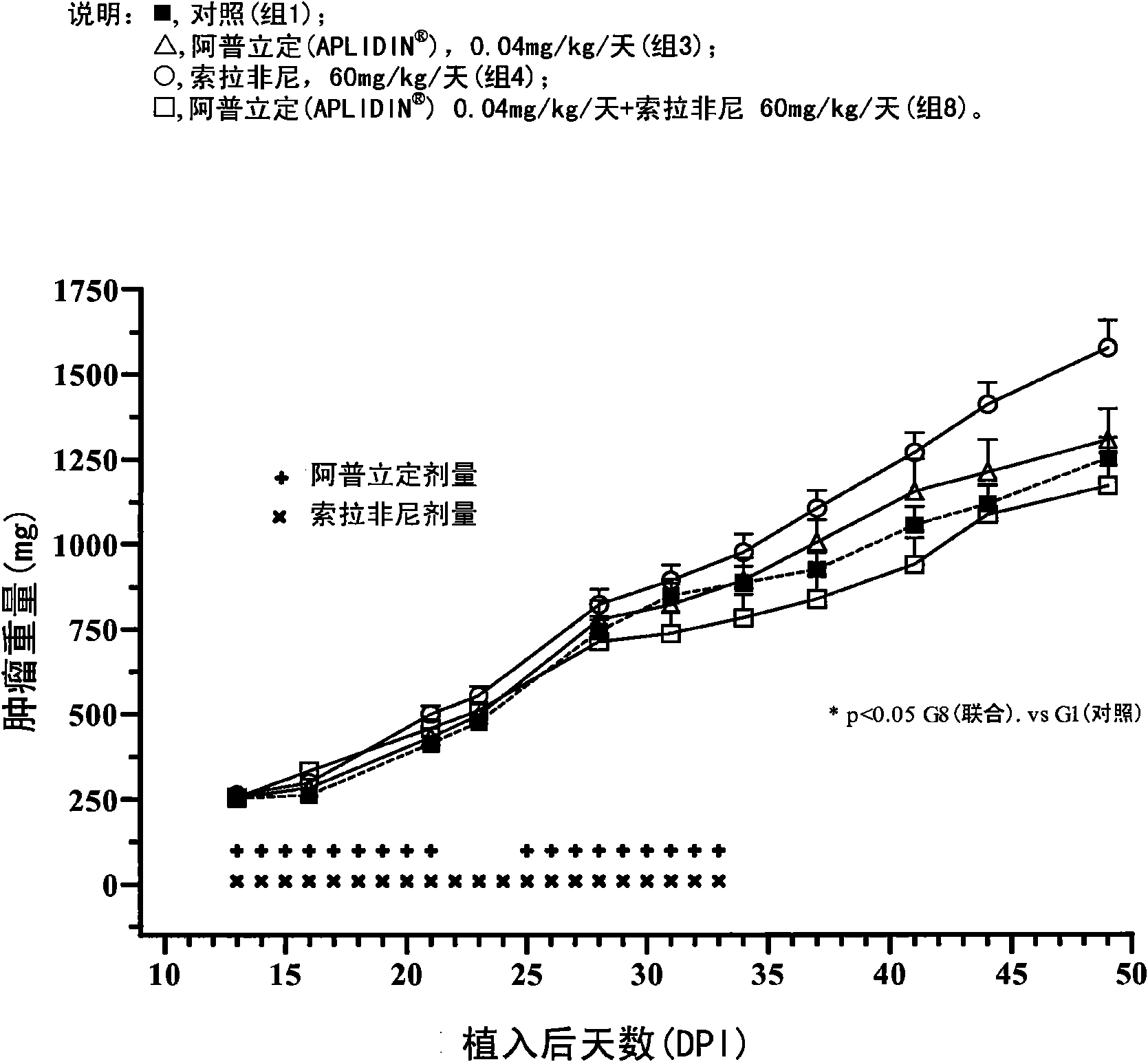
The present invention relates to combinations of aplidine with another anticancer drug selected from sorafenib, temsirolimus, and sunitinib, and the use of these combinations in the treatment of cancer.
Owner:PHARMA MAR U
Concentrated solution for temsirolimus injection and preparation method thereof
ActiveCN105687132ASubstance is smallOvercoming the problem of increasingOrganic active ingredientsPharmaceutical delivery mechanismOrganic acidAlcohol
The invention provides a preparation method of a concentrated solution for temsirolimus injection. The concentrated solution is mainly prepared from active component, benzyl alcohol, butyl hydroxybenzoate, organic acid or inorganic acid, pH regulator and absolute ethyl alcohol. The prepared preparation has few related substances, is stable in long-term storage process, and meanwhile solves the problem that due to the introduction of water, temsirolimus isomers and related substances are increased.
Owner:LUNAN BETTER PHARMA
Process for preparation of temsirolimus
The present invention provides two synthetic routes for the preparation of Temsirolimus (compound 1b and analog of Temsirolimus 1a). The first route includes the synthesis of CCI-779 by directly reacting rapamycin (4b) or Prolyl-rapamycin (4a) with substituent-2,2-bis(methoxy) propionic acid anhydride(11) in the presence of an organic base, followed by deprotection to give CCI-779 or Proline CCI-779. The second route includes a process involving a reaction of rapamycin-OH-31-sily ether (4d) or Prolyl-rapamycin-OH-31-sily ether (4c) with substituent-2,2-bis(methoxy) propionic acid anhydride(11) in the presence of an organic base and followed by subsequent hydrolysis step to obtain the desired CCI-779 or Proline CCI-779.Compound 11, as described in this invention, is stable at room temperature, cost effective and ease of processing.
Owner:CHUNGHWA CHEM SYNTHESIS & BIOTECH
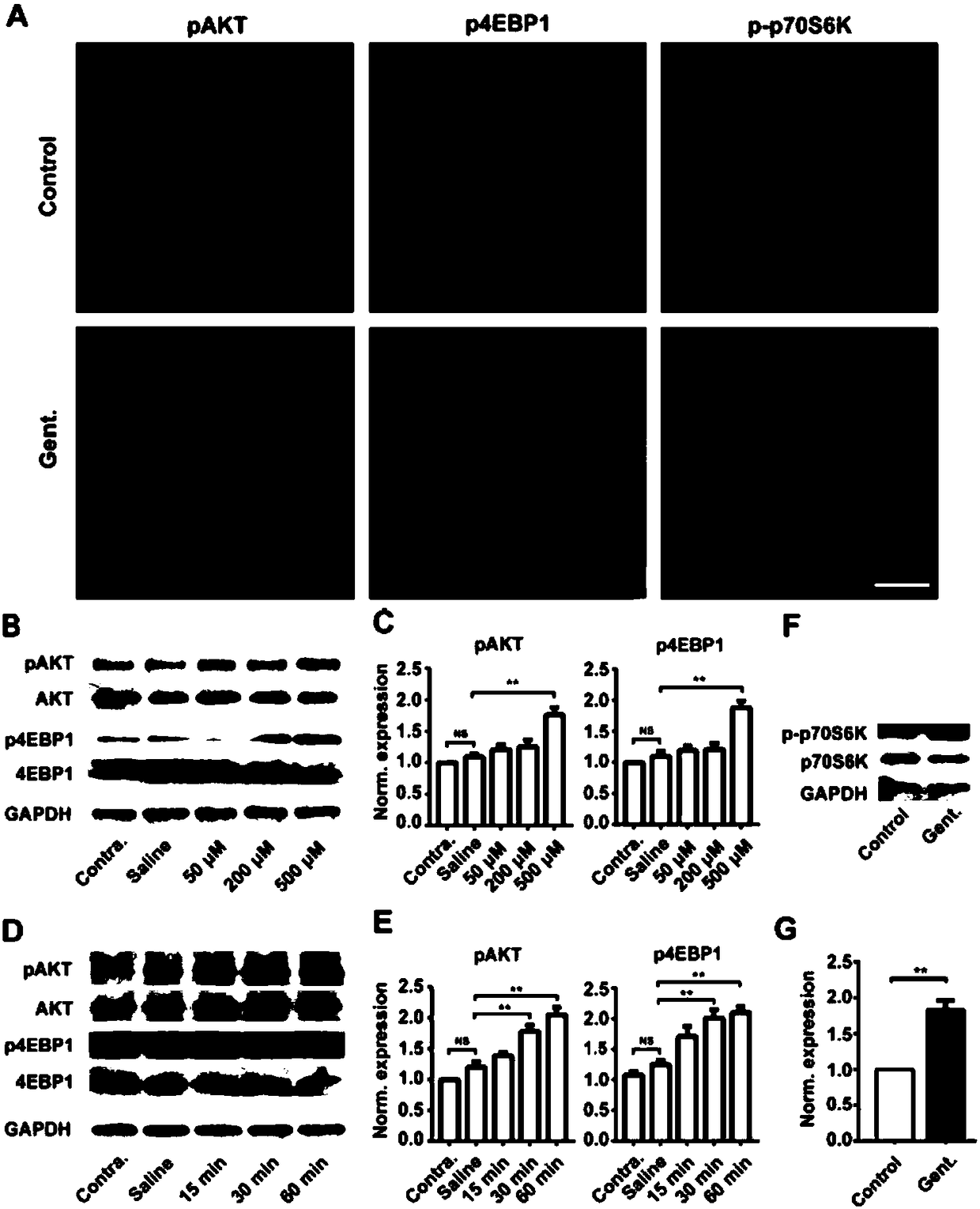
Application of mTOR signal path inhibitor to preparation of medicament for preventing or treating extragenetic hearing impairment
InactiveCN108310386AAvoid damageImprove hearingOrganic active ingredientsSenses disorderEverolimusMTOR signaling pathway
The invention discloses application of a mTOR signal path inhibitor to preparation of a medicament for preventing or treating extragenetic hearing impairment. The mTOR signal path inhibitor is selected from at least one of sirolimus, sirolimus analogue and second-generation mTOR signal path inhibitor; the sirolimus analogue is selected from at least one of everolimus, temsirolimus and Deforolimus;the second-generation mTOR signal path inhibitor is selected from at least one of PI3K / mTOR double inhibitor, selective mTORC1 / 2 inhibitor and ATP competitive mTOR kinase inhibitor. As found by the inventor of the application, the mTOR signal path inhibitor can effectively relieve damage to inner ear hair cells and spiral ganglion cells caused by non-genetic factors such as ototoxic drug, remarkably improve the impaired listening ability, and effectively prevent and treat extragenetic hearing impairment.
Owner:SOUTHERN MEDICAL UNIVERSITY
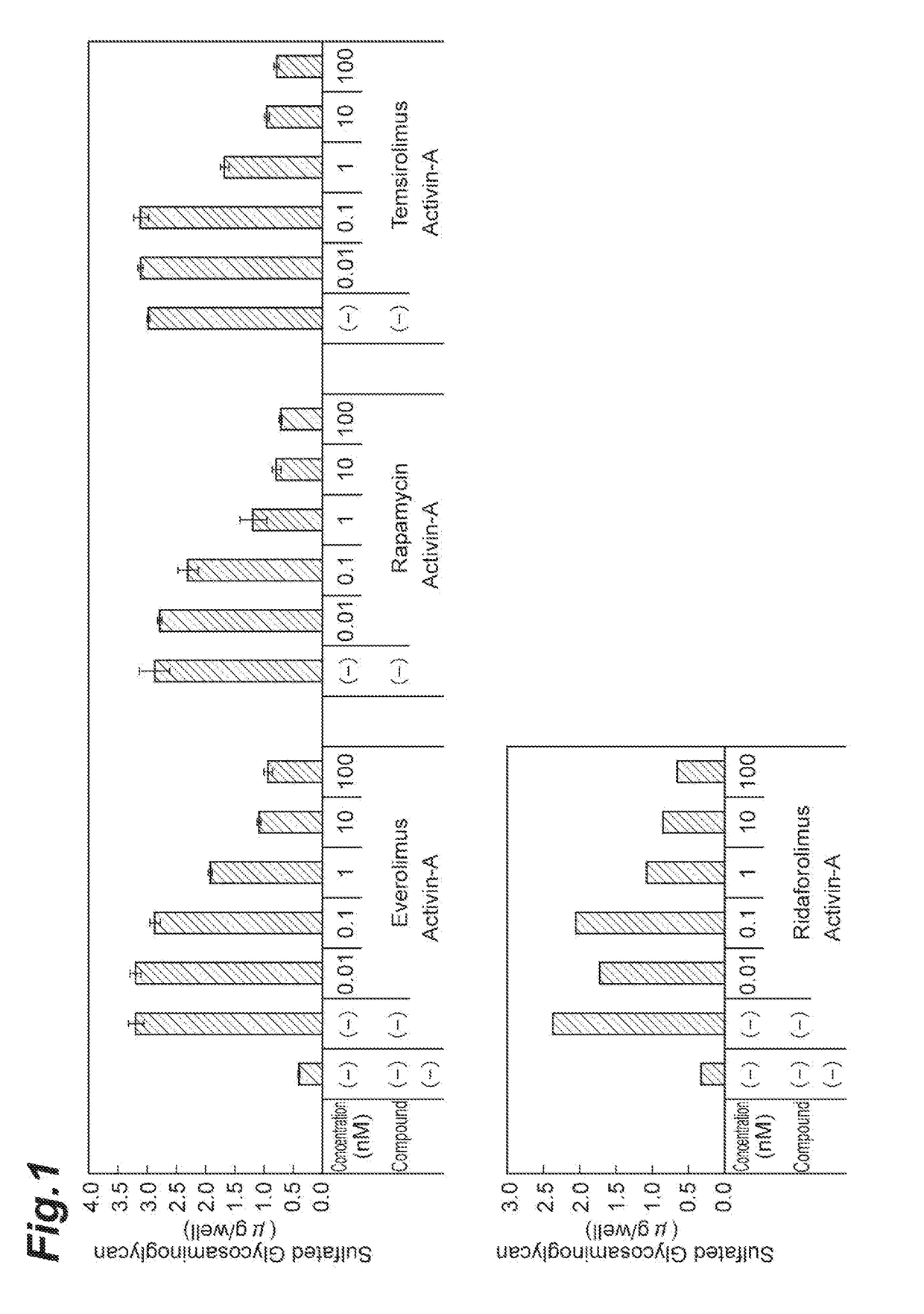
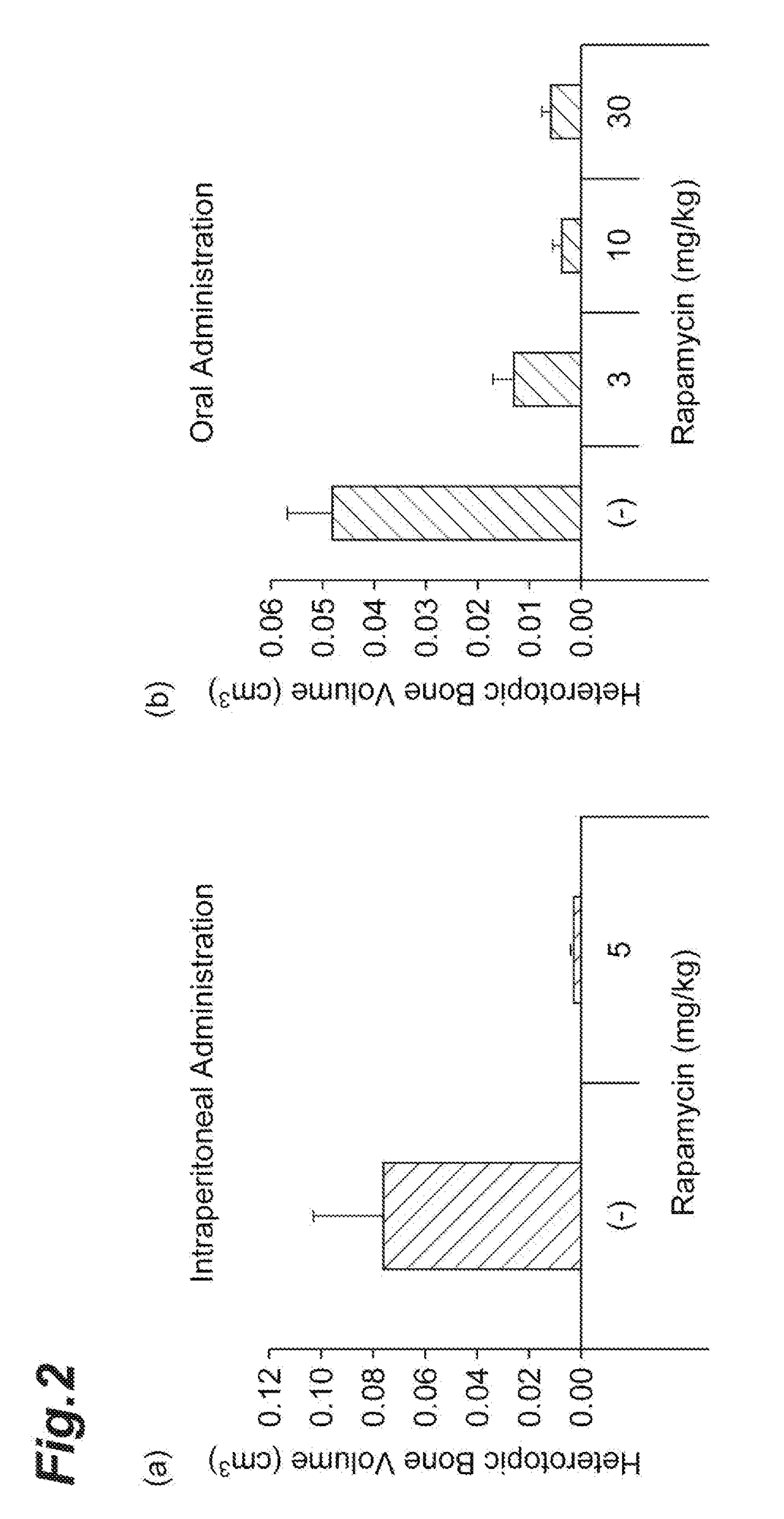
Therapeutic Agent for Fibrodysplasia Ossificans Progressiva
The present invention relates to a prophylactic or therapeutic agent for fibrodysplasia ossificans progressiva comprising as an active ingredient, at least one compound selected from the group consisting of rapamycin, temsirolimus, everolimus, ridaforolimus, TAFA93, umirolimus, olcorolimus, zotarolimus, and pharmaceutically acceptable salts thereof.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
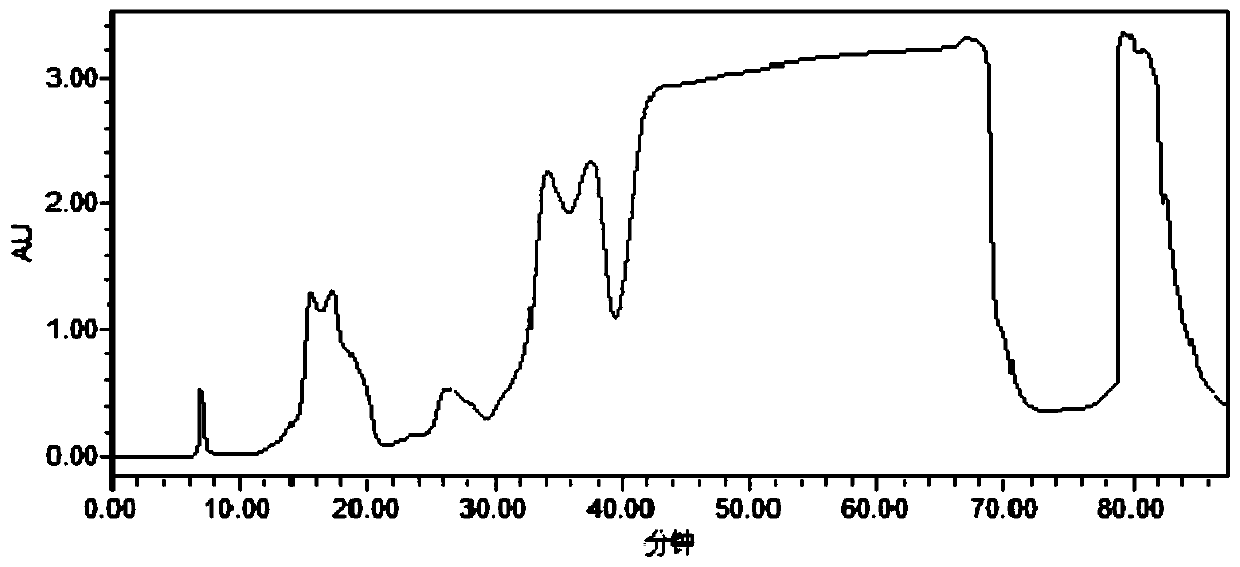
Method for separating and purifying temsirolimus
ActiveCN109851626AReduce the difficulty of purificationReduce contentOrganic chemistryPurification methodsTemsirolimus
The invention discloses a method for separating and purifying temsirolimus. The method comprises the following steps: (1) synthesis of a crude product; and (2) separation and purification. The specific separation and purification method has the advantages of great improvement of the purity and the yield of temsirolimus, reduction of the cost, and broad market application prospect.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
Improved anticancer treatments
The present invention relates to combinations of aplidine with another anticancer drug selected from sorafenib, temsirolimus, and sunitinib, and the use of these combinations in the treatment of cancer.
Owner:PHARMA MAR U
Method used for preparing temsirolimus and suitable for industrial production
InactiveCN102807571AHigh yieldEasy to operateOrganic chemistryBulk chemical productionCompound aOrganic solvent
The invention belongs to the technical field of methods for preparing temsirolimus. The method for preparing the temsirolimus comprises the following steps of: 1) performing catalytic reaction on 2,2-bis(hydroxymethyl)propionic acid and 4-methoxybenzaldehyde dimethylacetal in the presence of an organic solvent; 2) reacting a compound II with 2,4,6-trichlorobenzoyl chloride at normal temperature in the presence of an organic solvent under the alkaline condition, adding an organic solvent containing a compound A and 4-(N,N-dimethylamino)pyridine into the reaction solution, and reacting to obtain a compound B, wherein P is a protecting group; and 3) reacting the compound B with acid in the presence of a solvent to obtain the temsirolimus. The method is easy to operate, low in cost and suitable for industrial production, the synthetic route is short, and yield is high.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Temsirolimus albumin nano-composition as well as lyophilized preparation, preparation method and application thereof
ActiveCN107714652AReduce concentrationReduce usagePowder deliveryOrganic active ingredientsOrganosolvPharmaceutical Substances
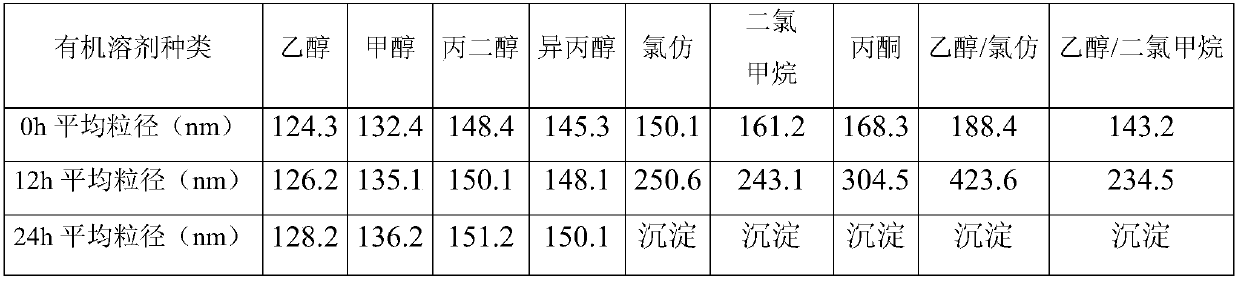
The invention belongs to the field of preparation of medicines, and particularly relates to a stable temsirolimus albumin nano-composition as well as a lyophilized preparation, a preparation method and application thereof. By adopting the temsirolimus albumin nano-composition disclosed by the invention, the technical defect that organic solvents such as chloroform / dichloromethane are not dissolvedwith water are required to use in the prior art is overcome. The temsirolimus albumin nano-composition is characterized in that temsirolimus or a derivative of the temsirolimus is dissolved by adopting an organic solvent mutually dissolved with the water, albumin is dispersed by adopting water-based medium, and temsirolimus is mixed with the albumin to prepare nano-suspension liquid, so that thenano-composition of which pH is 6.0 to 7.5 and the average particle size is not greater than 200nm is obtained. The nano-composition has nano-particles with excellent stability, so that the stabilityand the security of the medicine are improved; meanwhile, when the lyophilized preparation is prepared by adopting the nano-composition, even if a lyophilization protector and a protein stabilizationagent are not needed, the qualified lyophilized preparation also can be prepared. After the lyophilized preparation is prepared, zeta electrical potential of the lyophilized preparation after dispersion of the water-based medium is -2 to -40mv.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Combination therapy for treatment of restenosis
ActiveUS20180353488A1Cross sectional areaReduce apoptosisPowder deliveryOrganic active ingredientsCombined Modality TherapyPercent Diameter Stenosis
Described herein are methods for distributing a combination of a cell division inhibitor (e.g., temsirolimus or paclitaxel) and dexamethasone to a tissue surrounding a blood vessel for treating vascular diseases. Also disclosed are injectable compositions of a cell division inhibitor (e.g., temsirolimus or paclitaxel) and dexamethasone for delivery into the tissue surrounding a blood vessel for treating vascular diseases.
Owner:MERCATOR MEDSYST
Method for purifying temsirolimus
InactiveCN108948047ASmall single miscellaneousHigh purityOrganic chemistryChemical industryPurification methods
The invention belongs to the field of pharmaceutical and chemical industry, and specifically discloses a method for purifying temsirolimus. The method comprises the following steps: preliminarily purifying a crude temsirolimus product through a silica gel column; then dissolving above-mentioned obtained temsirolimus in a solvent a, carrying out decolorization with active carbon, and carrying out filtering; dropwise adding a solvent b, carrying out cooling, and carrying out crystallization under stirring; and carrying out filtering, washing and vacuum drying so as to obtain a finished temsirolimus product. The temsirolimus purified by using the method provided by the invention has a purity of larger than or equal to 98.5% and a maximum single impurity of less than 0.2%; and the method for purifying the temsirolimus provided by the invention has simple process and is applicable to industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing temsirolimus
InactiveUS9018373B2Simple processLow costOrganic chemistryBulk chemical productionPropanoic acidChloride
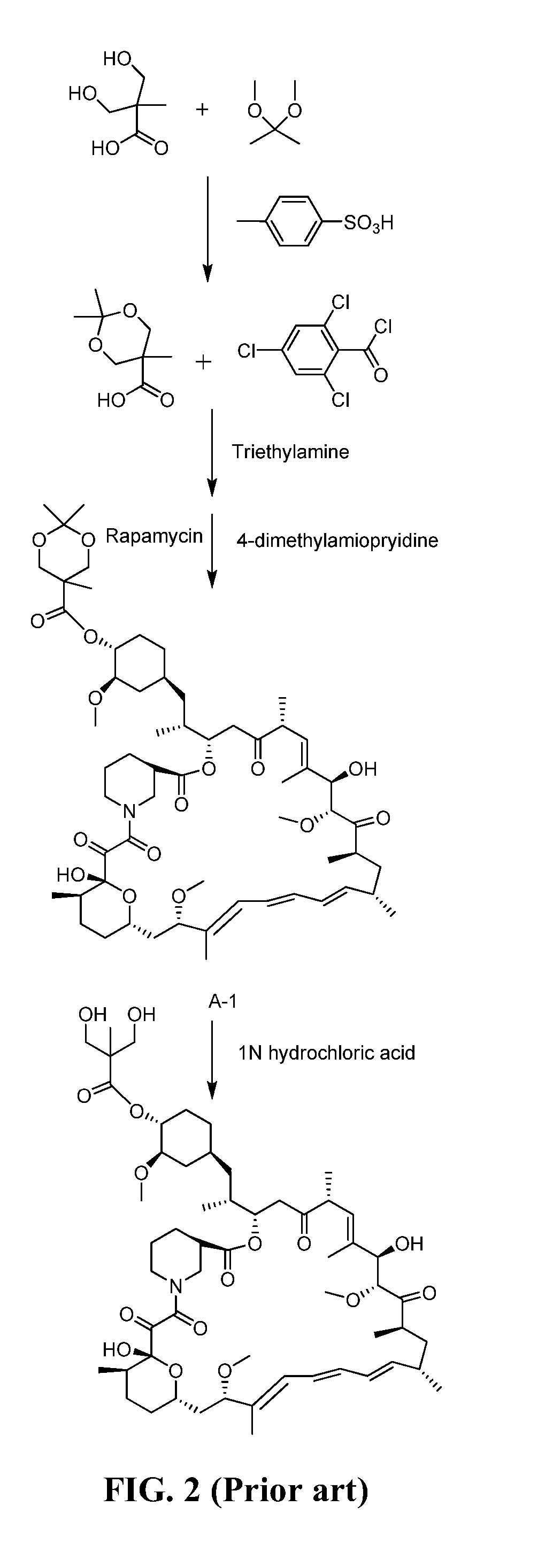
A method for preparing temsirolimus, the method including: using a substituted aromatic aldehyde to protect 2,2-dimethylol propionic acid to produce intermediate II; carrying out reaction between the intermediate II and 2,4,6-trichlorobenzoyl chloride; carrying out condensation reaction between a resulting product and rapamycin to produce intermediate III; and finally using sulfuric acid to remove a protecting group from the intermediate III to yield temsirolimus.
Owner:TIANJIN WEIJIE TECH
Combination of inotuzumab ozogamicin and torisel for the treatment of cancer
InactiveUS20140335109A1Achieve effectOrganic active ingredientsImmunoglobulinsAfter treatmentInotuzumab ozogamicin
The present invention relates to a therapeutic method for the treatment of cancer that comprises the use of a combination of inotuzumab ozogamicin (CMC-544) and temsirolimus. The enhanced antitumor of the combination therapy is particularly useful for patient population that are recalcitrant to inotuzumab ozogamicin or temsirolimus therapy, relapse after treatment with inotuzumab ozogamicin or temsirolimus or where enhanced antitumor effect reduces toxicities associated with treatment using inotuzumab ozogamicin or temsirolimus.
Owner:PFIZER INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com