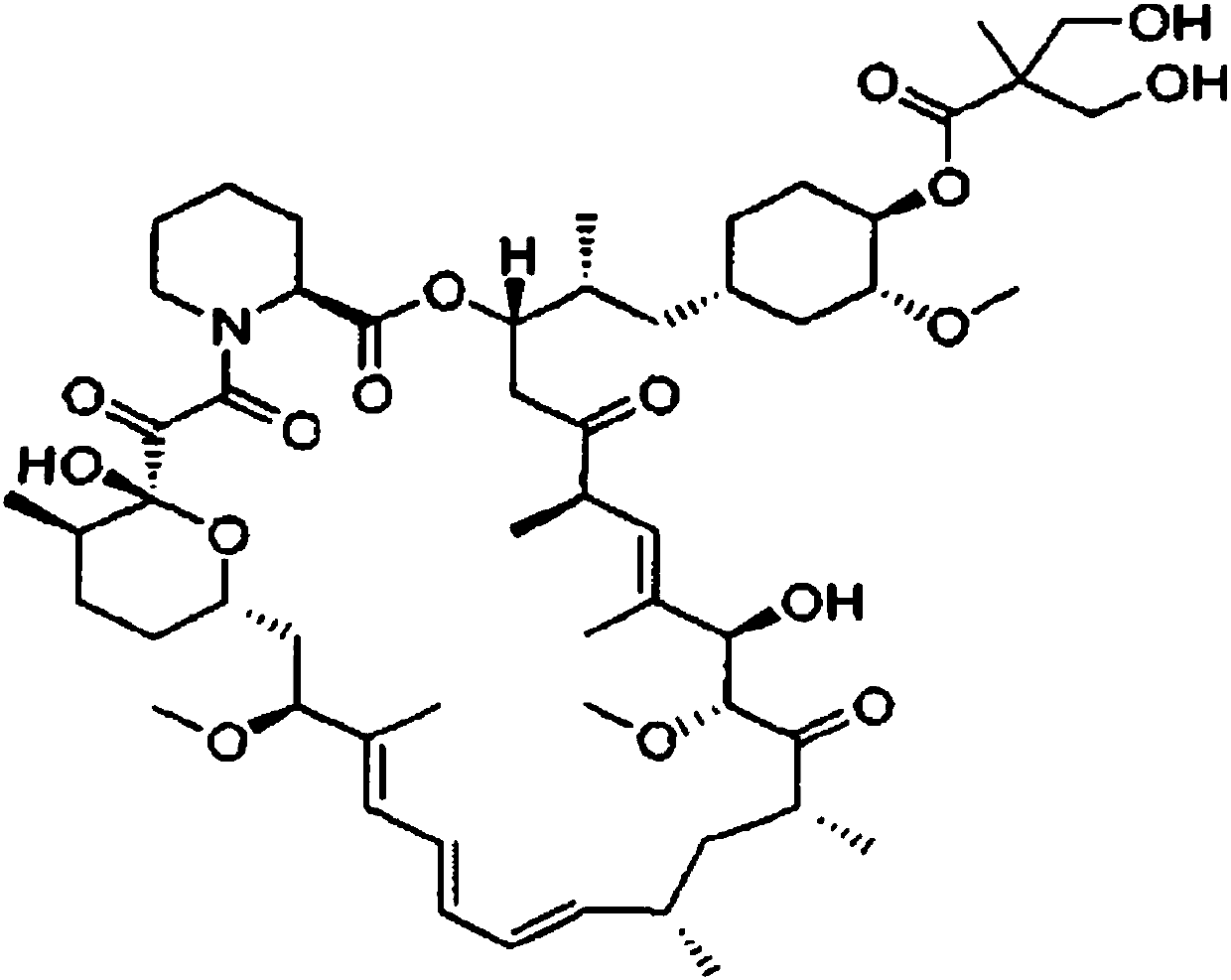
Temsirolimus albumin nano-composition as well as lyophilized preparation, preparation method and application thereof
A nanotechnology of temsirolimus and albumin, which is applied in the field of temsirolimus albumin nanocomposition and its freeze-dried preparation, to achieve the effect of increasing stability, safety and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
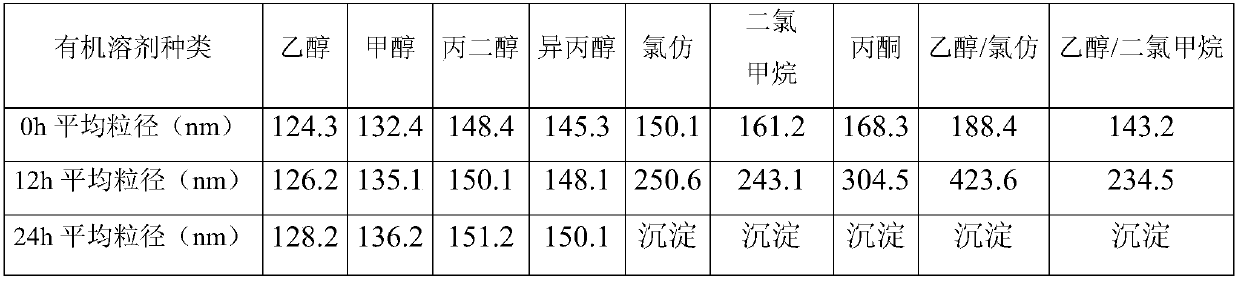
[0065] Dissolve 300mg of temsirolimus in 30ml of ethanol to form an organic phase, use 1000ml of 15mg / ml human serum albumin aqueous solution as the water phase, mix the water phase with the organic phase, and place in a high-speed shear After shearing at a speed of 10000rpm, it was transferred to a high-pressure homogenizer, homogenized under a pressure of 35000psi, and circulated 5 times to prepare particles of the composition, with an average particle size of 124.3nm and a pH of 6.7, passed through a 0.22μm Sterilize by filtration with a sterile filter head and freeze-dry for 60 hours. After freeze-drying was completed, physiological saline was used as the reconstitution medium, and the measured zeta potential was -5.7mv.
Embodiment 2
[0067] 300mg of temsirolimus was dissolved in 15ml of ethanol to form an organic phase, 100ml of human serum albumin (6mg / ml) was used as the water phase, the water phase was mixed with the organic phase, placed in a high-speed shearing machine to After shearing at a speed of 7000rpm, it was transferred to a high-pressure homogenizer, and homogenized under a pressure of 30,000psi for 5 cycles. The prepared composition was determined to have an average particle size of 139.4nm, a pH of 6.5, and a pass of 0.22 Filter and sterilize with a μm sterile filter head, and freeze-dry for 60 hours. After freeze-drying, using water as the reconstitution medium, the measured zeta potential was -23.6mv.
Embodiment 3
[0069] 300mg of temsirolimus was dissolved in 10ml of ethanol to form an organic phase, 20ml of human serum albumin (20mg / ml) was used as the water phase, the water phase was mixed with the organic phase, placed in a high-speed shearing machine to After shearing at a speed of 15000rpm, it was transferred to a high-pressure homogenizer, and homogenized under a pressure of 40000psi for 6 cycles. The prepared composition was measured to have an average particle size of 185.7nm, a pH of 7.0, and a particle size of 0.22μm Sterilize by filtration with a sterile filter head and freeze-dry for 60*h. After freeze-drying was completed, physiological saline was used as the reconstitution medium, and the measured zeta potential was -7.5mv.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com