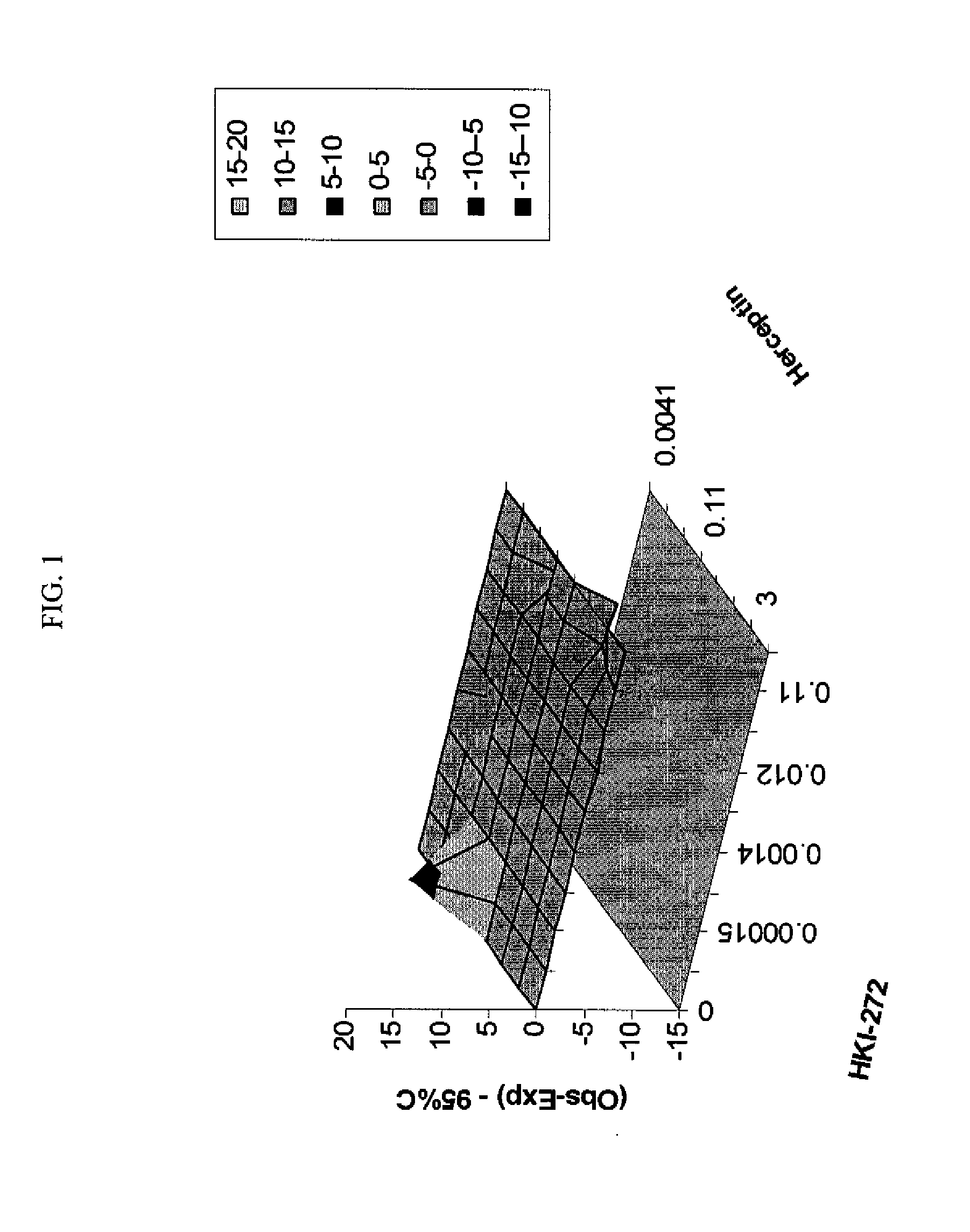
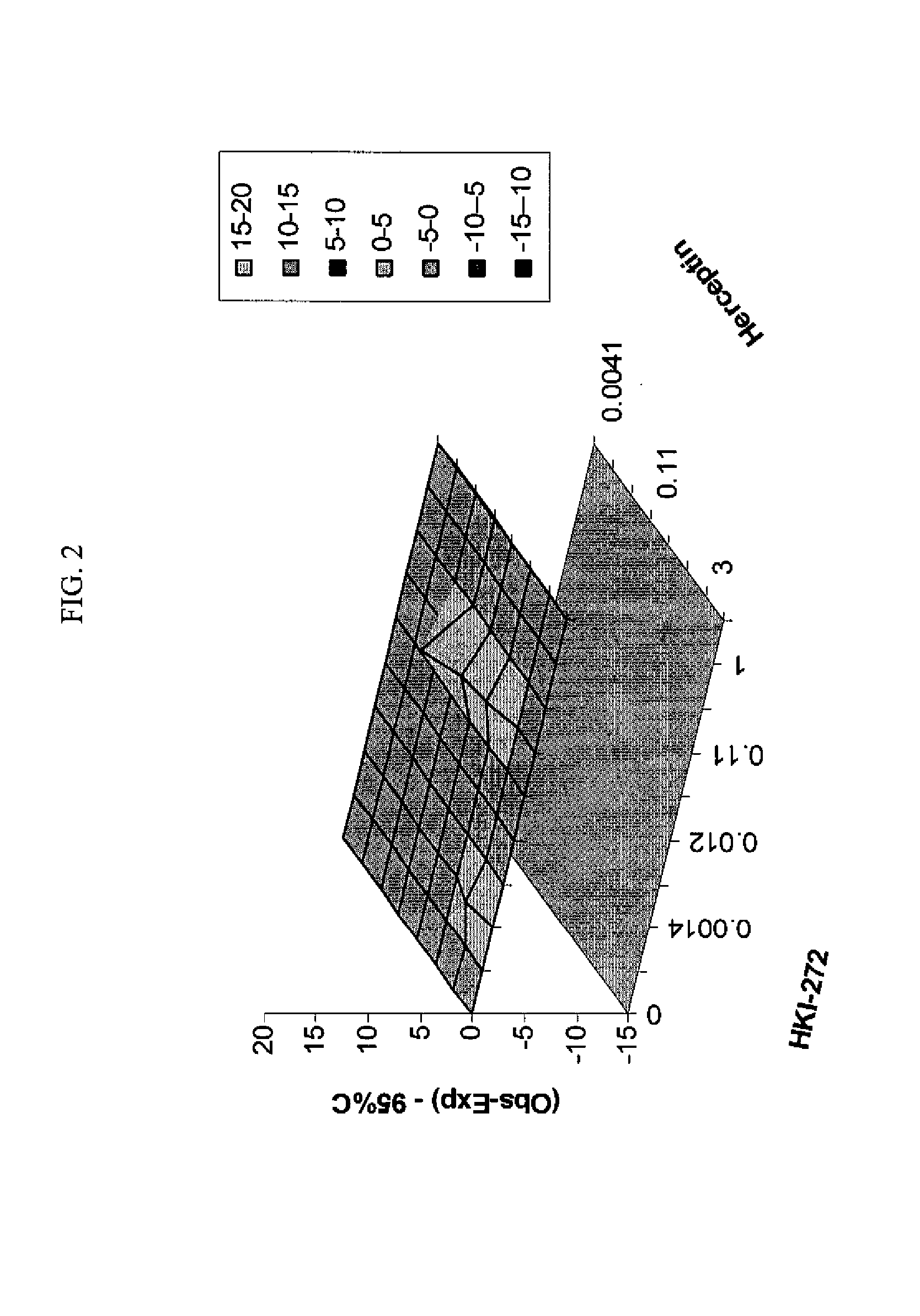
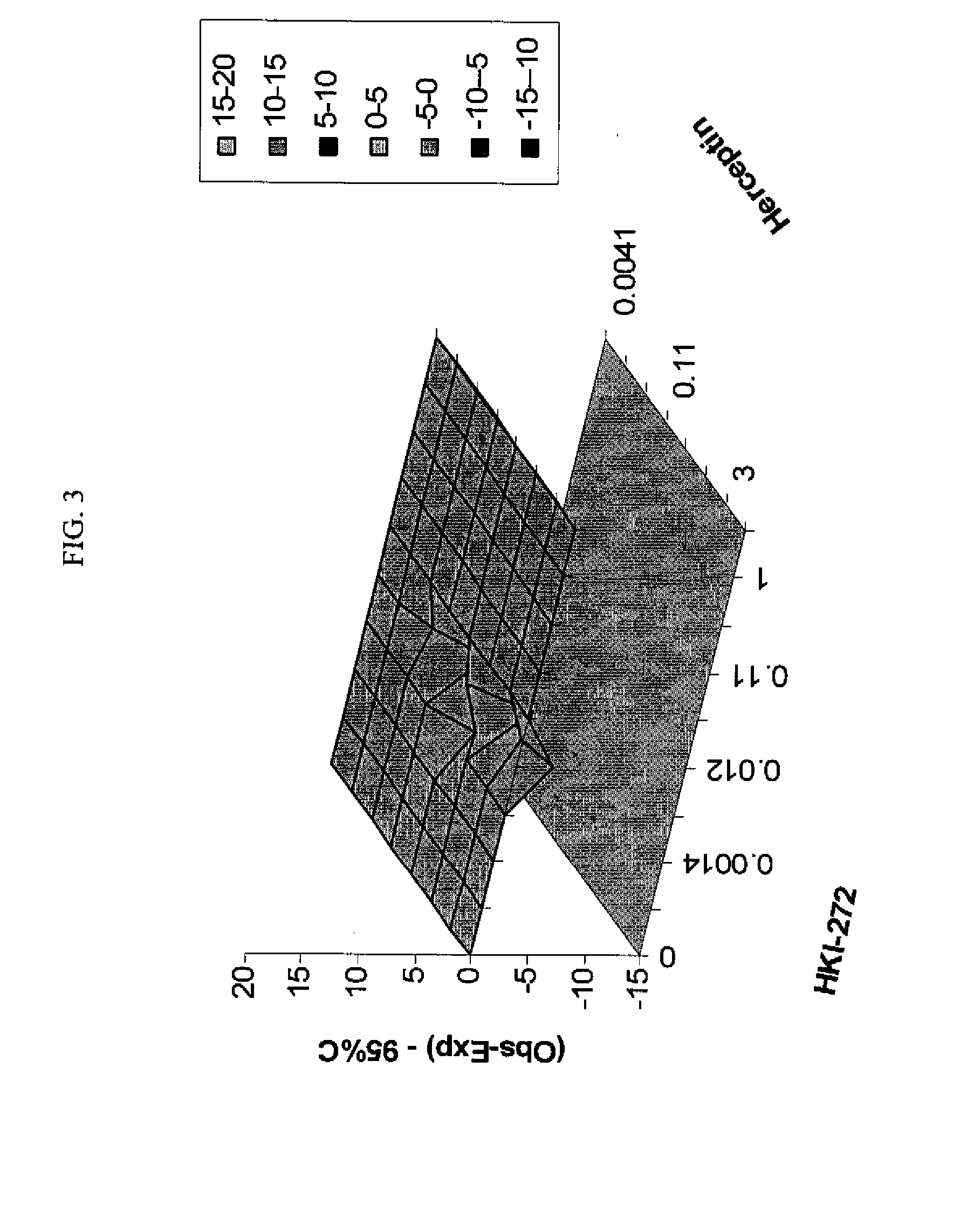
ANTINEOPLASTIC COMBINATIONS WITH mTOR INHIBITOR, TRASTUZUMAB, AND/OR HKI-272
a technology of mtor inhibitors and combinations, applied in the direction of antibody medical ingredients, extracellular fluid disorders, metabolic disorders, etc., can solve the problems of delayed tumor progression or tumor recurrence tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Combination Regimen of Temsirolimus (CCI-779) and Trastuzumab in Treatment of Neoplasms
[0073]Dosing begins at month 1, day 1 with weekly intravenous (IV) temsirolimus and trastuzumab (IV) at the dosages provided below.
[0074]Temsirolimus and trastuzumab can be administered simultaneously, consecutively, or on alternative days.
[0075]Temsirolimus is administered IV weekly over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to temsirolimus infusion.
[0076]A trastuzumab loading dose is administered IV weekly over a 90 minute period. Weekly doses are administered, which are typically half the amount of the loading dose. For example, a 4 mg / kg loading dose is typically followed by 2 mg / kg weekly doses. These amounts may be adjusted. In one embodiment, no loading dose is required and the same dose is administered throughout the course of treatment...
example 2
Use of a Combination Regimen of HKI-272 and Temsirolimus (CCI-779) in Treatment of Neoplasms
[0078]Dosing begins at month 1, day 1 with daily HKI-272 and weekly intravenous (IV) temsirolimus at the dosages provided below.
[0079]On month 1, day 1, HKI-272 is administered orally prior to temsirolimus. Temsirolimus is administered following HKI-272, preferably within 30 minutes.
[0080]Temsirolimus is administered IV weekly over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to temsirolimus infusion.
[0081]Thereafter, HKI-272 is taken orally once daily with food, preferably in the morning.
HKI-272 (mg)Temsirolimus Dose (mg)80151602524050
[0082]Dose adjustments and / or delays for HKI-272 and temsirolimus are permitted. For example, treatment may continue as described herein for six months, with daily doses of HKI-272 and weekly doses of temsirolimus....
example 3
Use of a Combination Regimen of HKI-272, Temsirolimus (CCI-779), and Trastuzumab in Treatment of Neoplasms
[0083]Dosing begins at month 1, day 1 with daily HKI-272 and weekly intravenous (IV) temsirolimus and trastuzumab (IV) at the dosages provided below.
[0084]On month 1, day 1, HKI-272 is administered orally prior to temsirolimus. Temsirolimus and trastuzumab are administered following HKI-272, preferably within 30 minutes.
[0085]Temsirolimus is administered IV weekly over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to temsirolimus infusion.
[0086]A trastuzumab loading dose is administered IV weekly over a 90 minute period. Weekly doses are administered, which are typically half the amount of the loading dose. For example, a 4 mg / kg loading dose is typically followed by 2 mg / kg weekly doses. These amounts may be adjusted. In one embodim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com