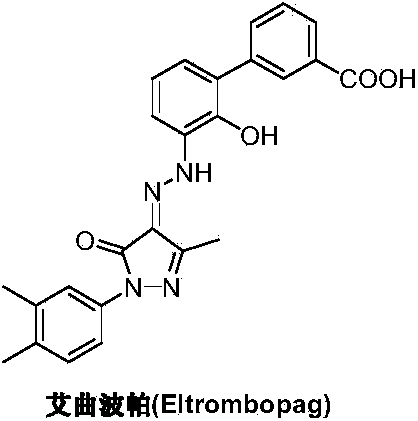
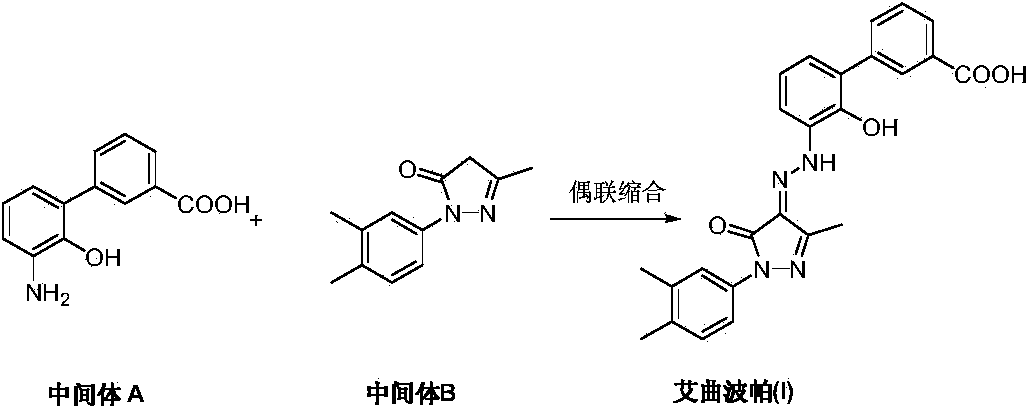
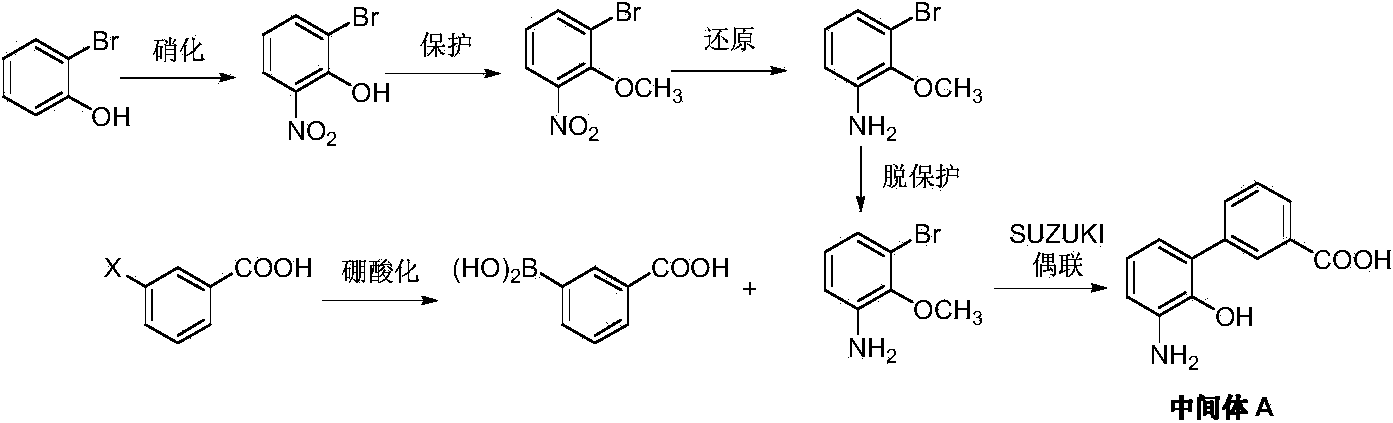
Preparation method of eltrombopag olamine
A technology of eltrombopag and alkyl acetoacetate, which is applied in the field of preparation of the therapeutic drug eltrombopag, can solve problems such as many reaction steps, high cost, and high pressure on environmental protection, and achieve easy availability of raw materials and high product yield And the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 3'-amino-2'-hydroxybiphenyl-3-carboxylic acid (II) (2.29 g, 10 mmol) and 50 mL of 1N hydrochloric acid into the reaction flask, and stir to dissolve. Add 25mL aqueous solution of sodium nitrite (0.76g, 11mmol) dropwise under ice bath, after dropping, add ethyl acetoacetate (1.63g, 12.5mmol) and sodium acetate (8.2g, 0.1mol) ethanol 75mL solution under stirring , raised to room temperature, and reacted for 10 hours, and TLC detected that the reaction was complete. Filtration, the crude product was recrystallized with methanol to obtain a light yellow solid (Z)-2-[3'-(2'-hydroxy-3-carboxylic acid biphenyl)hydrazono]-3-oxobutanoic acid ethyl ester (III ) 2.7g, yield 73.0%.
Embodiment 2
[0029] At room temperature, add 3,4-dimethylphenylhydrazine (0.75 g, 5.5 mmol) and glacial acetic acid (25 mL) into the reaction flask, and stir to dissolve. Add (Z)-2-[3'-(2'-hydroxyl-3-carboxybiphenyl)hydrazono]-3-oxobutanoic acid ethyl ester (III) (1.85g, 5mmol) and heat up to 100° C., stirred for 24 hours, and TLC detected that the reaction was complete. The solvent was removed under reduced pressure. The crude product was recrystallized from methanol to obtain 1.8 g of off-white solid Eltrombopag (I), with a yield of 81.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com