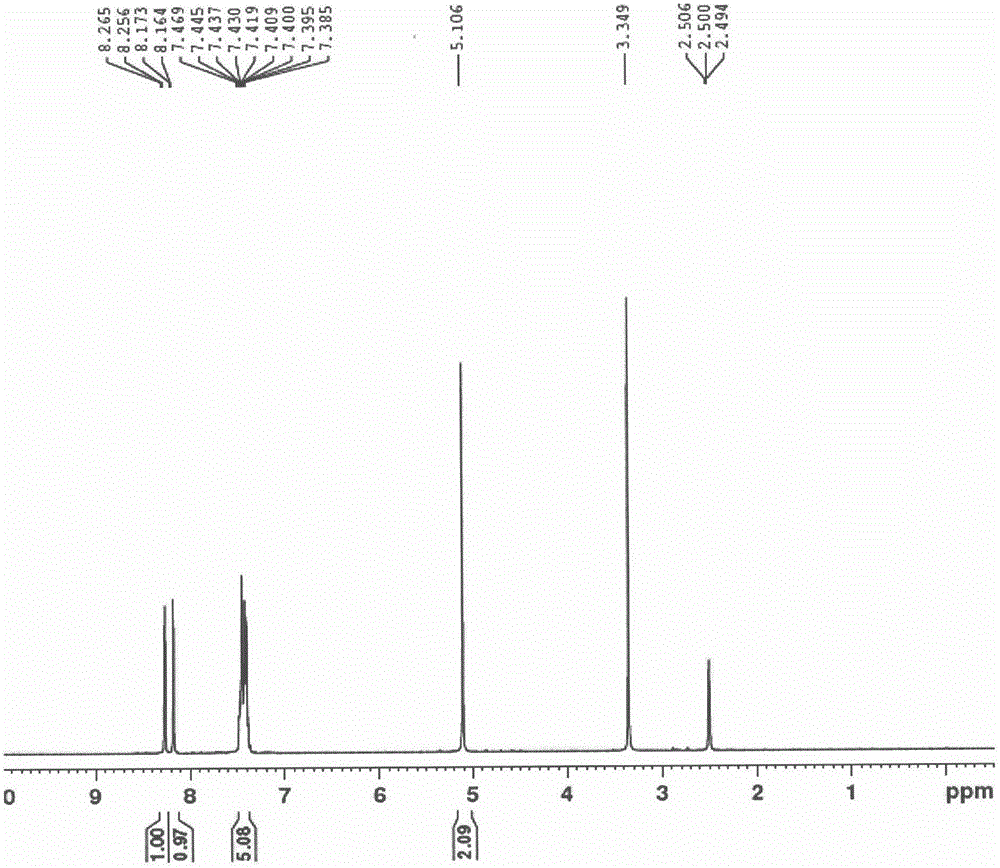
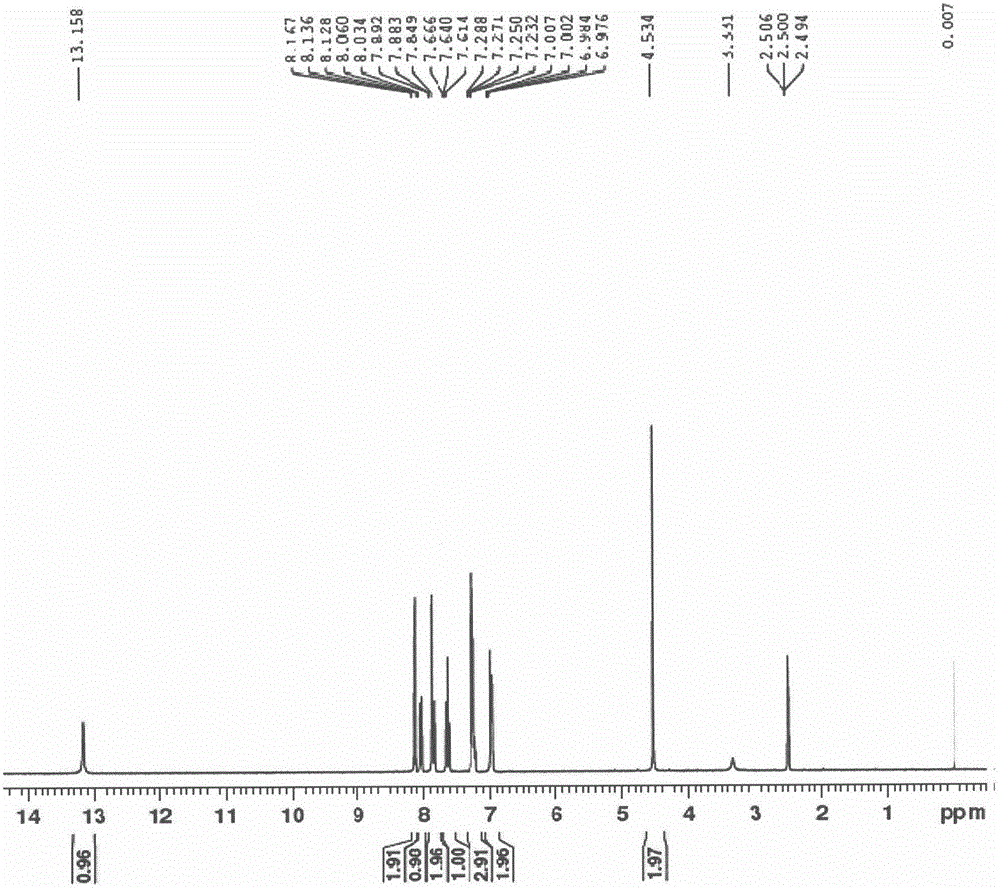
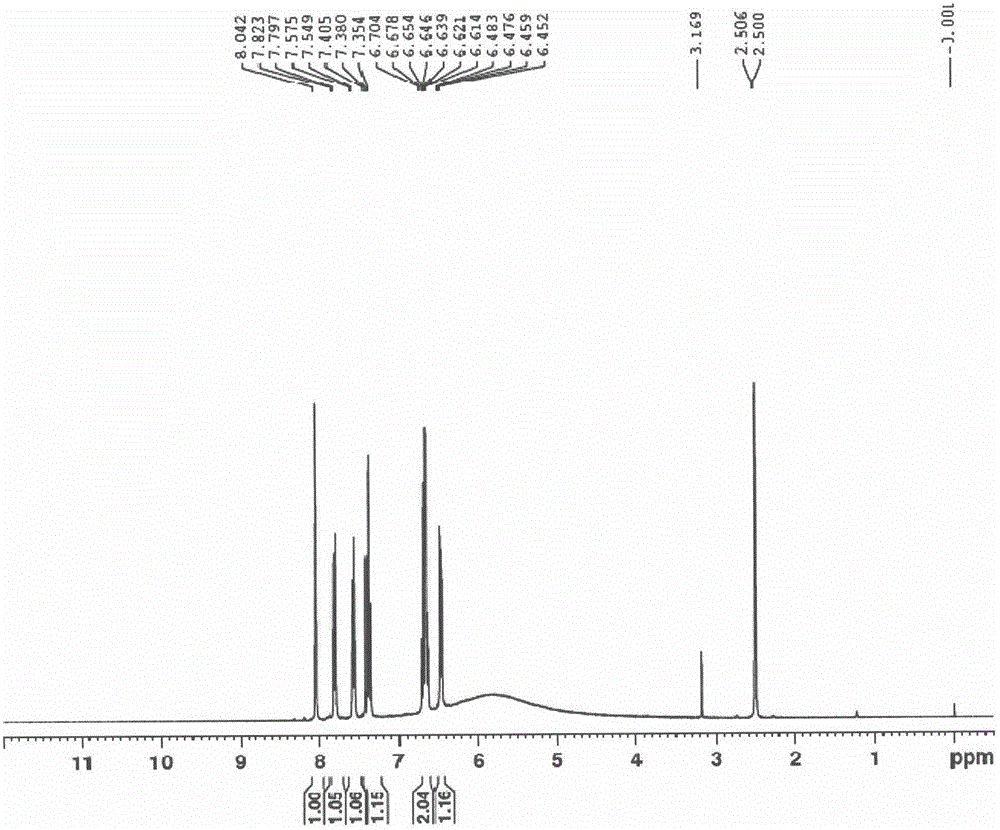
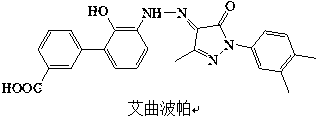
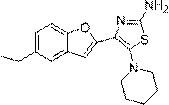
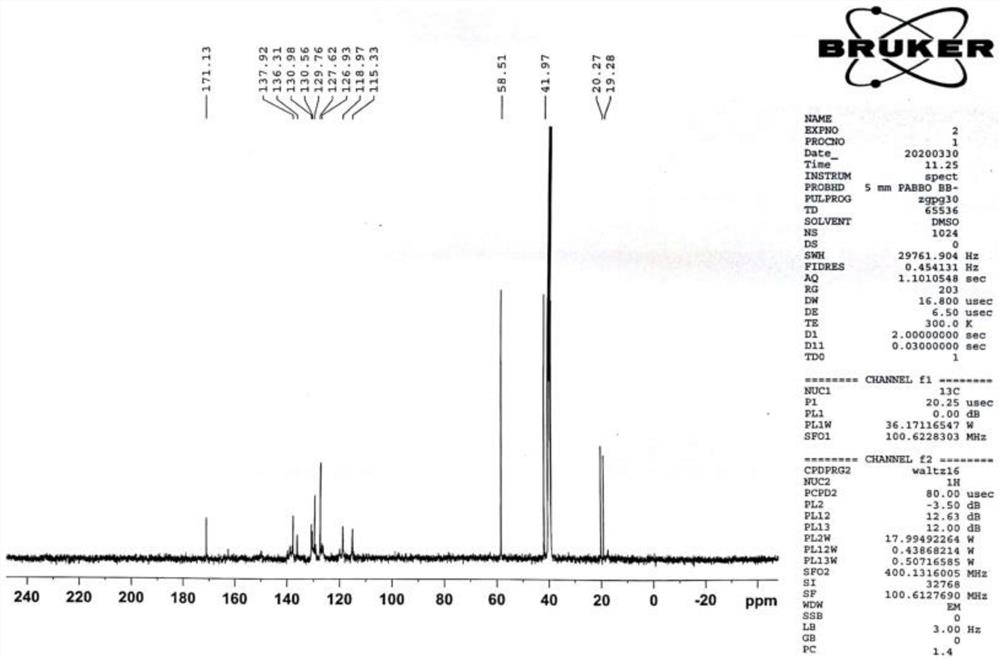
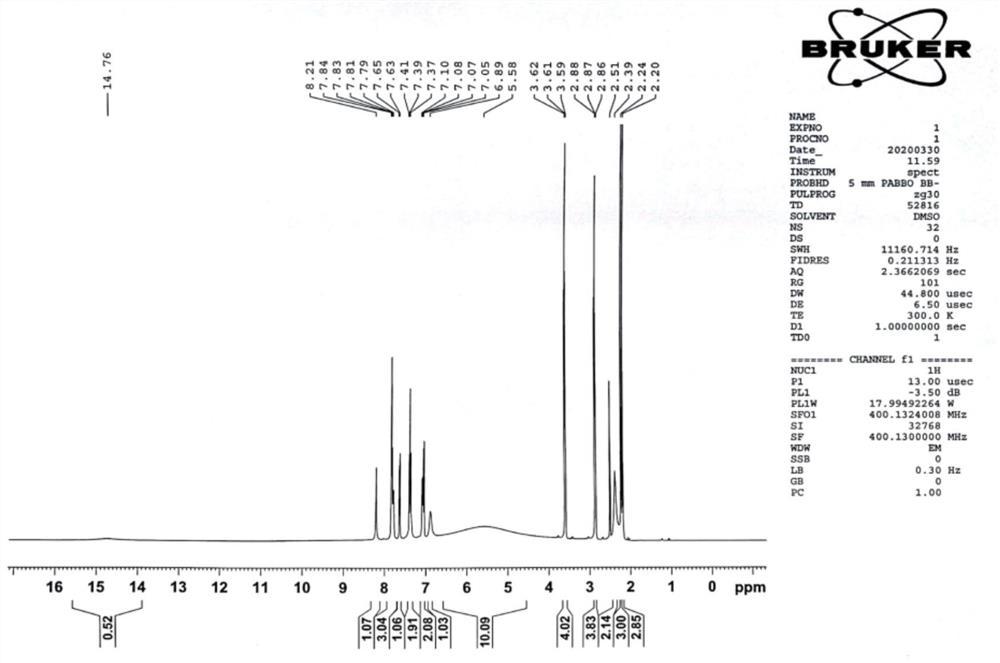
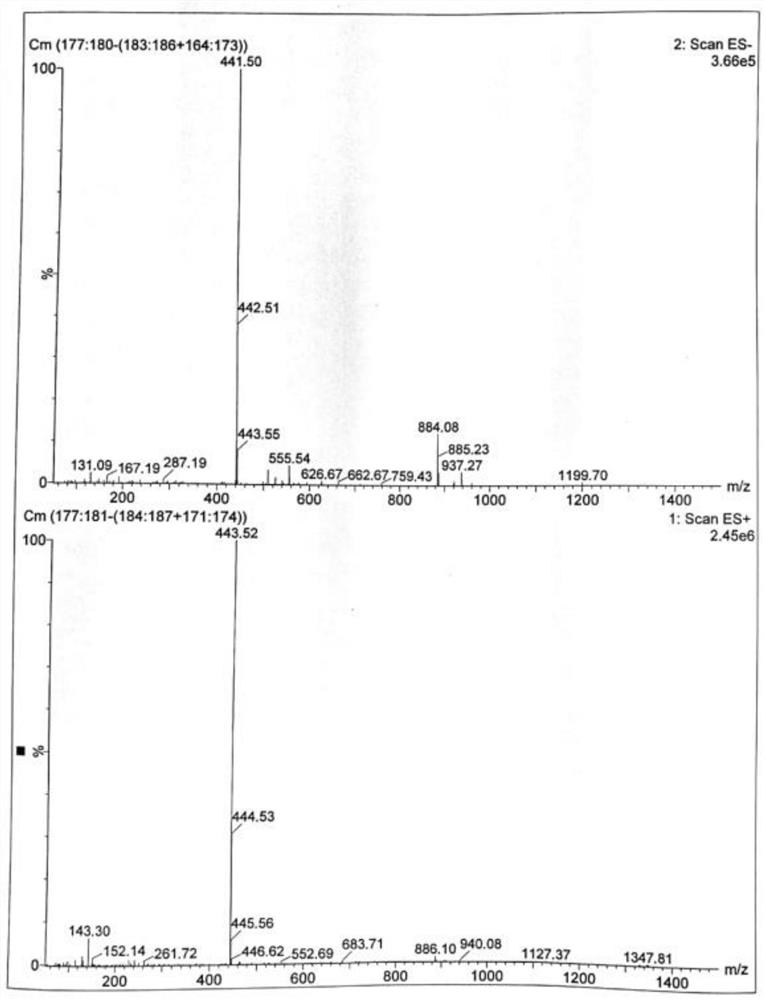
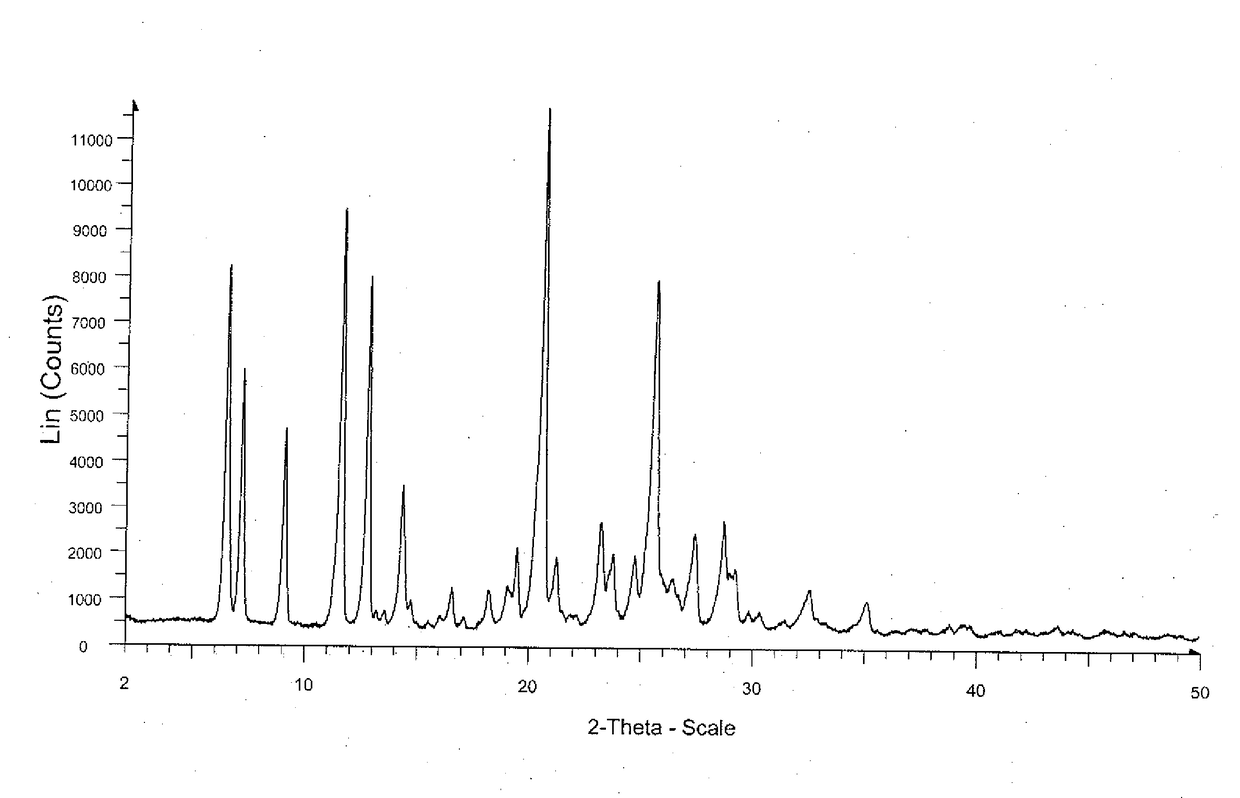
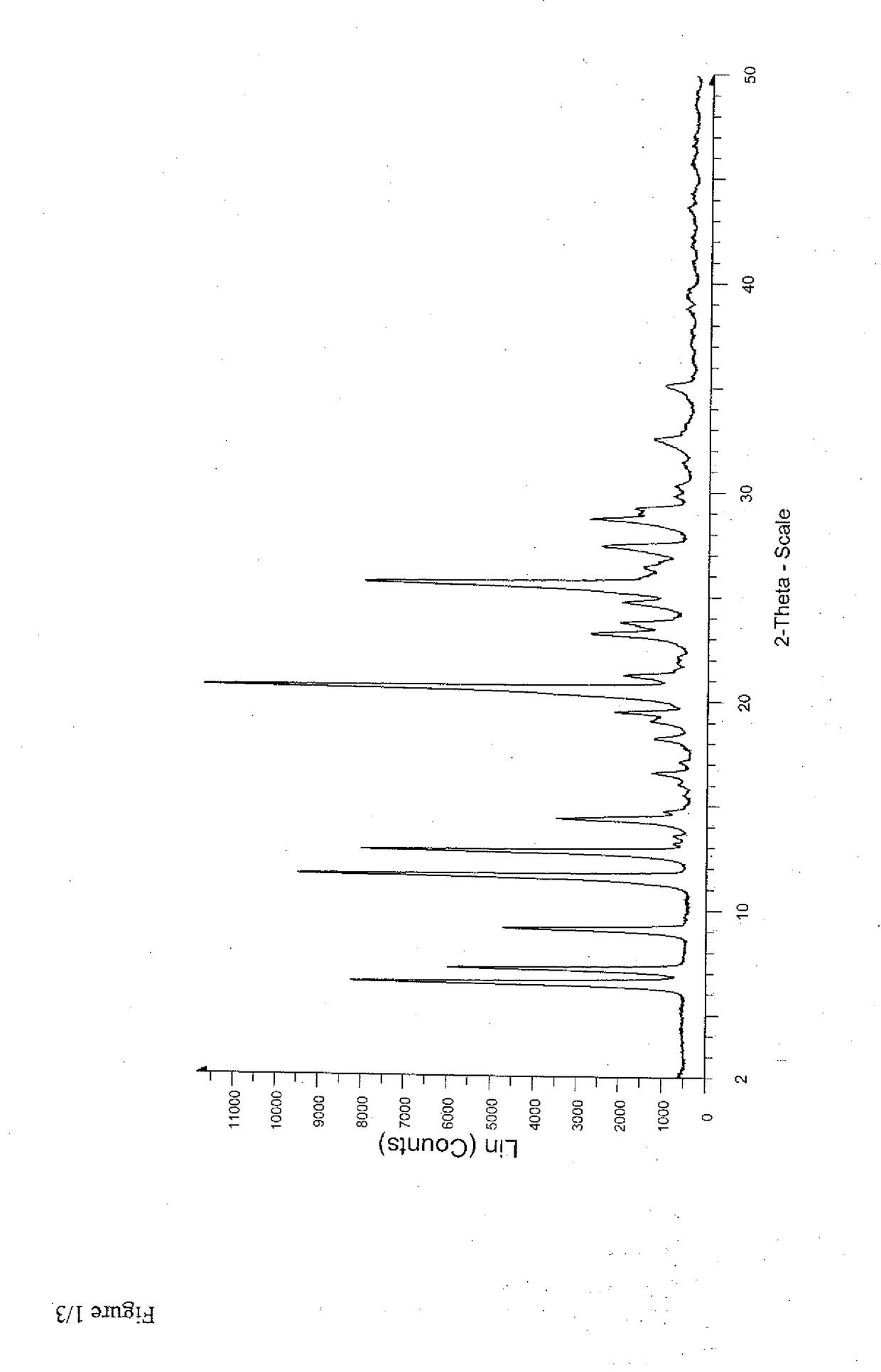
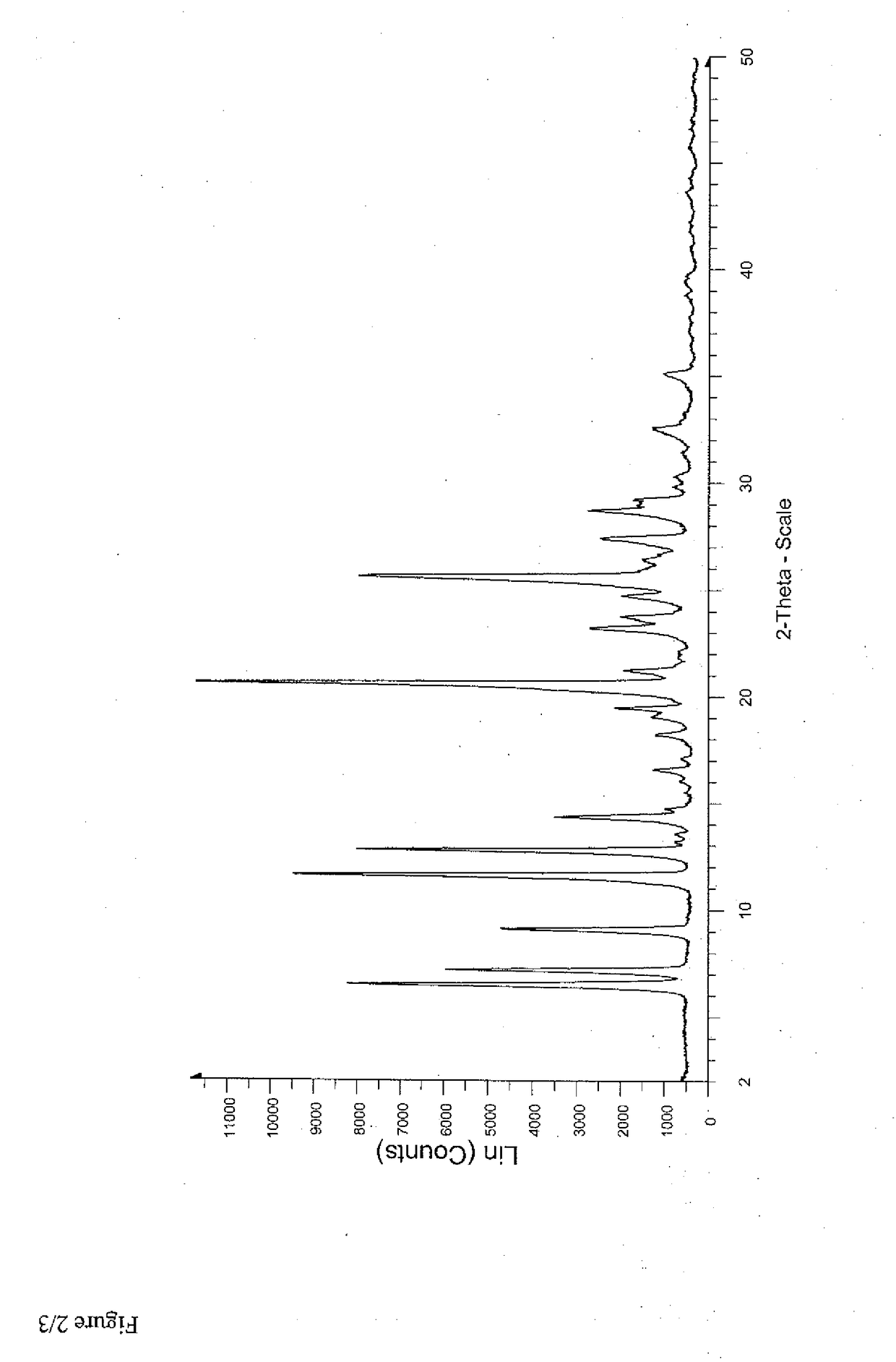
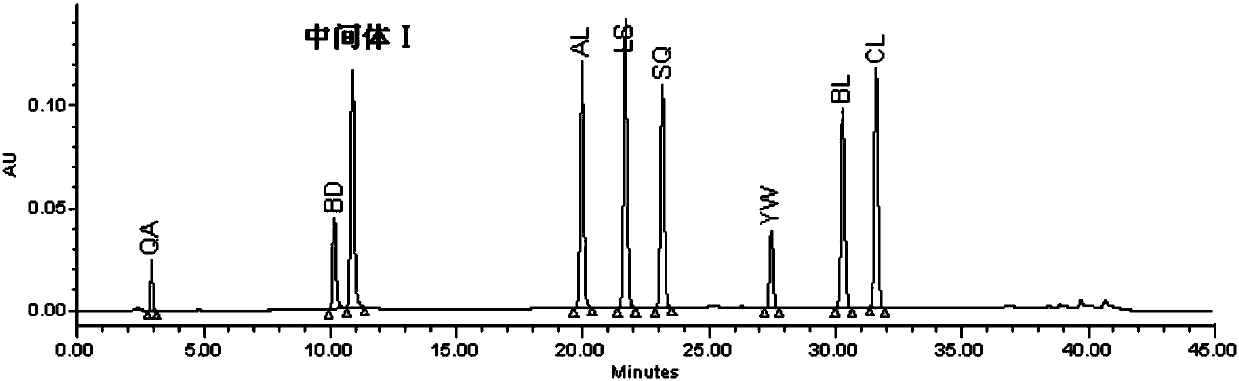
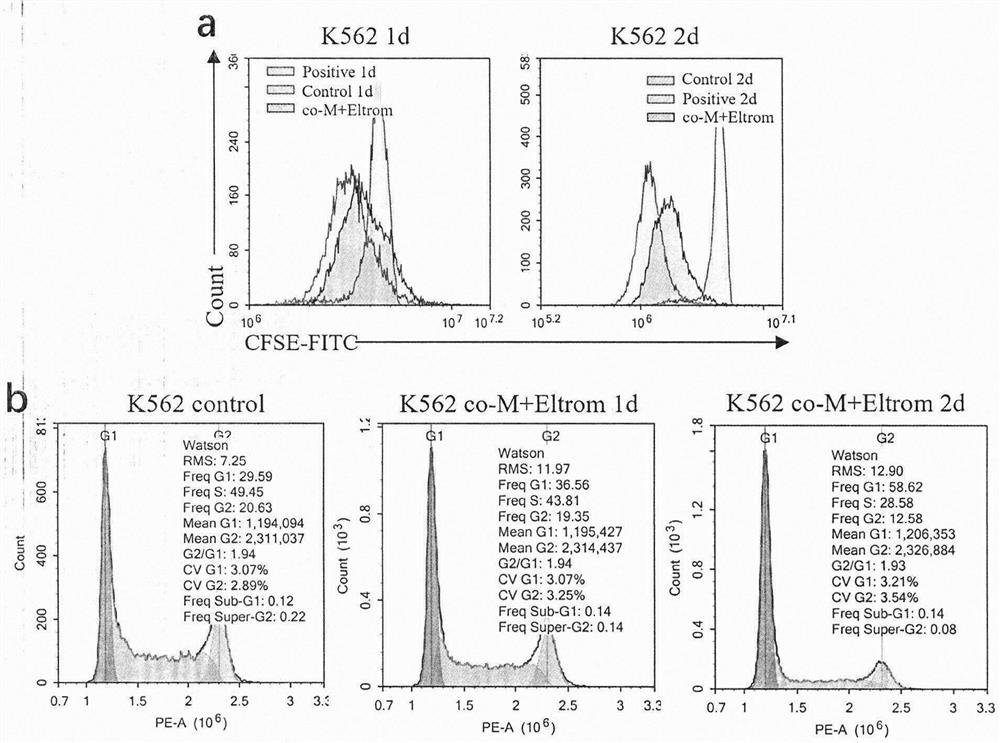
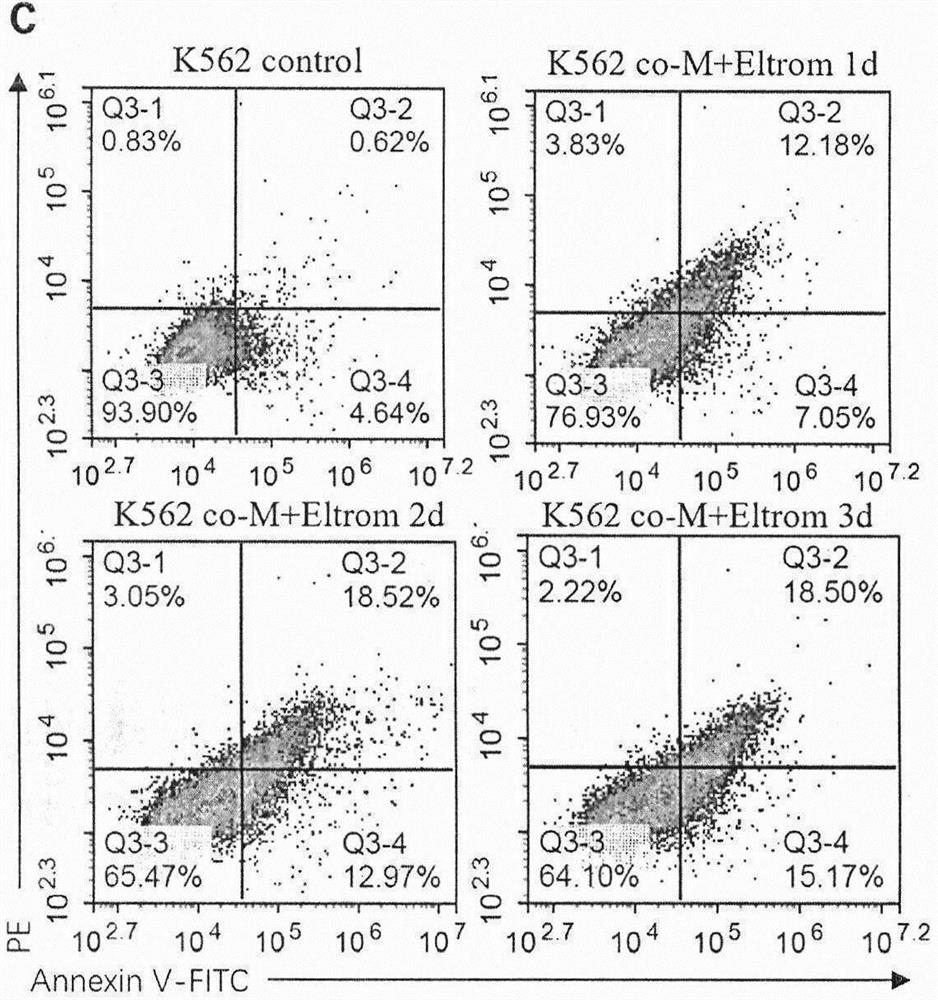
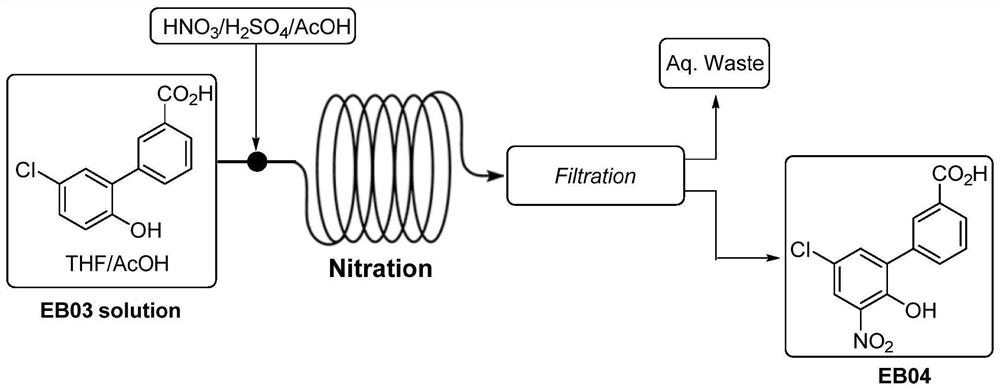
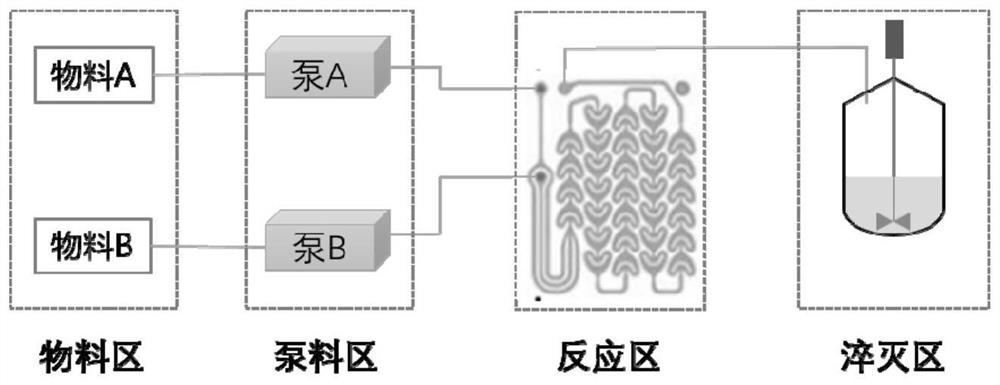
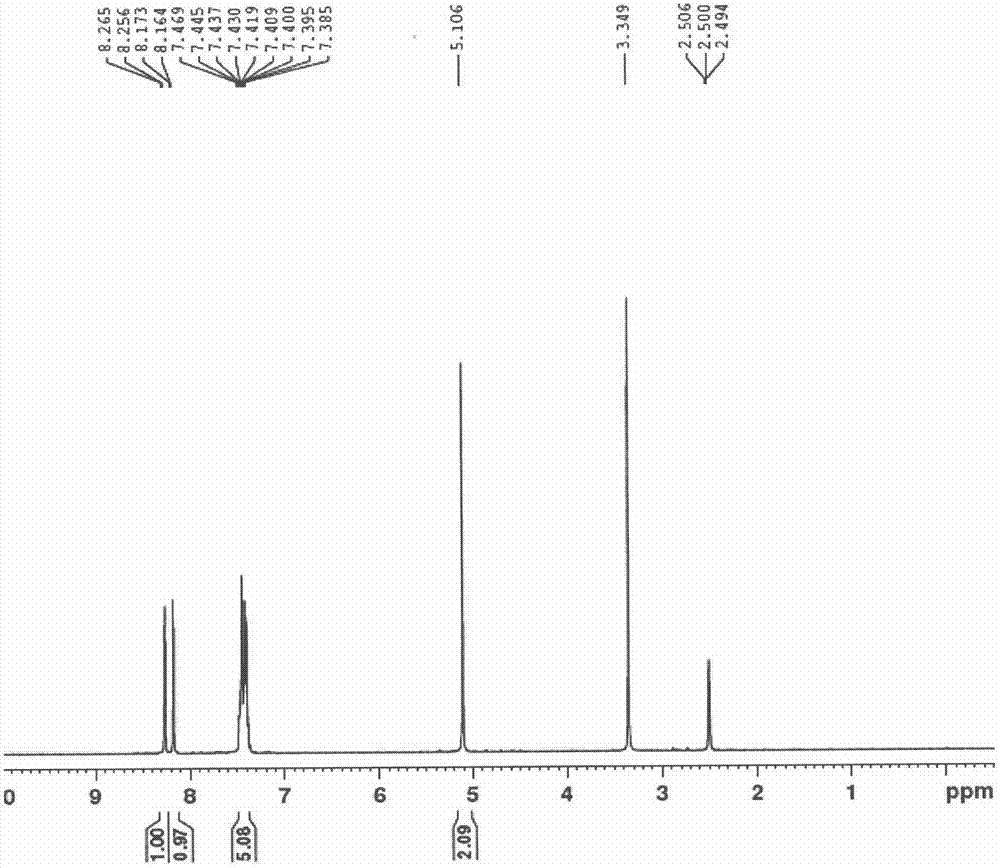
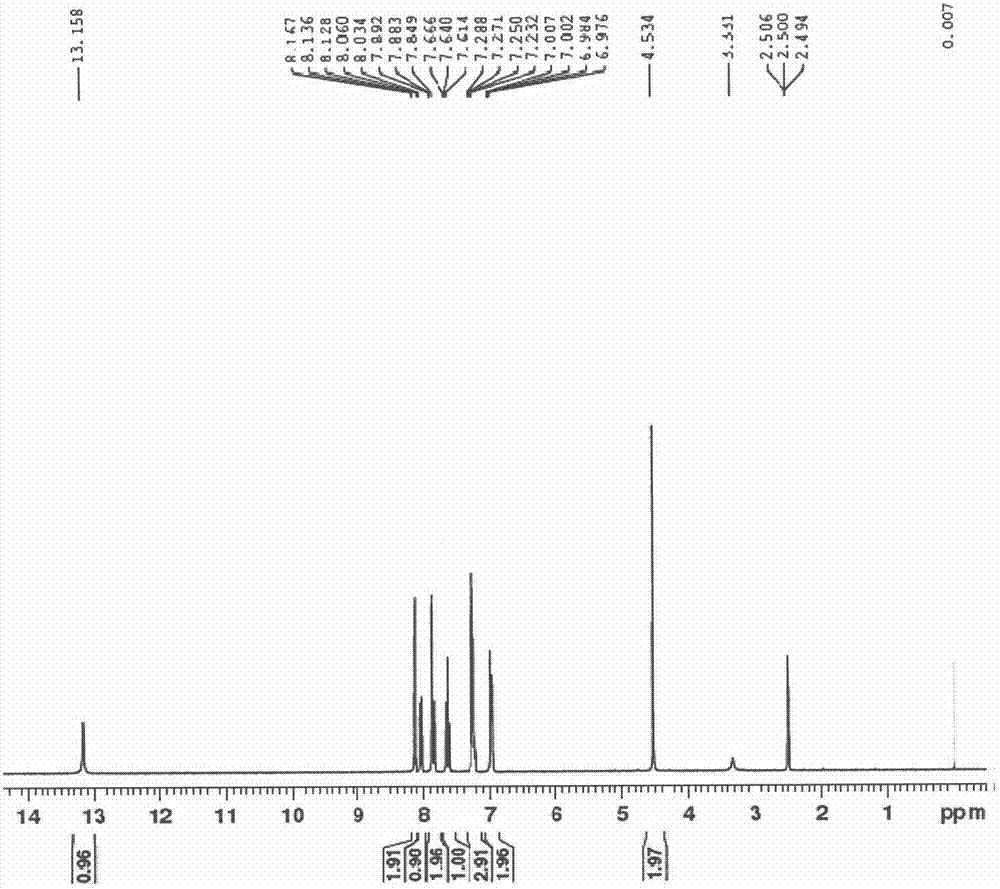
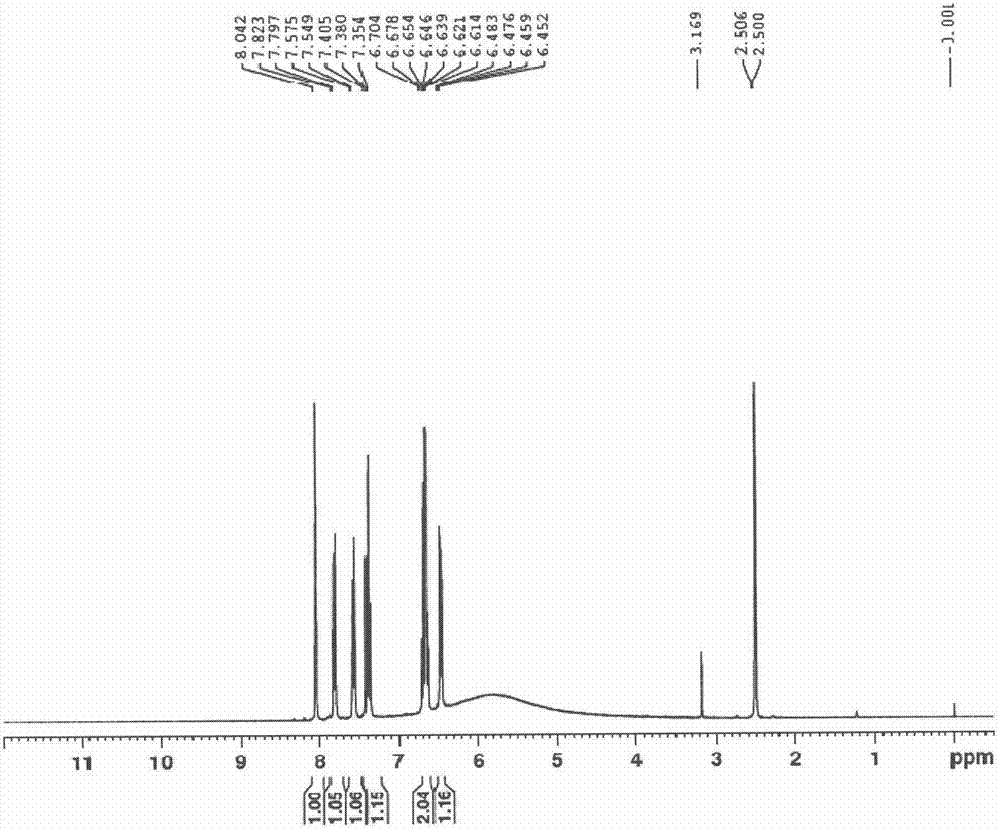
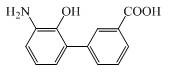
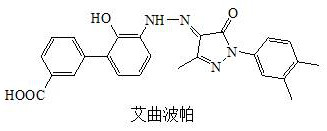
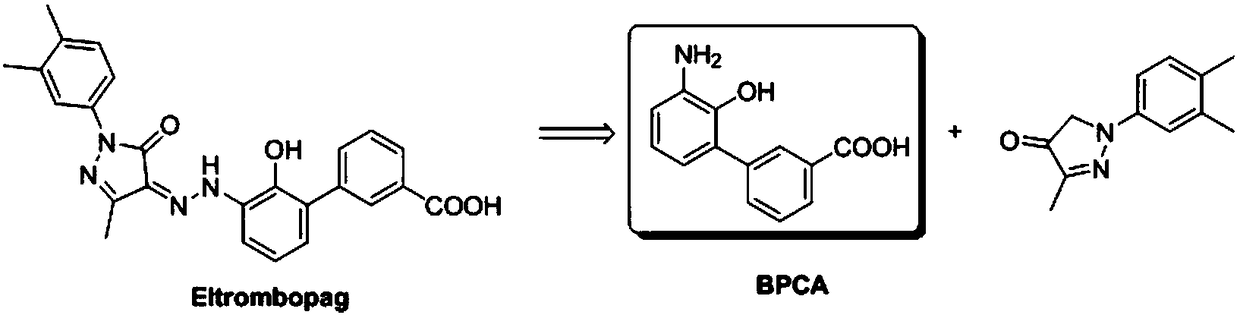
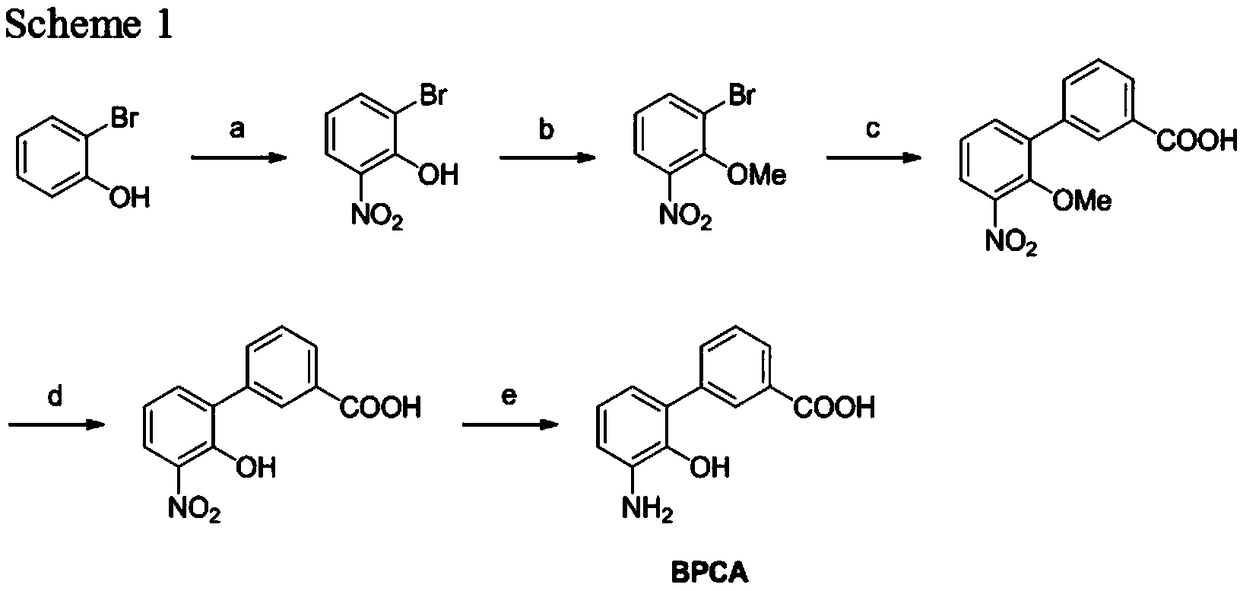
Patents
Literature
50 results about "Eltrombopag" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
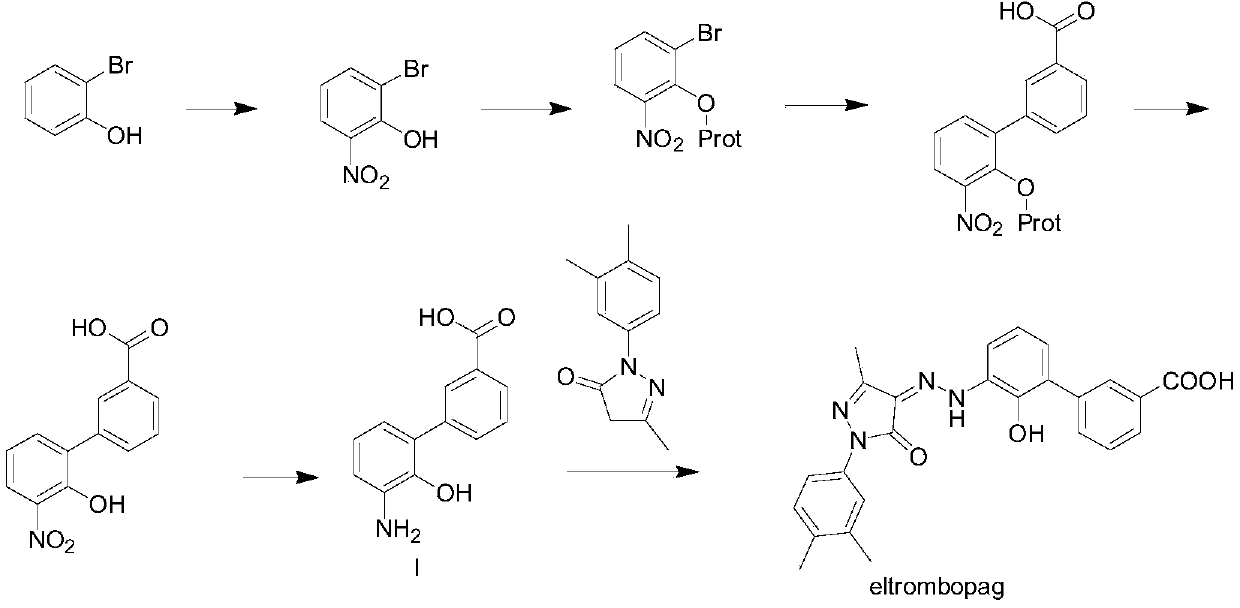
This medication is used to treat low platelet levels in people who have a certain blood disorder called chronic immune (idiopathic) thrombocytopenia purpura (ITP) or who have chronic hepatitis C. It may also be used to treat people with a certain blood disorder (aplastic anemia).
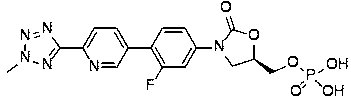
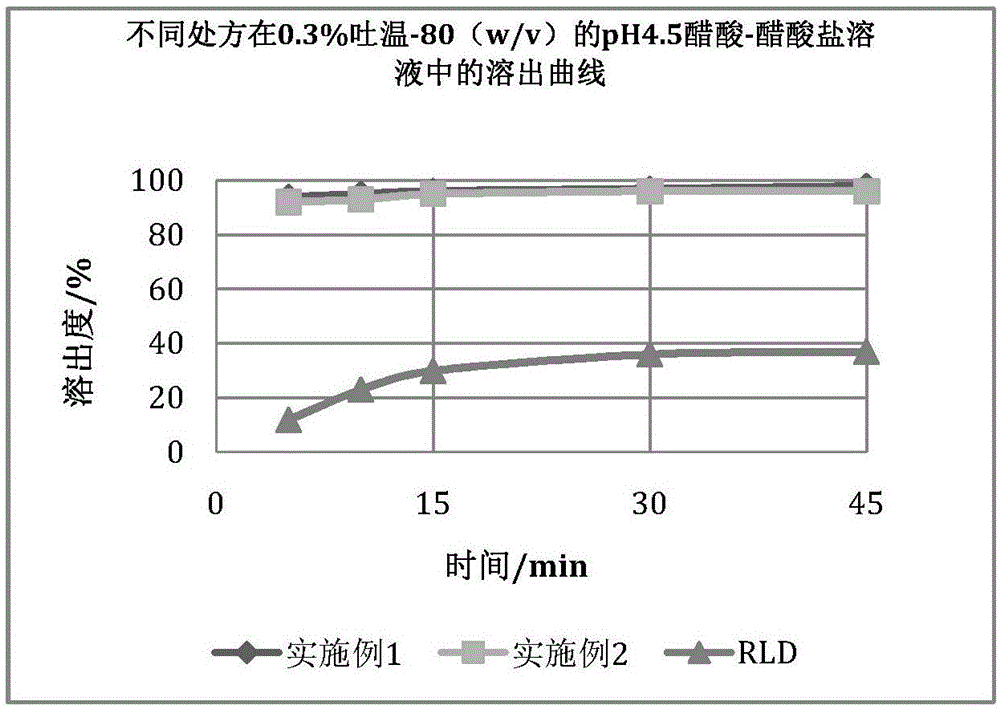
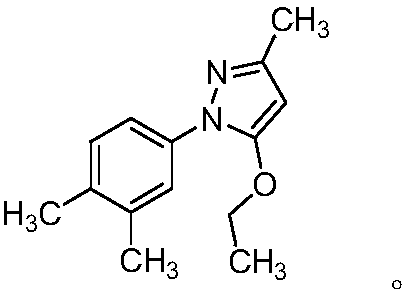
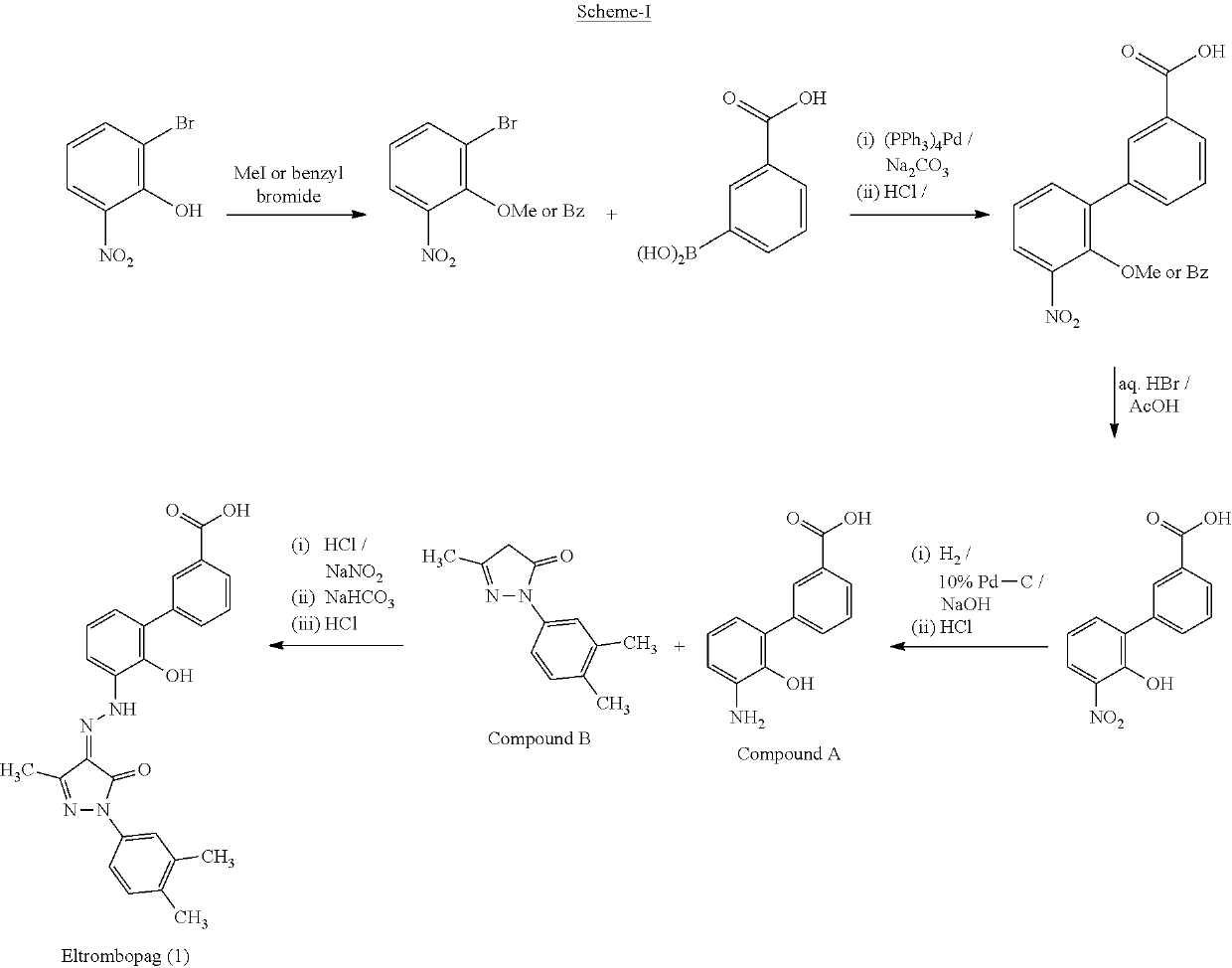
Eltrombopag tablet and preparation method thereof
InactiveCN105496976AImprove stabilityIncrease production costAntibacterial agentsOrganic active ingredientsGram-positive bacteriumPharmacology
The invention provides a preparation method of an eltrombopag tablet, belongs to the field of medicine new technologies, and relates to the eltrombopag tablet and the preparation method of the eltrombopag tablet. Eltrombopag serves as the main component of the tablet, and the tablet can be used for treating skin and skin tissue infection caused by gram-positive bacteria.
Owner:BEIJING VENTUREPHARM BIOTECH
Eltrombopag intermediate and preparation method therefor and application thereof
InactiveCN105085276ARaw materials are easy to getImprove securityOrganic chemistryOrganic compound preparationState of artReaction step
The present invention provides a novel preparation method for an eltrombopag intermediate. The raw materials of the intermediate is easily available, and the preparation method therefor is simple and high in safety; According to a reaction step section for preparing eltrombopag by using a novel intermediate, the yield is as twice as that in the prior art, so that the cost is lowered significantly, and the method is suitable for large-scale industrial production application. (img file='DDA0000503880400000011.TIF'wi='488'he='336' / ).
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Preparation method of eltrombopag olamine
ActiveCN103819406ARaw materials are easy to getHigh purityOrganic active ingredientsOrganic chemistryHydrazine compoundEltrombopag Olamine
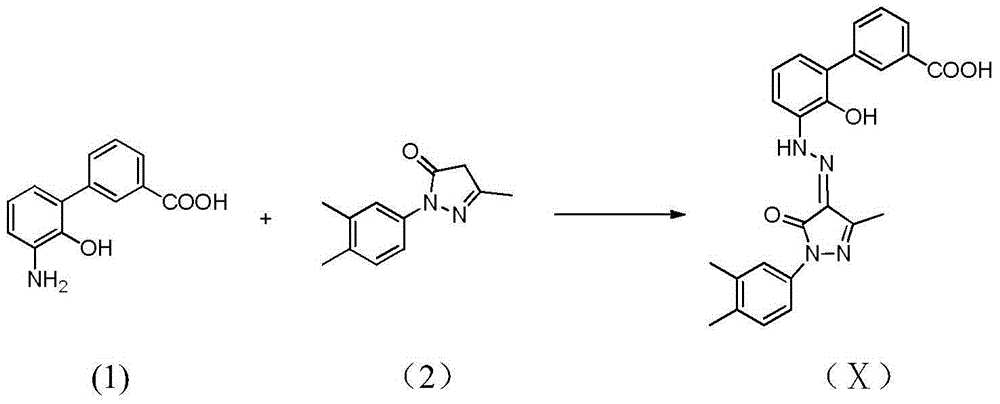
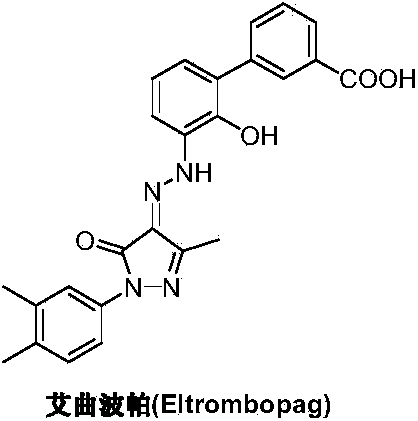
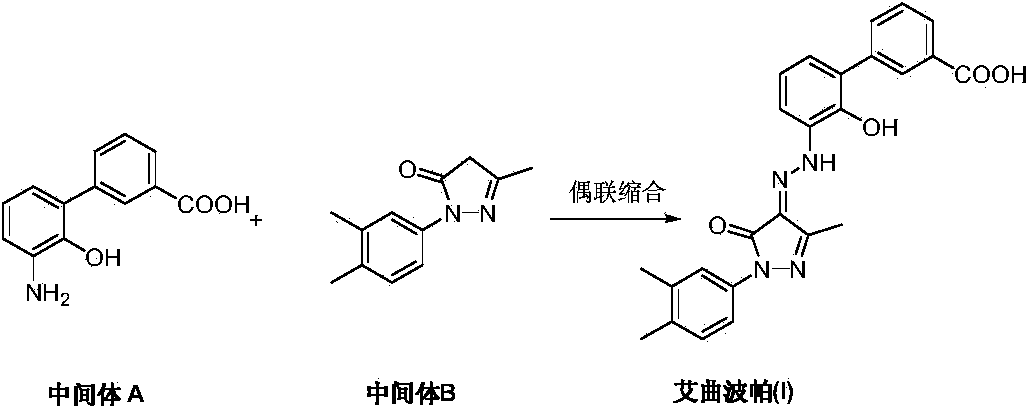
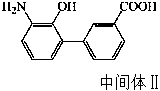
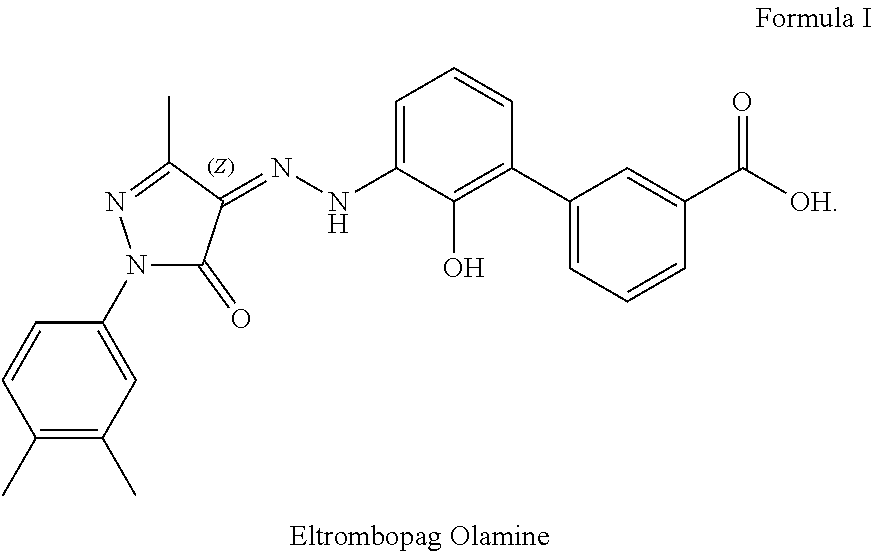
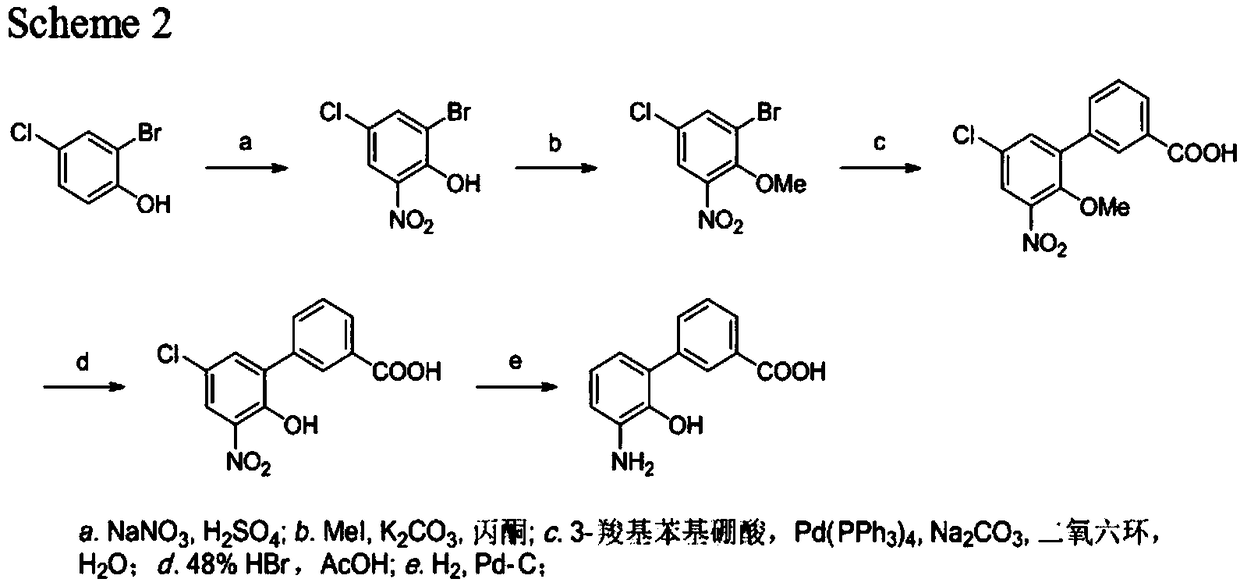
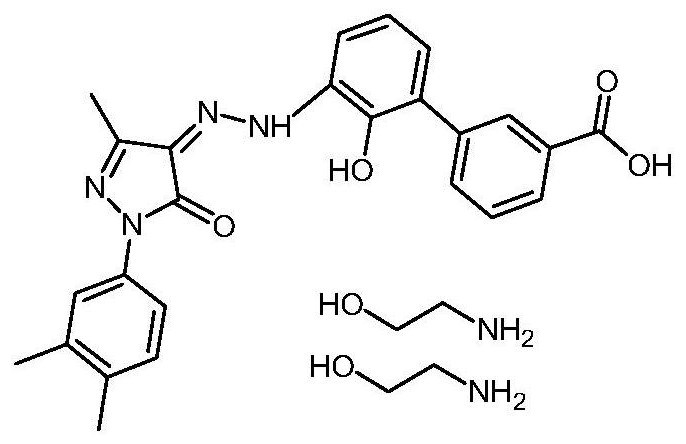
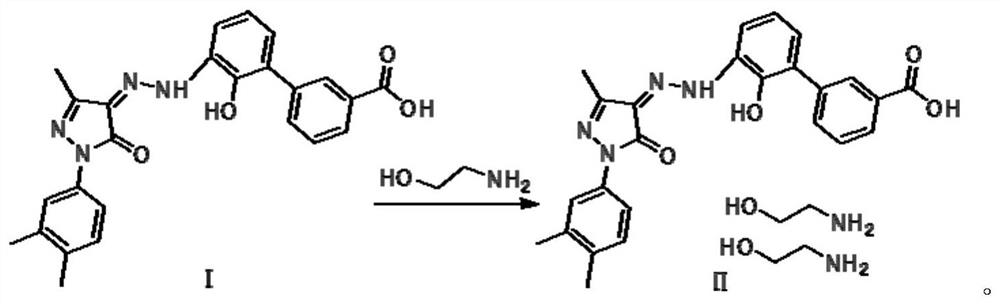
The invention discloses a preparation method of eltrombopag olamine (Eltrombopag). The preparation method comprises the following steps: carrying out azo coupling reaction by using 3'-amino-2'-hydroxy-biphenyl-3-carboxylic acid (II) and acetoacetic acid alkyl ester to obtain (Z)-2-[3'-(2'-hydroxyl-3-hydroxy-biphenyl) hydrazono]-3-oxo acid alkyl ester (III); carrying out condensation cyclization reaction on an intermediate (III) and 3,4-dimethyl benzene hydrazine to prepare the eltrombopag olamine (I). The method is concise in process, available in raw material, economical and environmental friendly, and beneficial to achievement of industrialization, and economic and technological development of the eltrombopag olamine crude drug can be facilitated.
Owner:山东佰盛能源科技有限公司
Novel method for preparing Eltrombopag intermediate
ActiveCN106146330AEasy to recycleWide variety of sourcesOrganic compound preparationAmino-carboxyl compound preparationHydrogenation processCombinatorial chemistry
The invention provides a method for preparing a compound shown as the formula I (please see the formula in the description). The method specifically comprises the following steps that 1, a compound shown as the formula (II) (please see the formula in the description) reacts with a compound shown as formula (V) (please see the formula in the description) under the alkaline condition to generate a compound shown as the formula (III) (please see the formula in the description); 2, the compound shown as the formula (III) (please see the formula in the description) reacts with a compound shown as the formula (VI) (please see the formula in the description) under the alkaline condition in the presence of palladium carbon to generate a compound shown as the formula (IV) (please see the formula in the description); 3, the compound shown as the formula (IV) (please see the formula in the description) reacts in the presence of palladium carbon and a hydrogen source under the alkaline condition to generate the compound shown as the formula (I) (please see the formula in the description). According to the method, design is ingenious, protecting group removal, dechlorination and nitro reduction are together completed in the final hydrogenation process, and the purity of the obtained compound shown as the formula (I) (please see the formula in the description) is high; the most important thing is that compared with other Suzuki coupling agents, cost of palladium carbon is lower, a source of palladium carbon is wide and easy to obtain, palladium carbon can be directly recycled and reused after being simply filtered and separated, and therefore the material cost is greatly reduced; meanwhile, emission of three wastes is reduced, and the method is quite suitable for industrialized production.
Owner:JIANGSU VCARE PHARMATECH
Senolytic compounds
PendingCN110678187AHalogenated hydrocarbon active ingredientsCyclic peptide ingredientsDiseaseNitrofurazone
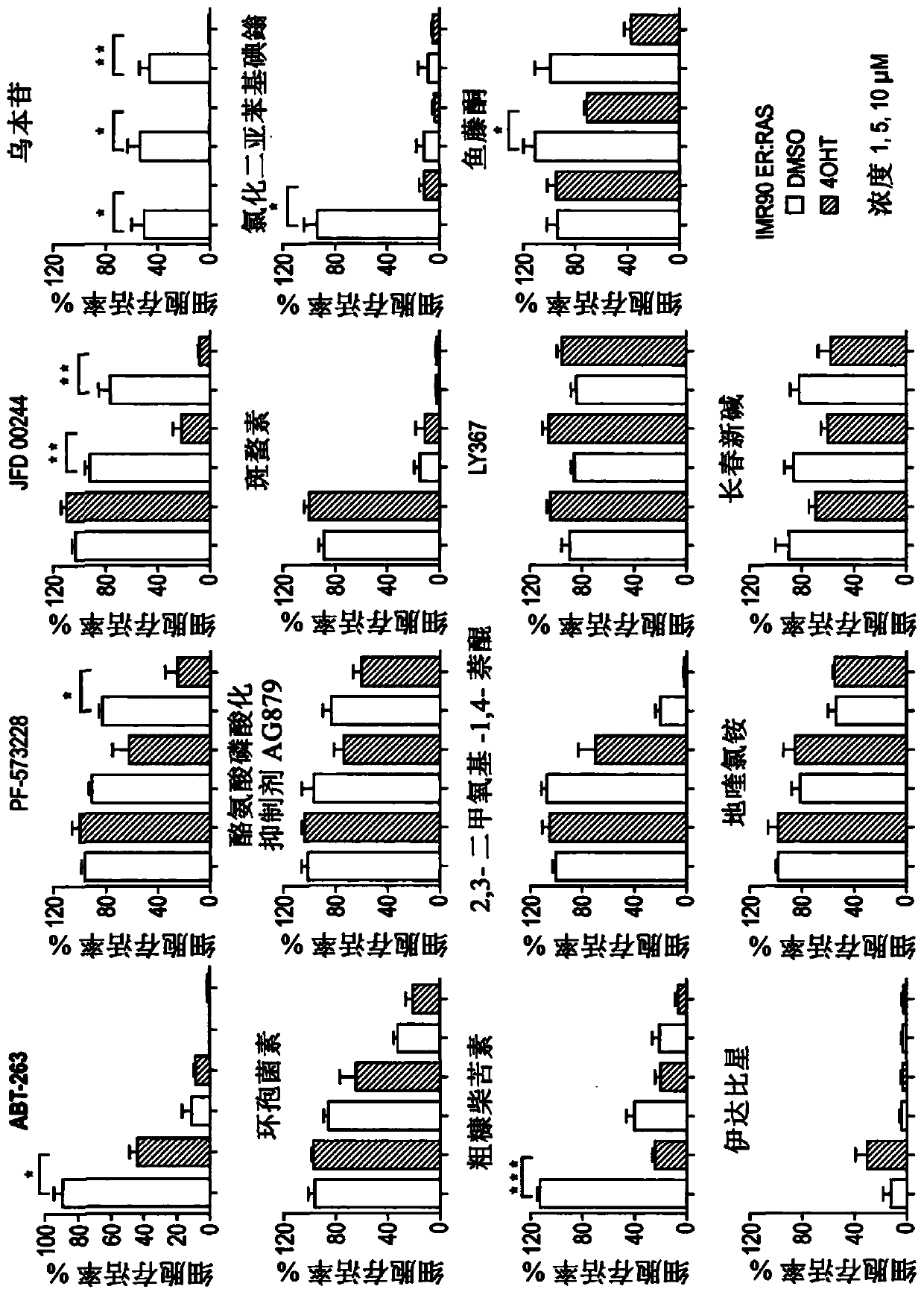
The present invention relates to an agent for use in selectively killing one or more senescent cells, wherein the agent is selected from the following: a cardiac glycoside or alglycone, a focal adhesion kinase (FAK) inhibitor, an HMG-CoA reductase inhibitor, JFD00244, Cyclosporine, Tyrphostin AG879, Cantharidin, Diphenyleneiodonium chloride, Rottlerin, 2,3-Dimethoxy-1,4-naphthoquinone, LY-367,265,Rotenone, Idarubicin, Dequalintum chloride, Vincristine, Nitazoxanide, Nitrofurazone, Temsirolimus, Eltrombopag, Adapalene, Azacyclonol, Enoxacin and Raltegravir, and pharmaceutically acceptable salts thereof. Another aspect relates to compounds for use in treating or preventing a senescence- associated disease or disorder, and methods relating thereto.
Owner:英国研究与创新公司
Eltrombopag inclusion compound and preparations and preparation method thereof
PendingCN107913411AIncrease flexibilityImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsEfficacyTherapeutic effect
The invention provides an Eltrombopag inclusion compound and preparations and a preparation method thereof. The Eltrombopag inclusion compound contains: Eltrombopag or a pharmaceutically acceptable salt thereof and an inclusion material, and the inclusion material is cyclodextrin or a derivative thereof. The inclusion compound provided by the invention can reduce the contact between polyvalent metal ions and Eltrombopag, thus reduce the formation of chelate, and increase medicine solubility. The preparations prepared from the inclusion compound and other pharmaceutically acceptable excipientswhich are provided by the invention can all increase the speed of drug dissolution, are favorable for the absorption of Eltrombopag drugs, increase the flexibility and compliance of medication by patients, and can also prevent a decrease in therapeutic effect or even ineffectiveness as the result of improper medication, thus ensuring the normal exertion of efficacy. In addition, the preparation method of the Eltrombopag inclusion compound provided by the invention has the advantages of high inclusion rate, low production cost, simple process and suitability for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of eltrombopag medicine for treating idiopathic thrombocytopenic purpura
InactiveCN107915678AReduce usagePromote the development of economy and technologyOrganic chemistryBlood disorderHigh volume manufacturingThrombocytopenic purpura
The invention discloses a preparation method of Eltrombopag used for treating idiopathic thrombocytopenic purpura. The chemical name of Eltrombopag is 3‑{(2Z)‑2‑[1‑(3 ,4‑xylyl)‑3‑methyl‑5‑oxo‑1,5‑dihydro‑4H‑pyrazole‑4‑ylidene]hydrazino}‑2‑hydroxy‑3‑biphenylcarboxylic acid‑ 2-amino ethanol salt; the preparation process of the present invention is simple, the raw material is easy to get, avoids the use of highly toxic methyl iodide, is economical and environmentally friendly, is conducive to the realization of industrialization, can promote the economic and technological development of Eltrombopag raw material medicine, and reduces the production cost. cost, suitable for mass production.
Owner:孙婷婷
Preparation method of eltrombopag diethanolamine salt
The invention provides a preparation method of eltrombopag diethanolamine salt; the preparation method is simple to perform and suitable for industrial production; active pharmaceutical ingredients of the eltrombopag diethanolamine salt prepared herein meet provisions in ICH (international council for harmonisation) guidelines.
Owner:CHANGZHOU PHARMA FACTORY
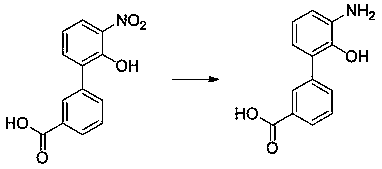
Preparation method of eltrombopag intermediate 2-hydroxy-3-(m-carboxyl phenyl)aniline
ActiveCN109704982AOrganic compound preparationAmino-carboxyl compound preparationCombinatorial chemistryAniline
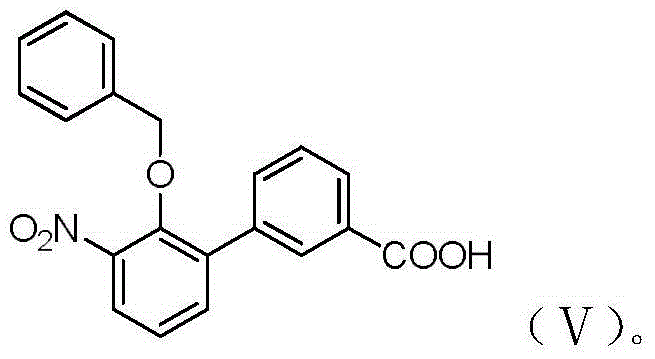
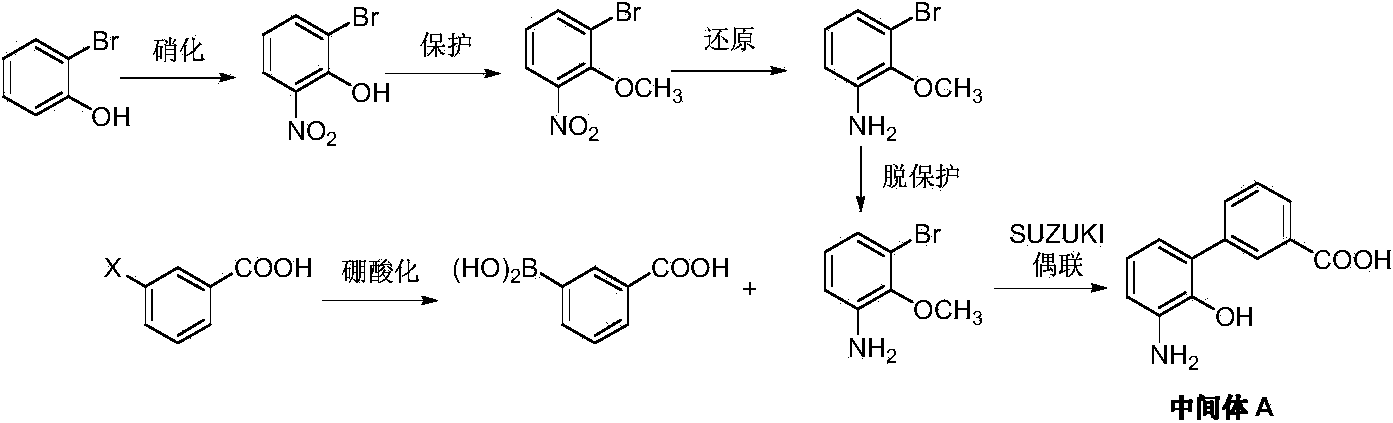
The invention provides a preparation method of eltrombopag intermediate 2-hydroxy-3-(m-carboxyl phenyl)aniline. According to the preparation method, 2-amino-4-chlorophenol is taken as a raw material,urea is adopted for protection, bromination, benzylation, Suzuki coupling reaction, hydrolysis deprotection of urea protection, hydrogenation dechlorinating and debenzylation are carried out so as toobtain 2-hydroxy-3-(m-carboxyl phenyl)aniline. Compared with the prior art, the advantages are that: operation is simple; conditions are mild and easily controllable; the preparation method is safe, and is friendly to the environment; yield is high; and the preparation method is suitable for industrialized large scale production.
Owner:SHANGHAI TIANCI INT PHARMA
Eltrombopag liquid capsule and preparation method thereof
InactiveCN106361719AEasy to produceQuick effectOrganic active ingredientsInorganic non-active ingredientsHard CapsuleSolvent
The invention relates to an eltrombopag liquid capsule and a preparation method thereof. The eltrombopag liquid capsule is prepared by dissolving eltrombopag in an auxiliary material to obtain content, and then filling a hard capsule shell with the content. The auxiliary material includes a solvent, a surfactant, a co-surfactant, an anti-oxidant, a pH regulator, a proper amount of water, etc. The liquid capsule overcomes the defects that the eltrombopag is low in dissolubility and is difficult to dissolve, and has many specifications and complex production process, thereby bringing better selection on medicine dosage forms for patients.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
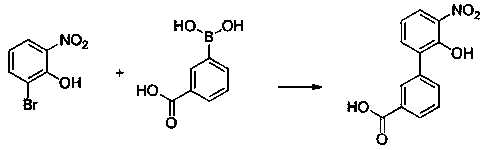
Preparation method of Eltrombopag key intermediate 3'-amino-2'-hydroxybiphenyl-3-carboxylic acid
PendingCN110407702AMild responseHigh yieldOrganic compound preparationAmino-carboxyl compound preparationPalladium on carbonNitration
The invention discloses a preparation method of an Eltrombopag key intermediate 3'-amino-2'-hydroxybiphenyl-3-carboxylic acid. The preparation method comprises the following steps: (1) nitrating o-bromophenol with a nitrating reagent by using water as a solvent; (2) carrying out a Suzuki coupling reaction on 2-bromo-6-nitrophenol and 3-carboxyphenylboronic acid in the presence of an alkali by using metallic palladium or its metal salt as a catalyst; and (3) carrying out nitro reduction on obtained 3'-nitro-2'-hydroxybiphenyl-3-boronic acid in the presence of a proper hydrogen source by using the metallic palladium or its metal salt as the catalyst. The Eltrombopag key intermediate 3'-amino-2'-hydroxybiphenyl-3-carboxylic acid is obtained through the preparation method of the nitration, Suzuki coupling and nitro reduction three-step reaction of the o-bromophenol used as the raw material. The Suzuki reaction uses the cheap and readily available palladium on carbon as the catalyst. The hydrogen source used in the reduction step is easy to operate. The preparation method has the advantages of simple reaction route in the whole process, mild conditions, simplicity in post-treatment operation, and suitableness for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method of eltrombopag
The invention relates to the technical field of medicine manufacturing, and discloses a preparation method of eltrombopag. The preparation method comprises the following steps: 1, sequentially carrying out a continuous reaction on a starting material I, glacial acetic acid and hydrobromic acid until the reaction is completed to obtain an intermediate I; 2, reducing the intermediate I by hydrazinehydrate under catalytic action of palladium to obtain an intermediate II; and 3, carrying out diazotization addition on the intermediate II to obtain the eltrombopag. According to the method, nitryl is prevented from being directly reduced by hydrogen on the premise that the medicine quality is guaranteed, so that production can be safely amplified, and market competitiveness of the variety is improved.
Owner:TIANJIN LISHENG PHARM CO LTD
(Trifluoromethoxy) pyrrolidine benzamide compound and application of compound in treating thrombocytopenia
The invention discloses a (trifluoromethoxy) pyrrolidine benzamide compound. A structural formula of the compound is as shown in the specification. A pharmacological experiment confirms that the compound has a better proliferation effect on 32D-MPL (myeloproliferative leukemia) cells containing a TPO (thrombopoietin) receptor; in-vivo activity and in-vitro activity are higher than those of a positive drug, eltrombopag; and the compound can be concluded to have an effect of a TPO receptor agonist. After the compound is administrated to a blood loss mouse, data analysis finds that data of the compound 6 in a test group on the seventh day and on the fourteenth day have significance as compared with a model group; a platelet concentration of tail blood on the fourteenth day is higher than thatof orbit blood; and the deeper research can be conducted in the aspect of thrombocytopenia related diseases.
Owner:窦玉玲
Preparation method of eltrombopag intermediate and preparation method of eltrombopag diethanolamine salt
ActiveCN112979481AHigh yieldHigh synthetic yieldOrganic compound preparationAmino-carboxyl compound preparationPhenylboronic acidNitration
The invention provides a preparation method of an eltrombopag intermediate and a preparation method of eltrombopag diethanolamine salt, and relates to the technical field of medicinal chemistry. According to the preparation method of the eltrombopag intermediate, p-bromophenol is taken as a raw material, and the problem of poor selectivity of nitration reaction is solved. After iodination, the coupling reaction yield of the intermediate compound as shown in a formula (I) and phenylboronic acid is high. Subsequently reduction reaction is carried out, the debromination reaction is complete, the yield is 90% or more, the refining is simpler, and the synthesis yield of the compound of the formula (I) is higher and the operation is simple. Through the improvement, the yield of the key compound shown as the formula (I) is relatively high, the subsequent treatment operation is simple, the process is easy for amplified production, the yield of the whole synthesis process of the eltrombopag diethanolamine salt is 48.1%, the operation is simple, less three wastes are generated, and the synthesis process is suitable for amplified production.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Crystalline form of eltrombopag free acid
ActiveUS20170275255A1Avoid pollutionOrganic chemistryOrganic compound preparationMedicinal chemistryFree acid
The present invention relates to crystalline form of Eltrombopag free acid and its process for preparation.
Owner:HETERO RES FOUND
Detection method of eltrombopag intermediate related substances and impurity reference substance used in method
ActiveCN107870217AEfficient separationAccurate monitoringComponent separationFiller ExcipientPhosphoric acid
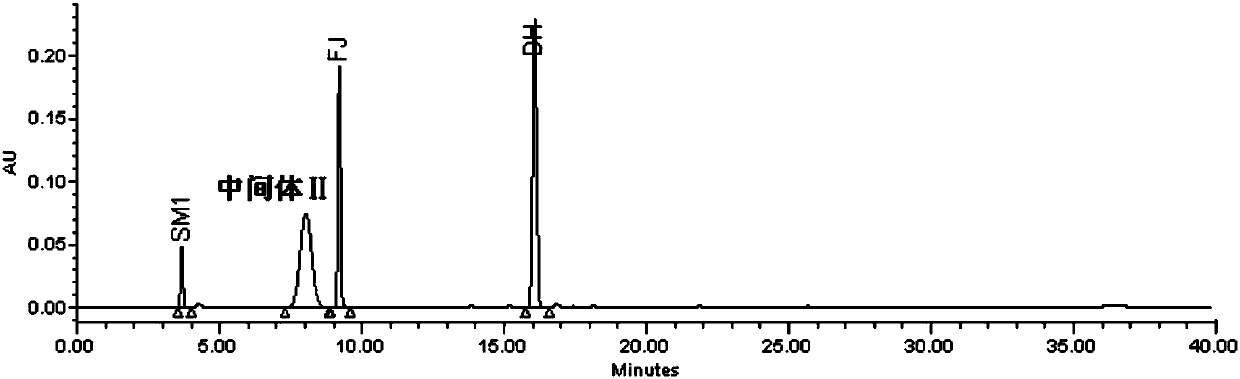
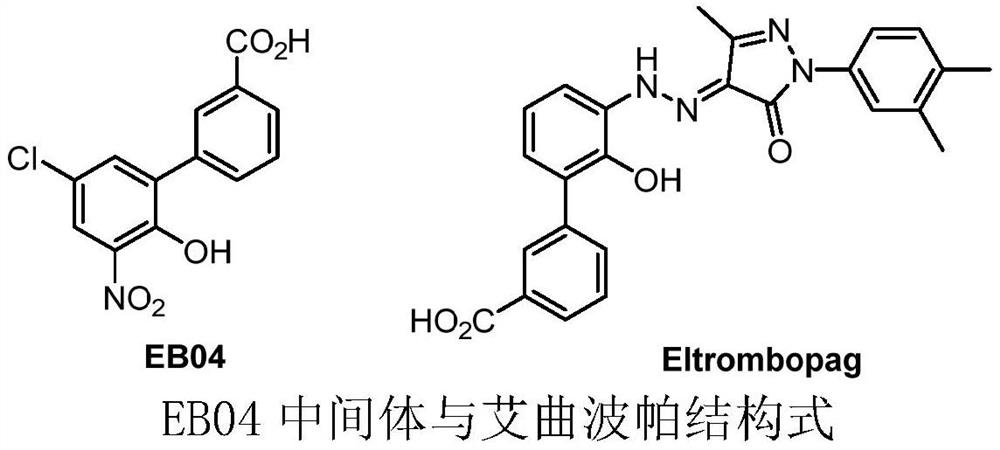
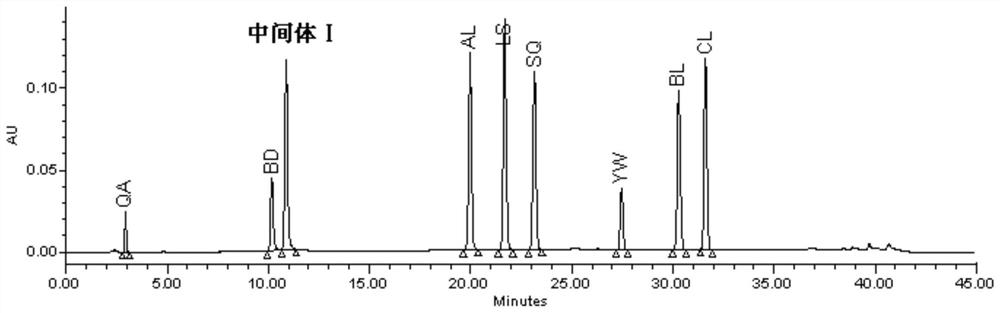
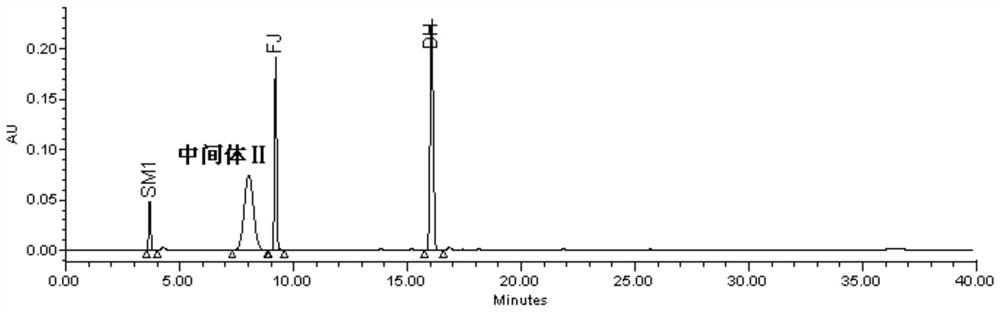
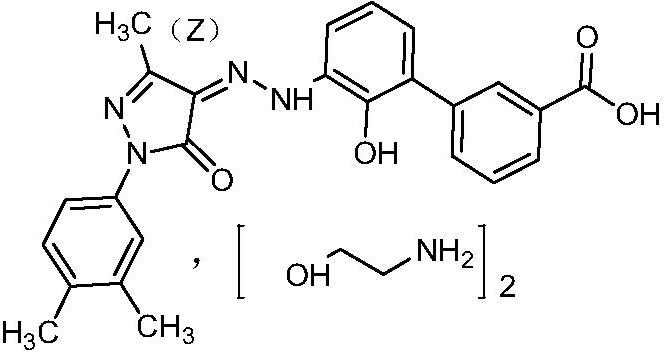
The invention discloses a detection method of eltrombopag intermediate related substances and an impurity reference substance used in the method and belongs to the technical field of pharmaceutical analysis. A chromatographic column taking phenyl and octadecyl silane bonded silica gel as filler is adopted, a phosphate buffer solution and an acetonitrile or phosphate-acetonitrile solution are adopted as mobile phases, a gradient elution manner is adopted, two established HPLC (high performance liquid chromatography) analysis methods can effectively determine the content of eltrombopag intermediate I 2'-hydroxyl-3'-amino-diphenyl-3-carboxylic acid and intermediate II 2-(3,4-dimethyl-phenyl)-5-methyl-2,4-dihydro-pyrazole-3-one as well as intermediate related impurities, the method is good inspecificity, high in sensitivity and convenient to operate, and quality control of a final eltrombopag product is effectively guaranteed. The invention also discloses a novel impurity reference substance compound.
Owner:QILU PHARMA
Preparation method of eltrombopag
The invention relates to the technical field of pharmaceutical manufacturing and discloses a preparation method of eltrombopag. The method comprises the following steps: 1) 12%-20% of 2-bromo-6-nitrophenol, 5%-7.5% of potassium carbonate and 7%-15% of benzyl bromide are taken sequentially and poured into 120 ml of an acetonitrile solution for a reflux reaction, temperature is increased to 90 DEG Cfor the reflux reaction and kept at 90 DEG C for continuous reflux for 3 h, then a reflux device is cooled to room temperature, an obtained solution is filtered and concentrated, dry solids are obtained and placed into a 100ml glass dish, and 40 ml of a triethylamine liquid is added. According to the preparation method of eltrombopag, the triethylamine liquid is added to the solids prepared in step 1, the triethylamine liquid and benzyl bromide can be subjected to a chemical reaction, white crystals of quaternary ammonium are generated by the reaction, the white crystals can be washed by clear water, so that benzyl bromide in the solids is eliminated, toxin components of the drug are reduced, follow-up manufacture of the drug is facilitated, product quality is guaranteed, and competitiveness of an enterprise is enhanced.
Owner:ABA CHEM SHANGHAI
Pharmaceutical composition comprising eltrombopag olamine
PendingUS20210308104A1Eliminates negative propertyDissolve fastOrganic active ingredientsGranular deliveryDrug capsuleEltrombopag Olamine
The present invention relates to a pharmaceutical capsule composition comprising eltrombopag olamine or a pharmaceutically acceptable salt thereof for use in the treatment of thrombocytopenia. Furthermore, the present invention relates to an improved, simple, rapid, cost-effective, time-saving and industrially available method of preparing the capsule composition comprising eltrombopag olamine.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Application of mesenchymal stem cells combined with TPO and analogues thereof in treatment of chronic myeloid leukemia
PendingCN113750220APrevent proliferationPromote apoptosisOrganic active ingredientsPeptide/protein ingredientsMyeloid leukemiaReceptor
The invention belongs to the technical field of medical biology, and particularly relates to application of umbilical cord-derived mesenchymal stem cells combined with TPO and analogues thereof in preparation of drugs for treating chronic myelogenous leukemia. The mesenchymal stem cells are human-derived umbilical cord mesenchymal stem cells (UC-MSCs), can efficiently induce differentiation of chronic myeloid leukemia cells after being combined with a TPO analogue Eltrombopag, and the capability is realized by secreting TPO by MSCs and inducing leukemia cells to express a TPO receptor (MPL); and compared with chemotherapy, mesenchymal stem cell transplantation is lower in toxic and side effects, has low immunogenicity and meets clinical requirements, and the Eltrombopag is a clinically approved drug. The method provides a new thought for stem cell transplantation treatment of myeloid leukemia, and can be applied to differentiation treatment of myeloid leukemia.
Owner:NANJING UNIV
Method for preparing eltrombopag nitration intermediate in micro-channel continuous flow reactor
InactiveCN112608239ANovel processChemical/physical/physico-chemical microreactorsNitro compound preparationChemical synthesisNitration
The invention discloses a method for preparing an eltrombopag nitration intermediate in a micro-channel continuous flow reactor, belongs to the field of organic chemical synthesis, and aims to improve a high-risk nitration process involved in an eltrombopag bulk drug production process and avoid the nitration reaction. The method comprises the following steps: injecting two reaction materials into a micro-channel at a certain flow rate respectively; injecting the mixed and reacted materials into a quenching kettle, and after quenching is completed, centrifuging, washing and drying to obtain a target product. The method for preparing the eltrombopag nitration intermediate in the micro-channel continuous flow reactor has the beneficial effects that a novel process technology is adopted, and the method has the characteristics of safety, high efficiency, environmental friendliness and the like.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Detection method for related substances of Eltrombopag intermediate I
The invention is named as a method for detecting related substances of Eltrombopag intermediate I, and belongs to the technical field of drug analysis. The present invention respectively adopts chromatographic columns with phenyl and octadecylsilane bonded silica gel as fillers, uses phosphate buffer solution and acetonitrile or phosphate acetonitrile solution as mobile phase, adopts gradient elution mode, and establishes two high-efficiency The liquid phase analysis method can effectively determine Eltrombopag synthetic intermediate Ⅰ 2'-hydroxy-3'-amino-biphenyl-3-carboxylic acid and intermediate Ⅱ 2-(3,4-dimethyl-phenyl) ‑5‑methyl‑2,4‑dihydro‑pyrazole‑3‑one and the content of related impurities in the above-mentioned intermediates, the method has good specificity, high sensitivity, and convenient operation. effective guarantee. The invention also discloses a new impurity reference substance compound.
Owner:QILU PHARMA CO LTD
A kind of method for preparing Eltrombopag intermediate
ActiveCN106146330BEasy to recycleWide variety of sourcesOrganic compound preparationAmino-carboxyl compound preparationHydrogenation processCombinatorial chemistry
The invention provides a method for preparing a compound shown as the formula I (please see the formula in the description). The method specifically comprises the following steps that 1, a compound shown as the formula (II) (please see the formula in the description) reacts with a compound shown as formula (V) (please see the formula in the description) under the alkaline condition to generate a compound shown as the formula (III) (please see the formula in the description); 2, the compound shown as the formula (III) (please see the formula in the description) reacts with a compound shown as the formula (VI) (please see the formula in the description) under the alkaline condition in the presence of palladium carbon to generate a compound shown as the formula (IV) (please see the formula in the description); 3, the compound shown as the formula (IV) (please see the formula in the description) reacts in the presence of palladium carbon and a hydrogen source under the alkaline condition to generate the compound shown as the formula (I) (please see the formula in the description). According to the method, design is ingenious, protecting group removal, dechlorination and nitro reduction are together completed in the final hydrogenation process, and the purity of the obtained compound shown as the formula (I) (please see the formula in the description) is high; the most important thing is that compared with other Suzuki coupling agents, cost of palladium carbon is lower, a source of palladium carbon is wide and easy to obtain, palladium carbon can be directly recycled and reused after being simply filtered and separated, and therefore the material cost is greatly reduced; meanwhile, emission of three wastes is reduced, and the method is quite suitable for industrialized production.
Owner:JIANGSU VCARE PHARMATECH
Preparation method of eltrombopag
PendingCN114507186AThe reaction route is simpleEasy to operateOrganic chemistryAcetic acidCombinatorial chemistry
The invention discloses a preparation method of eltrombopag. The preparation method comprises the following steps: 1, sequentially and continuously reacting a starting material I, glacial acetic acid and hydrobromic acid to obtain an intermediate I; 2, reducing the intermediate I through ammonium formate under the catalytic action of palladium to obtain an intermediate II; and 3, carrying out diazotization addition on the intermediate II to obtain eltrombopag. According to the method, on the premise of ensuring the medicine quality, direct reduction of nitryl by hydrogen is avoided, so that safe production amplification can be realized. Meanwhile, on the basis that the single maximum impurity of eltrombopag is controlled not to exceed 0.1% and the total impurity is controlled not to exceed 1.0%, the reaction can be completed more quickly, the time of the preparation process can be further shortened, the preparation speed of eltrombopag is increased, and the market competitiveness of the eltrombopag is enhanced.
Owner:TIANJIN LISHENG PHARM CO LTD
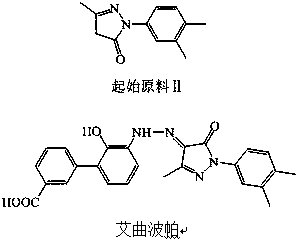
Synthetic method of eltrombopag intermediate
ActiveCN109485580AReduce processingFew reaction stepsOrganic compound preparationAmino-carboxyl compound preparationHalogenAmino derivatives
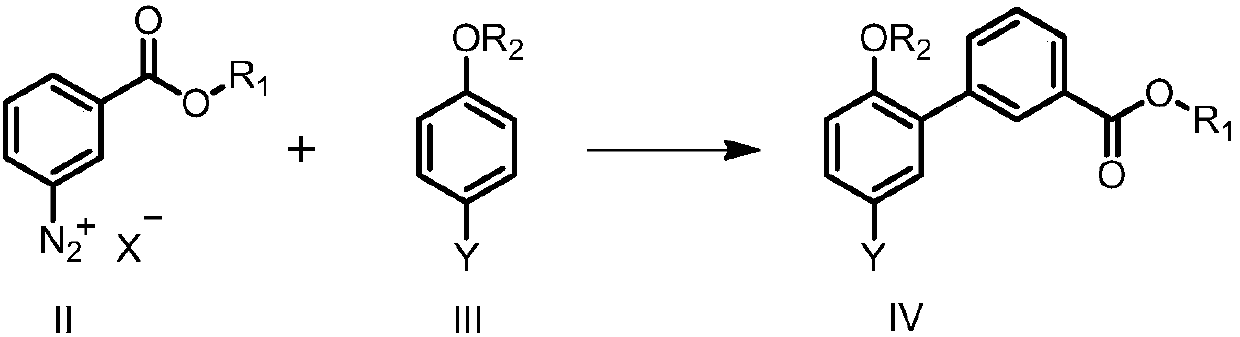
The application discloses a synthetic method of an eltrombopag intermediate, comprising: subjecting 2-aminophenol derivative shown as formula (II) as a raw material to react with an aromatic reagent shown as formula (III) to obtain an intermediate product shown as formula (IV); using the intermediate product shown as formula (IV) as a raw material to prepare the eltrombopag intermediate shown as formula (I), wherein R1 is selected from amino derivatives, and R2 is selected from halogen and sulfonyloxy group. The synthetic method has few reaction steps; the starting material is simple; the synthetic method is simpler, more economical and greener.
Owner:WUHAN WUYAO SCI & TECH
Preparation method of eltrombopag
The invention discloses a preparation method of eltrombopag, which includes steps of with a low-cost and easy-to-obtain amine or a salt thereof as a raw material, performing diazotization and couplingto form an intermediate IV; and performing nitration, reduction, coupling and hydrolysis to produce the eltrombopag. The preparation method is simple in operation and good in reaction selectivity, has gentle conditions and low production cost, and is easy to carry out in industrial production.
Owner:SUZHOU KELUN PHARMA RES CO LTD +1
Eltrombopag diethanolamine salt and preparation method thereof
The invention relates to the technical field of medicine preparation, and discloses an eltrombopag diethanolamine salt and a preparation method thereof. The preparation method comprises the followingsteps: dissolving ethanolamine in an organic solvent; heating to 60-75 DEG C, stirring to dissolve ethanolamine, dropwise adding a tetrahydrofuran solution of eltrombopag free acid into the ethanolamine solution, keeping the temperature, stirring, cooling after dropwise adding, and filtering, wherein the volume ratio of tetrahydrofuran to eltrombopag free acid to the organic solvent is (6-11): 1:(12-24). According to the method, in a homogeneous reaction system, sufficient salification is guaranteed, and the prepared eltrombopag diethanolamine salt is low in impurity content, simple in reaction operation, suitable for amplification and suitable for industrial production.
Owner:WUHAN WUYAO SCI & TECH
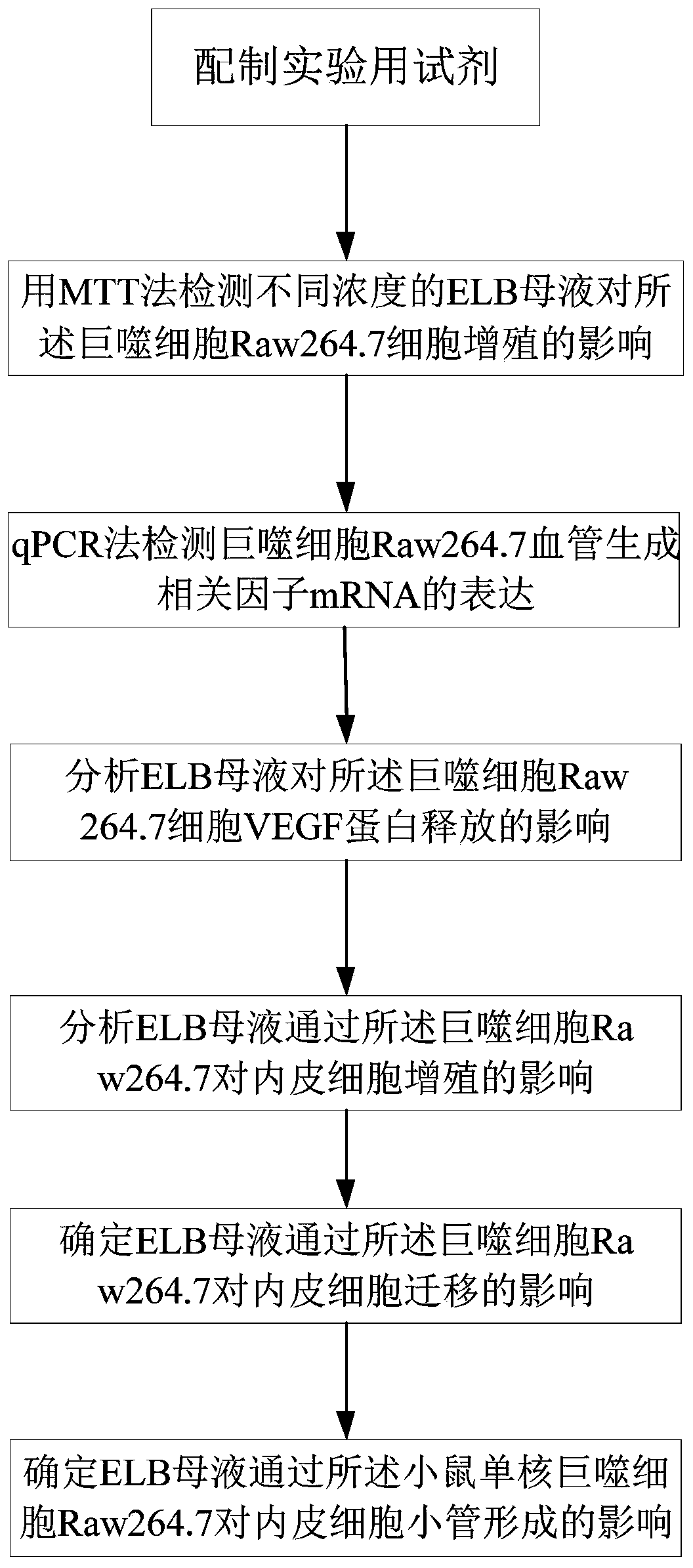
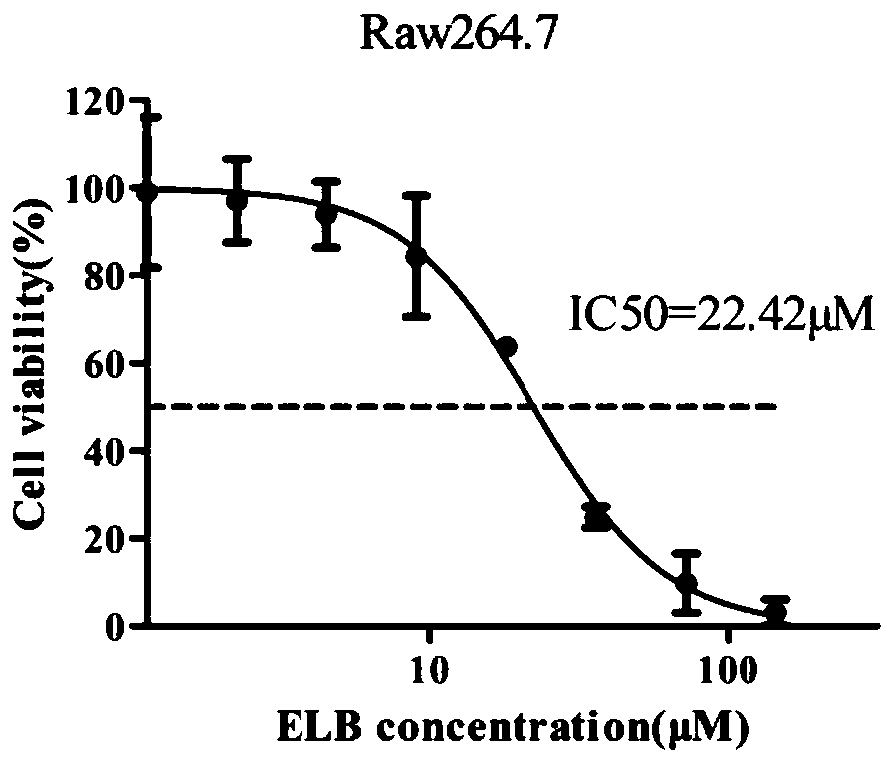
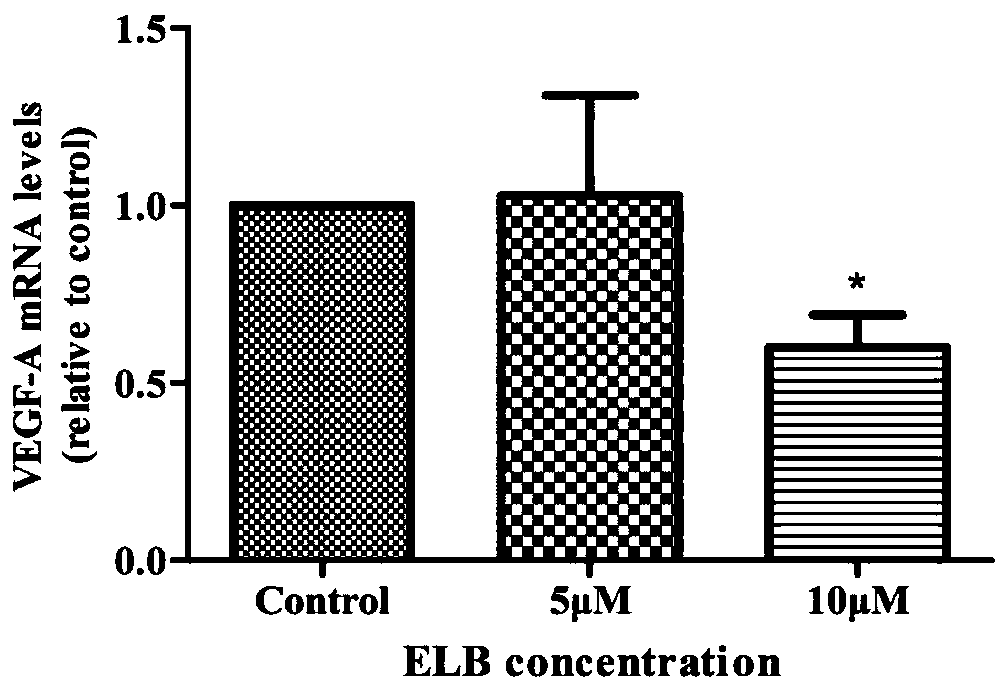
Method for verifying eltrombopag mediated anti-angiogenesis function of macrophage
InactiveCN110656152APrevent proliferationInhibition releaseCompound screeningApoptosis detectionMatrigelBlood vessel
The invention provides a method for verifying the Eltrombopag (ELB) mediated anti-angiogenesis function of macrophage. Through MTT assay, q-PCR and ELISA (enzyme linked immunosorbent assay), influenceof the ELB upon macrophage proliferation, expression of mRNA (messenger ribonucleic acid) of related angiogenesis factors (VEGF-A, bFGF, MMP-9) and VEGF (vascular endothelial growth factor) proteinscan be detected; and furthermore, through MTT experiments, cell wound scratch assay and Matrigel pipe formation experiments, influence of the ELB upon endothelial cell proliferation, migration and tube cavity formation through macrophage can be observed. The method verifies that the ELB is capable of inhibiting proliferation of the macrophage, inhibiting mRNA of the expression of related angiogenesis factors and inhibiting release of the VEGF proteins, and meanwhile, finds that the ELB acts on supernate of the macrophage and is capable of remarkably inhibiting proliferation, migration and tubule formation of the endothelial cells, and thus the macrophage can be mediated by the ELB and thus has the anti-angiogenesis function.
Owner:ZHENGZHOU UNIV
Application of hirsutine in medicine for treating thrombocytopenia
PendingCN114146087AAchieve therapeutic effectIncreased platelet countOrganic active ingredientsBlood disorderSide effectPharmaceutical drug
The invention relates to the field of medicinal chemistry, in particular to application of hirsutine in preparation of a medicine for treating thrombocytopenia. The hirsutine is shown as a formula I in the specification. According to the application of hirsutine in preparing the medicine, the platelet level in the body can be remarkably improved, the bleeding risk of a patient with thrombocytopenia and the probability that blood needs to be transfused are reduced, and the blood transfusion infection risk is reduced; and compared with the limitation of high treatment cost of TPO and TPO receptor agonists (such as eltrombopag and romiscine), the hirsutine has the advantages of easiness in acquisition, lower cost, small side effect and the like, and can greatly reduce the family burden of patients and reduce the social medical security expenditure pressure.
Owner:SOUTHWEST MEDICAL UNIVERISTY
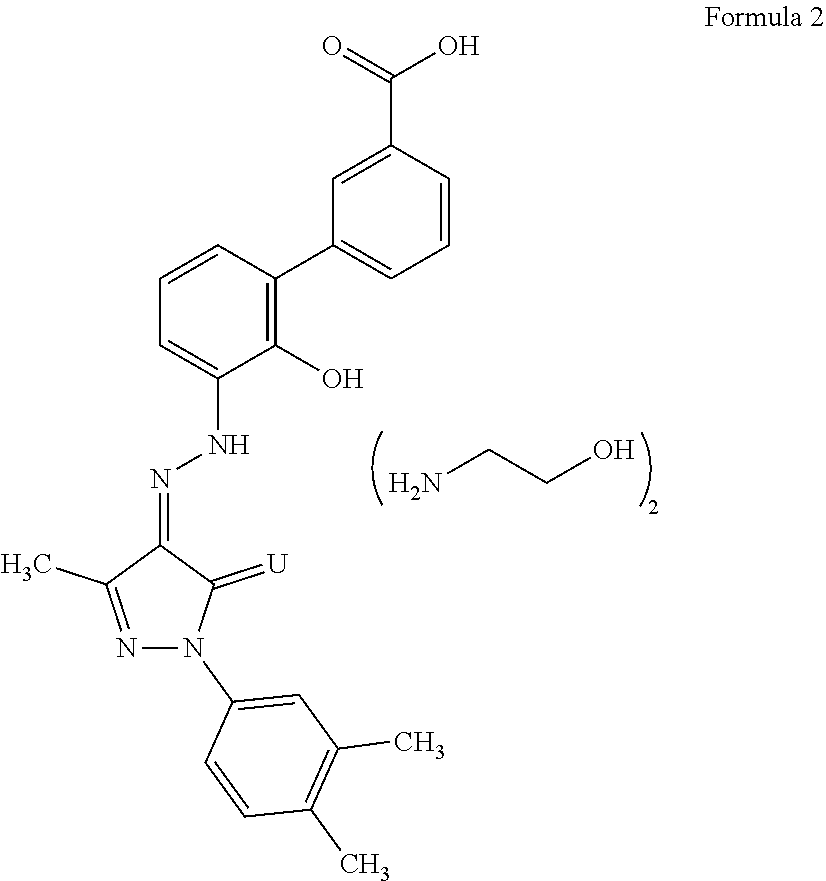
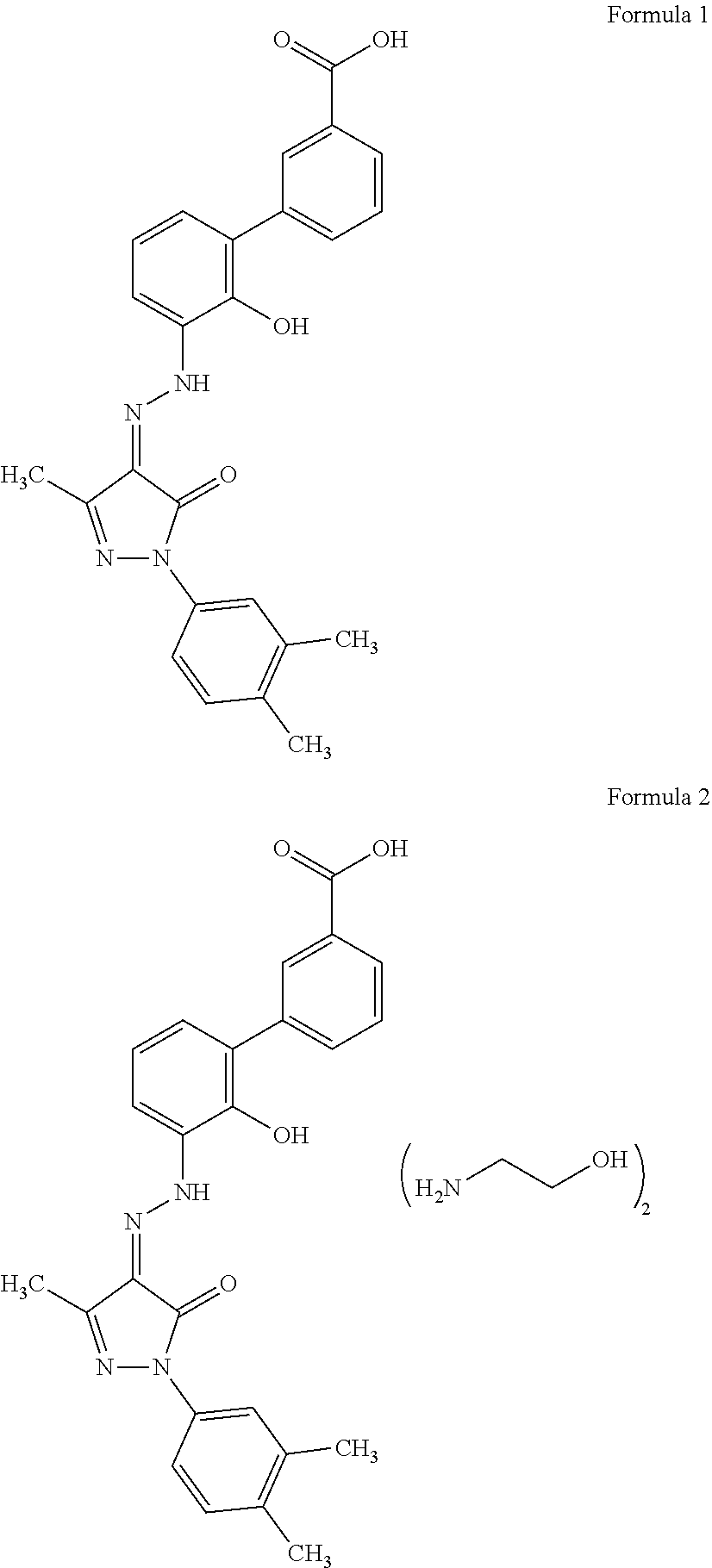
An improved process for the preparation of Eltrombopag Olamine and its intermediates
The present invention relates to an improved process for the purification of Eltrombopag olamine of compound of formula (2). The present invention also relates to an improved process for the preparation of Eltrombopag olamine intermediates and further conversion to Eltrombopag olamine of a compound of formula (2).
Owner:AUROBINDO PHARMA LTD
Preparation method of eltrombopag with stable performance
InactiveCN113024465ARaw materials are easy to getHigh yieldOrganic chemistrySodium acetateEthyl butyrate
The invention discloses a preparation method of eltrombopag with stable performance, which comprises the following steps: adding 3 '-amino-2'-hydroxybiphenyl-3-carboxylic acid (II) and hydrochloric acid into a reaction box, stirring and dissolving; dropwise adding a sodium nitrite aqueous solution under an ice bath, adding an ethanol solution containing ethyl acetoacetate and sodium acetate under stirring after dropwise adding, heating to room temperature, reacting for 10 hours, and completing TLC (Thin Layer Chromatography) detection reaction; and filtering, and recrystallizing the crude product with methanol to obtain an intermediate (Z)-2-[3'- (2'- hydroxy-3-carboxylic acid biphenyl) hydrazono ]-3-oxobutanoate (III), and carrying out condensation cyclization reaction on the intermediate (III) and 3, 4-dimethylphenylhydrazine to obtain Eltrombopag (I).
Owner:HANGZHOU HUANGSEN BIOLOGICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com