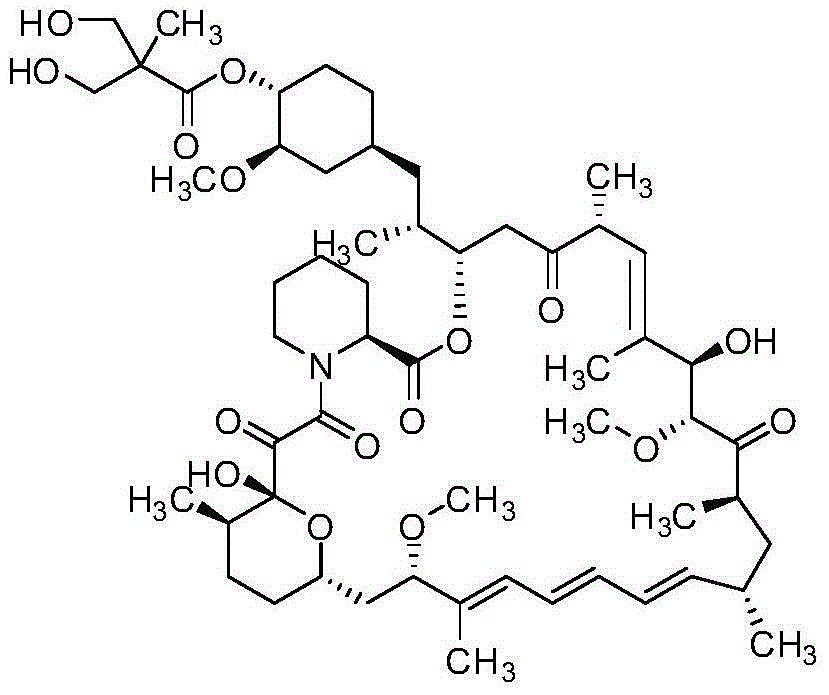
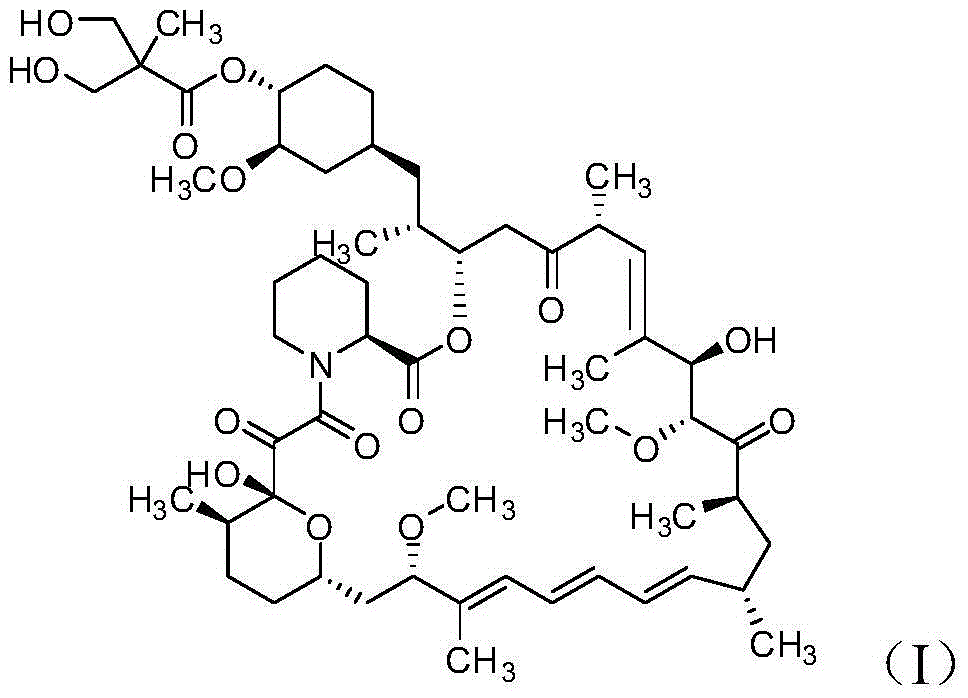
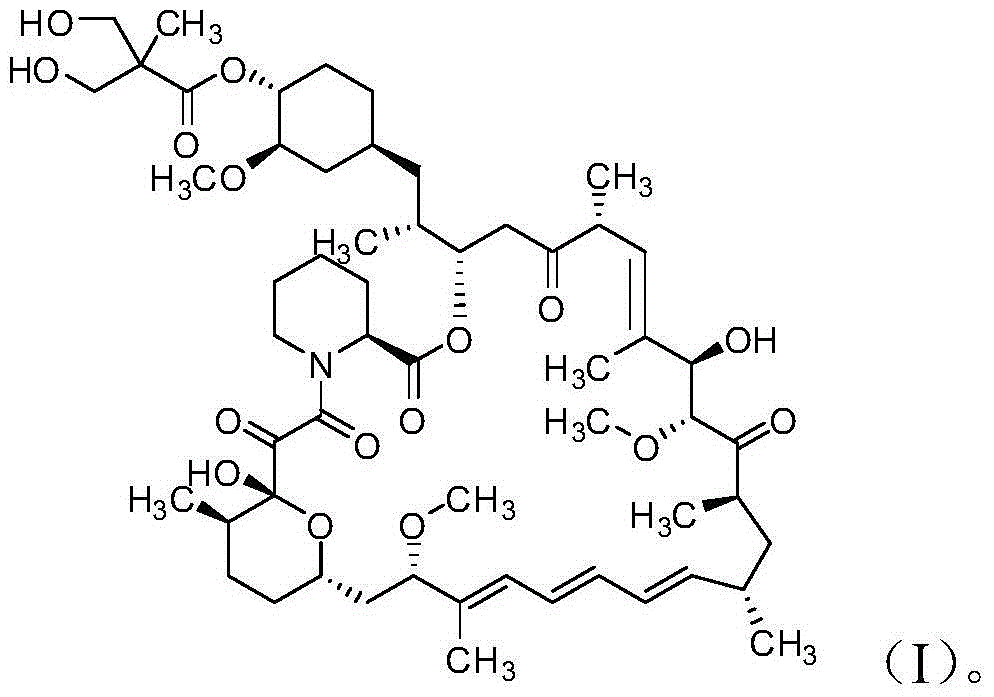
Method for preparing high-purity temsirolimus
A temsirolimus, high-purity technology, applied in the field of medicinal chemistry, can solve problems such as difficult to meet the quality requirements of injection forms, content control of starting material sirolimus, poor removal of impurities, etc., and achieve good industrial application prospects , the operator and the environment have little influence, and the effect of high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Preparation of semi-finished products of temsirolimus
[0041] Dissolve 8.3g of temsirolimus crude product (purity 75.68%, sirolimus 3.47%, isomer 16.48%, area normalization method, the same below) with 25mL ethyl acetate, and load it on normal phase silica spheres Packed column (Nanjing Baisai Biological Chromatography Technology Co., Ltd., the same below, particle size 5 μm), gradient elution with ethyl acetate: n-heptane mixed solvent system, 0 ~ 10min, ethyl acetate / n-heptane (v / v, the same below)=60:40, in 10~100min, ethyl acetate / n-heptane gradually changed from 60:40 to 100:0, the system temperature was 20°C, collected components, concentrated under reduced pressure at 30°C to After drying, 6.8 g of semi-finished temsirolimus (purity 84.78%, sirolimus 0.02%, isomer 13.23%) were obtained.
[0042] 2) Preparation of high-purity temsirolimus
[0043] After the above 6.8g temsirolimus semi-finished product was dissolved with 21mL acetonitrile, the sample was lo...
Embodiment 2
[0045] 1) Preparation of semi-finished products of temsirolimus
[0046] After dissolving 56g of temsirolimus crude product (purity 79.03%, sirolimus 2.54%, isomer 15.63%) with 560mL ethyl acetate, the sample was loaded on a normal-phase silica sphere packing column (10 μm), and the Gradient elution with mixed solvent system of n-hexane, 0-10min, ethyl acetate / n-hexane=60:40, 10-100min, ethyl acetate / n-hexane gradually changed from 60:40 to 100:0, system temperature 10°C, collected components, concentrated to dryness under reduced pressure at 20°C to obtain 46g semi-finished product of temsirolimus (purity 86.17%, sirolimus not detected, isomer 11.65%).
[0047] 2) Preparation of high-purity temsirolimus
[0048] After dissolving the above-mentioned 46g temsirolimus semi-finished product with 460mL acetonitrile, load the sample on a reversed-phase C6 packed column (10 μm), and carry out gradient elution with a mixed solvent system of acetonitrile and 20mmol / L potassium dihydr...
Embodiment 3
[0050] 1) Preparation of semi-finished products of temsirolimus
[0051] After dissolving 100g of temsirolimus crude product (purity 76.89%, sirolimus 1.95%, isomer 15.92%) with 500mL ethyl acetate, the sample was loaded on a normal-phase silica sphere packing column (20-45μm), washed with acetic acid Ethyl: n-heptane mixed solvent system for gradient elution, 0-10min, ethyl acetate / n-heptane = 60:40, 10-100min, ethyl acetate / n-heptane gradually changed from 60:40 to 100: 0, the system temperature was 15°C, the collected components were concentrated to dryness under reduced pressure at 30°C to obtain 84.4g semi-finished temsirolimus (purity 84.23%, sirolimus 0.01%, isomers: 13.47%).
[0052] 2) Preparation of temsirolimus finished product
[0053] Dissolve 84.4g of temsirolimus semi-finished product in 422mL of acetonitrile, load the sample on a reversed-phase C8 packed column (20-45μm), and use acetonitrile: 3.5μmol / L aqueous sodium bisulfate (pH5.5) mixed solvent system for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com