Eltrombopag liquid capsule and preparation method thereof
A technology of liquid capsules and capsule shells, which can be used in capsule delivery, blood diseases, pharmaceutical formulations, etc., and can solve problems such as increasing product complexity and not being authorized in China
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The content takes the hydrophilic solution type as an example
[0033] prescription
[0034] name Proportion / % effect Eltrombopag 10 Main drug PEG400 50 solvent Diethylene glycol monoethyl ether 23.4 solvent glycerin 5 co-surfactant Tween 80 2.5 Surfactant BHT 0.15 Antioxidants Monoethanolamine 0.5 pH regulator purified water Appropriate amount solvent
[0035] Process:
[0036] (1) Take PEG400 according to the prescription amount, place diethylene glycol monoethyl ether, glycerin, Tween 80, and monoethanolamine in a liquid mixing tank, stir and mix evenly;
[0037] (2) Weigh BHT and Eltrombopag according to the prescription amount, add to the above mixed solution and stir until dissolved to form a solution;
[0038] (3) Measure the moisture of the above solution, and add an appropriate amount of purified water according to the result to adjust the final moisture to about 8%;
[0039] ...
Embodiment 2
[0048] The content is self-emulsifying type as an example
[0049] prescription
[0050]
[0051]
[0052] Process:
[0053] (1) Weigh PPG-11, RH40, glyceryl triacetate, PEG400, and monoethanolamine according to the prescription quantity and place them in the liquid preparation tank, stir and mix evenly;
[0054] (2) Take tocopherol and eltrombopag according to the prescription amount, add to the above mixed solution and stir until dissolved to form a solution;
[0055] (3) Fill the above-mentioned solution as the content in a special hard capsule shell to obtain.
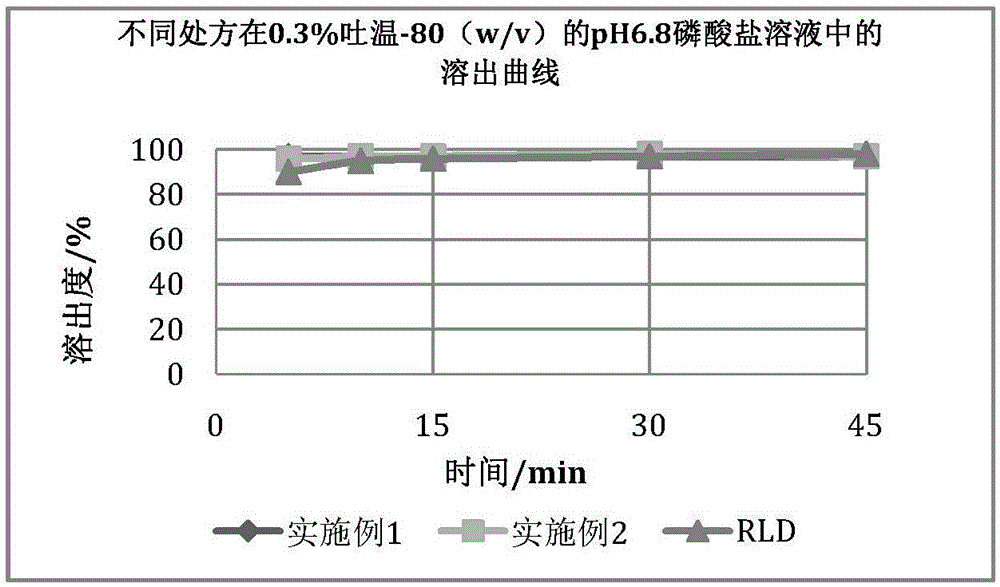
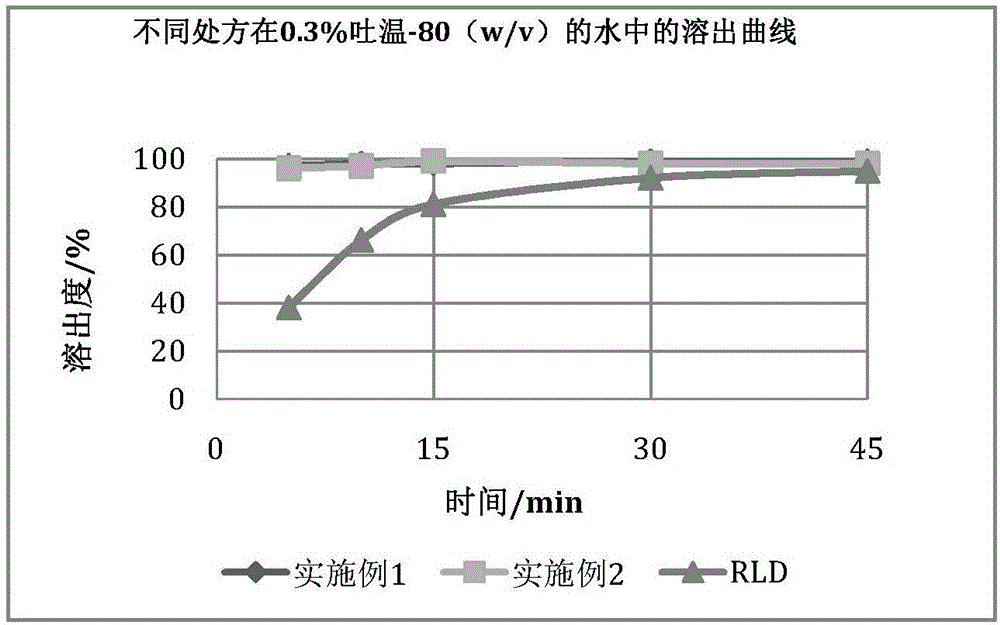
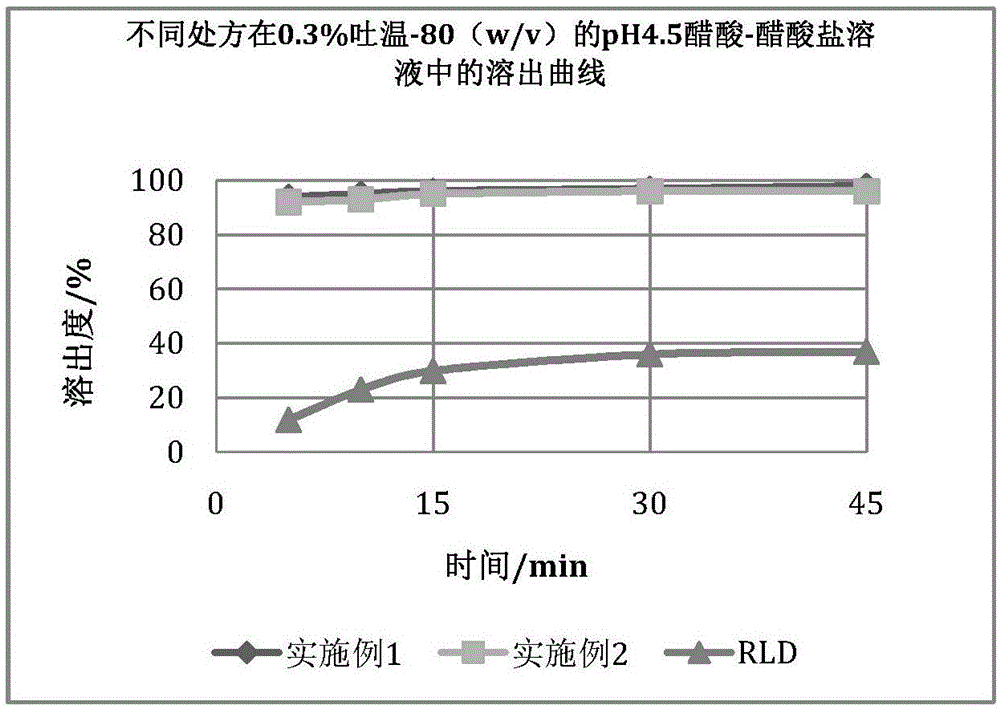
[0056] The samples prepared according to Examples 1 and 2 were compared with the original research product (RLD: Eltrombopag tablets were jointly developed by U.S. Ligand Pharmaceutical Co., Ltd. and U.K. Glaxo Smith Clay Pharmaceutical Co., Ltd.), and the results are as follows: Figure 1-4 . Due to the obvious solubilization effect of Tween-80, in order to obtain a dissolution profile with better distin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com