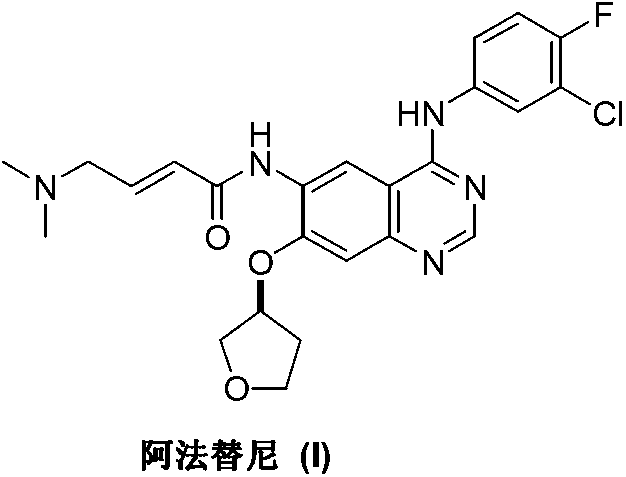
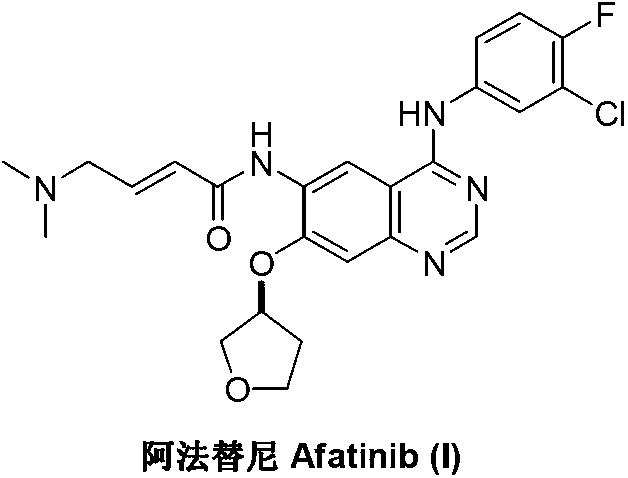
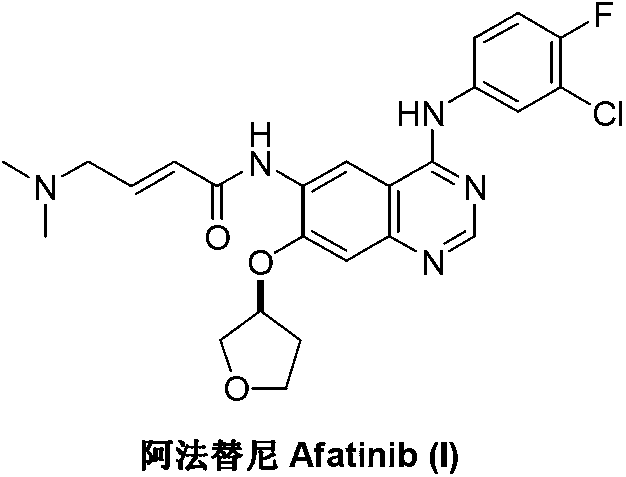
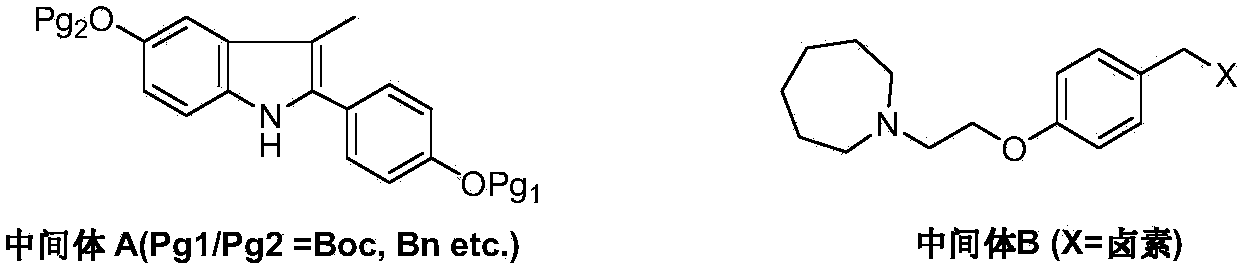Patents
Literature
83results about How to "Promote the development of economy and technology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
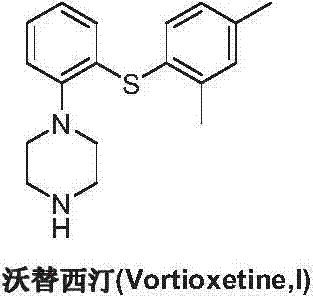
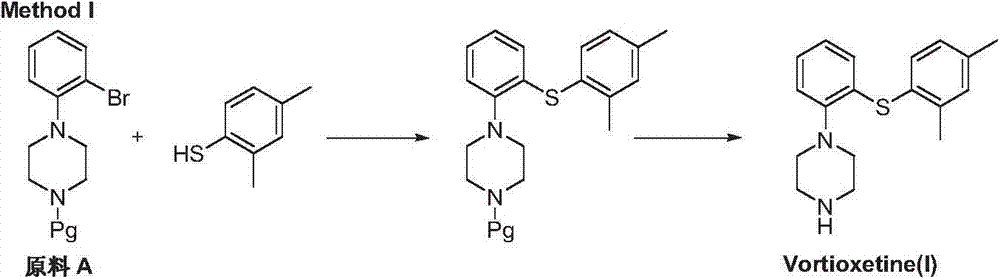
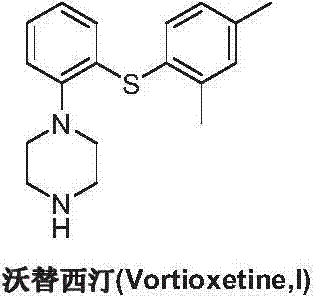
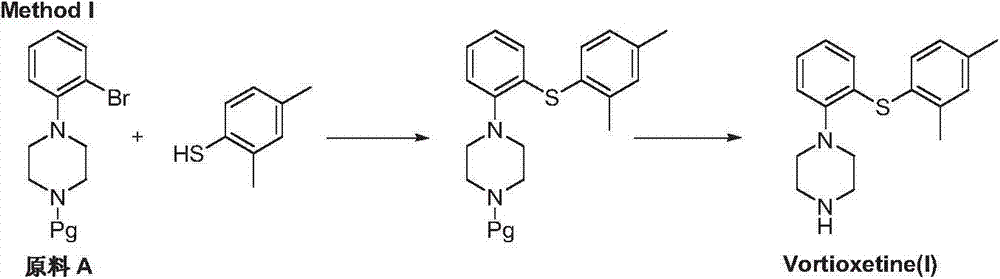
Preparation method of vortioxetine
ActiveCN103788020AEase of industrial productionEco-friendly economyOrganic chemistryNitrobenzeneAniline
The invention discloses a preparation method of vortioxetine (I). The preparation method comprises the following steps: subjecting a compound shown in a formula (II) as a raw material and 2,4-dimethylthiophenol (III) to condensation to generate 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV) or 2-(2,4-dimethylphenylthioalkyl)aniline (V) which is obtained by reducing the 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV), and subjecting the 2-(2,4-dimethylphenylthioalkyl)aniline (V) and a compound shown in a formula (VI) to cyclization under alkaline conditions to obtain the vortioxetine (I). The preparation method is accessible in raw materials, is concise in process, is economical and environment-friendly and is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
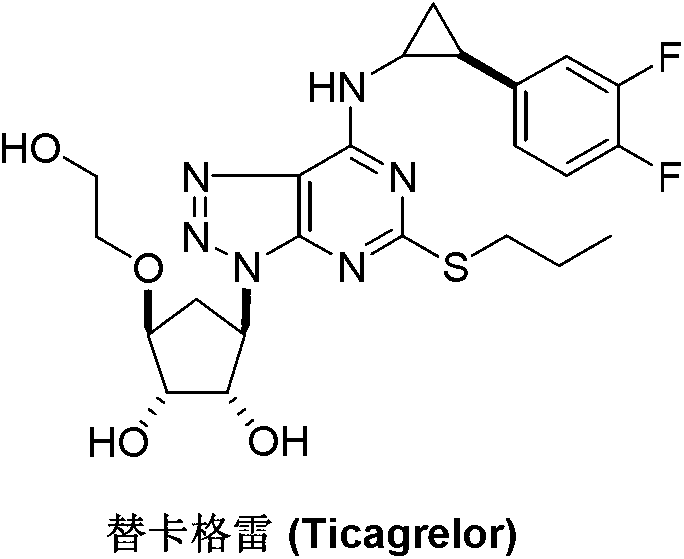
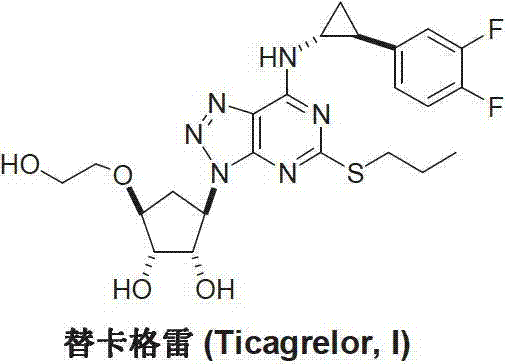
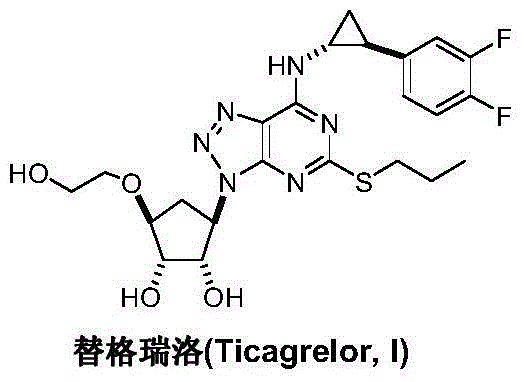
Preparation method of Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine
InactiveCN103130726AEase of industrial productionPromote the development of economy and technologyOrganic chemistryTicagrelorSodium sulfite
The invention discloses a preparation method of Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine. The preparation method of the Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine includes the following steps: using 4,6- dichloro-2-nitro-2-(pyridinecarboxylic) pyrimidine(V), enabling the nitro of a molecular structure to be reduced to amino through the reduction reaction of reducing agent sodium hydrosulfite, and obtaining the Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine. The preparation method of the Ticagrelor intermediate 4,6-dichloro-2-(pyridinecarboxylic)-5- aminopyrimidine is easy, convenient to implement, economical and environment-friendly, beneficial to industrial production and capable of facilitating the development of economic technology of the bulk drug.
Owner:许学农
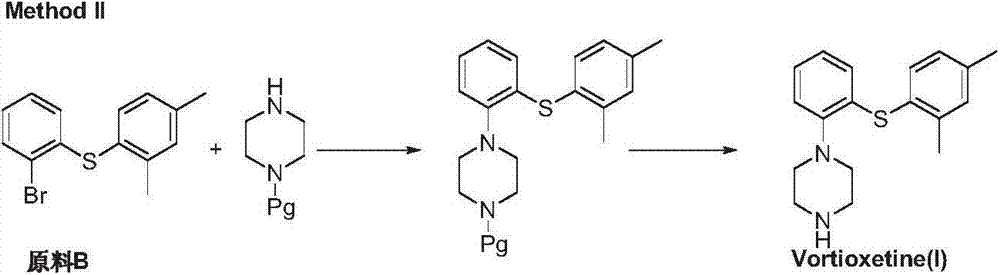
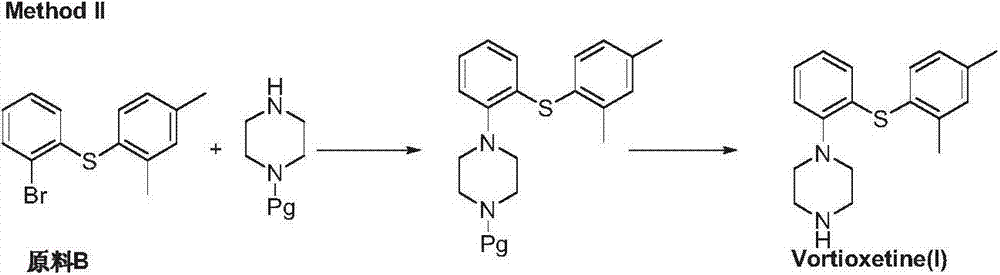
Preparation method of vortioxetine
ActiveCN103788019AEase of industrial productionEco-friendly economyOrganic chemistryNitrobenzeneAniline
The invention discloses a preparation method of vortioxetine (I). The preparation method comprises the following steps: subjecting 2-substituted thiophenol shown in a formula (II) and 2,4-dimethylphenyl halide shown in a formula (III) to condensation to generate 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV) or 2-(2,4-dimethylphenylthioalkyl)aniline (V) which is obtained by reducing the 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV), and subjecting the 2-(2,4-dimethylphenylthioalkyl)aniline (V) and a compound shown in a formula (VI) to cyclization under alkaline conditions to obtain the vortioxetine (I). The preparation method is accessible in raw materials, is concise in process, is economical and environment-friendly and is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
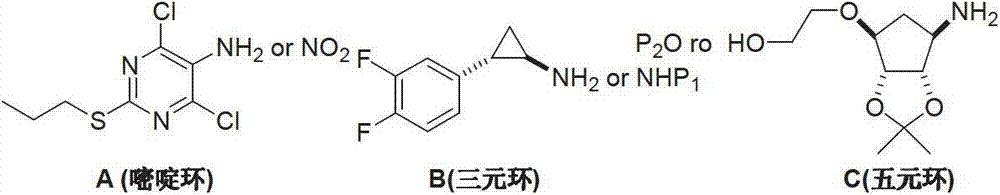
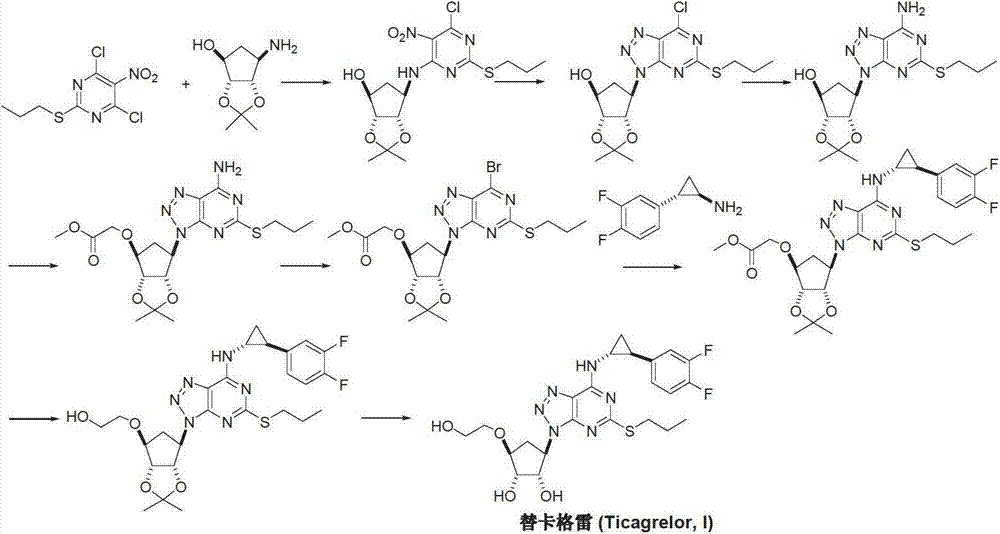
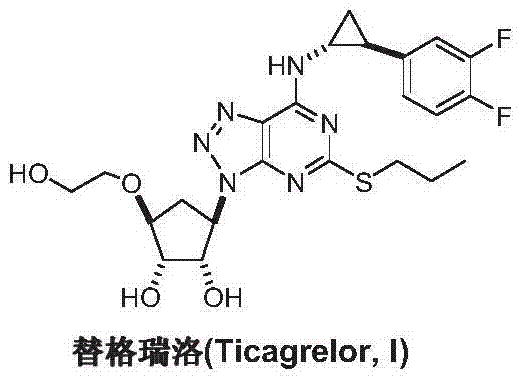
Preparation method of ticagrelor
ActiveCN103288836AEase of industrial productionPromote the development of economy and technologyOrganic chemistryTicagrelorAmination
The invention discloses a preparation method of ticagrelor. The preparation method comprises the following steps of: carrying out a cyclization reaction between 5-amino-1,4-di-substituted-1,2,3-triazole (II) and a dialkyl carbonate (III), thereby obtaining 9-substituted-2,6-dihydroxy-8-azaguanine (IV), chlorinating the intermediate (IV) to obtain 9-substituted-2,6-dichloro-8-azaguanine (V), carrying out an amination reaction between the intermediate (V) and trans-(1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine (VI) to generate 9-substituted-6-amino substituent-2-chloro-8-azaguanine (VII), and carrying out a propylthiolation reaction between the intermediate (VII) and propanethiol (VIII) to obtain the ticagrelor (I). The preparation method disclosed by the invention is simple in process, and high in chemical and chiral purity, and provides a new preparation way for industrial production of the ticagrelor.
Owner:鄄城县人民医院
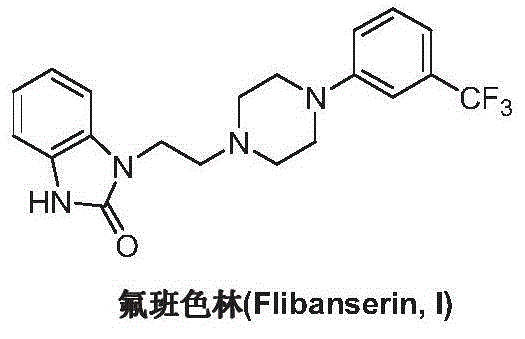
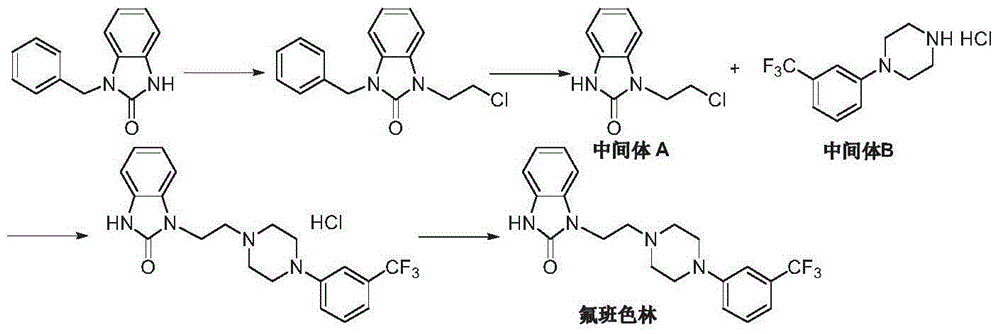
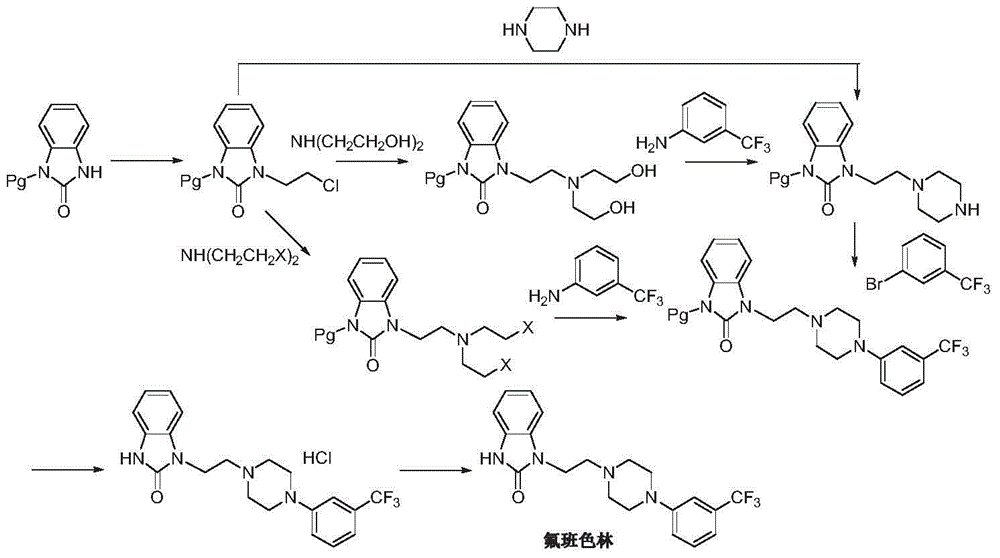
Preparation method of flibanserin
ActiveCN104926734AThe preparation process is convenientHigh yieldOrganic chemistryOrtho-nitroanilineHalogen
The invention discloses a preparation method of flibanserin. The preparation method uses trifluoromethylbenzene, triamine (2-halogen ethyl) and ortho-nitroaniline which are easy to obtain as raw materials and adopts classical elementary reactions such as cyclization, substitution, reduction and condensation, so that the flibanserin is prepared. The raw materials of the preparation method are easy to obtain, the technology is succinct, the yield is high, the preparation method is economical and environment-friendly, and a new preparation way is provided for the industrial production of the flibanserin.
Owner:SUZHOU LIXIN PHARMA
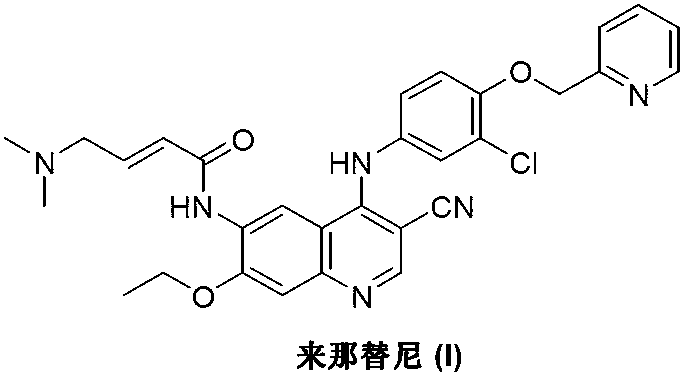
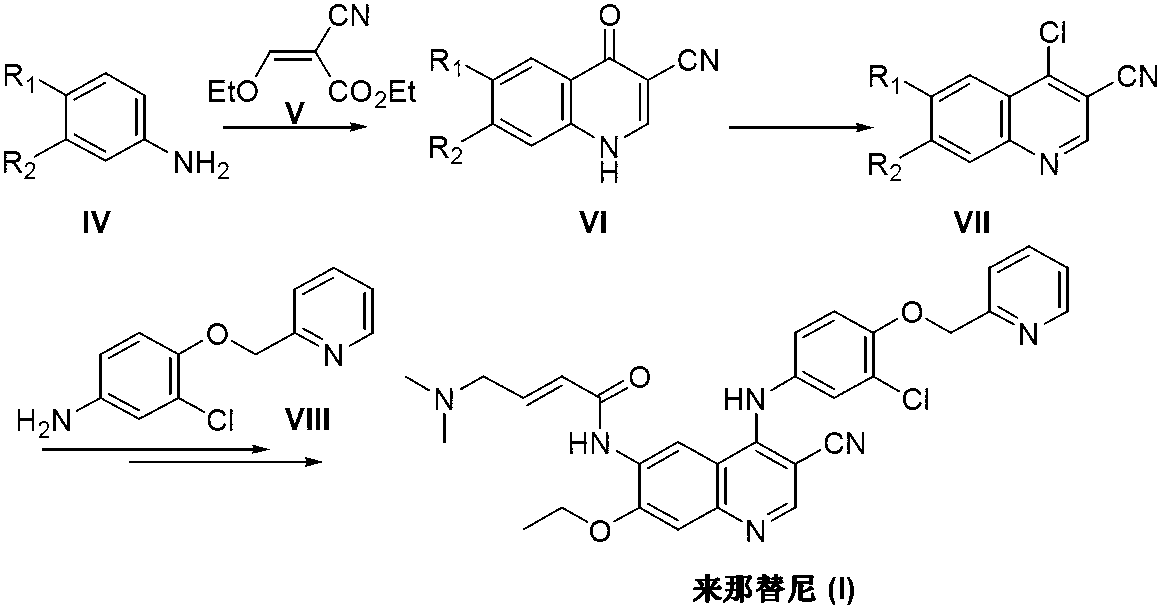
Preparation method of neratinib
InactiveCN103265530AEase of industrial productionRaw materials are easy to getOrganic chemistryBenzaldehydePyridine
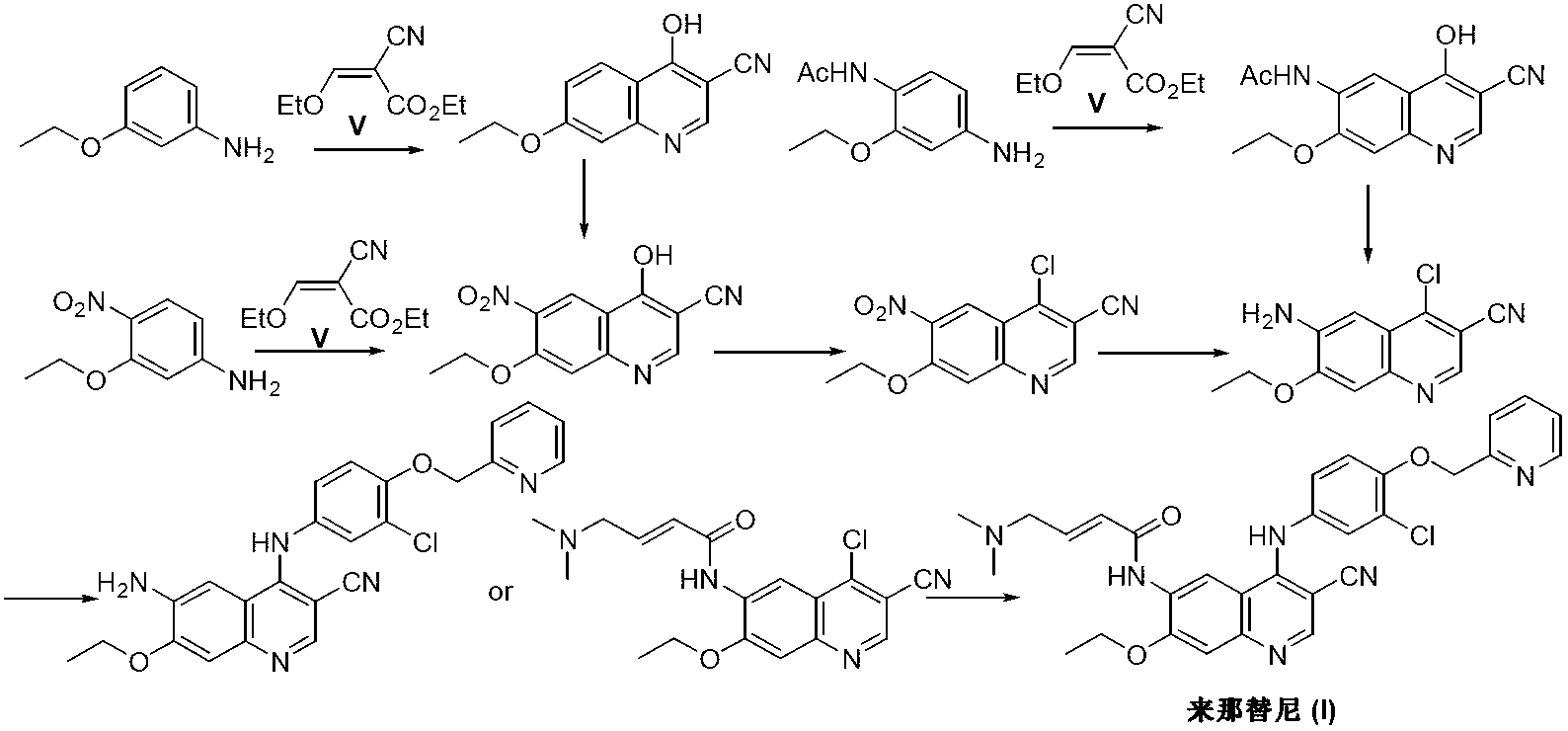
The invention discloses a preparation method of neratinib (I). The preparation method comprises the step that 6-[(E)-4-(dimethylamino)-2-butenamide]-7-ethoxy-4-amino-3-quinolinecarbonitrile (II) and 3-chloro-4-[(pyridine-2-yl)methoxy]-benzaldehyde (III) carry out condensation and reduction reactions to obtain neratinib (I). The preparation method is easy in obtainment of raw materials, concise in process, economical and environment-friendly and suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH +1
Preparation method of Olaparib intermediate
InactiveCN105085408ARaw materials are easy to getSimple processOrganic chemistryBenzoic acidIsobenzofuran
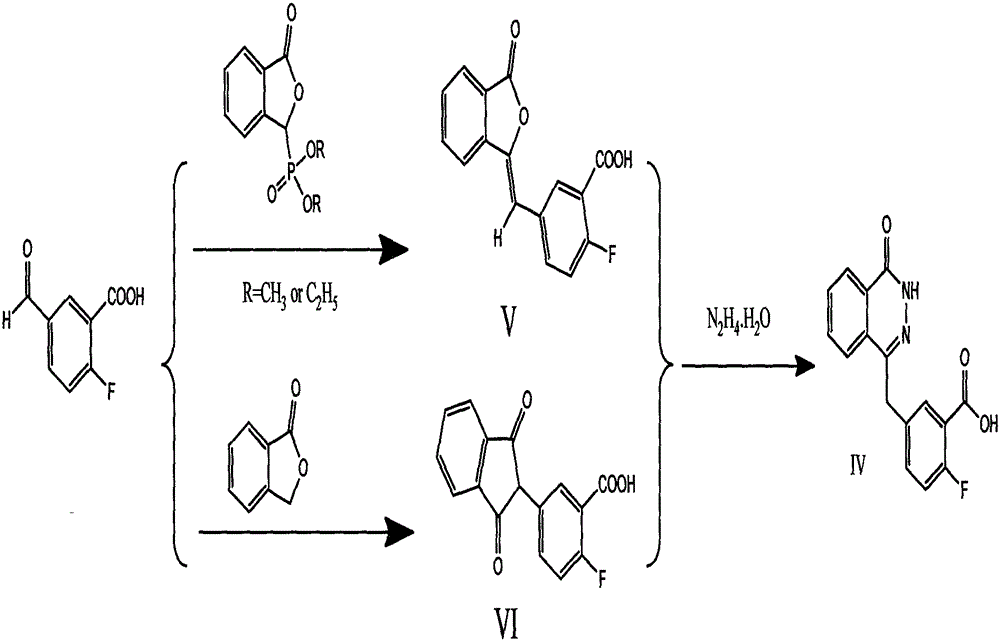
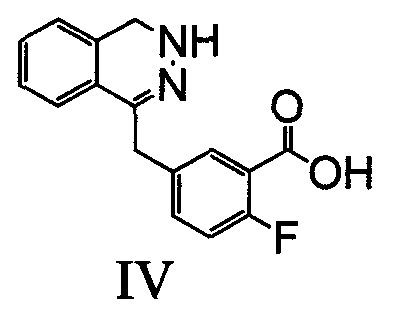
The invention relates to a preparation method of an Olaparib intermediate (IV), namely, 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid. The method comprises steps as follows: 1, 2-fluoro-5-formyl benzoic acid is used as a raw material and reacts with (3-oxo-1,3-dihydro-isobenzofuran-1-yl)dialkyl phosphate to produce 2-fluoro-5-(3-oxo-3H-isobenzofuran-1-yl-methylene)benzoic acid, namely, an intermediate (V); or 2-fluoro-5-formyl benzoic acid reacts with phthalide to produce 5-(2,3-dihydro-1,3-dioxo-1H-indene-2-yl)-2-fluobenzoic acid, namely, an intermediate (VI); 2, the intermediate V or the intermediate VI reacts with hydrazine hydtaye to produce an Olaparib intermediate (IV). The preparation method is concise in process, environment-friendly, economical and suitable for the industrial key Olaparib intermediate, raw materials are easy to obtain, and purification is easy.
Owner:GUANGZHOU YOUMIJIAN PHARMA TECH CO LTD
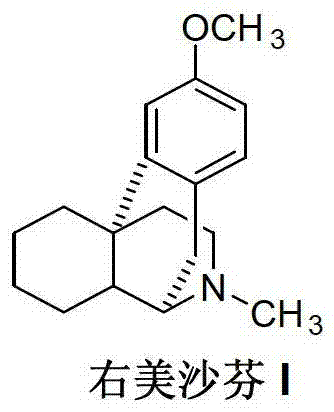
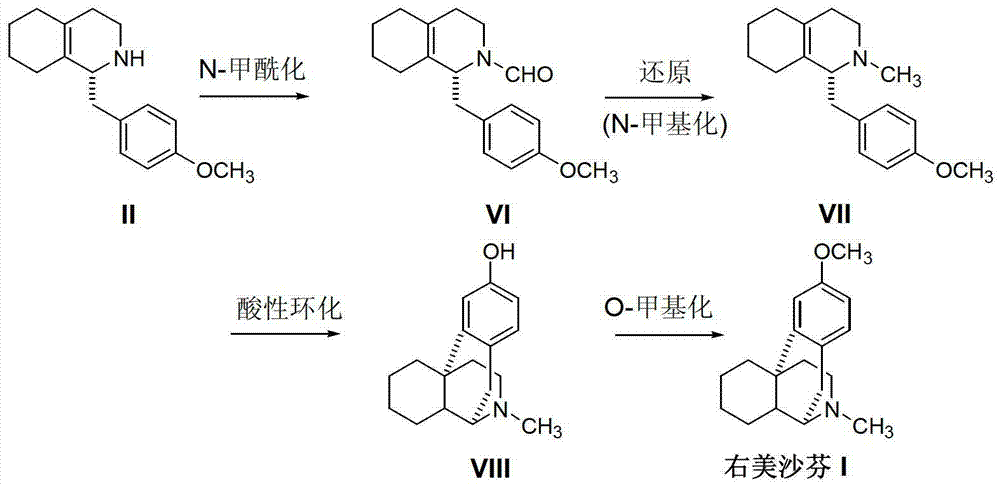
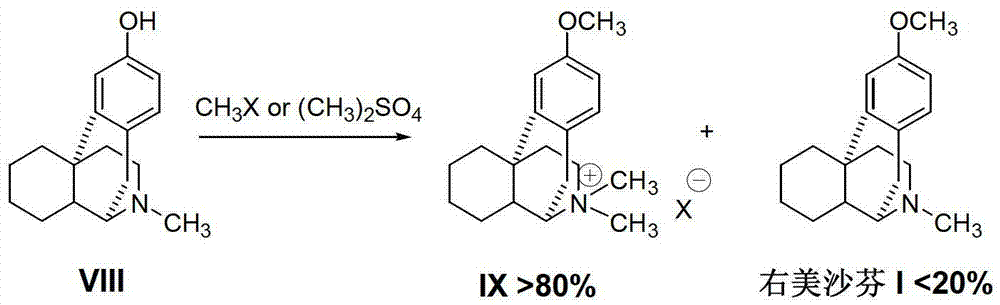
Preparation method of dextromethorphan
ActiveCN103044327AReduce manufacturing costPromote the development of economy and technologyOrganic chemistryIsoquinolineN methylation
The invention discloses a preparation method of dextromethorphan ((+)-3-methoxy-17-methyl-(9 alpha,13 alpha,14 alpha)-levorphane, I). The method comprises the following steps that a dextromethorphan intermediate (+)-1-(4-methoxy) benzyl-1,2,3,4,5,6,7,8-octahydro-isoquinoline (II) conducts N-benzylation reaction with a benzylation reagent under alkaline conditions to form (+)-1-(4-methoxy) benzyl-N-benzyl-1,2,3,4,5,6,7,8-octahydro-isoquinoline (III); the intermediate (III) is subjected to acid cyclization reaction to form (+)-3-hydroxy-17-benzyl-(9 alpha,13 alpha,14 alpha)-levorphane, (IV); the intermediate (IV) reacts with dimethyl sulfate or methine halide, and is subjected to O-methylation and N-methylation reaction to form (+)-3-methoxy group-17-benzyl-17-methyl-(9 alpha,13 alpha,14 alpha)-levorphane quaternary ammonium salt (V); (V) is subjected to catalytic hydrogenation reaction; benzyl is removed; and dextromethorphan (I) is obtained. Therefore, the preparation method can use a common and cheap methylation reagent to substitute an unusual methylation reagent such as phenyltrimethylammonium hydroxide, and the yield of reaction can be increased.
Owner:SUZHOU LIXIN PHARMA
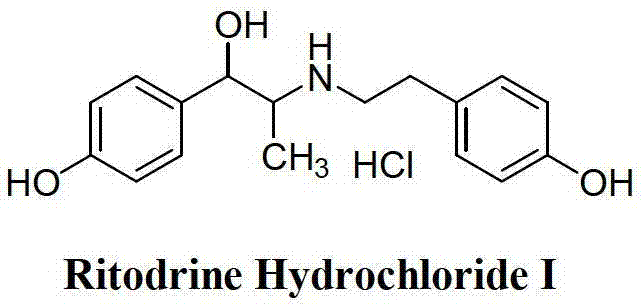
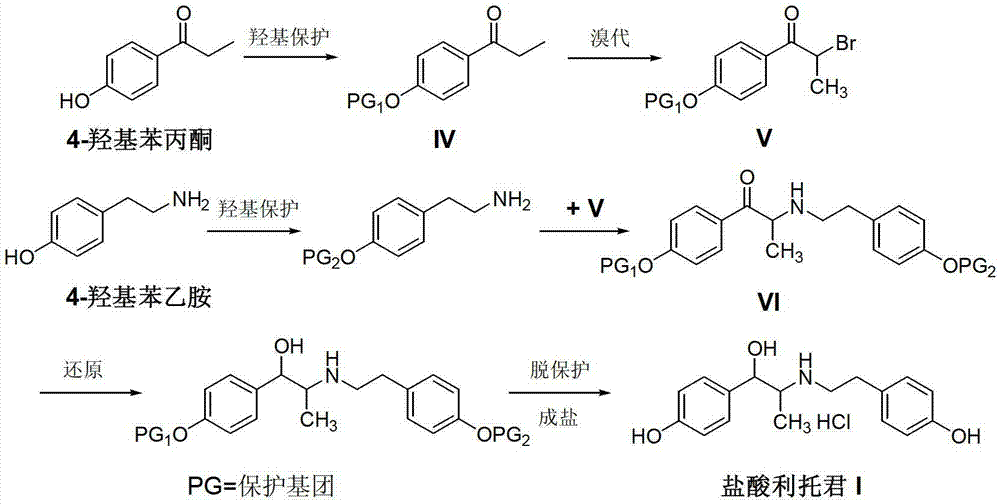
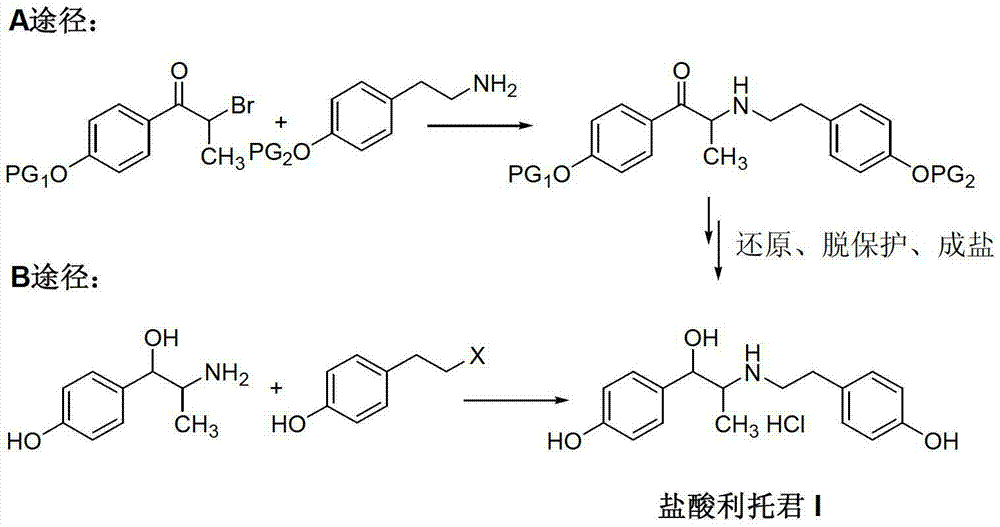
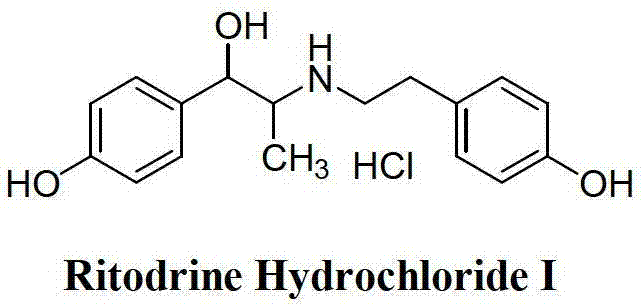
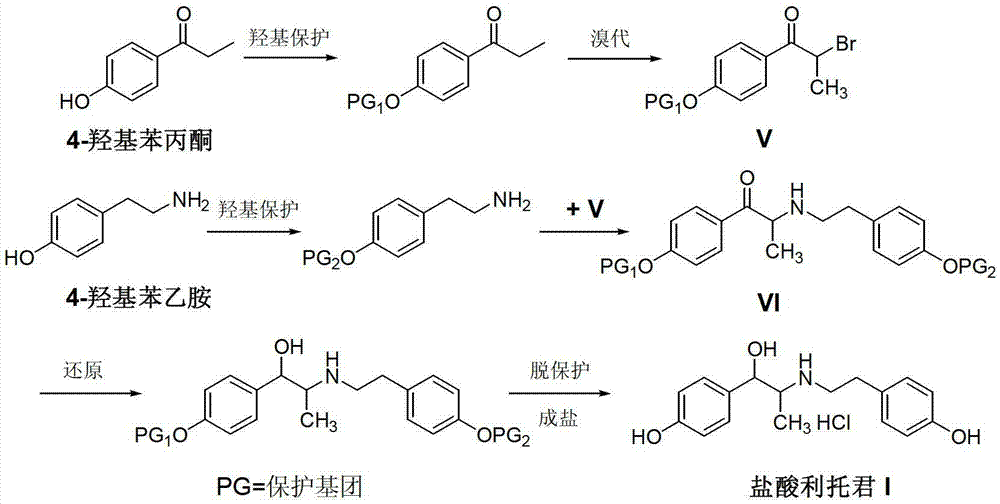
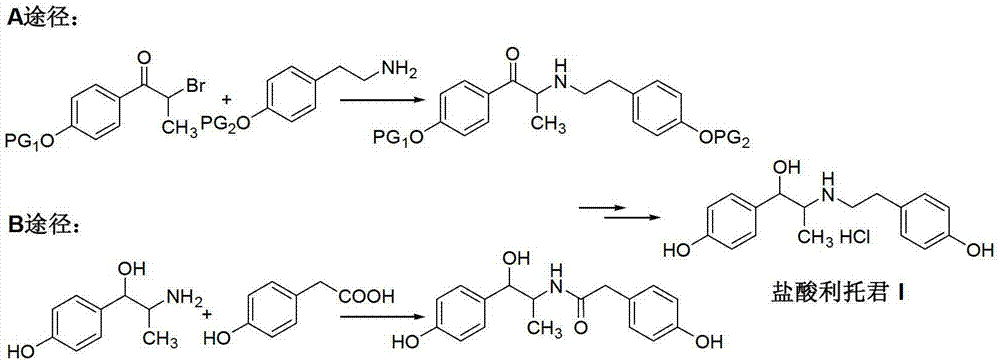
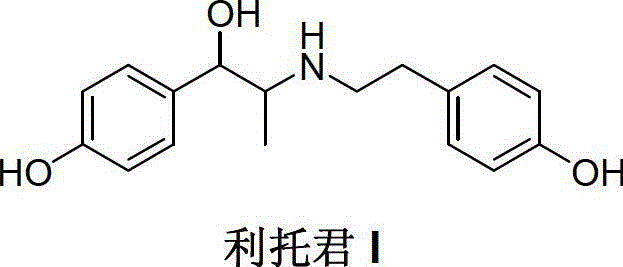
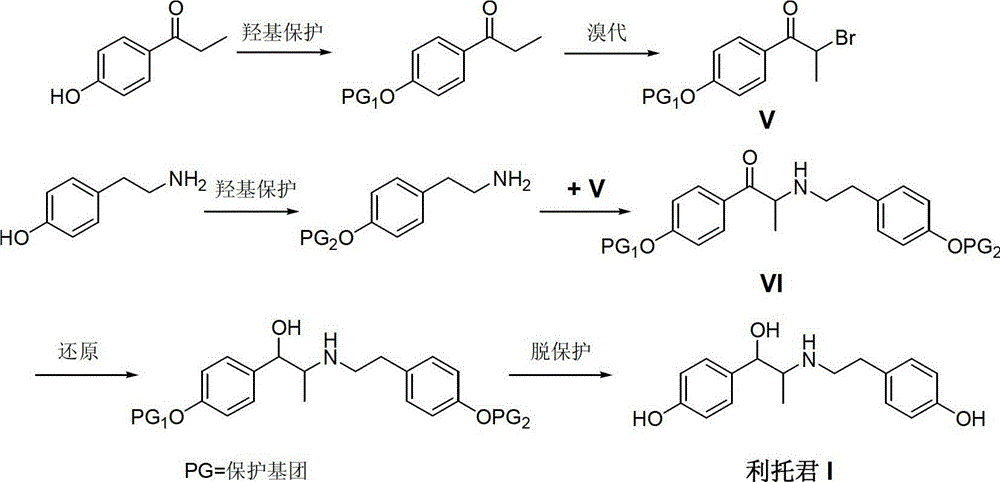
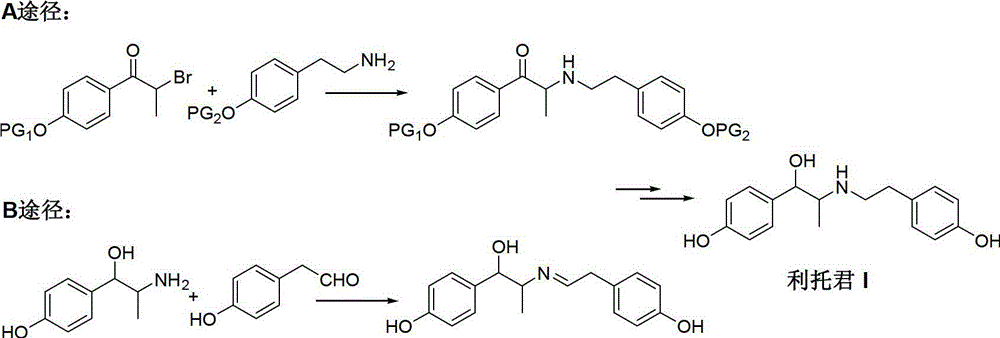
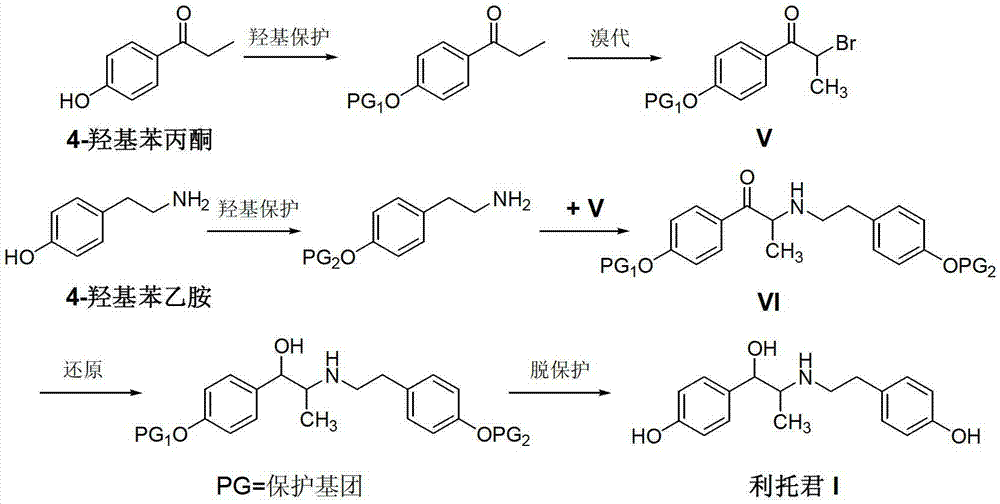
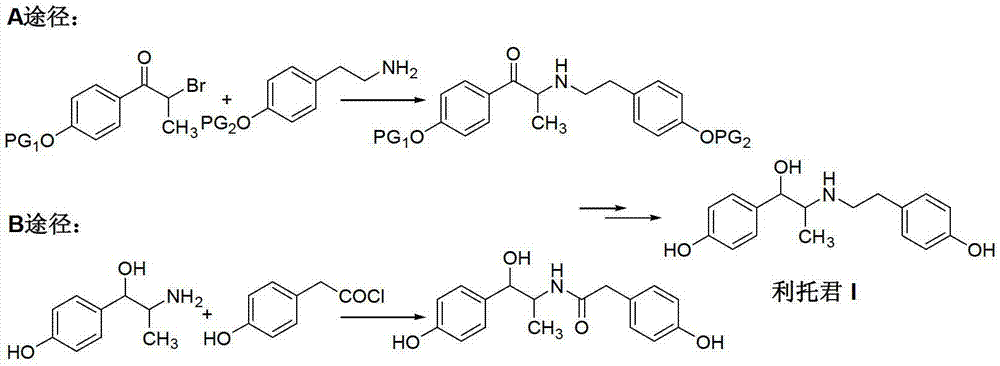
Preparation method of ritodrine hydrochloride
ActiveCN103113239AHigh chemoselectivityLow production costOrganic compound preparationAmino-hyroxy compound preparationPropanolHalogen
The invention discloses a preparation method of ritodrine hydrochloride (I). The preparation method comprises the following steps of: subjecting 2-amino-1-(4-hydroxyphenyl) propanol hydrochloride (II) and 4-(2-halogen ethanol) phenol (III) to condensation reaction in the presence of a catalyst to obtain ritodrine and then forming salt with ritodrine and hydrochloric acid to obtain ritodrine hydrochloride (I). The preparation method has the advantages that the production cost of ritodrine hydrochloride can be effectively controlled, the product quality is substantially improved and economic and technical development of the active pharmaceutical ingredient is promoted.
Owner:SUZHOU LIXIN PHARMA
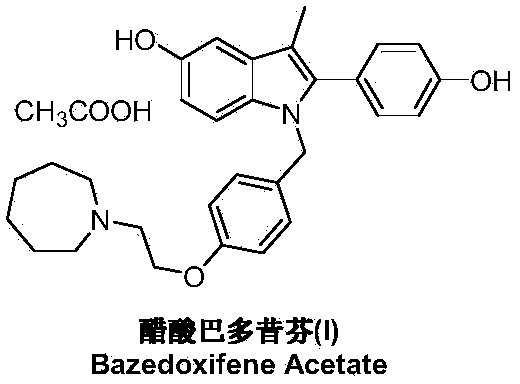
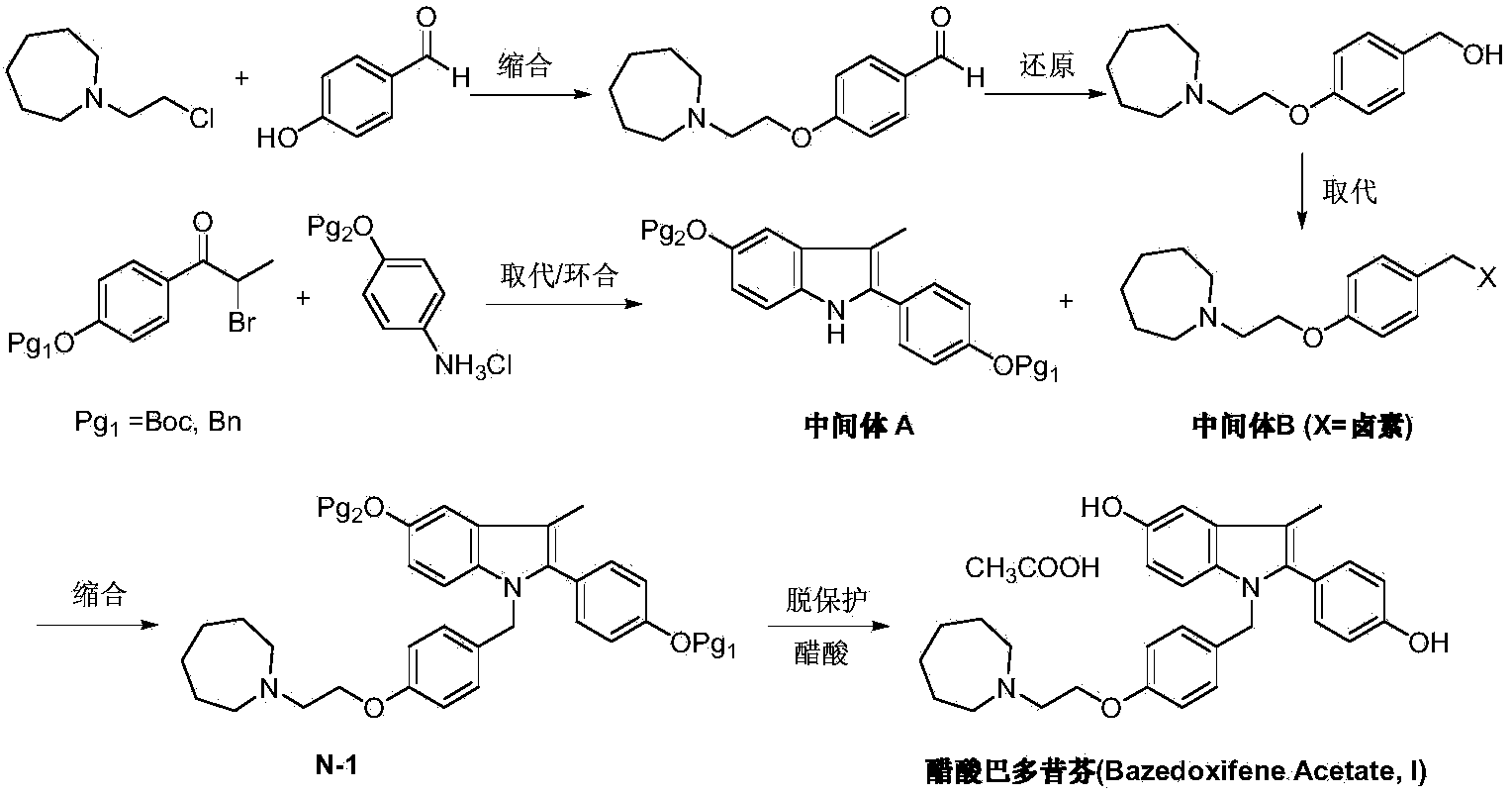
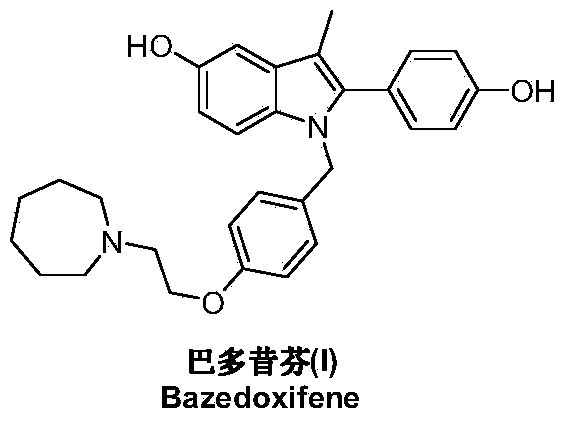
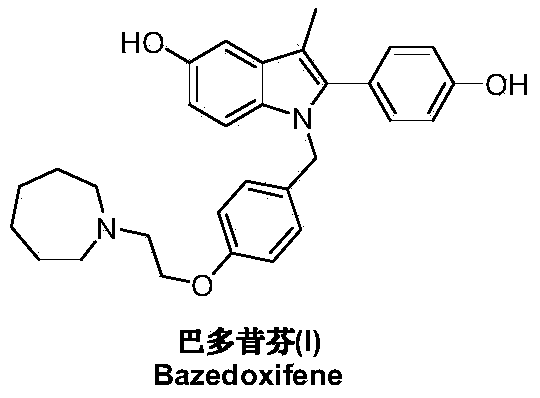
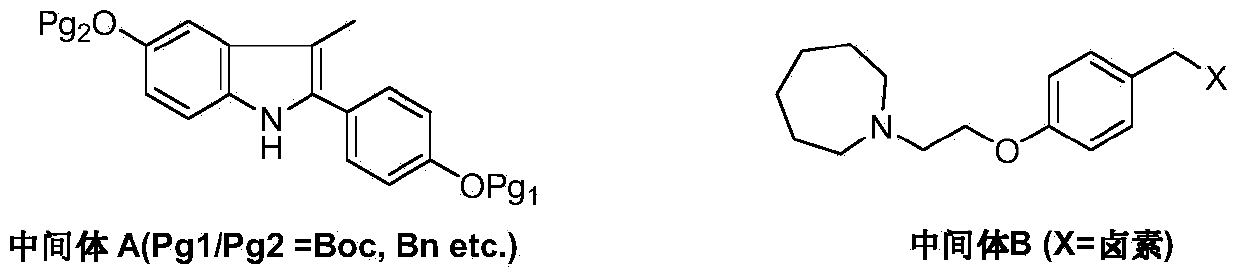
Preparation method of bazedoxifene acetate
ActiveCN103864665AEase of industrial productionPromote the development of economy and technologyOrganic chemistryAcetic acidAlcohol
The invention discloses a preparation method of bazedoxifene acetate. The method comprises the following steps: carrying out a condensation cyclization reaction on 1-(4-Pg1 oxygroup phenyl) propyl alcohol (II) and N-{4-(2-azacycloheptane-1-yl-ethyoxyl-benzyl)}N-{4-(Pg2 oxygroup phenyl)} hydrazine (III) so as to obtain 1-{4-(2-azacycloheptane-1-yl-ethyoxyl-benzyl)}-2-(4-Pg1 oxygroup-phenyl)-3-methyl-5-(Pg2 oxygroup)-1H-benzpyrole (IV); and carrying out deprotection on the intermediate (IV) and salifying the intermediate (IV) with acetic acid so as to obtain the bazedoxifene acetate (I). The preparation method is concise in process, high in yield, economical and environment-friendly, thereby providing a novel preparation way for the industrial production of the bazedoxifene acetate.
Owner:苏州特瑞药业股份有限公司
Preparation method of ritodrine hydrochloride
ActiveCN103113237ALow production costPromote the development of economy and technologyOrganic compound preparationAmino-hyroxy compound preparationPropizochlorPtru catalyst
The invention discloses a preparation method of ritodrine hydrochloride (I). The preparation method comprises the following steps of: subjecting 2-amino-1-(4-hydroxyphenyl) propanol hydrochloride (II) and 4-hydroxyphenylacetic acid (III) to amidation and condensation reactions under the action of a catalyst to obtain an intermediate N-(2-(4-hydroxyphenyl)-2-hydroxy-1-methylethyl)-4-hydroxy phenylacetamide (IV) and obtaining ritodrine hydrochloride (I) through reduction reaction and salt forming reaction of the intermediate (IV). The preparation method has the advantages that the production cost of ritodrine can be effectively controlled, the product quality is substantially improved and economic and technical development of the active pharmaceutical ingredient is promoted.
Owner:SUZHOU LIXIN PHARMA
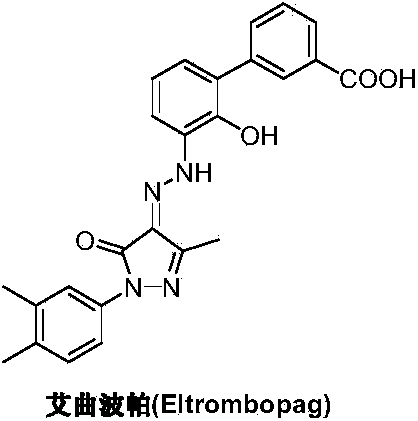
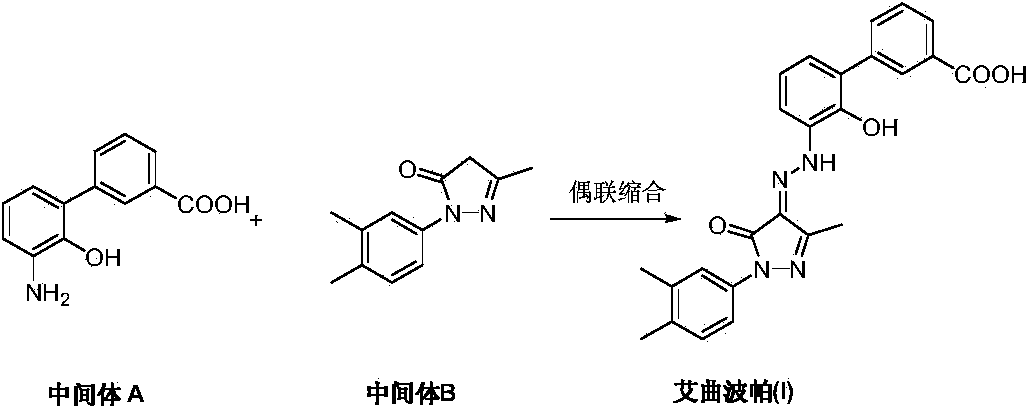
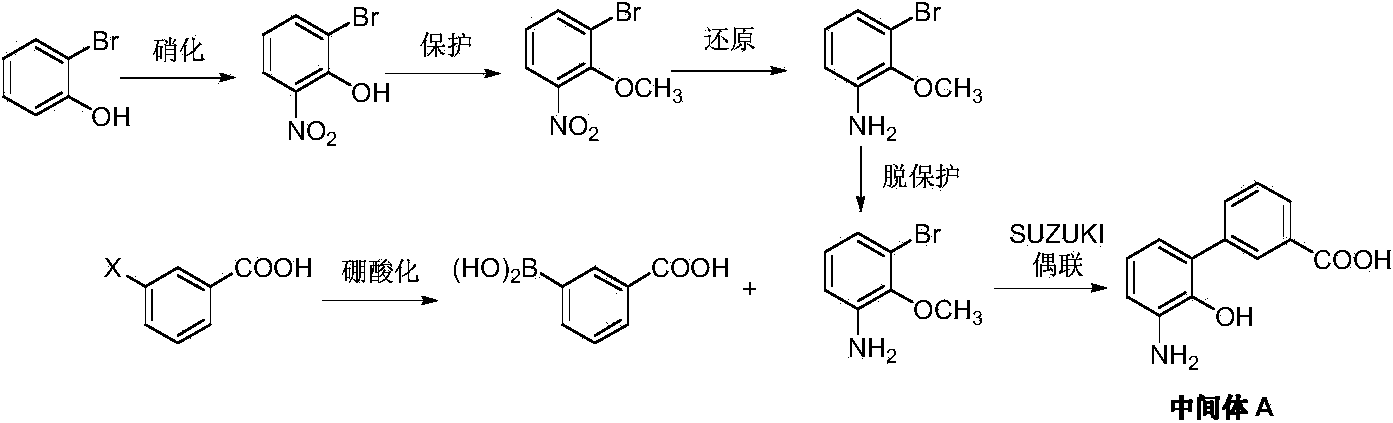
Preparation method of eltrombopag olamine
ActiveCN103819406ARaw materials are easy to getHigh purityOrganic active ingredientsOrganic chemistryHydrazine compoundEltrombopag Olamine
The invention discloses a preparation method of eltrombopag olamine (Eltrombopag). The preparation method comprises the following steps: carrying out azo coupling reaction by using 3'-amino-2'-hydroxy-biphenyl-3-carboxylic acid (II) and acetoacetic acid alkyl ester to obtain (Z)-2-[3'-(2'-hydroxyl-3-hydroxy-biphenyl) hydrazono]-3-oxo acid alkyl ester (III); carrying out condensation cyclization reaction on an intermediate (III) and 3,4-dimethyl benzene hydrazine to prepare the eltrombopag olamine (I). The method is concise in process, available in raw material, economical and environmental friendly, and beneficial to achievement of industrialization, and economic and technological development of the eltrombopag olamine crude drug can be facilitated.
Owner:山东佰盛能源科技有限公司
Preparation method of bazedoxifene
ActiveCN103772261AEasy to prepareHigh yieldOrganic chemistryBulk chemical productionMedicinal chemistryBazedoxifene
The invention discloses a preparation method of bazedoxifene. The preparation method of the bazedoxifene comprises the following steps: taking 1-(4-Pg1oxy-phenyl)-allylene (II) and N-[4-(2-azacycloheptane-1-yl- ethyoxyl-benzyl)]-N-[4-(Pg2 oxyphenyl)] hydrazine (III) to perform addition cyclization reaction and acquire 1-[4-(2-azacycloheptane-1-yl- ethyoxyl-benzyl)]-2-(4-Pg1oxy-phenyl)-3-methyl-5-(Pg2oxy)-1H-benzpyrole (IV); performing deprotection on an intermediate (IV) to prepare bazedoxifene (I). The preparation method is simple in process, high in yield, economical and environmental-friendly, so that a novel preparation method for industrial production of the bazedoxifene.
Owner:SUZHOU LIXIN PHARMA
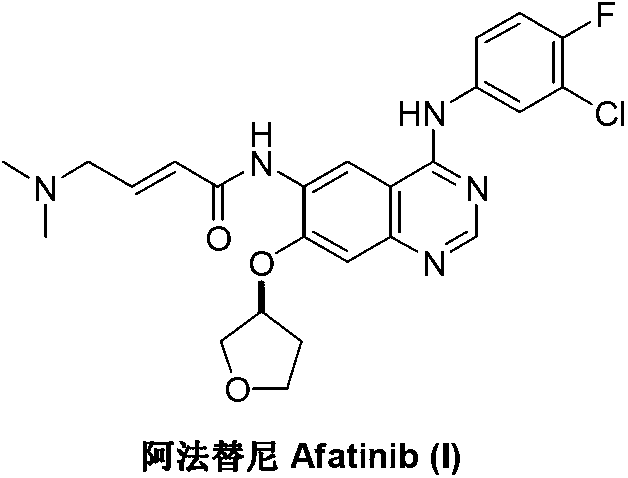
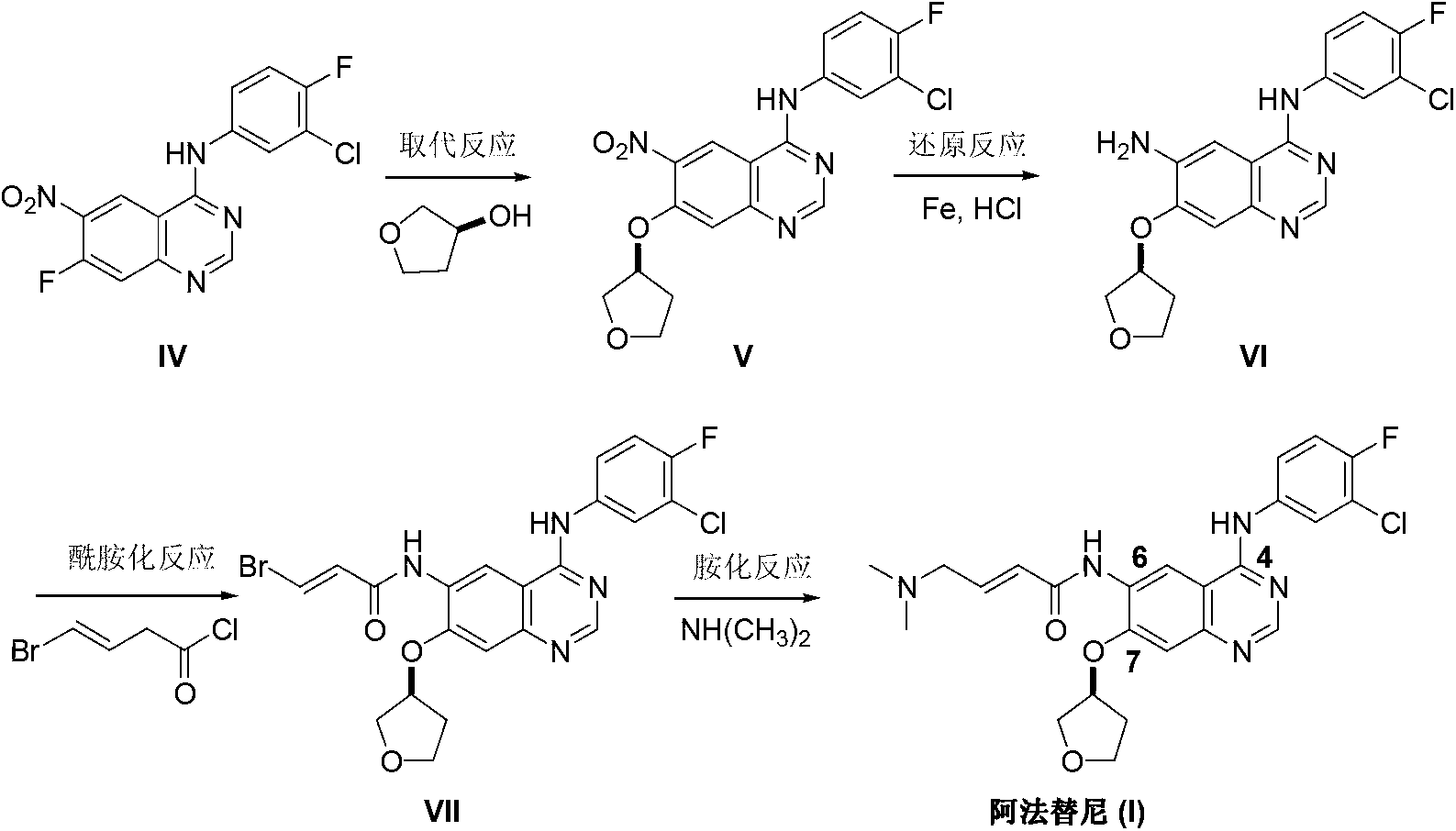
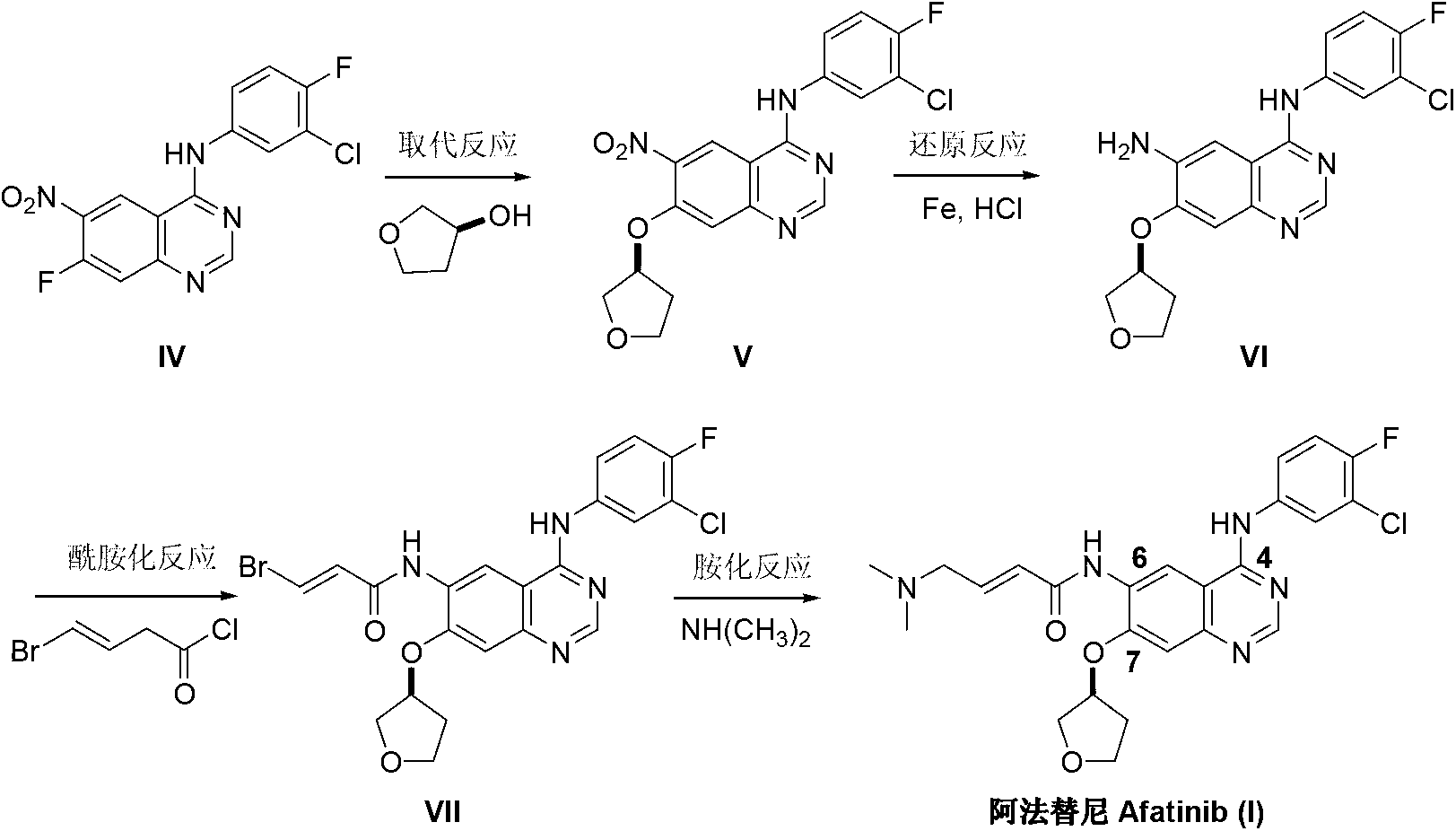
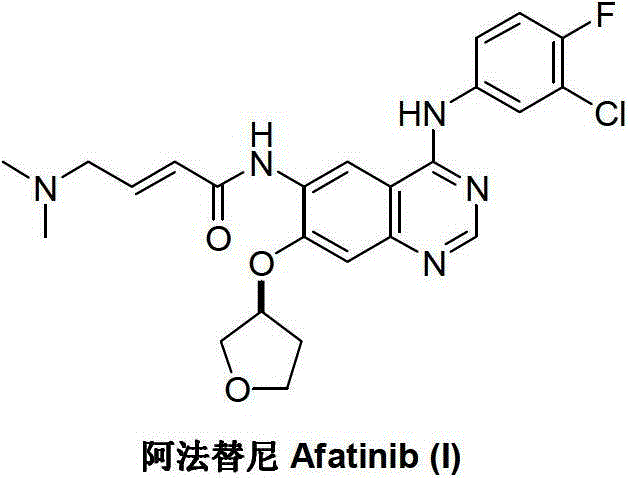
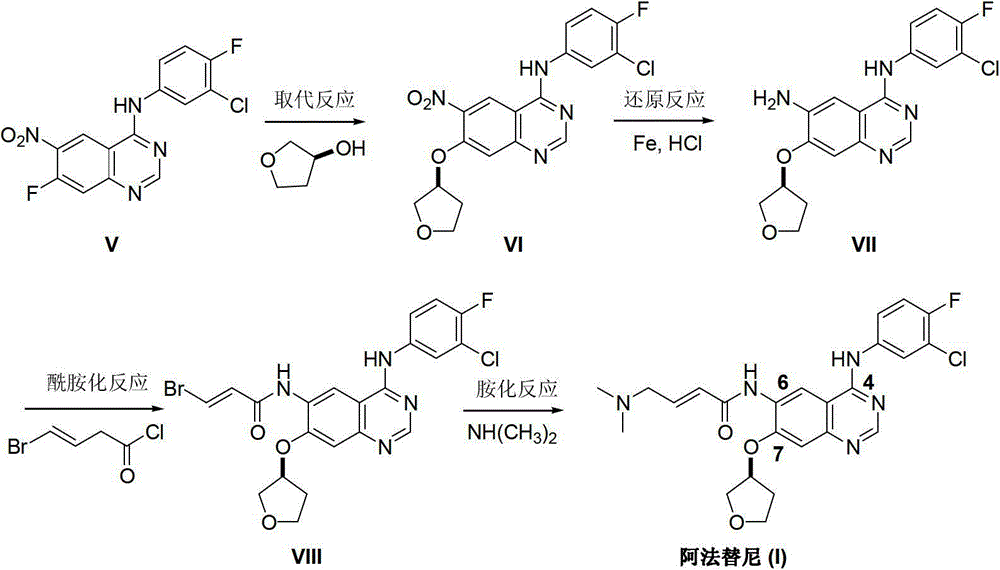
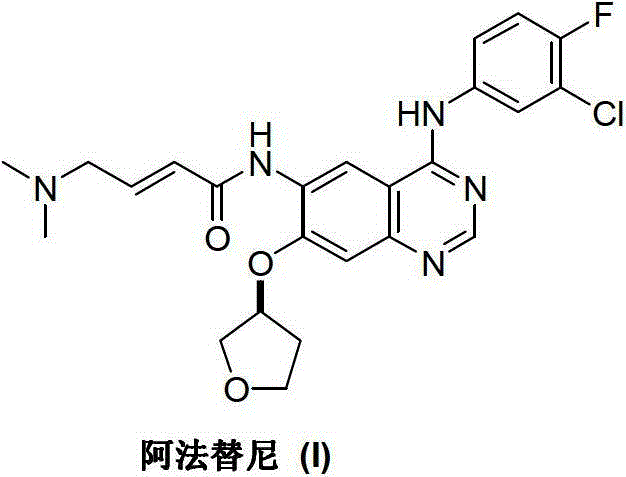
Method for preparing Afatinib
ActiveCN103254183AEase of industrial productionPromote the development of economy and technologyOrganic chemistryN dimethylformamideAniline
The invention discloses a method for preparing Afatinib (I). The method comprises the following steps: 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)-(tetrahydrofuran-3-yl)oxy]aniline (II) and N,N-dimethylformamide dimethylacetal (DMF-DMA) are subjected to condensation reaction so as to produce an intermediate (III), and the intermediate (III), without to the need of separation, is directly subjected to cyclization reaction with 4-fluoro-3-chloroaniline so as to prepare Afatinib (I). According to the method, the steps for preparing Afatinib are reduced obviously, and the cost is reduced greatly.
Owner:铜陵尚东高新科创有限公司
Method for preparing Afatinib
InactiveCN103254182AEase of industrial productionPromote the development of economy and technologyOrganic chemistryN dimethylformamideAniline
The invention discloses a method for preparing Afatinib. The method comprises the following steps: 4-fluoro-3-chloroaniline and N,N-dimethylformamide dimethylacetal (DMF-DMA) are subjected to condensation reaction so as to produce a Schiff base (III), and the Schiff base (III), without to the need of separation, is subjected to cyclization reaction with 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)-(tetrahydrofuran-3-yl)oxy]aniline so as to prepare Afatinib. According to the method, the steps for preparing Afatinib are reduced obviously, and the cost is reduced greatly.
Owner:SUZHOU MIRACPHARMA TECH +1
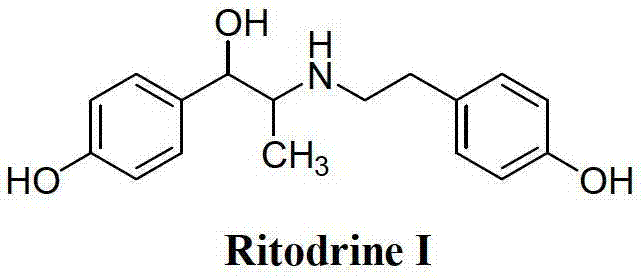
Preparation method of ritodrine
ActiveCN102976959ALow costImprove qualityOrganic compound preparationAmino-hyroxy compound preparationPropanolNitrite
The invention discloses a preparation method of ritodrine (1-(4-hydroxyphenyl)-2-[2-(4-hydroxyphenyl)ethylamino]propanol, I), which comprises the following steps: reacting 4-hydroxypropiophenone and alkyl nitrite to obtain an intermediate 2-oximino-4-hydroxypropiophenone (II); performing reduction reaction on the intermediate (II) to obtain 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (III); then, performing condensation reaction on the intermediate (III) and 4-hydroxyphenylacetaldehyde to generate a Schiff base intermediate (1-(4-hydroxyphenyl)-2-[2-(4-hydroxyphenyl)ethylimido]propanol, IV); and performing reduction reaction on the intermediate (IV) to obtain the ritodrine (I). The preparation method has high chemical selectivity and can be implemented without the protection of any functional group, so that the production cost and quality of the ritodrine (I) are greatly improved.
Owner:SUZHOU LIXIN PHARMA
Preparation method of ritodrine
ActiveCN103113238AHigh chemoselectivityControl production costsOrganic compound preparationAmino-hyroxy compound preparationHydrochlorideChloride
The invention discloses a preparation method of ritodrine (I). The preparation method comprises the following steps of: subjecting 2-amino-1-(4-hydroxyphenyl) propanol hydrochloride (II) and 4-hydroxy phenylacetyl chloride (III) to amidation reaction to obtain an intermediate N-(2-(4- hydroxyphenyl)-2-hydroxy-1-methylethyl)-4-hydroxy phenylacetamide (IV) and obtaining ritodrine (I) through reduction reaction of the intermediate (IV). The preparation method has the advantages that the production cost of ritodrine can be effectively controlled, the product quality is substantially improved and economic and technical development of the active pharmaceutical ingredient is promoted.
Owner:SUZHOU LIXIN PHARMA
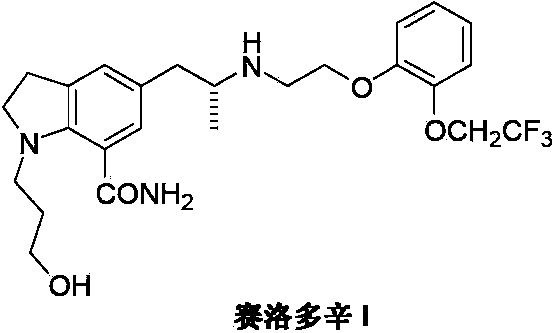
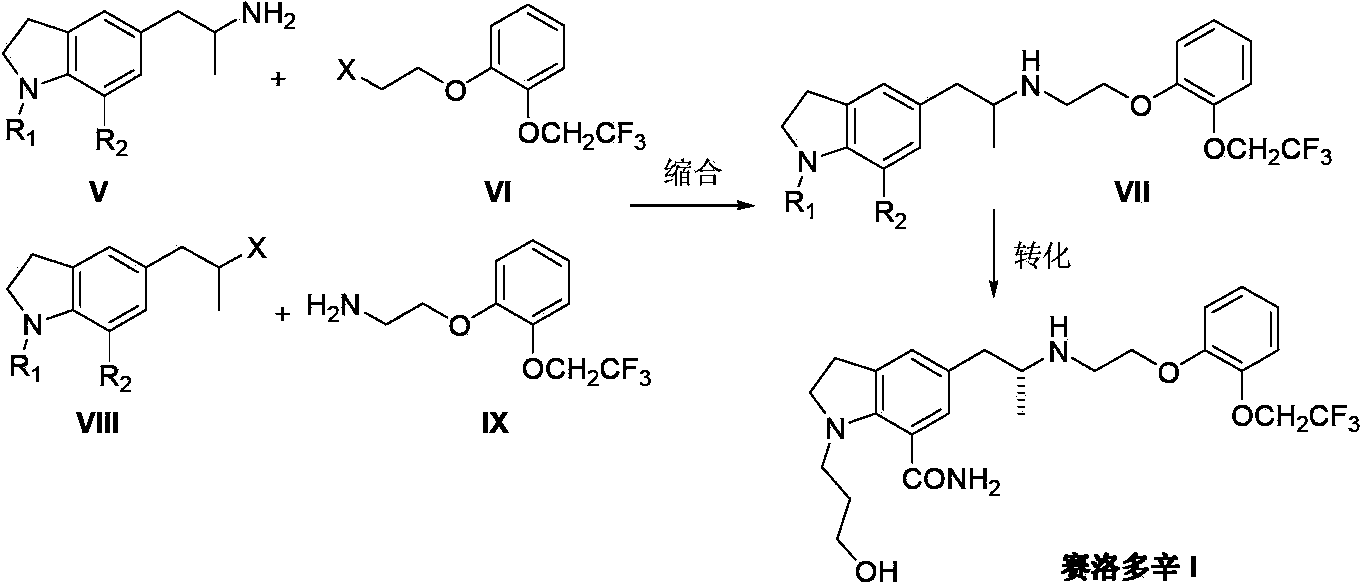
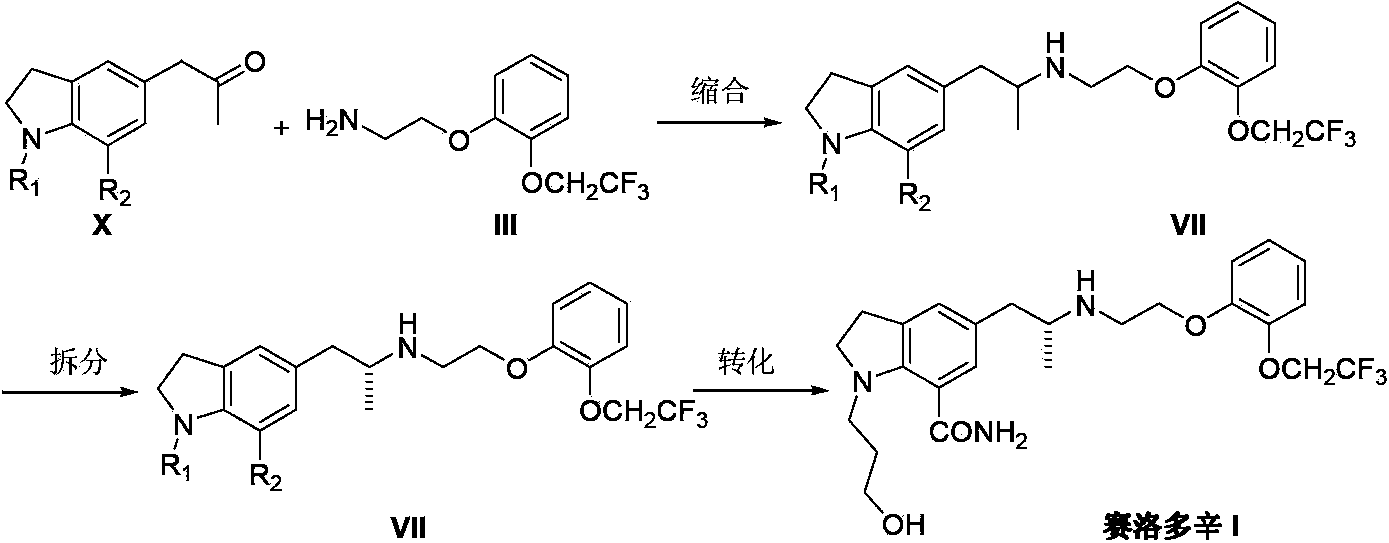
Preparation method of Silodosin
InactiveCN103396352APromote the development of economy and technologyRaw materials are easy to getOrganic chemistryChemistryEthylamines
The invention discloses a preparation method of Silodosin (I). The preparation method comprises the steps of: subjecting 1, 7-disubstituted-5-(2-oxopropyl) indoline (II) and 2-[2-(2, 2, 2-trifluoroethoxy)phenoxy]ethylamine (III) to an amination reduction reaction to obtain 2, 3-dihydro-1, 7-disubstituted-5-[2-[2-[2-(2, 2, 2-trifluoroethoxy)phenoxy]ethylamine]propyl]-1H-indole (IV), and subjecting the intermediate (IV) to splitting and conversion of the 1, 7- functional group, thus obtaining the Silodosin (I). The preparation method has high chemical selectivity, can effectively control the cost, and can improve the quality of the product, thus promoting the economic and technological development of the bulk drug.
Owner:南京联智医药科技有限公司
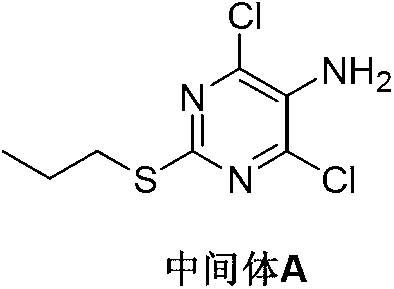
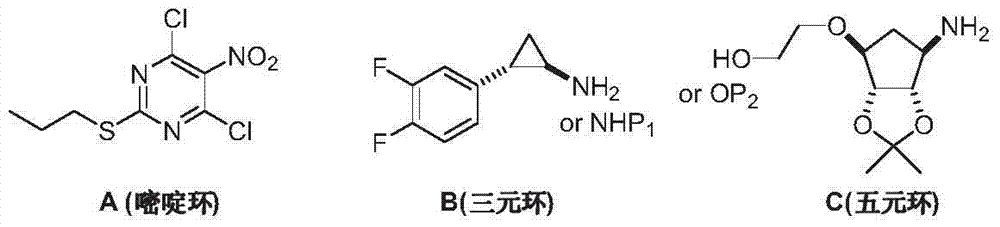
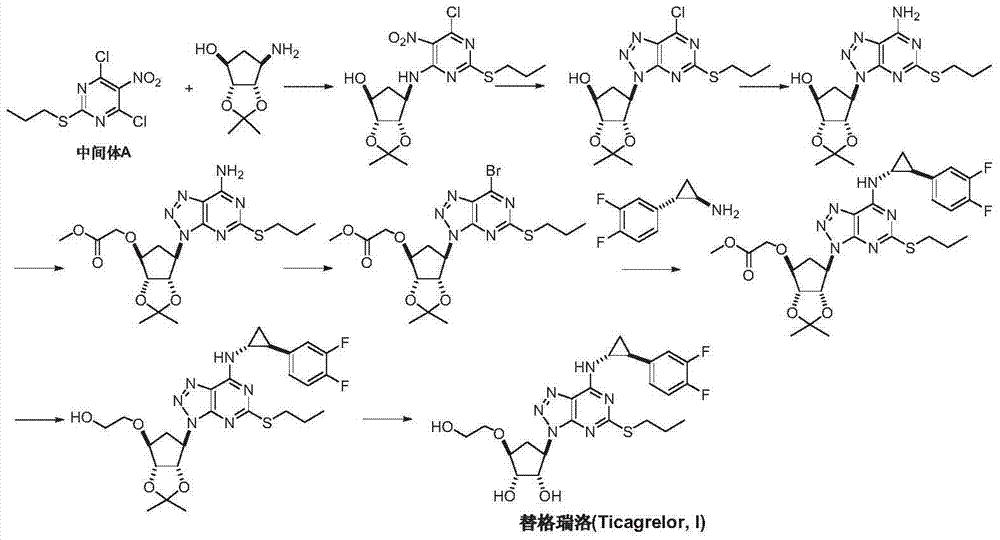
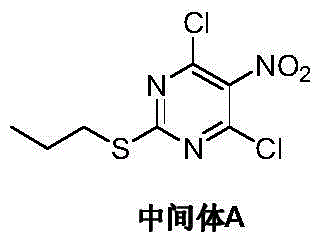
Preparation method of Ticagrelor intermediate 4, 6-dichloro-5-nitro-2-(propylthio) pyrimidine
ActiveCN103787984AEase of industrial productionPromote the development of economy and technologyOrganic chemistryPyrimidineMalonic acid
The invention discloses a preparation method of Ticagrelor intermediate 4, 6-dichloro-5-nitro-2-(propylthio) pyrimidine (intermediate A). The preparation method comprises the steps of carrying out ring-closure reaction on 2-nitro-1, 3 malonic acid alkyl ester (I) and thiourea (II) to generate 5-nitro-2-sulfo-barbituric acid (III); carrying out sulfur alkylation reaction on the obtained 5-nitro-2-sulfo-barbituric acid (III) and halogenated propane (IV) to obtain 4, 6-dyhydroxyl-5-nitro-2-(propylthio) pyrimidine (V); carrying out chlorination on the 4, 6-dyhydroxyl-5-nitro-2-(propylthio) pyrimidine (V) to obtain the Ticagrelor intermediate 4, 6-dichloro-5-nitro-2-(propylthio) pyrimidine (intermediate A). The preparation method is simple and convenient as well as economical and environmentally friendly, is beneficial to the industrial production of the medicine, and is capable of promoting the development of the economic technology of the raw material medicine, and raw materials are easily available.
Owner:苏州特瑞药业股份有限公司
Preparation method of cediranib
ActiveCN103275069AThe production process is easy to controlImprove product qualityOrganic chemistryState of artPyrrolidine
The invention discloses a preparation method of cediranib. The preparation method comprises the step that 6-methoxy-7-(3-pyrrolidine-1-yl-propoxy)-3,4-dihydroquinazoline-4-ketone (II) and 4-fluro-5-hydroxy-2-methylindole (III) carry out one-step condensation reaction under the actions of an organic alkali and a condensing agent to prepare cediranib (I). Compared with the prior art, the preparation method is easy in obtainment of raw materials, concise in process and mild in conditions, has the effects on optimizing the environment and improving the quality, is suitable for industrial production, and promotes development of the economic technology of active pharmaceutical ingredients.
Owner:迁安华韵知识产权服务中心
Afatinib preparation method
ActiveCN103242303BEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-Hydroxytetrahydrofuran2-Butene
The invention discloses an Afatinib (I) preparation method which comprises the following steps: performing etherification reaction on 4-chloro-6-amino-7-hydroxyquinazoline (II) and (S)-3-hydroxytetrahydrofuran to generate 4-chloro-6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (III); performing acylation reaction on the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride to generate 4-chloro-6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (IV); and performing condensation reaction on the compound (IV) and 4-fluoro-3-chloroaniline to obtain Afatinib (I). The preparation method is simple, economic and environment-friendly in process, and meets the requirements for large-scale industrialization.
Owner:铜陵尚东高新科创有限公司
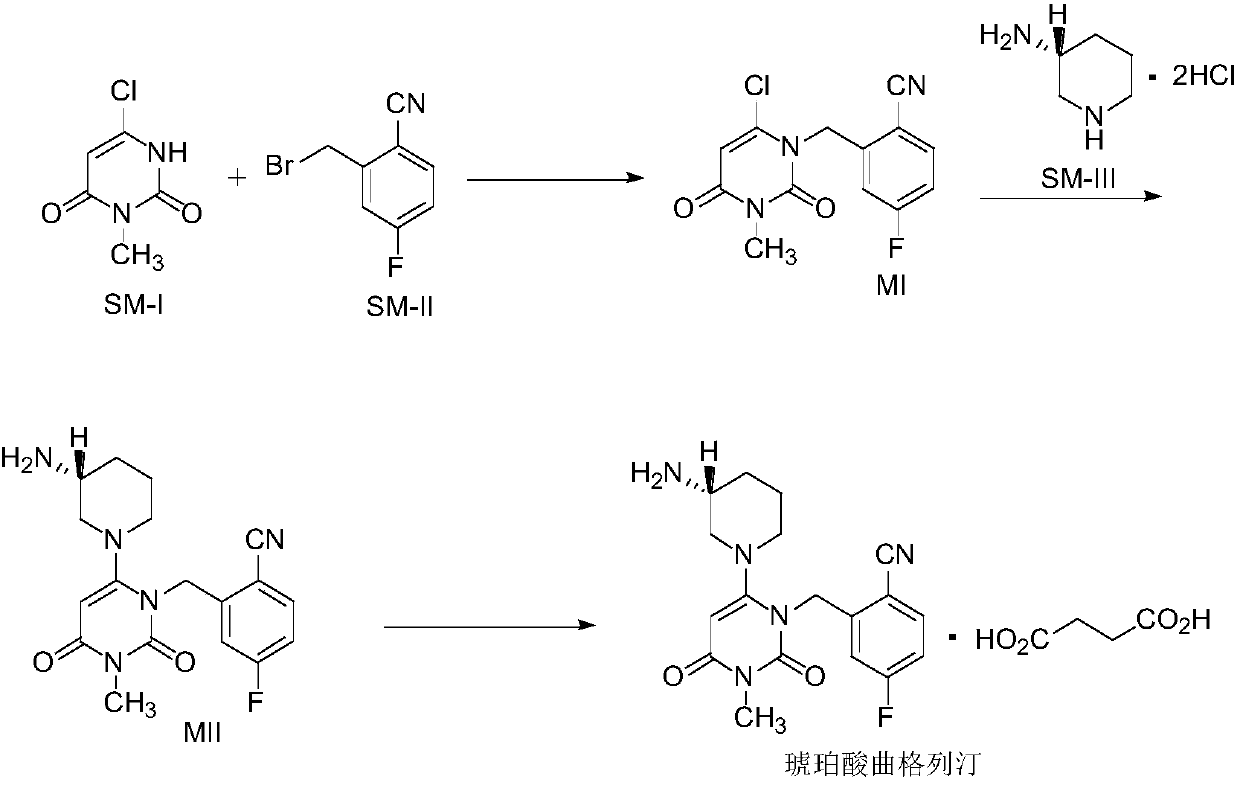
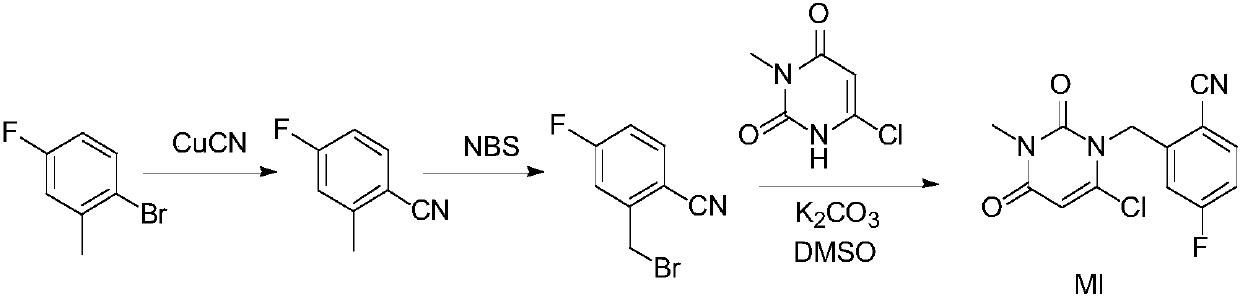
Preparation method of trelagliptin succinate
InactiveCN111349075AShort reaction timeEasy to operateCarboxylic acid salt preparationBiochemical engineeringProcess engineering
The invention provides an improved preparation method of trelagliptin succinate. The method comprises the following steps: by using 6-chloro-3-methyluracil and 2-cyano-5-fluorobenzyl bromide as initial raw materials, carrying out substitution reaction twice, refining, and carrying out a salifying reaction to obtain the trelagliptin succinate finished product. The method has the advantages of simple process, easily available raw materials, economy, environmental protection, high product yield, purity of 99% or above, and facilitation of realization of industrialization.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
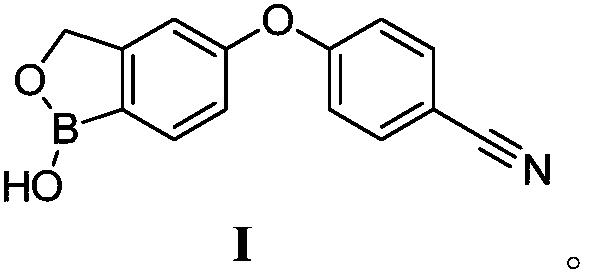
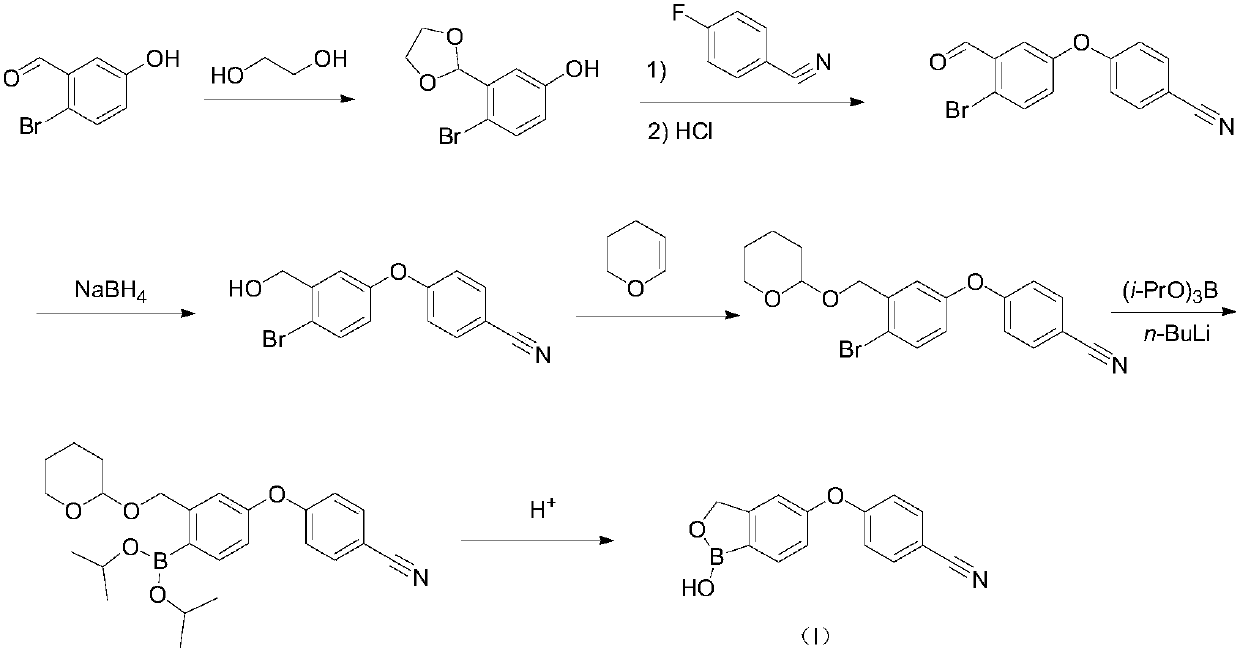
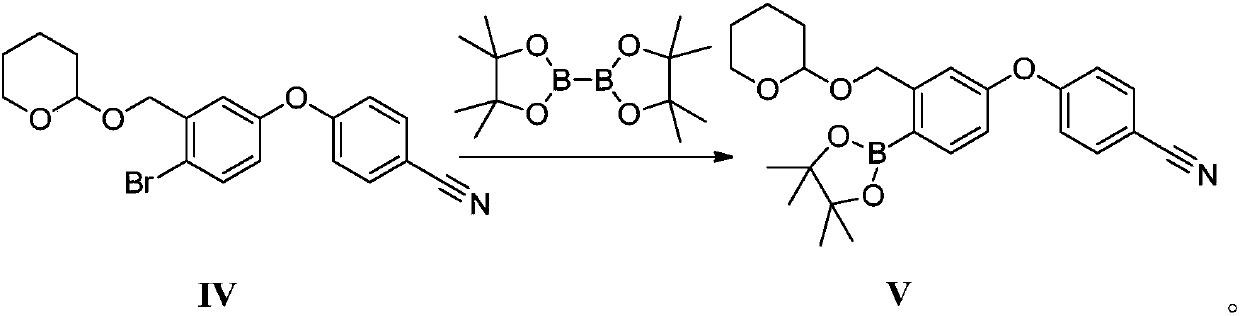
Method for preparing crisaborole
InactiveCN109517003ARaw materials are easy to getSimple stepsCarboxylic acid nitrile preparationOrganic compound preparationSolventStructural formula
The invention discloses a method for preparing crisaborole shown as a formula I. The preparation method disclosed by the invention comprises the following steps: carrying out the following reaction between a compound IV and bis(pinacolato)diboron in a solvent in the presence of alkali and a catalyst, thereby obtaining the compound V. The preparation method disclosed by the invention has the characteristics of being readily available in raw materials, simple in steps, mild in reaction conditions, controllable in quality, environmental-friendly, low in cost and the like. The industrial production of the bulk drug is facilitated, and development of the economic technology is promoted. The structural formula is as shown in the specification.
Owner:成都安满生物医药科技有限公司
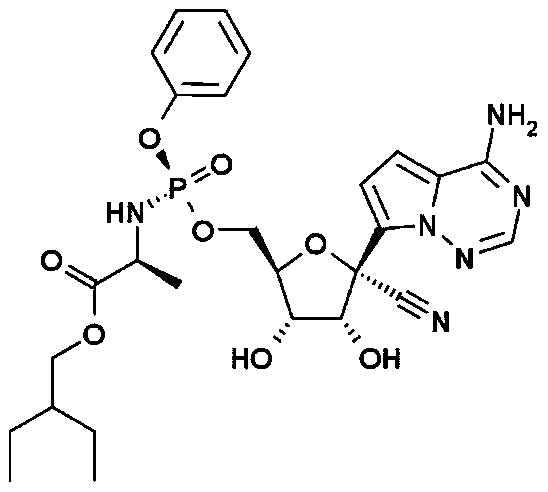
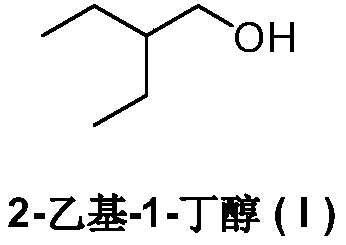
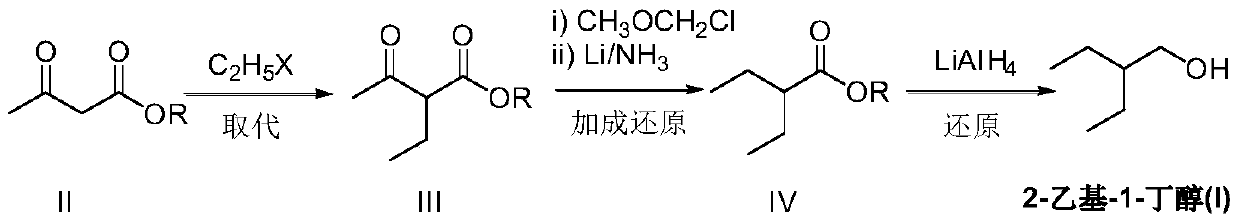
Preparation method of remdesivir intermediate 2-ethyl-1-butanol
ActiveCN111470946AEase of industrial productionRaw materials are easy to obtainOrganic compound preparationCarboxylic acid esters preparationAcetic acidButyrate
The invention relates to a preparation method of a remdesivir intermediate 2-ethyl-1-butanol. The preparation method comprises a step of substitution reaction, namely a step of carrying out a substitution reaction on alkyl acetoacetate and halogenated ethane under an alkaline condition to obtain alkyl 2-ethyl-3-oxo-butyrate; a step of addition reduction, namely a step of carrying out an addition reduction reaction on the alkyl 2-ethyl-3-oxo-butyate to obtain alkyl 2-ethylbutyrate; a step of reduction, namely a step of subjecting the alkyl 2-ethylbutyrate to a reduction reaction to prepare 2-ethyl-1-butanol (I). According to the preparation method of the remdesivir intermediate 2-ethyl-1-butanol, the alkyl acetoacetate and halogenated ethane serve as main raw materials, the raw materials are simple and easy to obtain, the 2-ethyl-1-butanol (I) is prepared through substitution reaction, addition reduction and reduction reaction, the process is simple, economical and environmentally friendly, the product is convenient to obtain, and industrial production of remdesivir bulk drugs is facilitated.
Owner:苏州特瑞药业股份有限公司
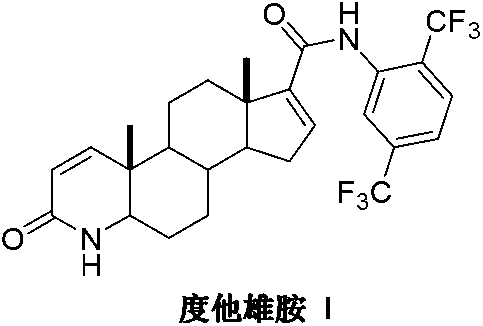
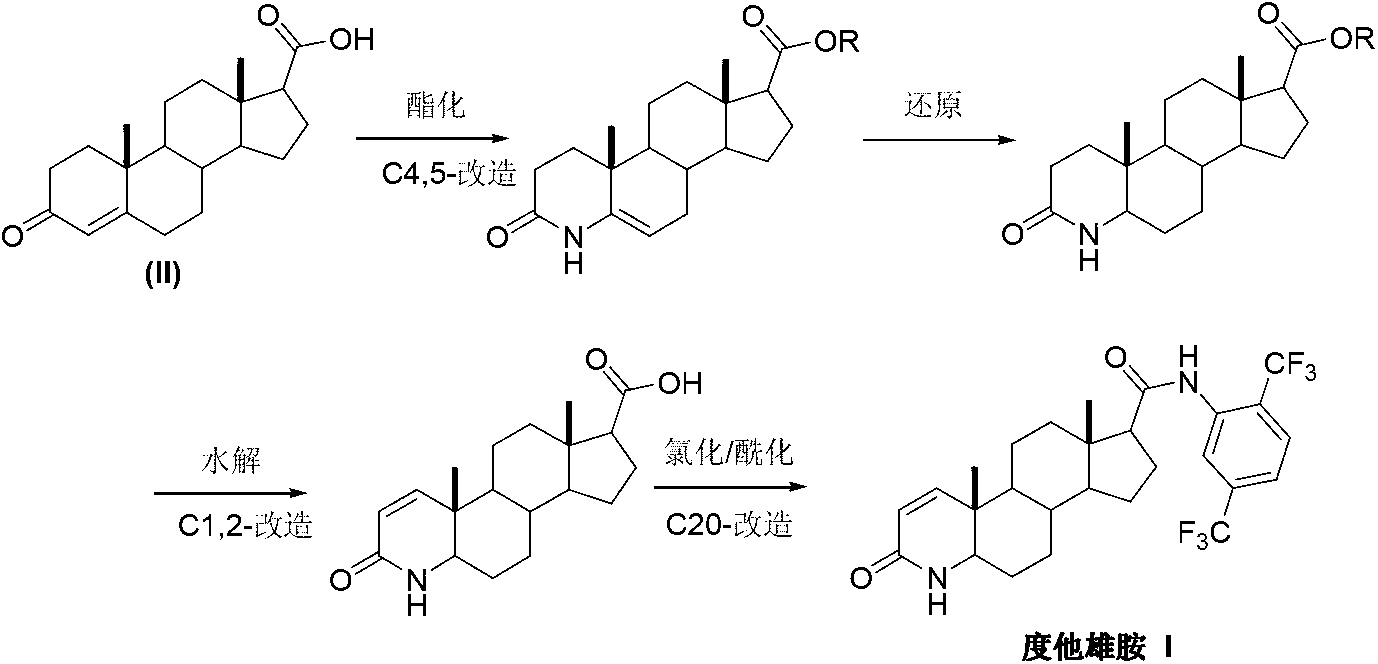
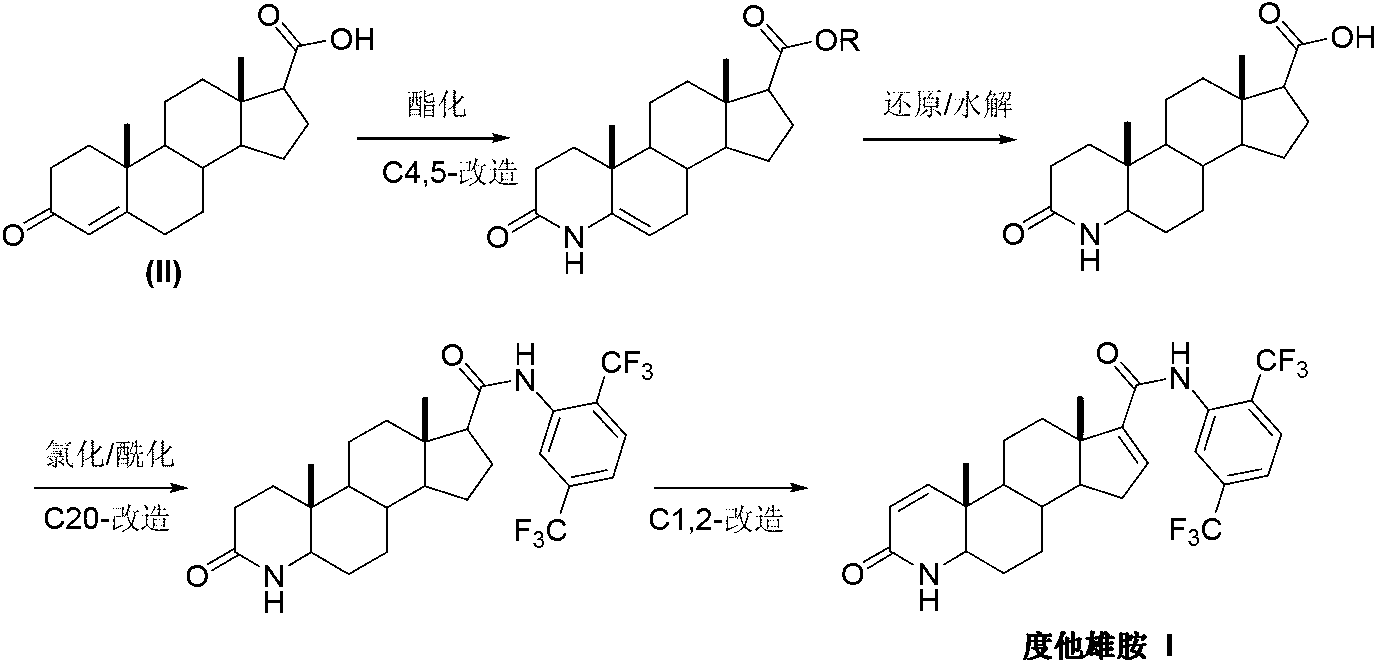
Preparation method of dutasteride
InactiveCN103254271AThe production process is easy to controlImprove product qualitySteroidsKetonic acidsDehydrogenation
The invention discloses a preparation method of dutasteride (N-(2,5-di(trifluoromethyl)phenyl)-4-aza-5alpha-androstane-1-ene-3-keto-17beta-formamide, I). The preparation method comprises the following steps that: pregnene ketonic acid (II) is taken as a raw material to convert carboxylic acid to amide through amidation to prepare androstane-4-ene-3-keto-17beta-formamide (III); the compound (III) is subjected to oxidative ring opening and ammonolysis cyclization to prepare 4-aza-androstane-5-ene-3-keto-17beta-formamide (IV); the compound (IV) is subjected to reductive hydrogenation reaction to generate 4-aza-5alpha-androstane-3-keto-17beta-formamide (V); the compound (V) is subjected to oxidative dehydrogenation to generate 4-aza-5alpha-androstane-1-ene-3-keto-17beta-formamide (VI); and the compound (VI) and 2,5-di(trifluoromethyl phenylamine) (VII) carry out amine exchange reaction in the presence of a catalyst to prepare dutasteride (I). The preparation method has the advantages of concise process, easiness in obtaining raw materials and controllable quality, and is suitable for industrial production.
Owner:ANHUI OURUIDA ELECTRICAL APPLIANCE TECH
Anti-HIV medicine containing indinavir and preparation method thereof
InactiveCN108324716APromote the development of economy and technologyHigh dissolution rateOrganic active ingredientsOrganic chemistryValeramideHigh volume manufacturing
The invention discloses anti-HIV medicine containing indinavir and a preparation method thereof. The anti-HIV medicine containing indinavir is prepared from indinavir and pharmaceutically acceptable carriers, wherein the chemical name of indinavir is (1(1S,2R),5(S))-2,3,5-tri-deoxy-N-(2,3-dihydro-2-hydroxyl-1H-indene-1-yl)-5-[2-[[(1,1-dimethyl ethyl) amino]carbonyl]-4-(3-picolyl)-1-piperazinyl]-2-(benzyl)-D-erythro-valeramide. The process of a preparation process is simple and compact; the raw materials can be easily obtained; economic performance and environment protection are realized; the industrialization can be favorably realized; the economic technology development of the indinavir raw medicine of the anti-HIV medicine can be promoted; the dissolving-out degree of the anti-HIV medicine containing indinavir is high; the effect is ideal; the medicine is suitable for mass production.
Owner:RIZHAO PUDA PHARMA TECH CO LTD
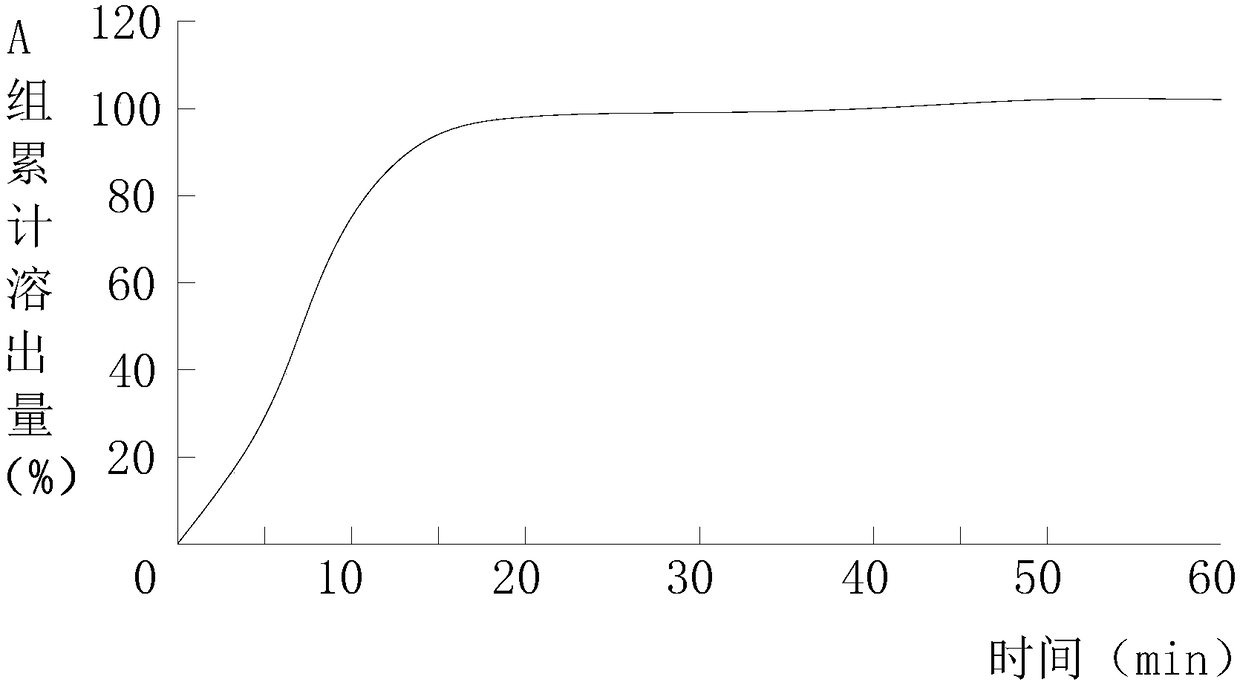
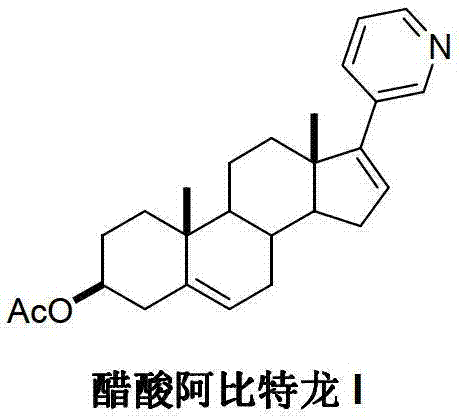
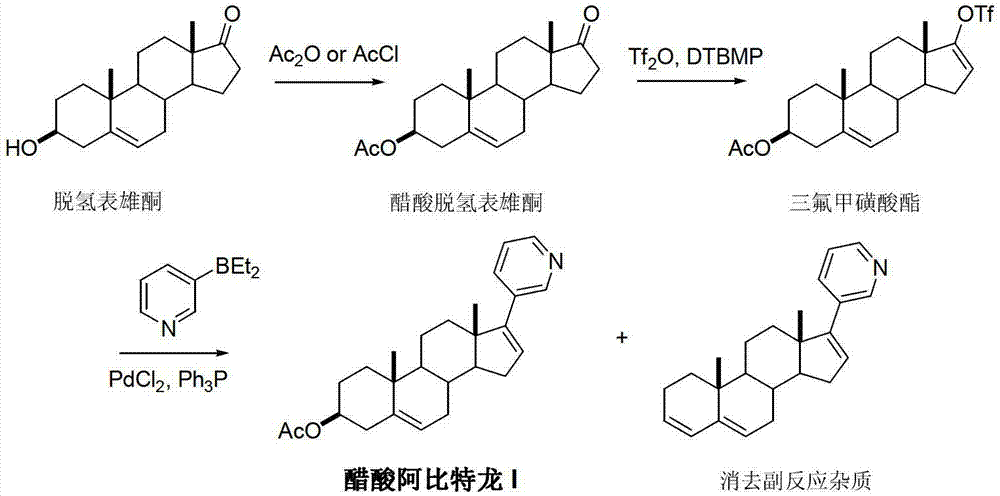
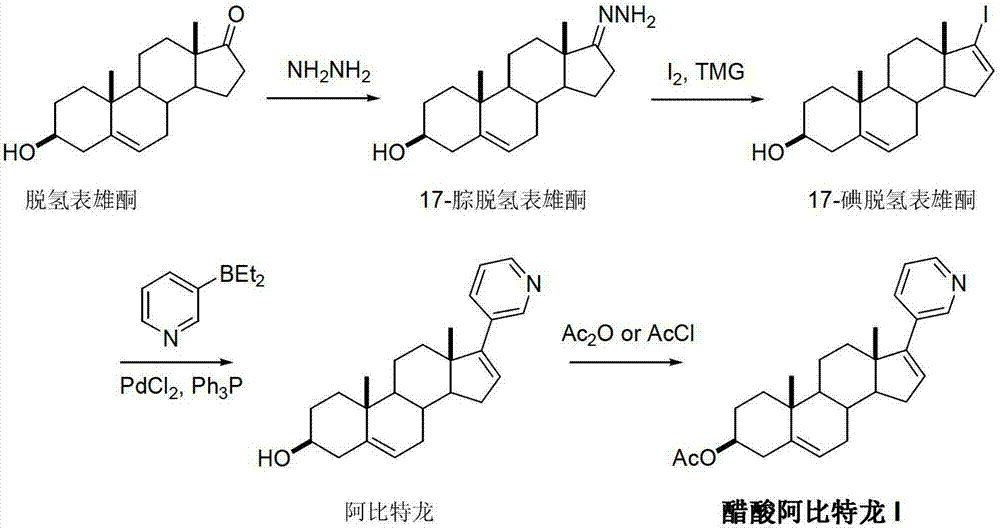
Preparation method of abiraterone acetate
ActiveCN103242410APromote the development of economy and technologyHigh chemoselectivitySteroidsHalogenAlcohol
The invention discloses a preparation method of abiraterone acetate (I) having a chemical name of 17-(3-pyridyl)-androstane-5,16-diene-3beta-alcohol acetate. The preparation method comprises the following steps of: taking 17-halogen-androstane-diene-3beta-alcohol acetate (II) and 3-pyridine boronic acid pinacol ester (III) as raw materials, and obtaining the abiraterone acetate (I) through a coupling reaction under the action of a catalyst. The preparation method has the advantages that the process is simple, raw materials are easily available, and quality is controllable; and therefore the preparation method is suitable for industrial production.
Owner:临沂经开财金投资发展有限公司
Preparation method of ticagrelor intermediate 4,6-dichloro-5-nitro-2-(propylthio)pyrimidine
ActiveCN103787984BEase of industrial productionPromote the development of economy and technologyOrganic chemistryAlkyl transferMalonic acid
The invention discloses a preparation method of Ticagrelor intermediate 4, 6-dichloro-5-nitro-2-(propylthio) pyrimidine (intermediate A). The preparation method comprises the steps of carrying out ring-closure reaction on 2-nitro-1, 3 malonic acid alkyl ester (I) and thiourea (II) to generate 5-nitro-2-sulfo-barbituric acid (III); carrying out sulfur alkylation reaction on the obtained 5-nitro-2-sulfo-barbituric acid (III) and halogenated propane (IV) to obtain 4, 6-dyhydroxyl-5-nitro-2-(propylthio) pyrimidine (V); carrying out chlorination on the 4, 6-dyhydroxyl-5-nitro-2-(propylthio) pyrimidine (V) to obtain the Ticagrelor intermediate 4, 6-dichloro-5-nitro-2-(propylthio) pyrimidine (intermediate A). The preparation method is simple and convenient as well as economical and environmentally friendly, is beneficial to the industrial production of the medicine, and is capable of promoting the development of the economic technology of the raw material medicine, and raw materials are easily available.
Owner:苏州特瑞药业股份有限公司
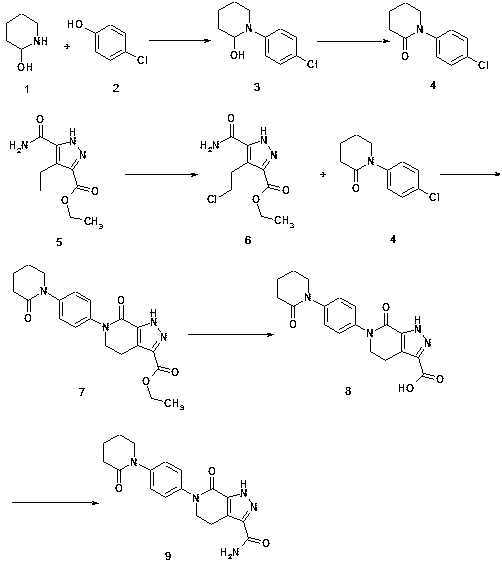
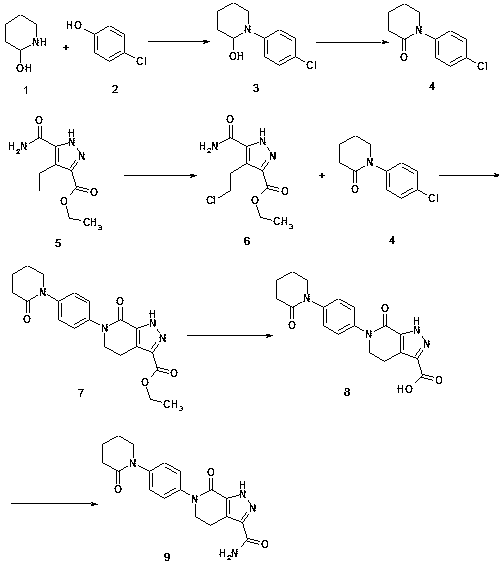
Preparation method for drug apixaban intermediate preventing venous thrombosis after hip and knee replacement surgeries
InactiveCN107936016APromote the development of economy and technologySimple processOrganic chemistryVeinThrombus
The invention discloses a preparation method for a drug apixaban intermediate preventing venous thrombosis after hip and knee replacement surgeries. The chemical name of the drug apixaban intermediatepreventing venous thrombosis after hip and knee replacement surgeries is 7-oxo-6-[4-(2-oxopipperidine-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazole[3,4-c]pyridine-3-forma-mide, and a structural formulathereof is as shown in the description. The simple preparation method uses raw materials easy to obtain, is economical and environmentally friendly, has high yield and produces highly pure product, is suitable for industrial production, can advance the economic technology of active ingredients of the apixaban intermediate, and lowers the production cost. The explored new intermediate preparationmethod is significant for the economic technology of apixaban.
Owner:董丹丹
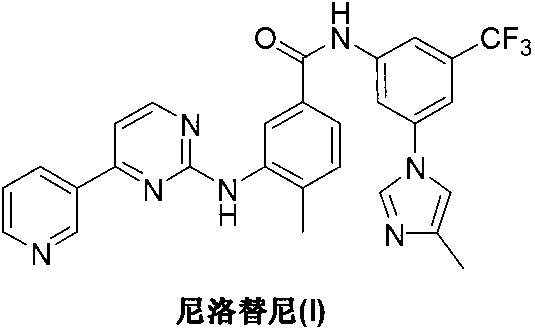
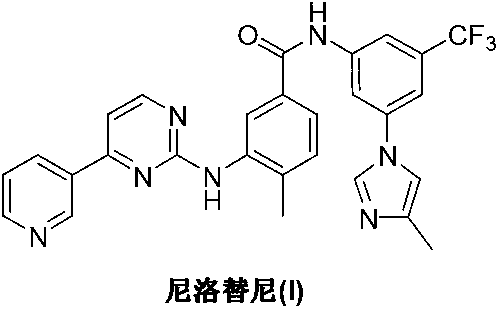
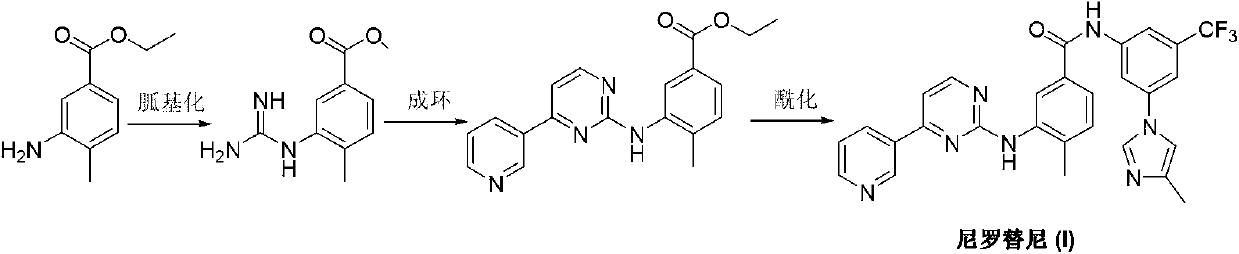
Preparation method of nilotinib
InactiveCN103275068AProduction is easy to controlImprove product qualityOrganic chemistryOrganic baseNilotinib
The invention discloses a preparation method of nilotinib, which comprises the following steps of: under the effect of organic base and a condensing agent, performing further condensation reaction between 4-(3-pyridyl)-2-pyrimidone (II) and 3-amino-4-methyl-N-[3-(4-methyl-1H-imidazole-1-yl)-5-trifluorotolylmethyl]benzamide (III) to obtain nilotinib (I). The preparation method disclosed by the invention has the advantages that the raw materials are easily available, the technology is simple, the conditions are mild, the environment is optimized, and the quality is improved; and moreover, the preparation method is suitable for industrial production and promotes the development of the economic technology of raw material medicines.
Owner:SUZHOU MIRACPHARMA TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com