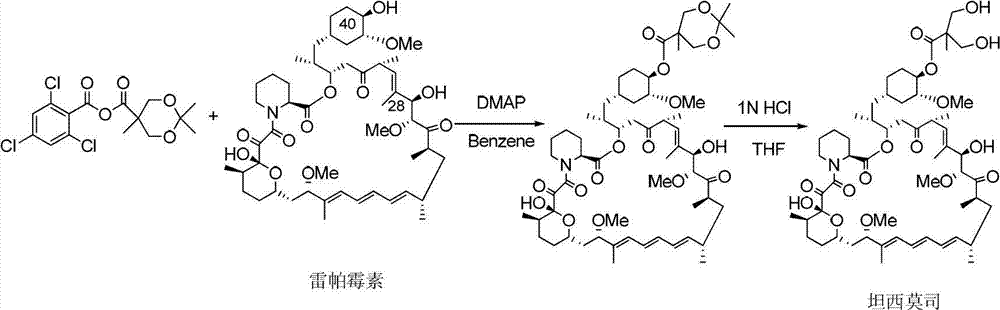
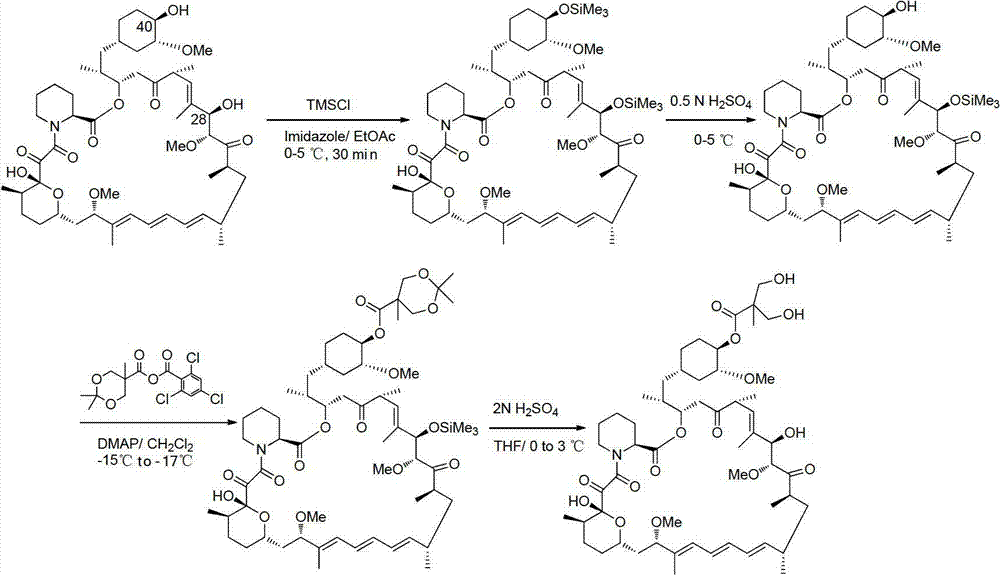
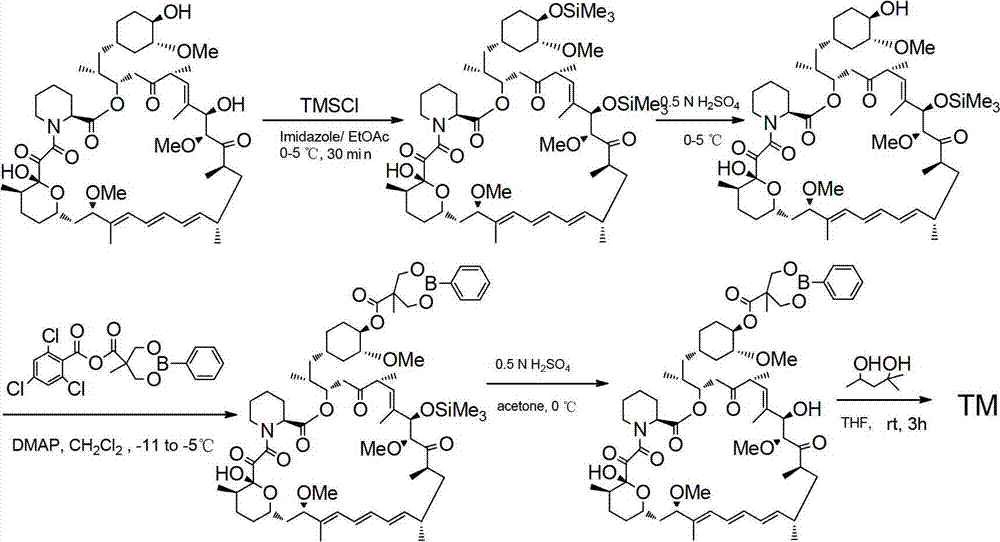
Method used for preparing temsirolimus and suitable for industrial production
A compound and catalyst technology, applied in the field of preparation of temsirolimus, can solve the problems of high price, non-compliance with environmental safety requirements, and highly toxic compounds, and achieve the effects of low cost, short synthetic route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 2,2-bis(hydroxymethyl)propionic acid (5 g, 37.3 mmol) and 4-methoxybenzaldehyde dimethyl acetal (8.2 g, 45 mmol) to 50 ml N,N-dimethylformamide , stirred and added p-toluenesulfonic acid monohydrate (1.8g, 9.4mmol), reacted at room temperature for 3~5h, then poured the reaction solution into 500ml of ice water and stirred for 5~10min, then directly suction filtered, and the filter cake was washed with a small amount of water and drained After that, it was directly dried in a vacuum oven at 50°C to 60°C to obtain 7.6g of compound II-1.
[0030]
[0031] MS: 253.14 (M+H), 1 HNMR (CDCl 3 ): δ7.397-7.375, 6.882-6.861 (dd, 4H); 7.257 (s, 1H, COOH); 5.443 (s, 1H); 4.589-4.560, 3.740-3.675 (dd, 4H, Ar-H); 3.791(s, 3H); 1.095(s, 3H).
Embodiment 2
[0033] Add 2,2-bis(hydroxymethyl)propionic acid (5 g, 37.3 mmol) and 4-methoxybenzaldehyde dimethyl acetal (8.2 g, 45 mmol) to 50 ml N,N-dimethylformamide , stirred and added zinc chloride (1.28g, 9.4mmol), reacted at room temperature for 3~5h, poured the reaction solution into 500ml of ice water and stirred for 5~10min, then directly sucked and filtered, washed the filter cake with a small amount of water and drained, and directly used Dry in a vacuum oven at 50°C~60°C to obtain 7.0 g of compound Ⅱ-1.
Embodiment 3
[0035] Add compound Ⅱ-1 (0.56g, 2.2mmol) and triethylamine (0.34g, 3.4mmol) into 5ml of dichloromethane, stir and add 2,4,6-trichlorobenzoyl chloride (0.54g, 2.2mmol ), after reacting at room temperature for 6 hours, add rapamycin (1g, 1.1mmol) and 4-(N,N-dimethylamino)pyridine (0.53g, 4.3mmol) in 5ml dichloromethane, and control the reaction temperature Compound B-1 (1.1 g) was obtained after reacting for 3 hours at 0°C to 5°C.
[0036]
[0037] MS: 1170.72 (M+Na), 13 CNMR (CDCl 3 ): δ17.74 & 19.20(CH3), 41.99 & 42.40(C), 57.53 & 57.88(OCH3), 101.66 & 101.77(CH), 113.51 & 113.68(ArCH), 127.39 & 127.60(ArCH), 173.56 &177. 47 (C=O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com