Patents
Literature
47 results about "Butenamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
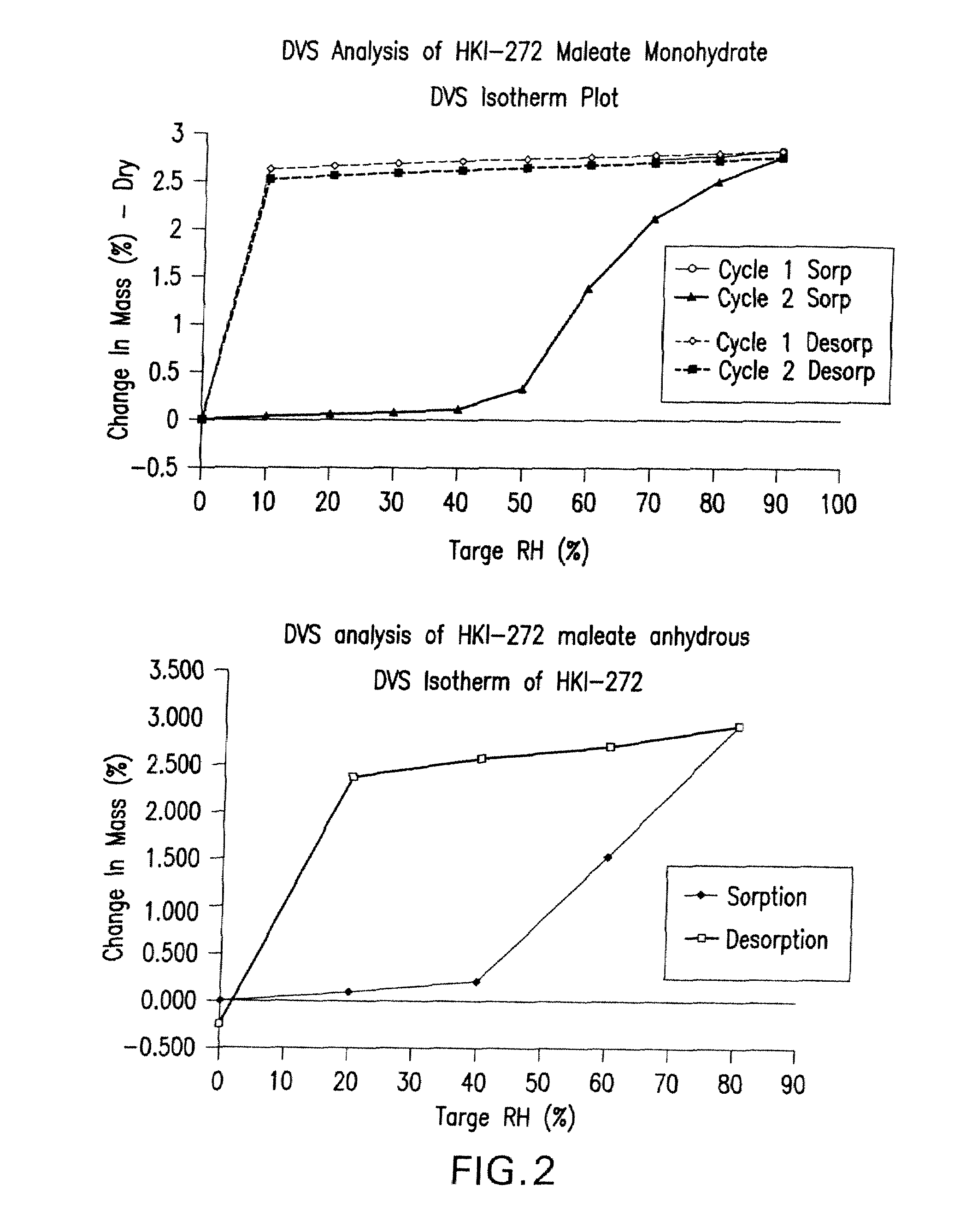
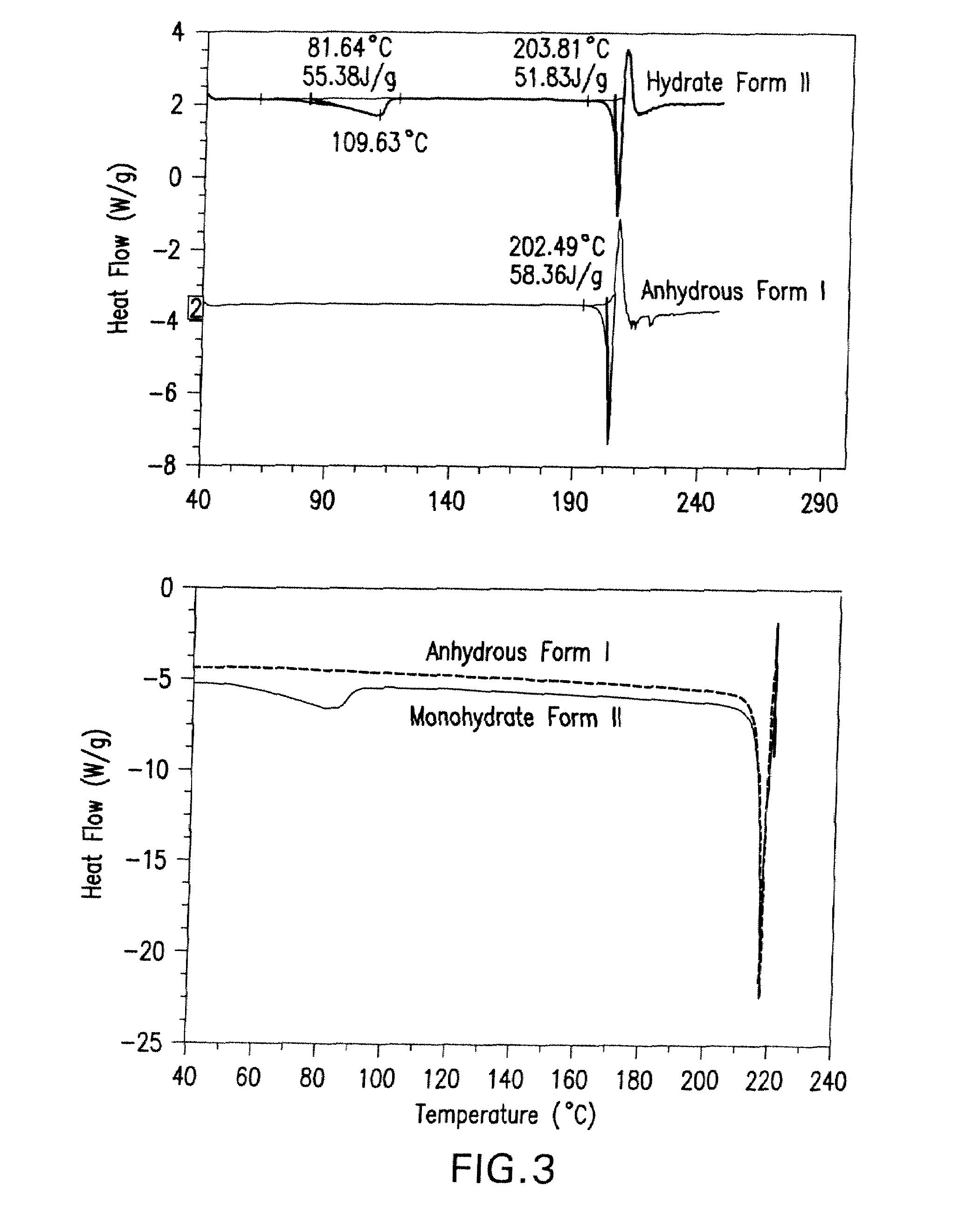
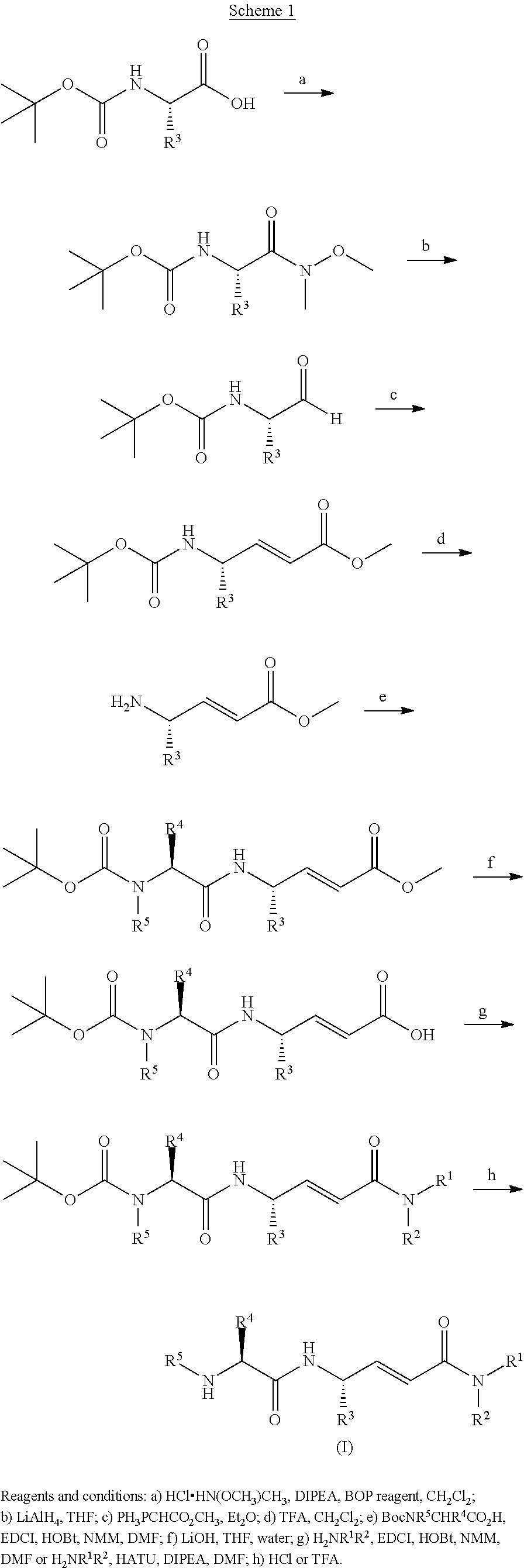
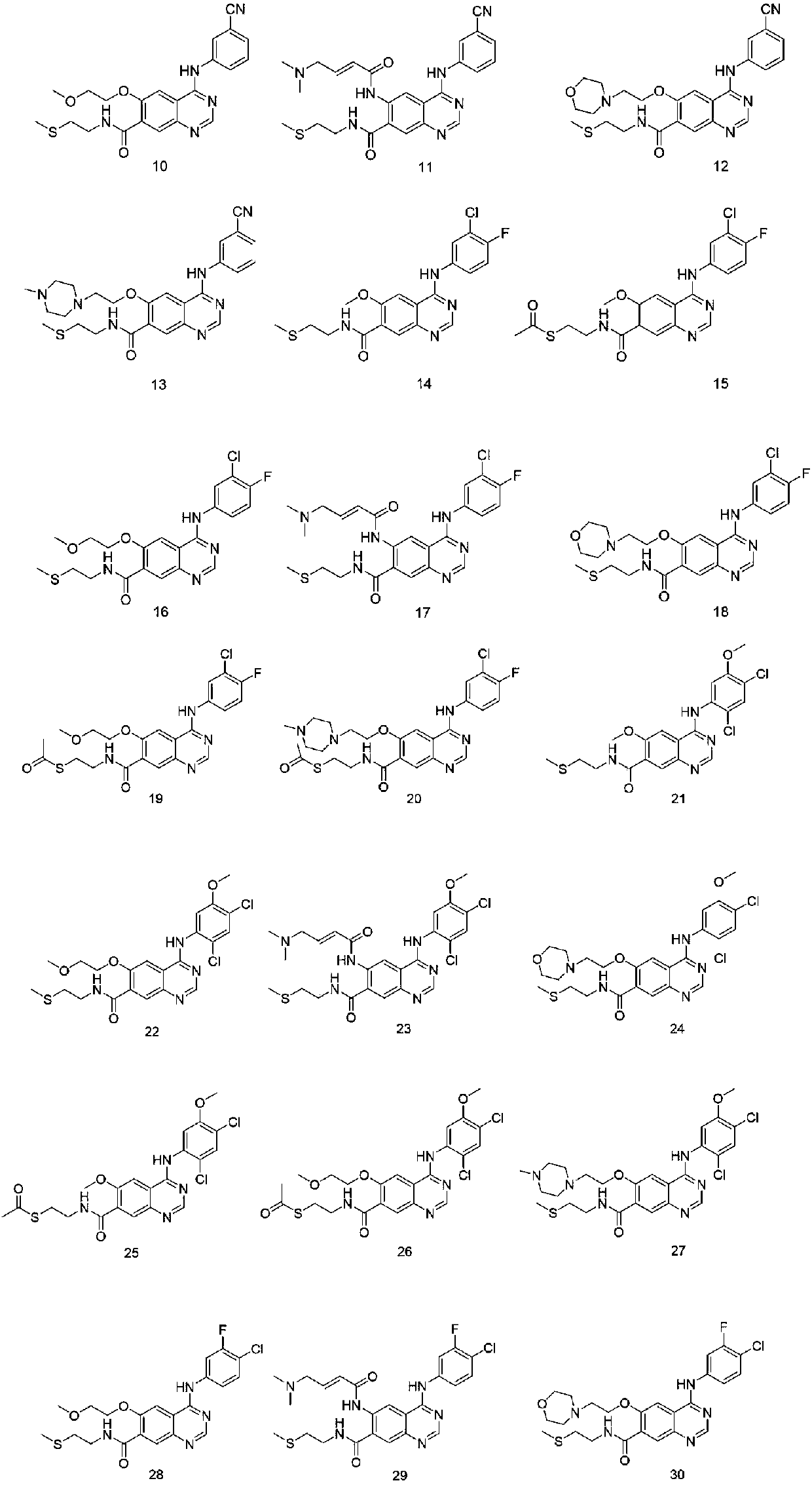
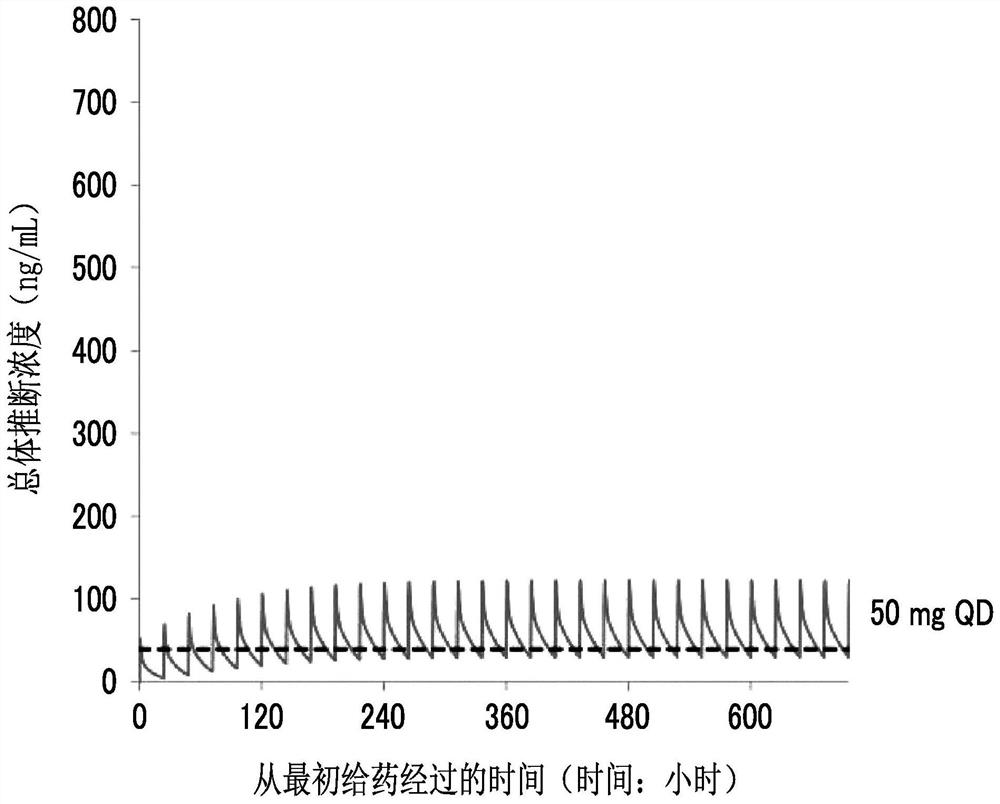
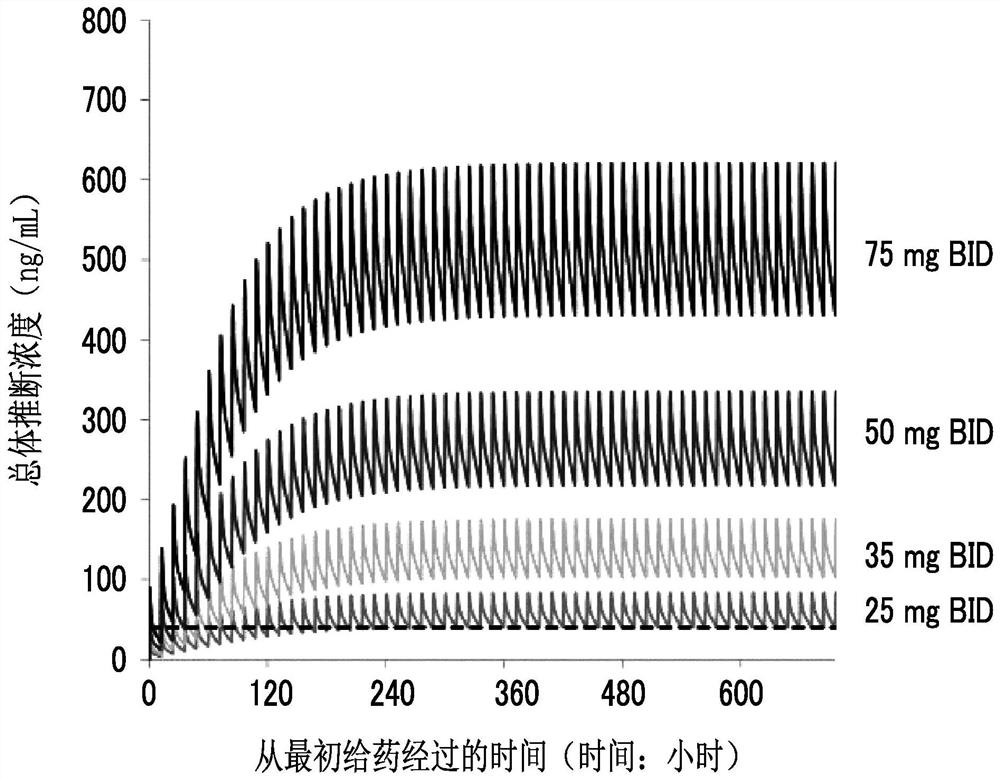
Maleate salts of (e)-n--4-(dimethylamino)-2-butenamide and crystalline forms thereof
ActiveUS20090176827A1Preventing and treating and inhibiting cancerBiocideOrganic compound preparationKinaseMedicinal chemistry
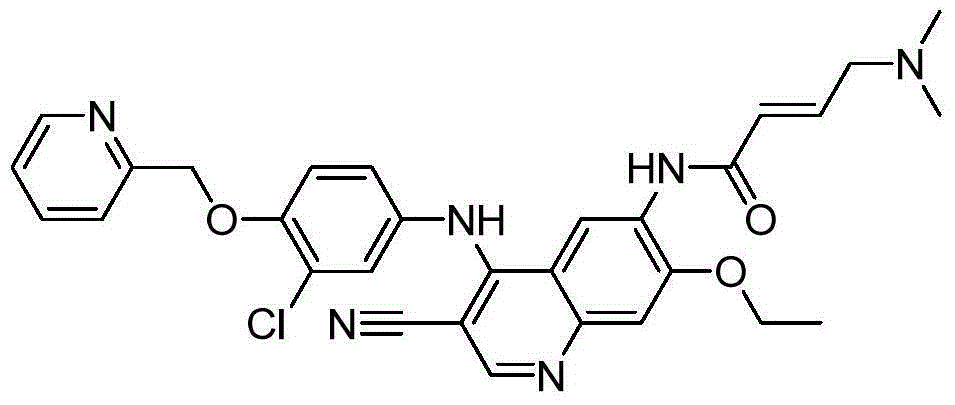
The present invention relates to maleate salt forms of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide, methods of preparing crystalline maleate salt forms, the associated compounds, and pharmaceutical compositions containing the same. The maleate salts are useful in treating cancers, particularly those affected by kinases of the epidermal growth factor receptor family.
Owner:WYETH LLC
Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof
The present invention relates to maleate salt forms of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide, methods of preparing crystalline maleate salt forms, the associated compounds, and pharmaceutical compositions containing the same. The maleate salts are useful in treating cancers, particularly those affected by kinases of the epidermal growth factor receptor family.
Owner:WYETH LLC
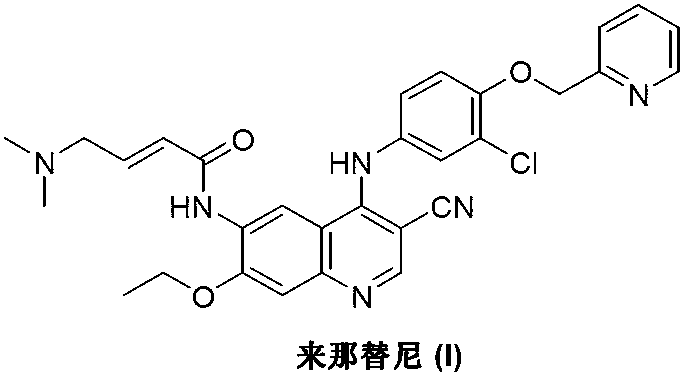
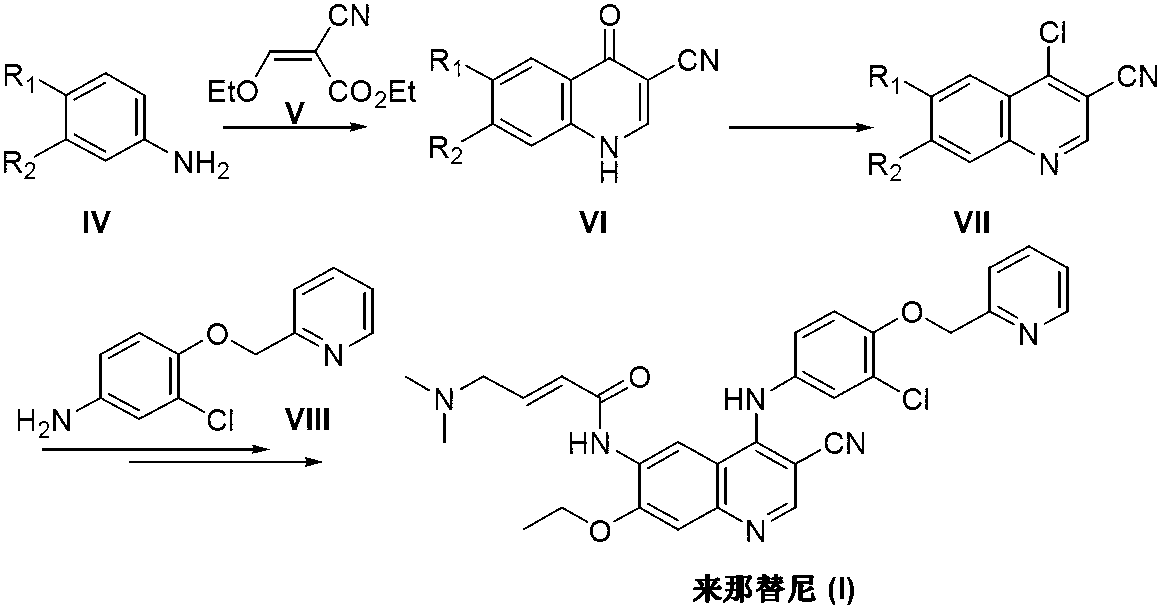
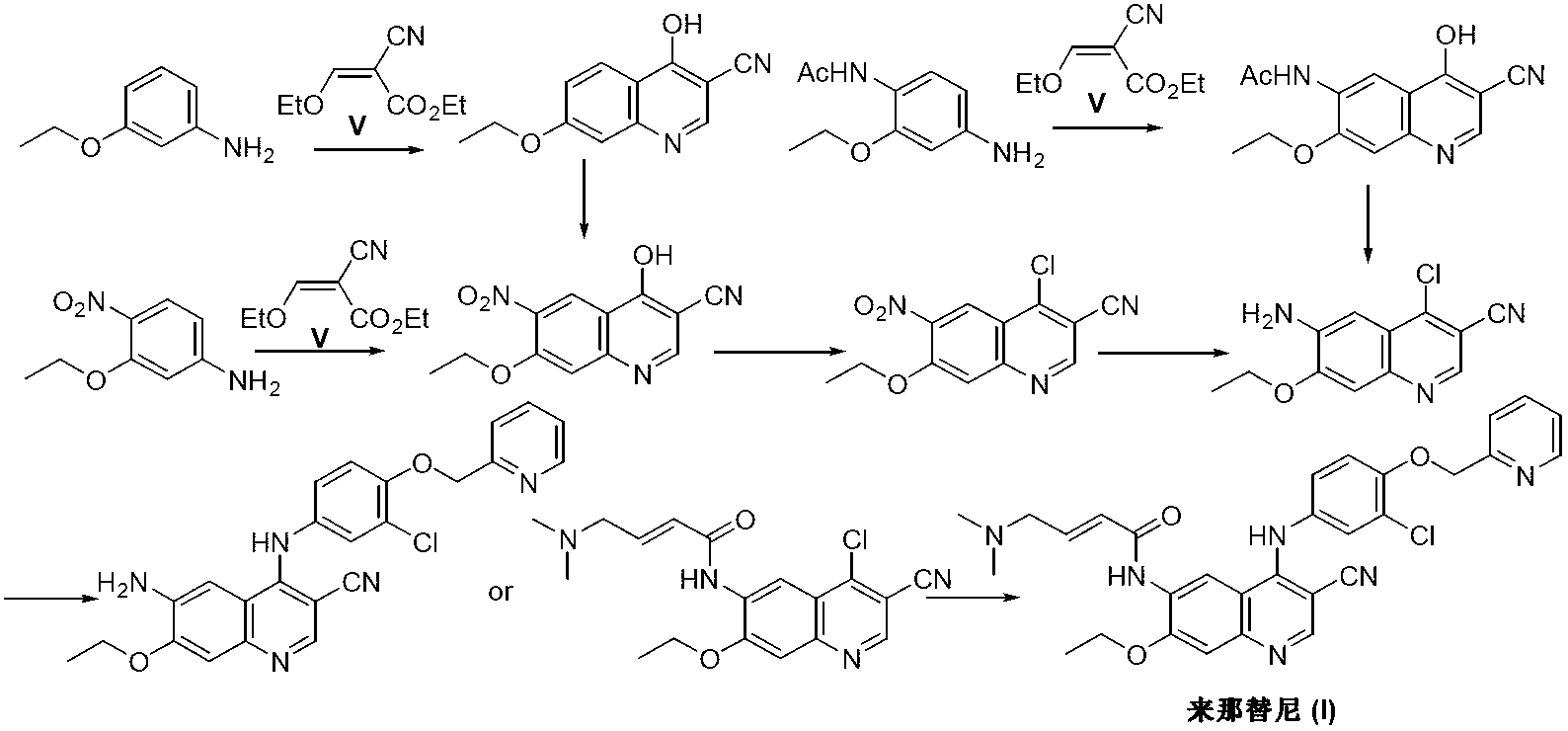
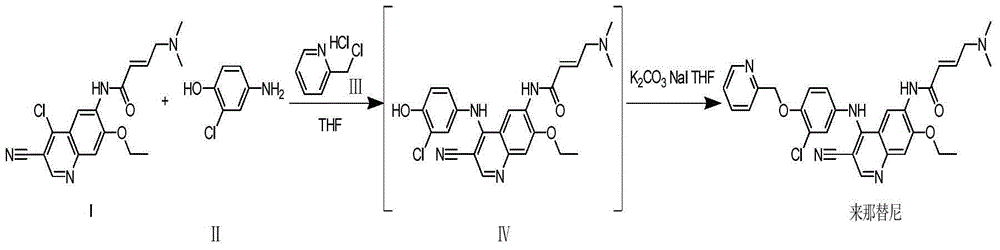
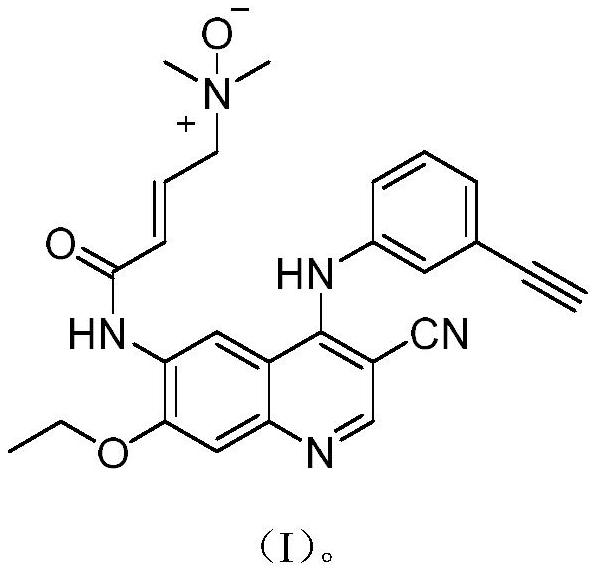
Preparation method of neratinib
InactiveCN103265530AEase of industrial productionRaw materials are easy to getOrganic chemistryBenzaldehydePyridine
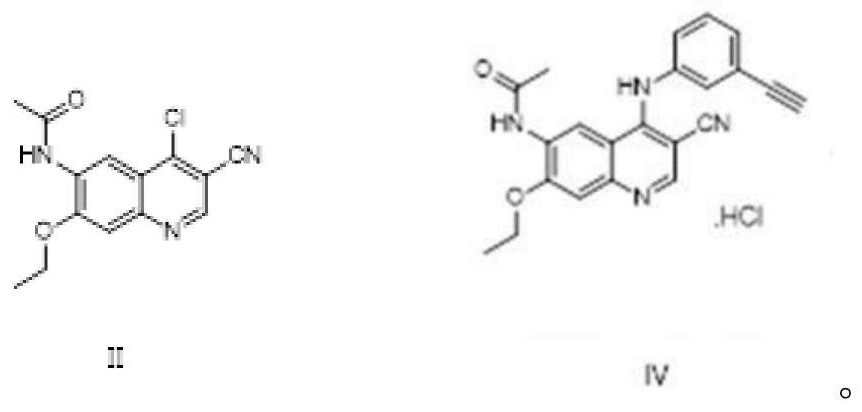
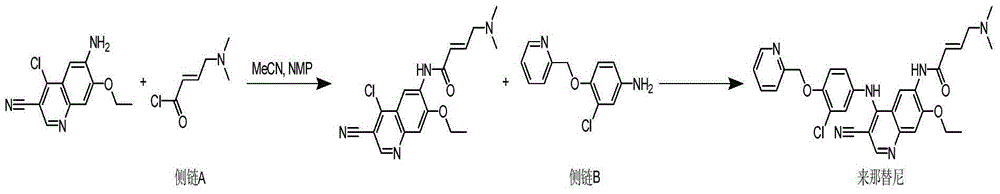
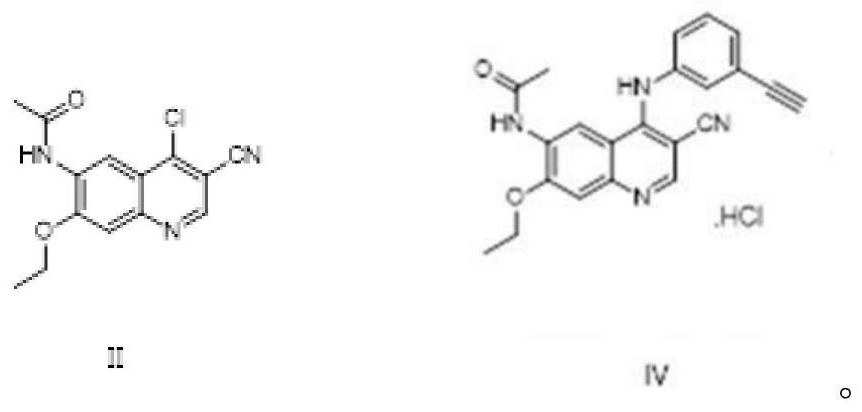
The invention discloses a preparation method of neratinib (I). The preparation method comprises the step that 6-[(E)-4-(dimethylamino)-2-butenamide]-7-ethoxy-4-amino-3-quinolinecarbonitrile (II) and 3-chloro-4-[(pyridine-2-yl)methoxy]-benzaldehyde (III) carry out condensation and reduction reactions to obtain neratinib (I). The preparation method is easy in obtainment of raw materials, concise in process, economical and environment-friendly and suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH +1
Quinazoline-7-ether compounds and methods of use
The invention provides quinazoline-7-ether derivatives, particularly 4-anilinyl-6- butenamido-quinazoline-7-ether derivatives that are inhibitors of the receptor protein tyrosine kinases (RTK). The compounds are useful in the treatment of diseases and disorders where RTK activity is implicated such as a hyperproliferative diseases (e.g., cancer). Also provided are methods of preparation of the quinazoline derivatives and methods of use as therapeutic agents alone or in a drug combination.
Owner:JIANGSU KANION PHARMA CO LTD
New use of quinazoline derivative tyrosine kinase inhibitor
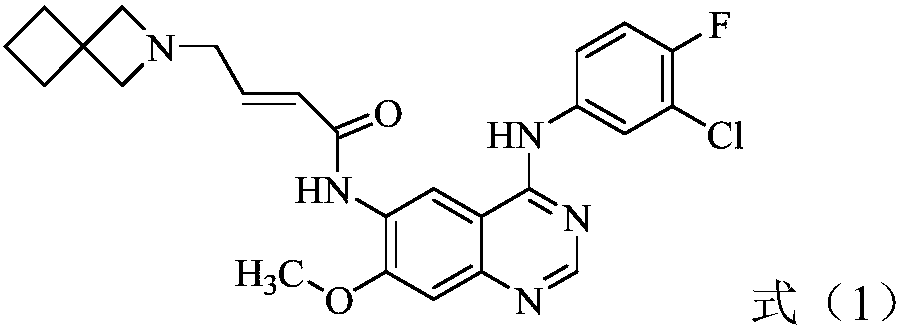
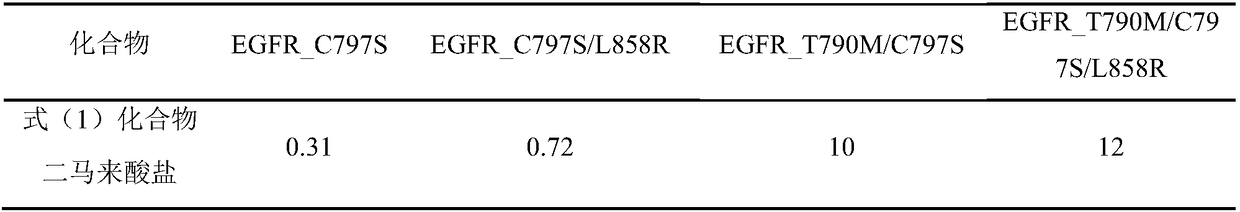
The present invention relates to new use of a quinazoline derivative tyrosine kinase inhibitor, and specifically relates to new use of a compound represented by the formula (1) (E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazoline-6-yl)-4-(2-azaspiro[3.3]heptane-2-yl)-2-butenamide and pharmaceutically acceptable salts thereof.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
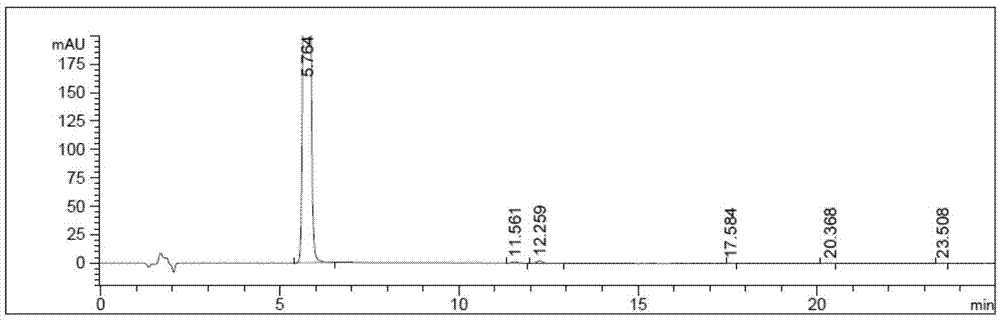
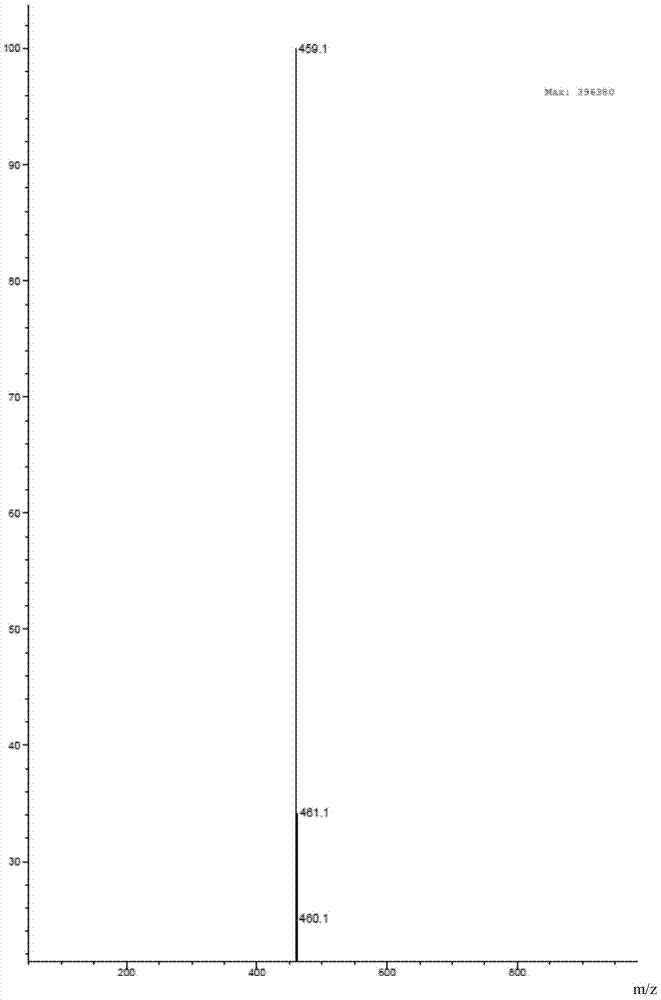
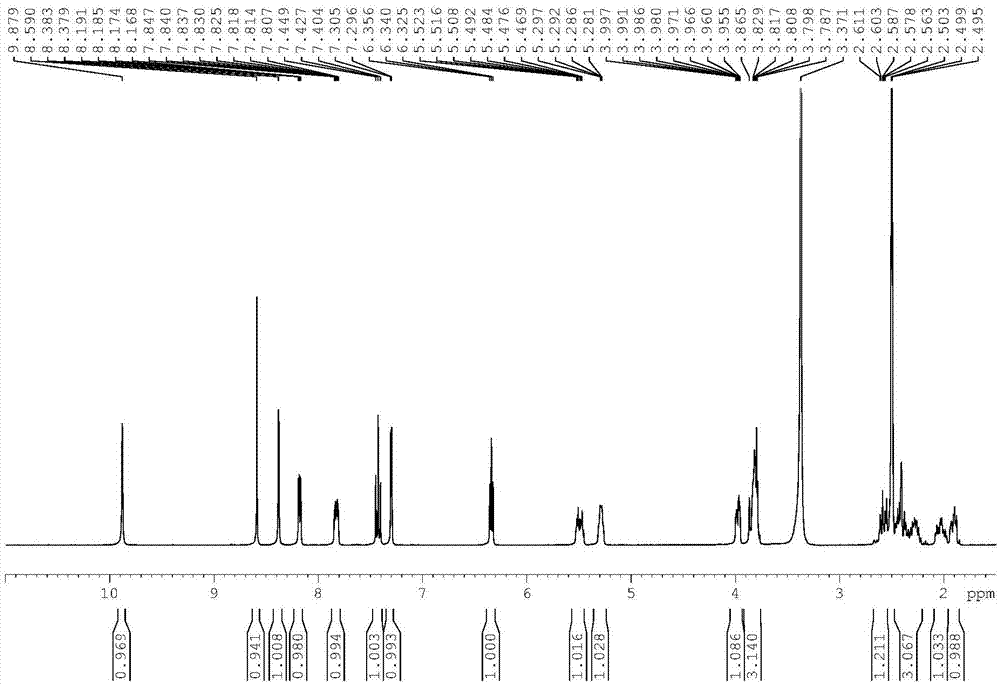
Preparation method of neratinib
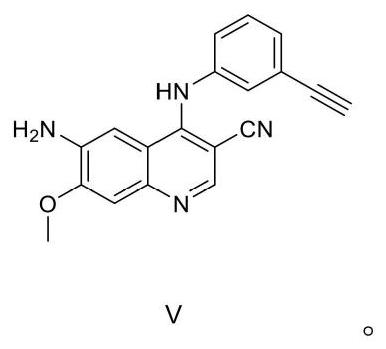
The invention relates to the field of pharmaceuticals, in particular to a preparation method of neratinib. The preparation method specifically includes: successively condensing 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-cyanoquinoline (I) with 2-chloro-4-aminophenol (II) and 2-(chloromethyl)pyridine hydrochloride (III) through an intermediate (E)-N-{4-[3-chloro-4-hydroxyanilino]3-cyano-7-ethoxy-6-quinoline}-4-dimethylamino-2-butenamide (IV). The materials in the preparation method are easy to obtain, and the preparation method is simple, green and economical and has greatly worthy of application.
Owner:HARBIN ZHENBAO PHARMA +1
Compound, and preparation method and application thereof
InactiveCN106916147AEfficient preparationStarting materials are cheap and readily availableOrganic chemistryHydrogenOrganic solvent
The invention relates to a compound, and a preparation method and application thereof. Specifically, the compound is as shown in a formula 1 which is described in the specification. The invention also provides the preparation method for the compound as shown in the formula 1. The preparation method comprises a step of contacting N-[4-[(3-chloro-4-fluorophenyl)amino]-7[[(3S)-tetrahydro-3-furyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butyleneamide with an alkaline aqueous solution in an organic solvent so as to obtain the compound as shown in the formula 1. The preparation method is simple to operate; a white powder product can be obtained through direct filtering after post-treatment; and the prepared compound has high purity, as high as 99% or above, and can be directly used as an impurity control substance for research on the quality of an afatinib bulk drug.
Owner:WATERSTONE PHARMA WUHAN
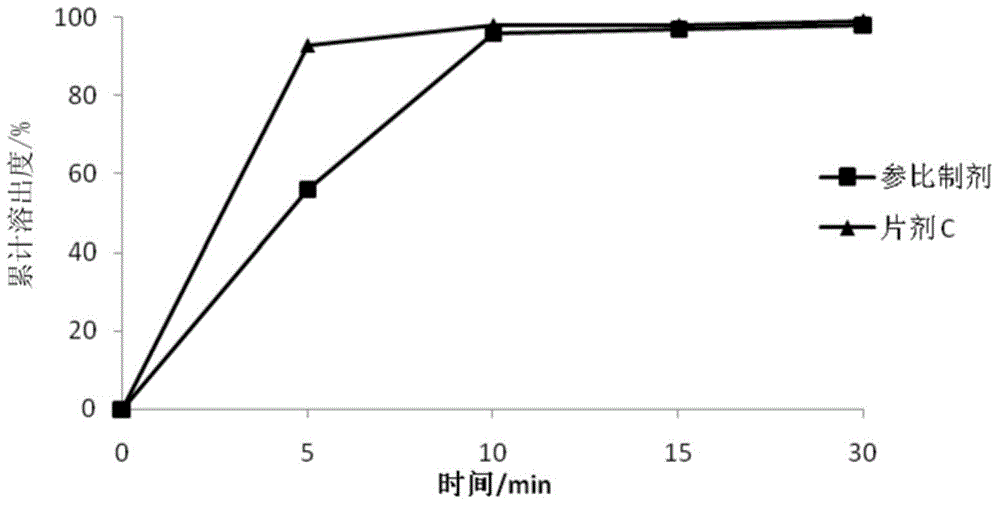
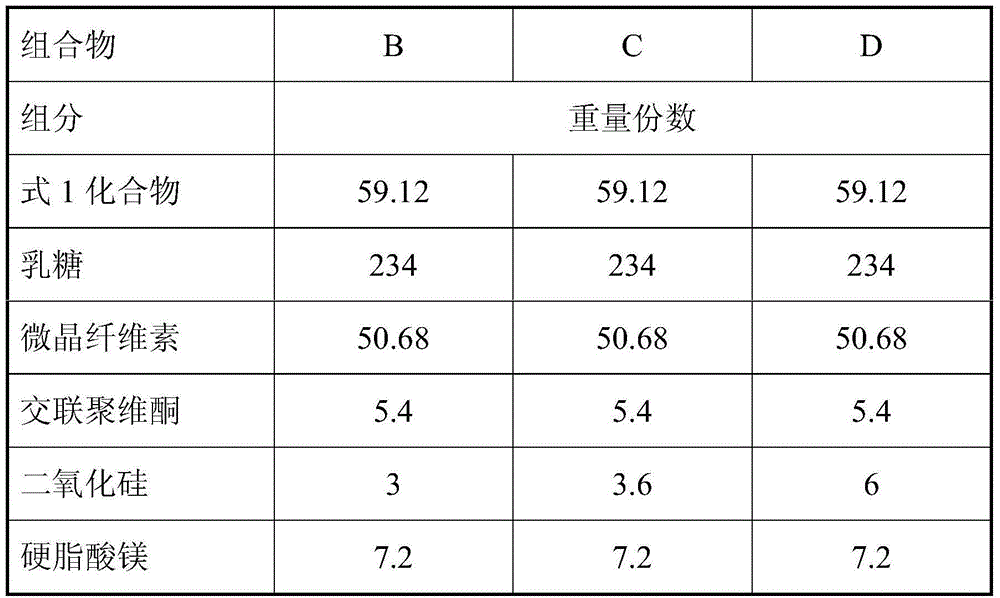
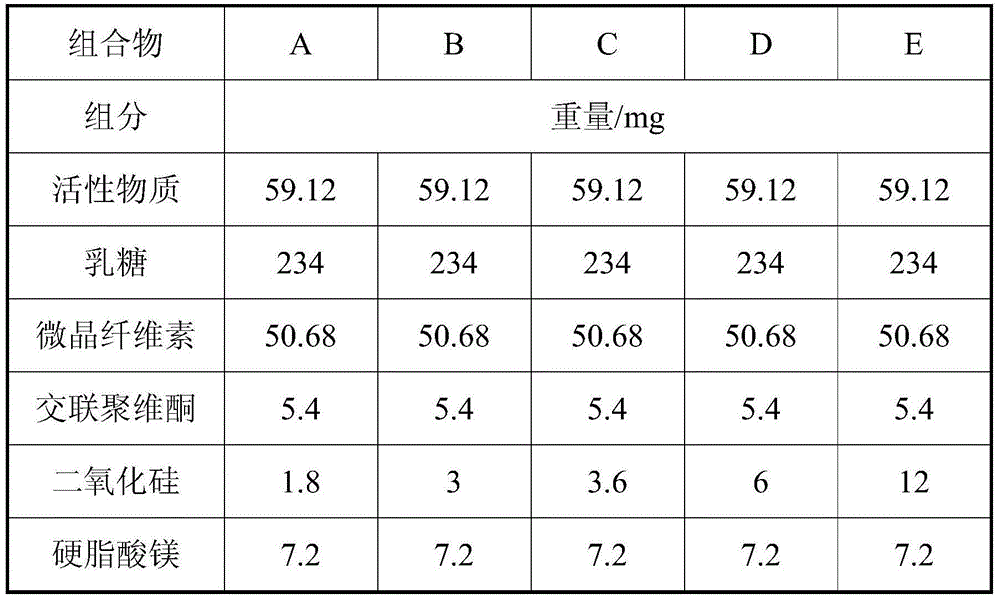
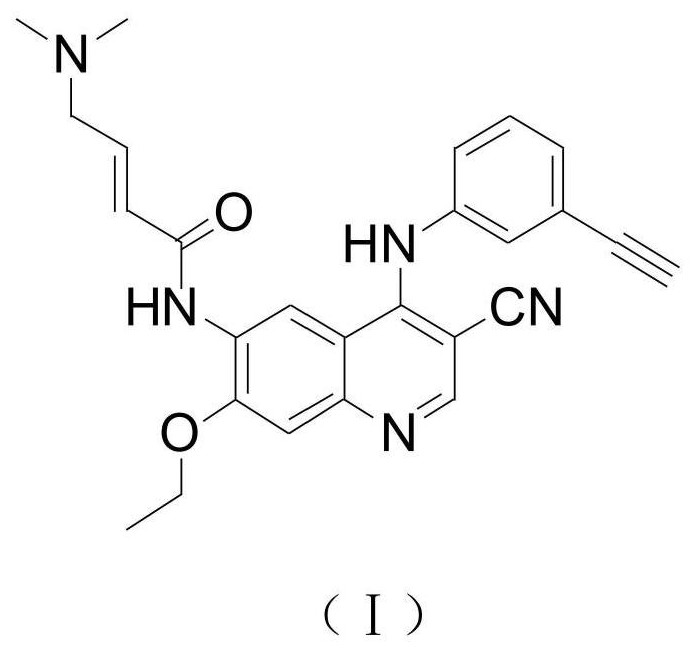
Pharmaceutical composition and preparation method thereof
The present invention discloses a pharmaceutical composition and a preparation method thereof, particularly to a pharmaceutical composition, which comprises an active substance (2E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-(((3S)-tetrahydro-3-furanyl)oxy)-6-quinazolinyl)-4-(dimethylamino)-2-butenamide dimaleate, a carrier, a disintegrant, a glidant and a lubricant. The composition of the present invention is hardly electrostatically charged, and is suitable for being directly prepared into the solid preparation. The present invention further discloses a solid preparation prepared from the composition.
Owner:JIANGSU HANSOH PHARMA CO LTD
Preparation method of EGFR molecular targeting antitumor drug
ActiveCN111875539ARaw materials are easy to getSimple processOrganic chemistryAntineoplastic agentsQuinolinePharmaceutical Substances
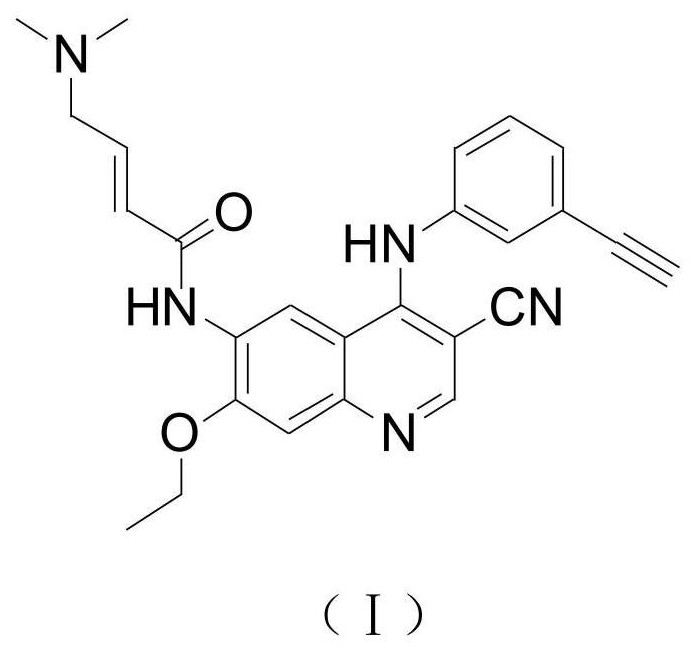
The invention discloses a preparation method of an EGFR molecular targeting antitumor drug. The EGFR molecular targeting antitumor drug is (E)-N-[4-(3-acetylenephenyl)-amino-3-cyano-7-ethoxy-6-quinolyl]-4-(dimethylamino)-2-buteneamide. The preparation method of the EGFR molecular targeting antitumor drug comprises the following step: carrying out a heating substitution reaction on N-(4-chloro-3-cyano-7-ethoxyquinoline-6-yl)acetamide and m-aminophenylacetylene under the condition that n-propanol is used as a reaction solvent. The method has the advantages of easily available raw materials, simple process, short reaction time, low impurity content, high yield, suitability for industrial production and the like.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
Cathepsin c inhibitors
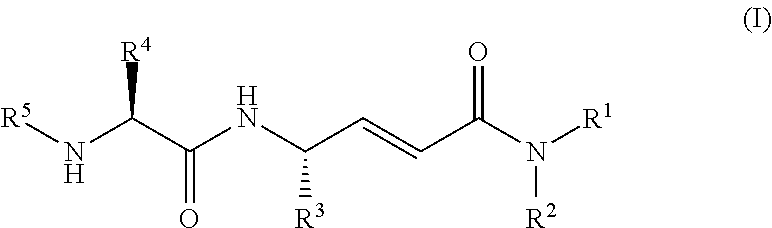
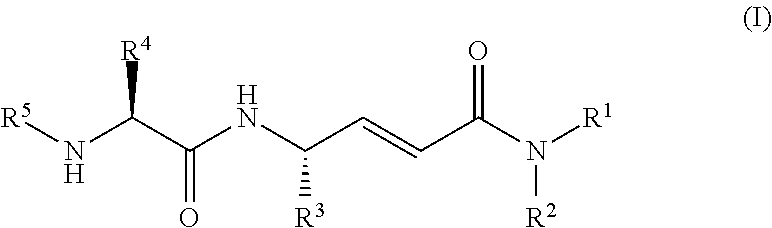
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXO GROUP LTD
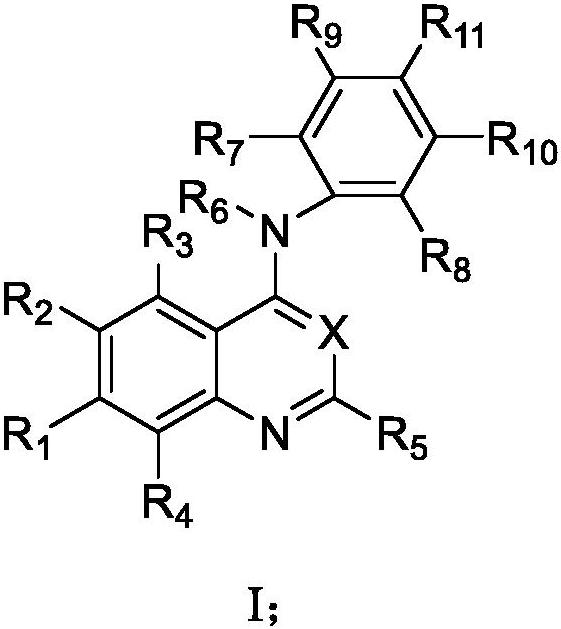
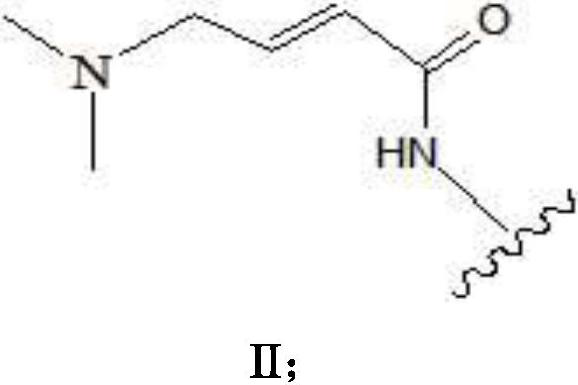
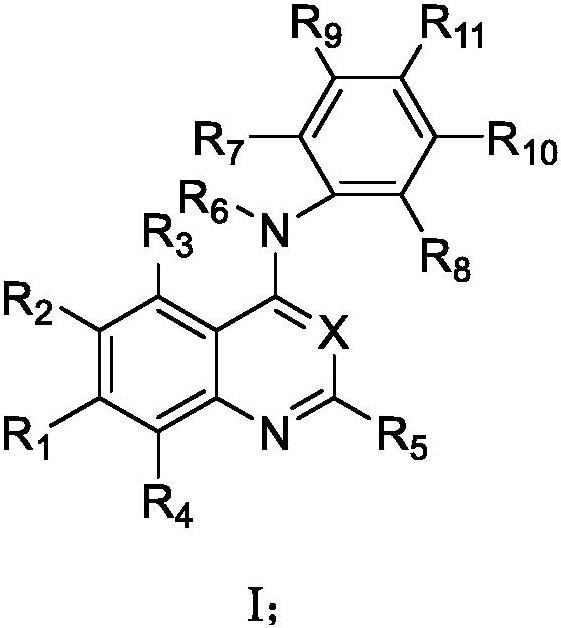
Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof
The present invention relates to an improved process for the preparation of N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) represented by the following structural formula:
Owner:MSN LAB PTE LTD
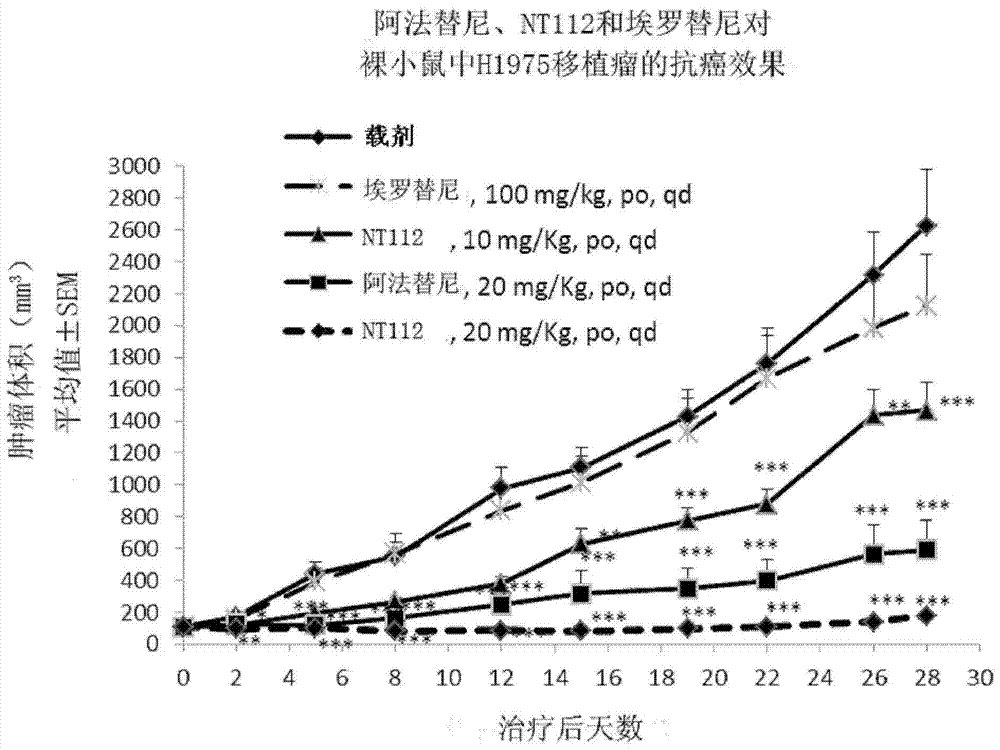
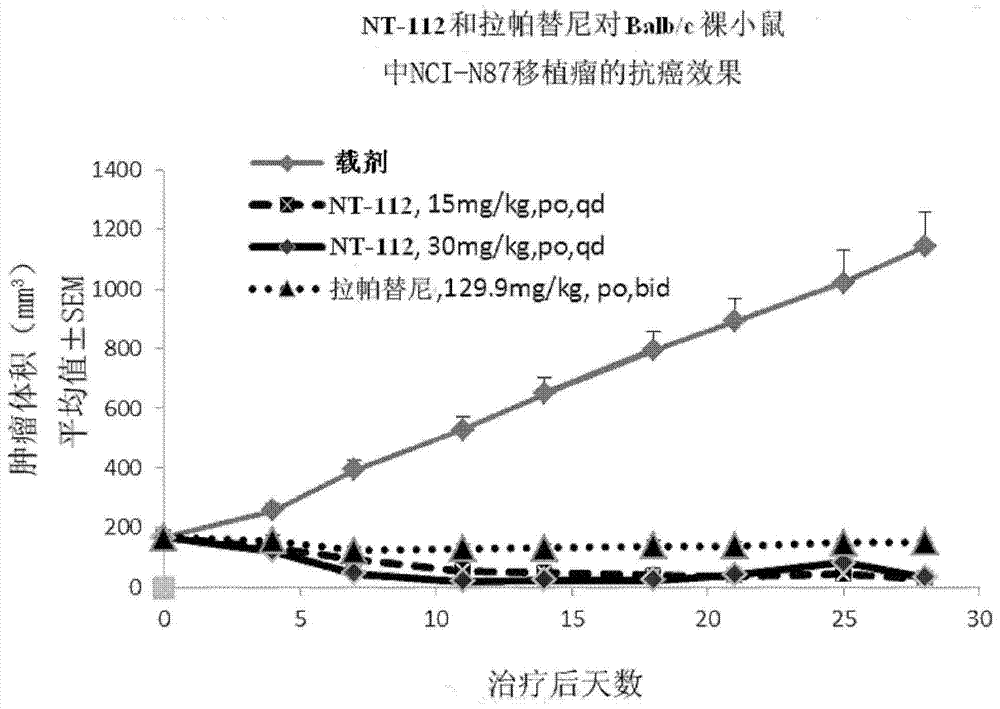
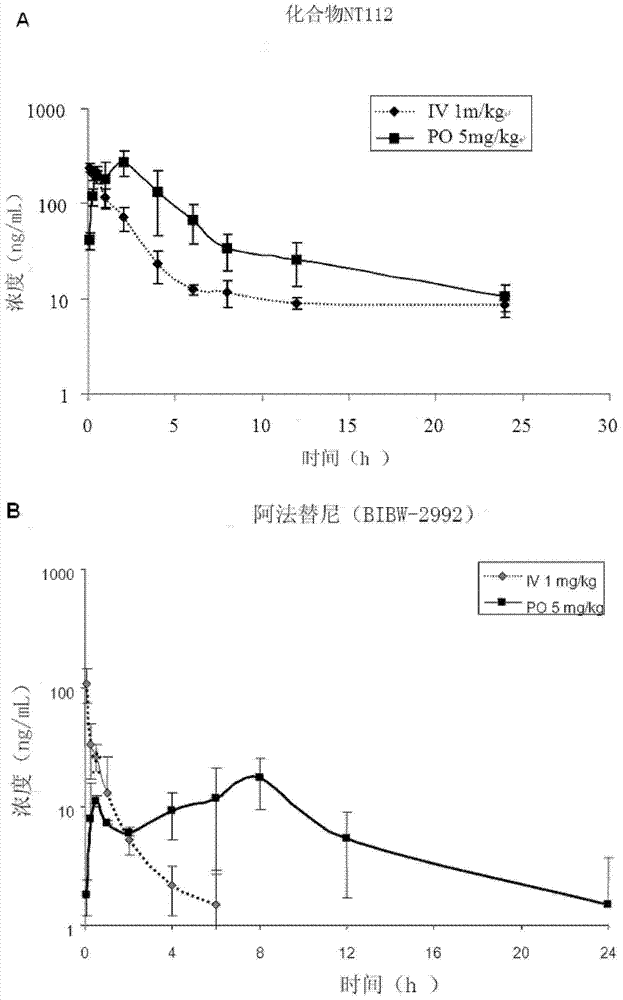
Pharmaceutical composition combining substituted butene amide and mTOR inhibitor as well as application of pharmaceutical composition
ActiveCN111617081APrevent proliferationGood synergyOrganic active ingredientsOrganic chemistryMultiple cancerButenamide
The invention discloses a pharmaceutical composition combining substituted butene amide and a mTOR inhibitor as well as application of the pharmaceutical composition, and particularly relates to a composition or kit containing at least one substituted butene amide or a pharmaceutically acceptable salt or solvate thereof and at least one mTOR inhibitor as well as application of at least one substituted butene amide or a pharmaceutically acceptable salt or solvate and at least one mTOR inhibitor in preparation of a cancer treating medicine or kit. Compared with the prior art, the pharmaceuticalcomposition has the following advantages: the substituted butene amide or the medicinal salt thereof and the mTOR inhibitor have proliferation inhibition effects on multiple cancers, and have significant synergistic effects through combined use.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
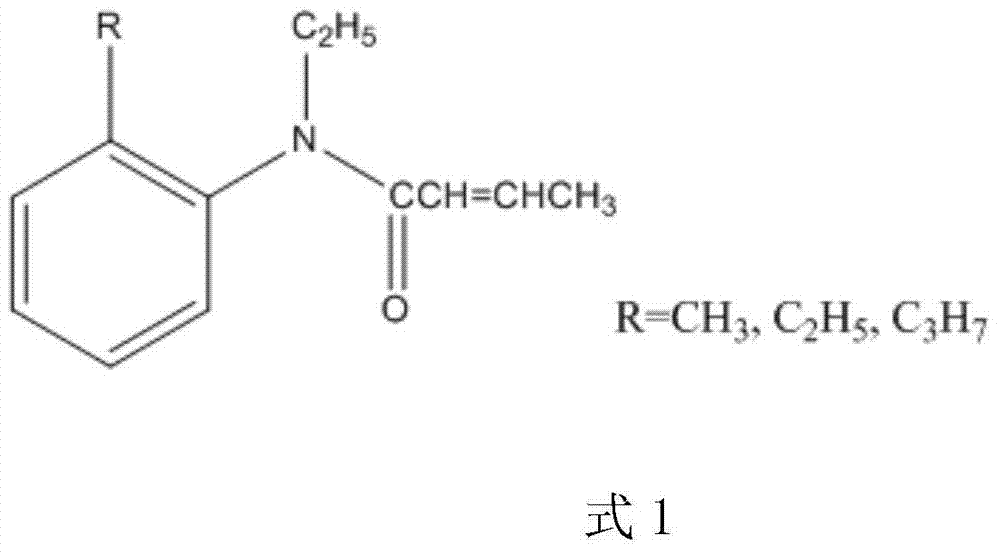
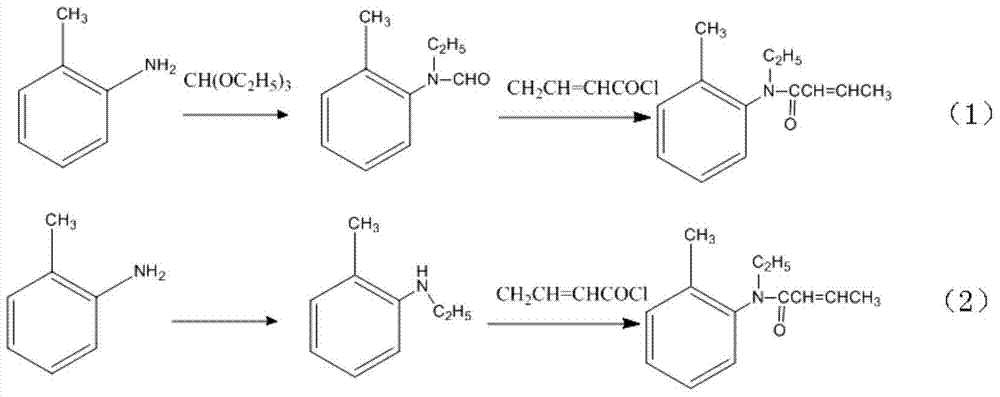
Synthesis method for trans N-ethyl-N-(2'-alkyl phenyl)-2-butenamide
ActiveCN104326936AHigh puritySimple methodOrganic compound preparationCarboxylic acid amides preparationAlkaneDistillation
The invention relates to a synthesis method for trans N-ethyl-N-(2'-alkyl phenyl)-2-butenamide. The method comprises the steps of: 1) adding trans 2- butenoic acid into a reaction bottle, conducting dilution with an alkane solvent, performing cooling to 0-10DEG C, adding thionyl chloride dropwise, and then carrying out reaction at 10-50DEG C for 3-4h for standby use; 2) adding N-ethyl-2-alkyl aniline into the reaction bottle, conducting dilution with an alkane solvent, adding an alkaline aqueous solution, subjecting the mixed solution to stirring reaction at room temperature, adding the mixed solution obtained in step 1) slowly in a dropwise manner, and then carrying out reaction at 60-90DEG C for 5-6h at the end of dropwise adding; and 3) at the end of the reaction, separating out the organic phase, and washing the organic phase to neutral by water, conducting room temperature distillation to remove the solvent, and then performing pressure reduced distillation to separate the product. The method is simple, has high product yield, and can produce the high purity trans-structure product.
Owner:ZHEJIANG HUADIE CHEM
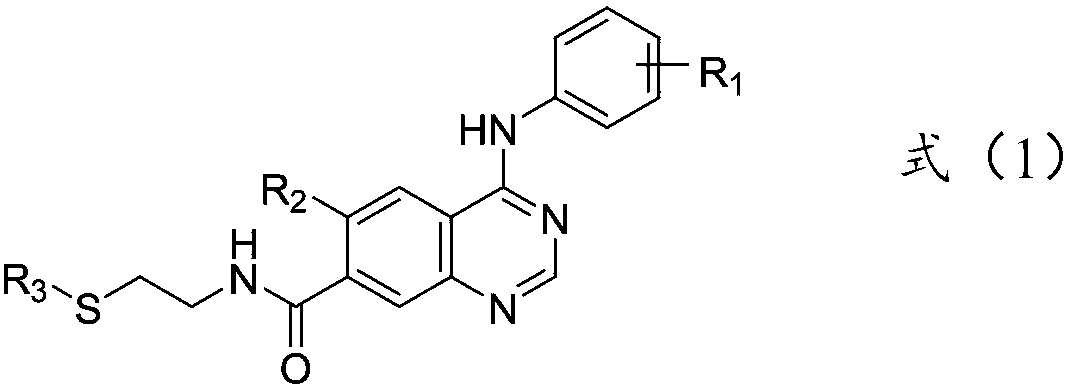
Quinazoline derivative and application thereof
ActiveCN110642796AInhibition selectivityStrong inhibitory activityOrganic active ingredientsNervous disorderMorpholineEthyl group
The invention relates to a quinazoline derivative shown in a formula (1) and a pharmaceutically acceptable salt thereof. In the formula (1), R<1> is selected from one or more halogens, trifluoromethyl, trifluoromethoxy, nitro, cyano, hydroxyl or sulfonyl isopropyl; R<2> is selected from methoxy, ethoxy, 3-methyl ether ethoxy, 4-methylpiperazine ethoxy, 4-(N,N-dimethyl)amino-2-butene acylamino or 2-morpholine ethoxy; and R<3> is selected from methyl, ethyl or acetyl. The quinazoline derivative provided by the invention, pharmaceutically acceptable salts, solvates, stereoisomers and prodrugs ofthe quinazoline derivative all have remarkable EGFR inhibitory activity, have high in-vitro inhibitory activity on EGFR, T790M and C797S drug resistance mutation, and have remarkable curative effectsin treatment of EGFR-related diseases.
Owner:烟台药物研究所
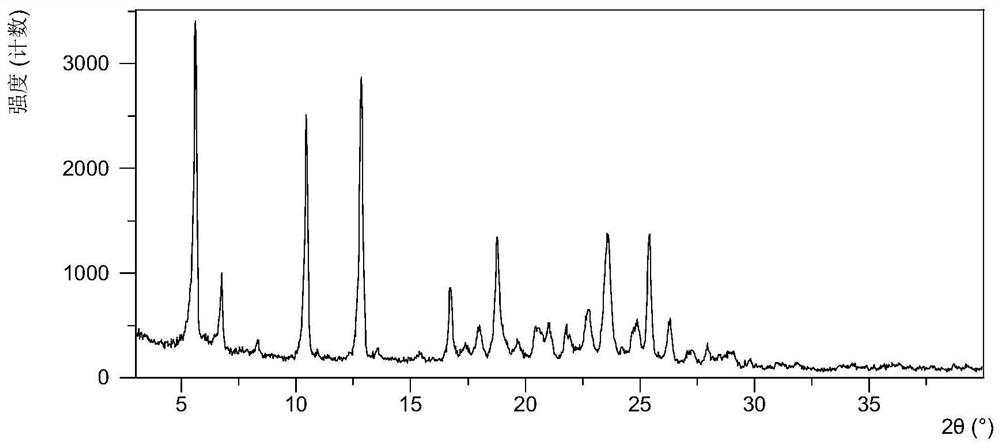
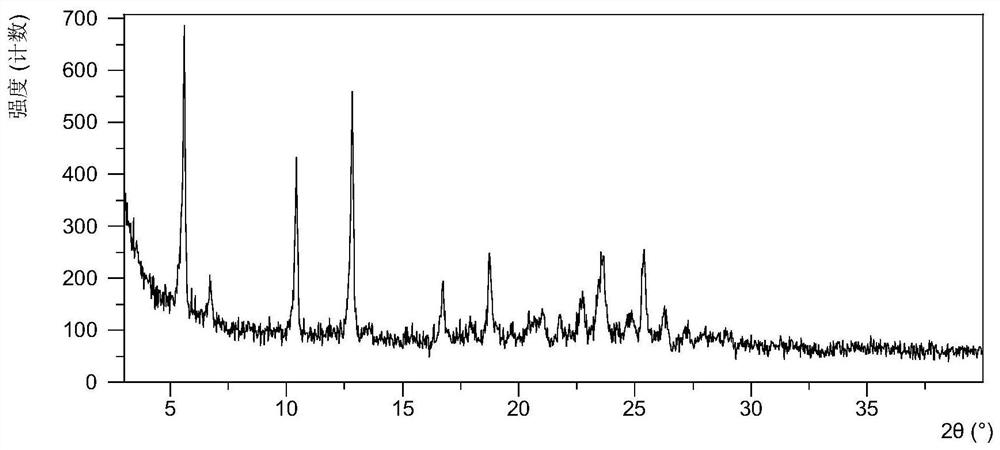
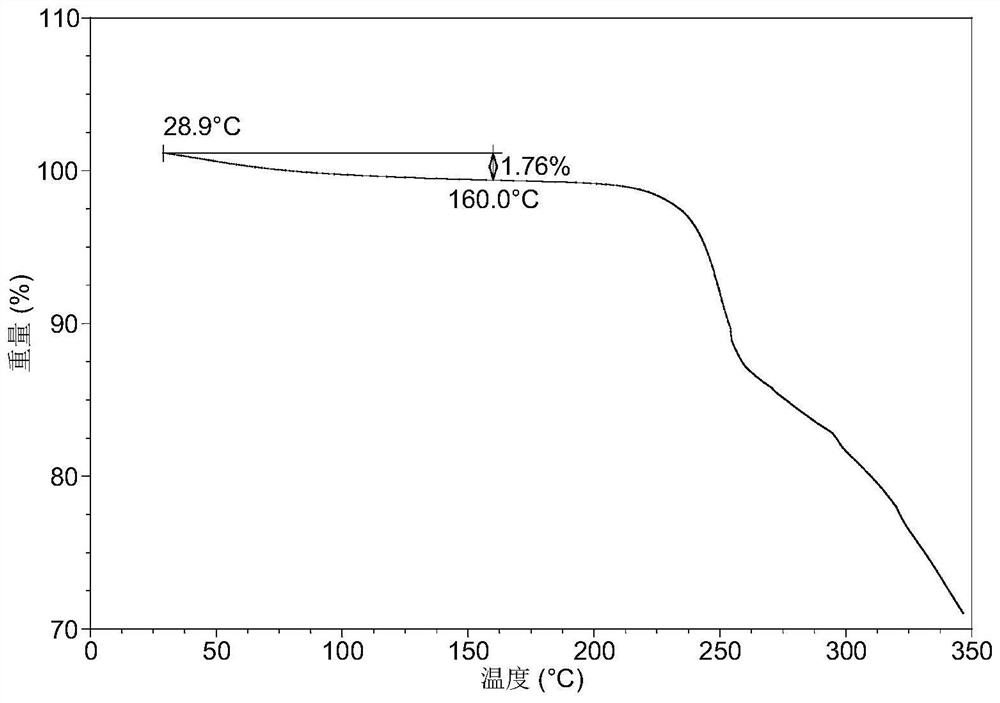
Crystal form of quinazoline compound and preparation method thereof
PendingCN113717111AEfficient selectionImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityPharmaceutical Substances
The invention relates to a crystal form C of (2E)-N-[4-[(3-chloro-4-fluorophenyl) amino]-7-methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-buteneamide, a preparation method thereof and a medicine containing the crystal form C. The crystal form C is an anhydrous substance, Cu-K alpha radiation is used, and the crystal form C has an X-ray powder diffraction pattern with characteristic peaks at diffraction angles 2theta of 5.6 + / - 0.2 degrees, 10.5 + / - 0.2 degrees and 12.8 + / - 0.2 degrees. The crystal form C is the anhydrous substance and has obvious advantages in medicine development compared with an existing solvate, in addition, the solubility of the crystal form C is remarkably improved compared with that of the existing solvate on the premise that good stability is kept.
Owner:CRYSTAL PHARMATECH CO LTD
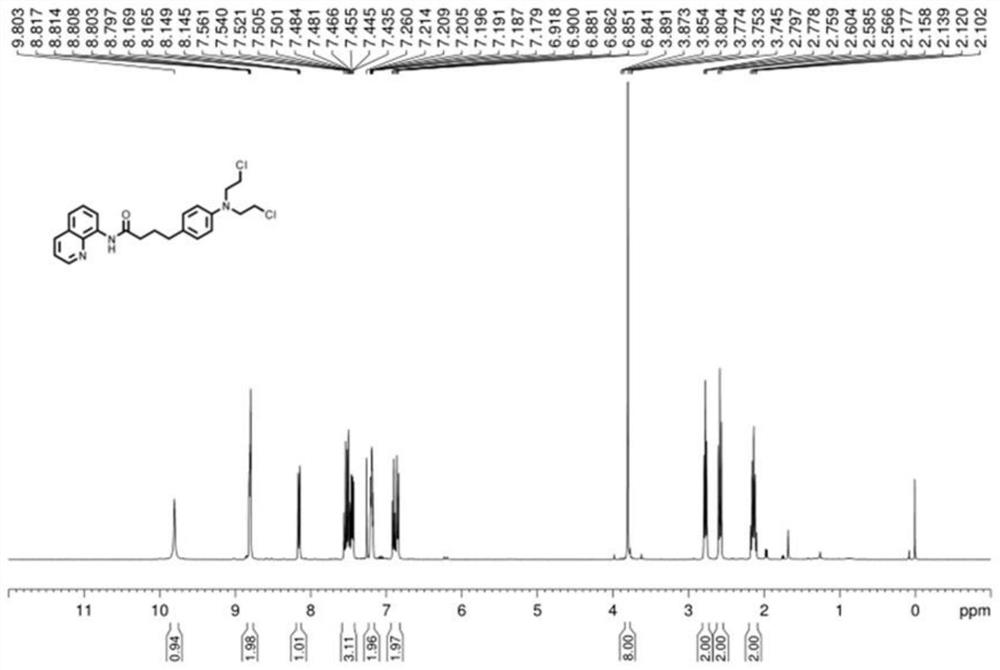
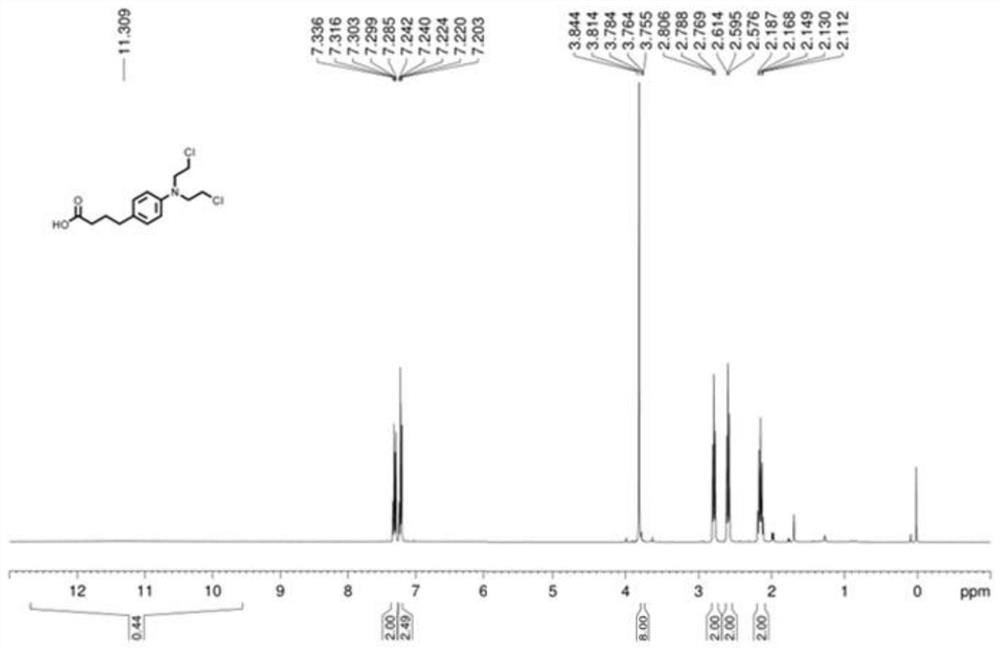
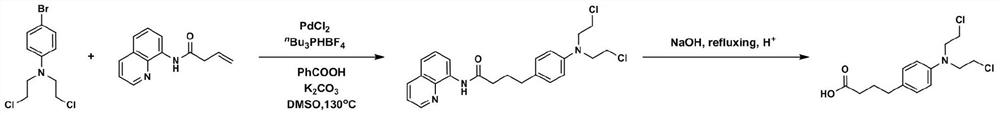
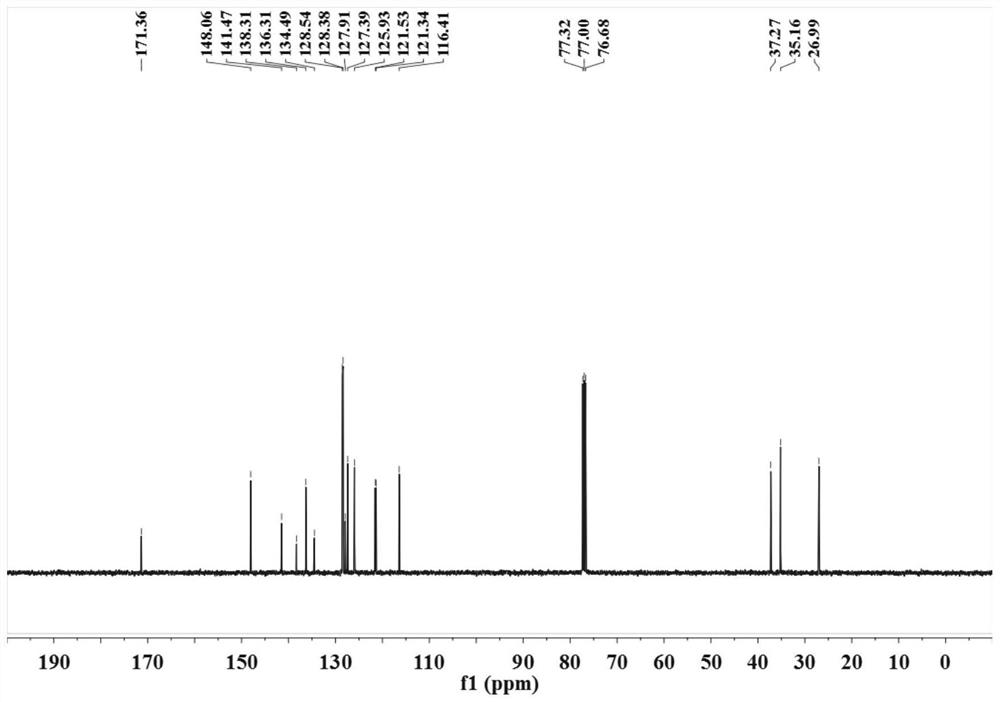
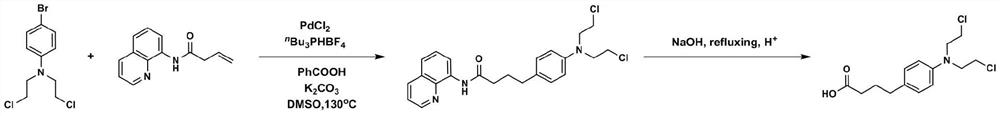
Method for synthesizing chlorambucil
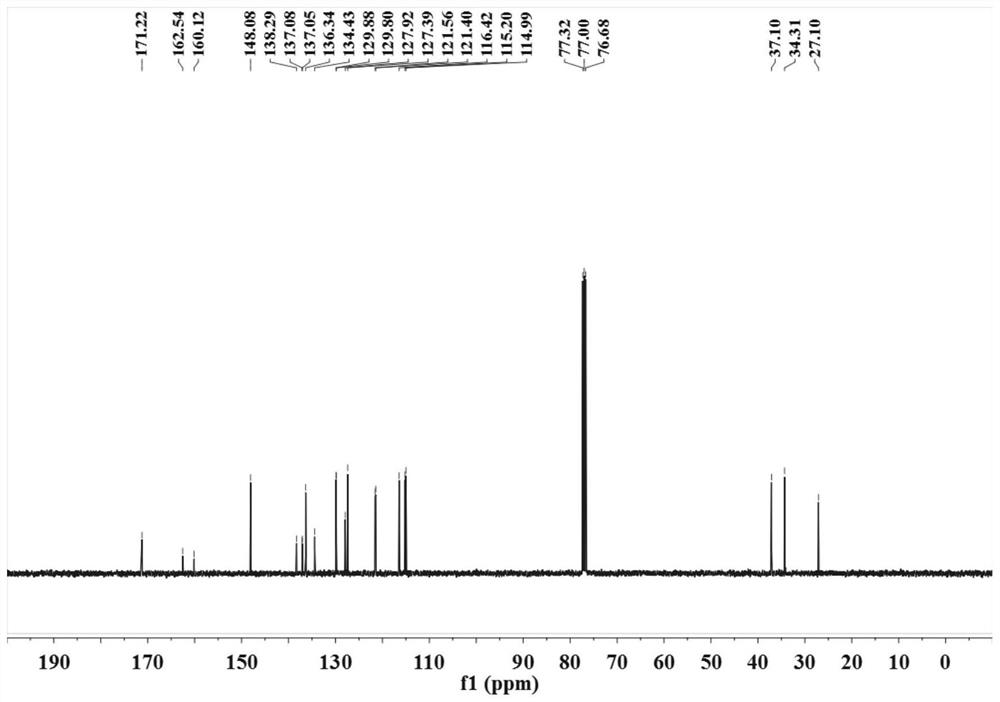
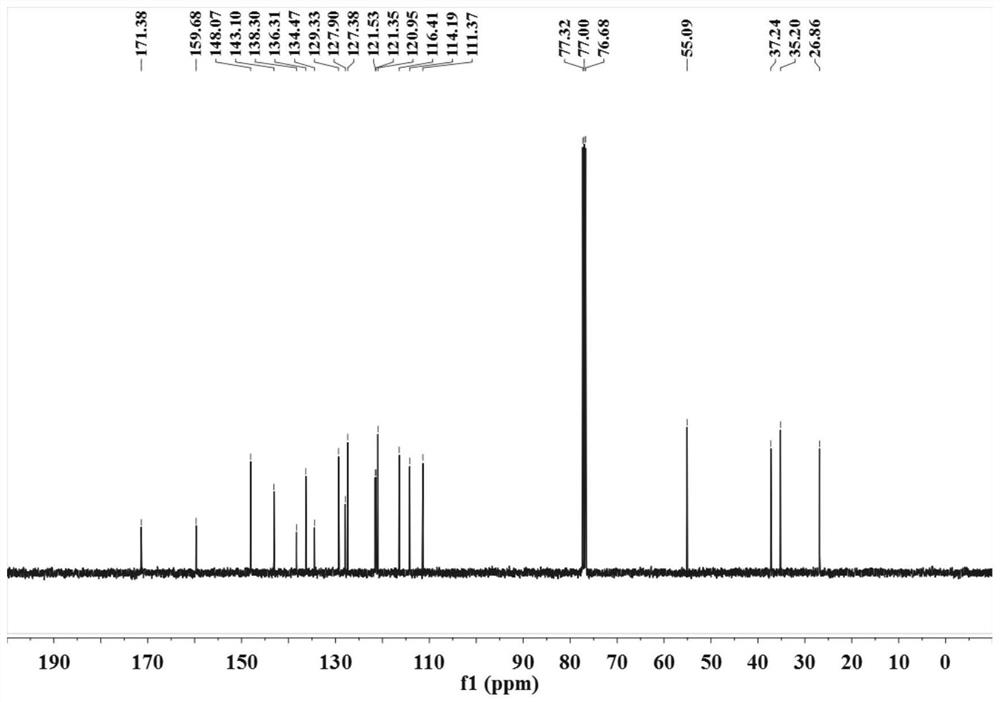
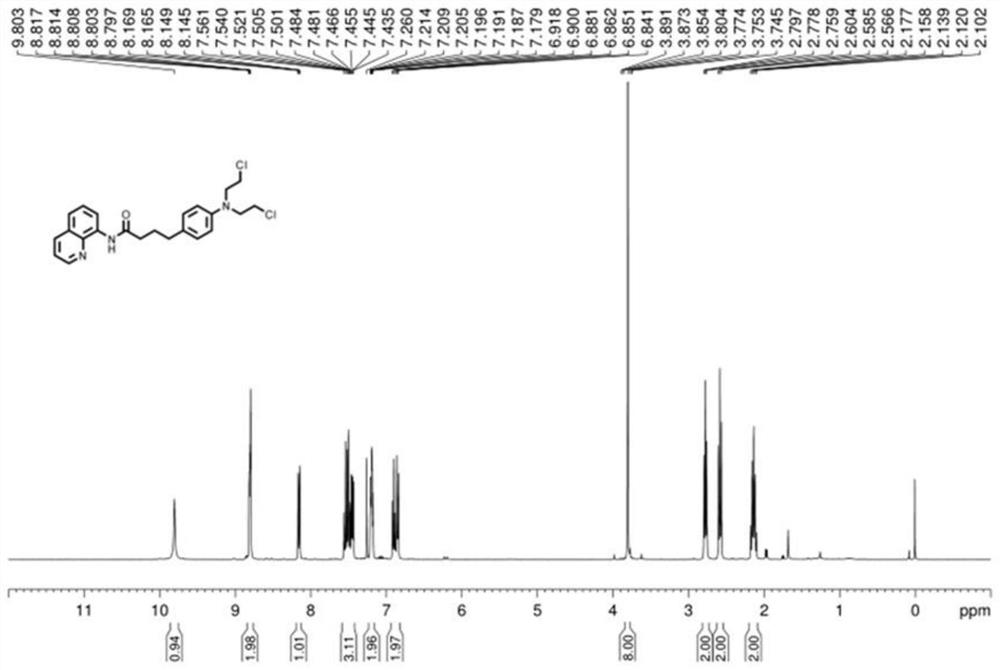
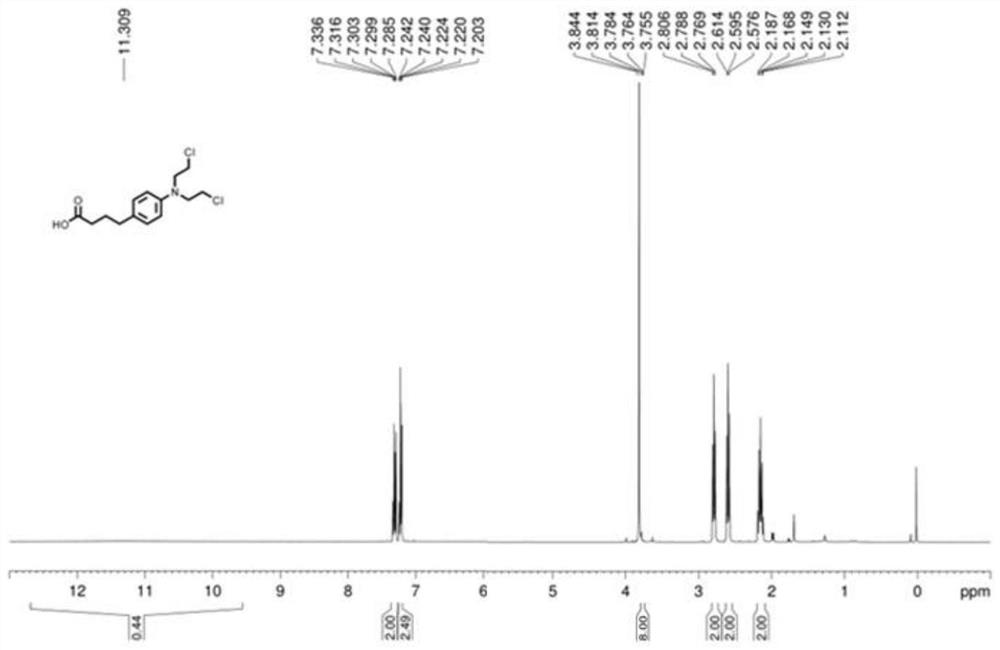
ActiveCN113121373ASolve the problem of too many stepsHigh selectivityOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidTetrafluoroborate
The invention discloses a method for synthesizing chlorambucil, which relates to the technical field of organic chemistry, and comprises the following steps: S1, adding a mixture of 4-bromo-N, N-bis (2-chloroethyl) aniline, N-(quinolin-8-yl) butyl 3-enamide, palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate and benzoic acid at ratio of 10.83%: 10.83%-32.50%: 0.22%-2.17%: 0.43%-2.17%: 10.83%-54.17%: 10.83%-54.17% and dimethyl sulfoxide at molar volume ratio of 0.1 mmol: 2 mL to a reaction vessel for uniform mixing; and S2, placing the reaction container in an oil bath at 135-145 DEG C, and violently stirring to react for 24 hours. According to the invention, through the coupling reaction, 8-aminoquinoline is designed as a compound of a leaving group to control regioselectivity and chemical selectivity in the reaction, so that the problem of excessive steps in the existing synthesis process of chlorambucil is effectively solved; the method has the characteristics of high reaction region selectivity and yield, mild reaction conditions and simple reaction and post-treatment purification processes.
Owner:NANJING TECH UNIV
A simple and efficient method for synthesizing 4-arylbutyric acid derivatives
ActiveCN112142660BSolve the problem of cumbersome steps in the synthesis processHigh yieldOrganic chemistryDiabrezideButenamide
The invention discloses a simple and efficient method for synthesizing 4-arylbutyric acid derivatives. Removable 8-aminoquinoline is used as a guiding group to control the regioselectivity of the reaction, and non-activated 3-butenamide is used. As a reaction raw material, it reacts with cheap, readily available, stable and efficient aryltrimethoxysilane to prepare and synthesize a series of functionalized 4-arylbutyric acid derivatives concisely and efficiently. The method has the advantages of simple operation, simple and easy-to-obtain reaction raw materials, easy separation and purification of products, high product yield and good reaction zone selectivity. The invention can provide related products for treating urea cycle disorder, novel anti-type II diabetes drugs, angiotensin-converting enzyme inhibitors and the like, and has good market potential and application value.
Owner:HUAIYIN TEACHERS COLLEGE
A kind of method of synthesizing chlorambucil
ActiveCN113121373BSolve the problem of too many stepsHigh selectivityOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidTetrafluoroborate
The invention discloses a method for synthesizing chlorambucil, which relates to the technical field of organic chemistry, and comprises the following steps: S1, the proportion content is 10.83%: 10.83% - 32.50%: 0.22% - 2.17%: 0.43% - 2.17%: 10.83%-54.17%: 10.83%-54.17% of 4-bromo-N,N-bis(2-chloroethyl)aniline, N-(quinolin-8-yl)but-3-enamide, Add the mixture made of palladium chloride, tri-n-butylphosphine tetrafluoroborate, potassium carbonate, benzoic acid and dimethyl sulfoxide in a molar volume ratio of 0.1mmol: 2mL to the reaction vessel and mix well; S2. The reaction vessel was placed in an oil bath at 135-145° C. and stirred vigorously for 24 hours. The present invention controls the region and chemoselectivity in the reaction by designing 8-aminoquinoline as a compound with a leaving group through a coupling reaction, effectively solving the problem of too many steps in the synthesis process of the existing chlorambucil problem, and the present invention has the characteristics of high reaction regioselectivity and yield, mild reaction conditions, and simple reaction and post-treatment purification process.
Owner:NANJING TECH UNIV
Granulated product containing antitumor agent
PendingUS20210244733A1Improve stabilityIncrease is suppressedOrganic active ingredientsGranular deliveryCompound aSuccinic acid
An object of the present invention is to provide a granulated product containing a succinate salt of (S,E)-N-(1-((5-(2-((4-cyanophenyl)amino)-4-(propylamino)pyrimidin-5-yl)-4-pentyn-1-yl)amino)-1-oxopropan-2-yl)-4-(dimethylamino)-N-methyl-2-butenamide (hereinafter, referred to as Compound A), which has excellent stability. According to the present invention, there is provided a granulated product containing a succinate salt of (S,E)-N-(1-((5-(2-((4-cyanophenyl)amino)-4-(propylamino)pyrimidin-5-yl)-4-pentyn-1-yl)amino)-1-oxopropan-2-yl)-4-(dimethylamino)-N-methyl-2-butenamide and at least one or more additives of the group consisting of magnesium stearate, sodium stearyl fumarate, and hydrogenated oil.
Owner:FUJIFILM CORP
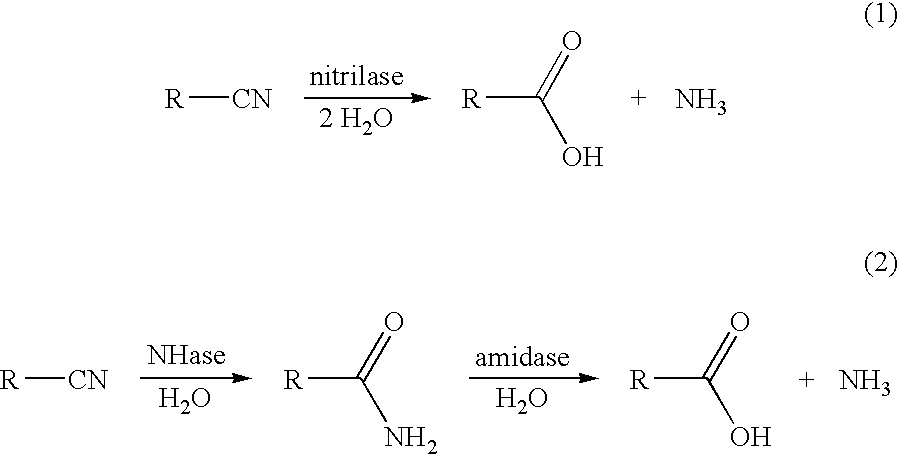
Preparation of (E)- and (Z)-2-methyl-2-butenoic acids
InactiveUS20060115883A1High regional selectivityHigh purityBacteriaHydrolases2 Butenoic AcidsRegioselectivity
A method has been developed to prepare (E)- and (Z)-2-methyl-2-butenoic acids (2M2BA) from a mixture of (E,Z)-2-methyl-2-butenenitriles (2M2BN) by the regioselective hydrolysis of (E)-2M2BN to (E)-2-methyl-2-butenoic acid (2M2BA) using enzyme catalysts having either a nitrilase activity or a combination of nitrile hydratase and amidase activities. The method provides high yields without significant conversion of (Z)-2M2BN to (Z)-2M2BA. The regioselective hydrolysis of (E)-2M2BN to (E)-2M2BA makes possible the facile separation of (E)-2M2BA from (Z)-2M2BN or (Z)-2-methyl-2-butenamide (2M2BAm), and the subsequent conversion of (Z)-2M2BN or (Z)-2M2BAm to (Z)-2M2BA.
Owner:EI DU PONT DE NEMOURS & CO
Synthesis method of sulfur-containing gamma, gamma-bis-arylamine butyramide compound
ActiveCN113861086ASimple processSimple and fast operationOrganic compound preparationChemical recyclingBenzoic acidRotary evaporator
The invention relates to a method for synthesizing a sulfur-containing gamma, gamma-bis-arylamine butyramide compound, which comprises the following steps of: stirring N-(2-(ethylthio)phenyl)butyl-3-acrylamide serving as a reaction substrate, arylamine serving as a nucleophilic reagent, palladium acetate serving as a catalyst, ferric trichloride and ferrous acetate serving as oxidants, benzoic acid serving as an additive and acetonitrile serving as a solvent, at 90 DEG C to react for 24 hours; and after the reaction is finished, filtering the reaction liquid, removing the solvent from the filtrate by using a rotary evaporator to obtain a residue, carrying out column chromatography separation on the residue by using a silica gel column, leaching the residue by using an eluent, collecting effluent containing a target product, combining the effluent, and carrying out vacuum concentration to remove the solvent to obtain the target product. The method has the advantages of simple and easily available raw materials, relatively mild reaction conditions, novel and simple preparation process, less pollution and low energy consumption.
Owner:WENZHOU UNIVERSITY
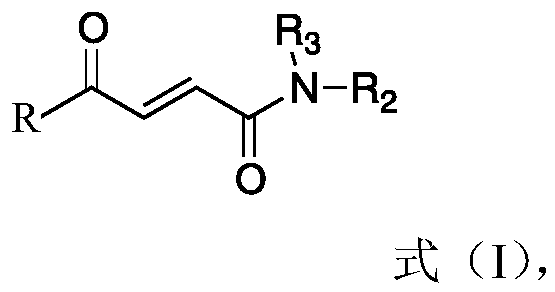
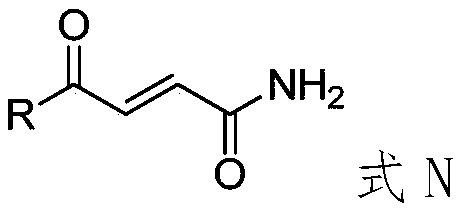
Application of 4-oxo-2-butenamide derivatives in the preparation of antibacterial agents
ActiveCN106860441BAntibacterial agentsOrganic compound preparationMethicillin sensitiveMethicillin-resistant Staphylococcus epidermidis
The invention discloses application of a 4-oxo-2-crotonamide derivative to preparation of bacteriostatic agents. The structure of the 4-oxo-2-crotonamide derivative is shown as a formula (I). The 4-oxo-2-crotonamide derivative has a bacteriostatic effect; good antibacterial activity can be realized on methicillin-resistant staphylococcus aureus, methicillin-resistant staphylococcus epidermidis, vancomycin drug-resistant enterococcus faecalis, vancomycin drug-resistant enterococcus faecium, methicillin-sensitive staphylococcus aureus, methicillin-sensitive staphylococcus epidermidis, vancomycin-sensitive enterococcus faecalis and vancomycin-sensitive enterococcus faecium. The formula I is shown in the description.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
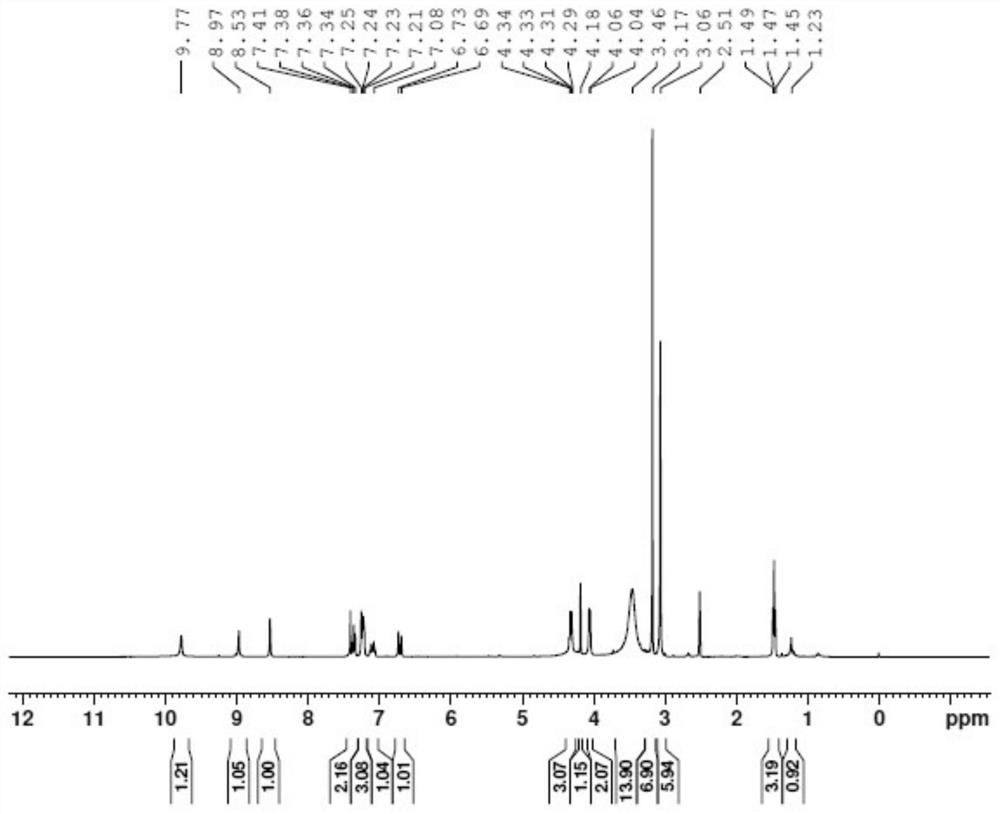
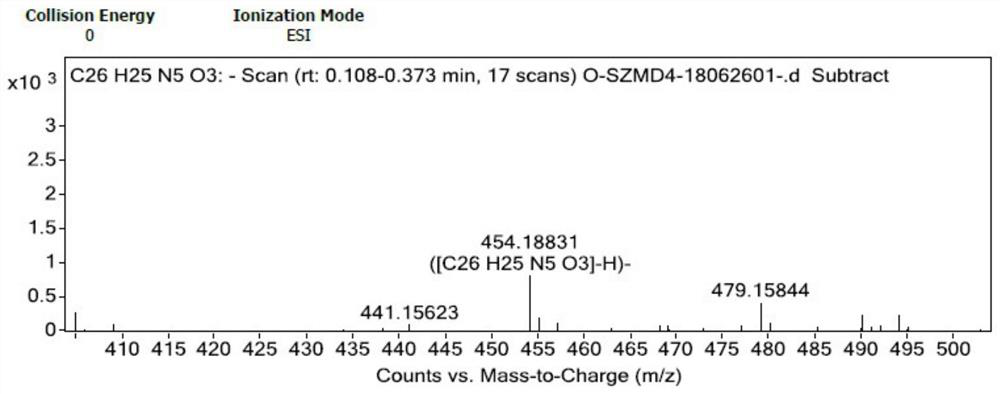
Substituted butenamide-N-oxide and preparation method and application thereof
PendingCN113493413AGood anticancer effectEasy to operateOrganic active ingredientsOrganic chemistryAnticarcinogenic EffectQuinoline
The invention discloses a substituted butenamide-N-oxide as well as a preparation method and application thereof. The compound is prepared by carrying out oxidation reaction on a compound (E)-N-[4-(3-ethynylphenyl) amino-3-cyano-7-ethoxyquinoline-6-yl]-4-(dimethylamino) butyl-2-alkenyl amide or a salt thereof in a solvent. A pharmaceutical composition containing the compound can be used as a targeted drug for treating patients with tumors. Compared with the prior art, the N-oxide has a good anti-cancer effect, the application prospect of an anti-cancer treatment drug is increased, the process preparation and operation are relatively simple, the reaction time is short, few three wastes are generated, and the process is environmentally friendly.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
Cathepsin C inhibitors
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXO GRP LTD
Antitumor agent for acute myeloid leukemia
The present invention addresses the problem of providing an antitumor agent for acute myeloid leukemia, the antitumor agent exhibiting practical effects on acute myeloid leukemia. According to the present invention, provided is an antitumor agent for acute myeloid leukemia, the antitumor agent containing a compound, such as (S,E)-N-(1-((5-(2-((4-cyanophenyl)amino)-4-(propylamino)pyrimidin-5-yl)-4-pentyn-1-yl)amino)-1-oxopropan-2-yl)-4-(dimethylamino)-N-methyl-2-butenamide, represented by general formula [1] specified in the present specification, or a salt thereof, wherein the amount of the antitumor agent administered per dose is within a predetermined range.
Owner:FUJIFILM CORP
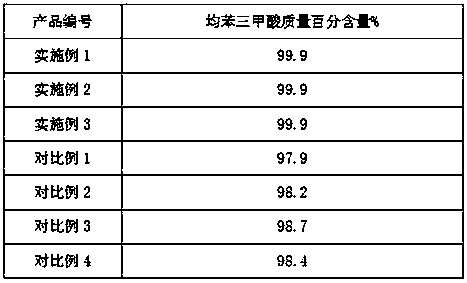
Preparation method for refined material of crude trimesic acid
InactiveCN108187637AImprove corrosion resistanceImprove heat resistanceOther chemical processesAlkali metal oxides/hydroxidesFuranWax
The invention relates to a preparation method for a refined material of crude trimesic acid. The preparation method comprises the following steps: adding, by weight, maleic anhydride, polyvinyl alcohol, 1,5,9,13-tetradeca-tetraene, 3-isopentyl(2-methyl-3-furyl)disulfide, furan-3-ylethynyltrimethylsilane, N-hydroxy-5-norbornene-2,3-dicarboximide perfluorobutylsulfonate, (2Z)-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide, benzoyl peroxide, liquid wax and water into a reaction kettle, and carrying out uniform mixing under stirring; then carrying out heating for a reaction; after completion of the reaction, carrying out washing with gasoline so as to remove the liquid wax; and then successively carrying out washing with water and drying to obtain the refined material of crude trimesic acid.
Owner:孝感市锐思新材科技有限公司
Cathepsin c inhibitors
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Maleate salts of (E)-N-(3-cyano-7-ethoxy-4-((4-phenoxyphenyl)amino) quinolin-6-yl)-4-(dimethylamino)but-2-enamide and crystalline forms thereof
The present application relates to maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)aniline]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide, crystalline forms thereof, to processes for its preparation, to pharmaceutical compositions comprising it and to its use in the control of disorders.
Owner:TELIGENE LTD
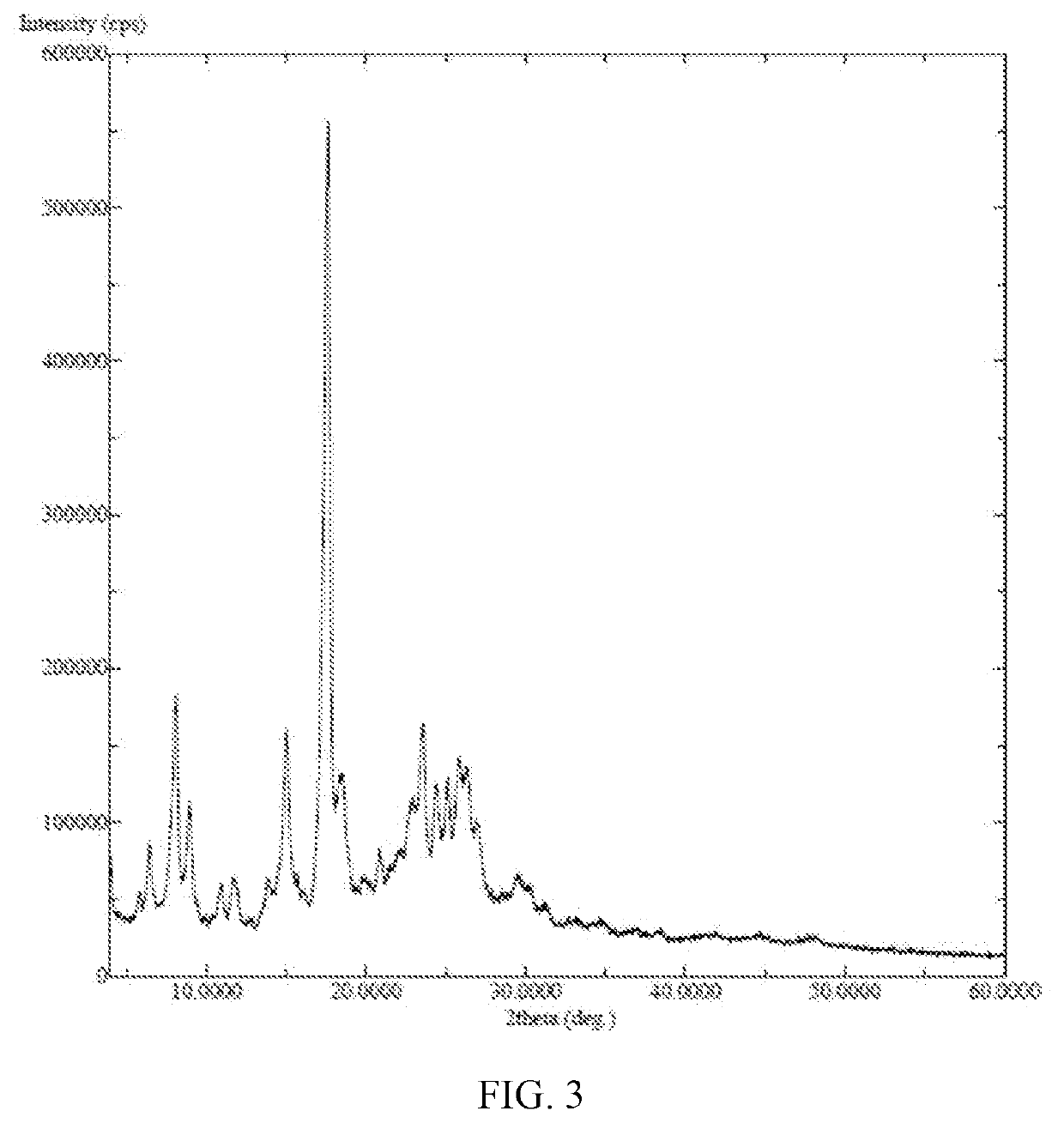
PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF
ActiveUS20180297989A1Easy to handleOrganic chemistryCrystallization auxillary selectionFuranPolymer science
The present invention relates to an improved process for the preparation of N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) represented by the following structural formula:
Owner:MSN LAB PTE LTD
A kind of preparation method of EGFR molecular targeted anti-tumor drug
ActiveCN111875539BRaw materials are easy to getSimple processOrganic chemistryAntineoplastic agentsQuinolinePharmaceutical Substances
The invention discloses a preparation method of an EGFR molecule-targeted antitumor drug, and the EGFR molecule-targeted antitumor drug is (E)-N-[4-(3-ethynylphenyl)amino-3- Cyano-7-ethoxy-6-quinolyl]-4-(dimethylamino)-2-butenamide. The preparation method of the EGFR molecular targeting antineoplastic drug comprises the following steps: N-(4-chloro-3-cyano-7-ethoxyquinoline-6-yl) acetamide and m-aminophenylacetylene Under the condition of n-propanol as reaction solvent, heating substitution reaction was carried out. The method has the advantages of easy-to-obtain raw materials, simple process, short reaction time, low impurity content, high yield, and suitability for industrialized production.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof](https://images-eureka.patsnap.com/patent_img/8c7c36da-d7d9-4031-a8c0-e4932a79e5d2/US08022216-20110920-D00001.png)
![Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof](https://images-eureka.patsnap.com/patent_img/8c7c36da-d7d9-4031-a8c0-e4932a79e5d2/US08022216-20110920-D00002.png)
![Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof Maleate salts of (E)-N-{4-[3-chloro-4-(2-pyridinylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide and crystalline forms thereof](https://images-eureka.patsnap.com/patent_img/8c7c36da-d7d9-4031-a8c0-e4932a79e5d2/US08022216-20110920-D00003.png)

![Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof](https://images-eureka.patsnap.com/patent_img/961d9db9-b5f7-48b5-9302-2478e8391503/US10550107-D00001.png)
![Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof](https://images-eureka.patsnap.com/patent_img/961d9db9-b5f7-48b5-9302-2478e8391503/US10550107-D00002.png)
![Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof Process for the preparation of N-[4-[(3-chloro-4-fluoro phenyl) amino]-7-[[(3s-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethyl amino)-(2E)-2-butenamide (2Z)-2-butenedioate (1:2) and its polymorphs thereof](https://images-eureka.patsnap.com/patent_img/961d9db9-b5f7-48b5-9302-2478e8391503/US10550107-D00003.png)



![PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF](https://images-eureka.patsnap.com/patent_img/98792f57-15f1-4e3f-bef4-8939abd3f8a0/US20180297989A1-D00000.png)
![PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF](https://images-eureka.patsnap.com/patent_img/98792f57-15f1-4e3f-bef4-8939abd3f8a0/US20180297989A1-D00001.png)
![PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF PROCESS FOR THE PREPARATION OF N-[4-[(3-CHLORO-4-FLUORO PHENYL) AMINO]-7-[[(3s-TETRAHYDRO-3-FURANYL]OXY]-6-QUINAZOLINYL]-4-(DIMETHYL AMINO)-(2E)-2-BUTENAMIDE (2Z)-2-BUTENEDIOATE (1 :2) AND ITS POLYMORPHS THEREOF](https://images-eureka.patsnap.com/patent_img/98792f57-15f1-4e3f-bef4-8939abd3f8a0/US20180297989A1-D00002.png)