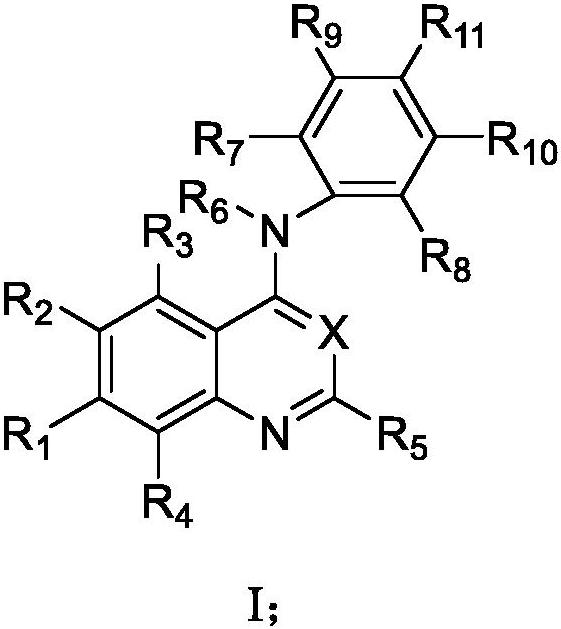
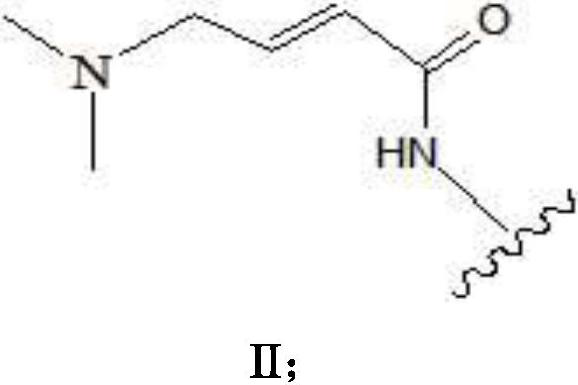
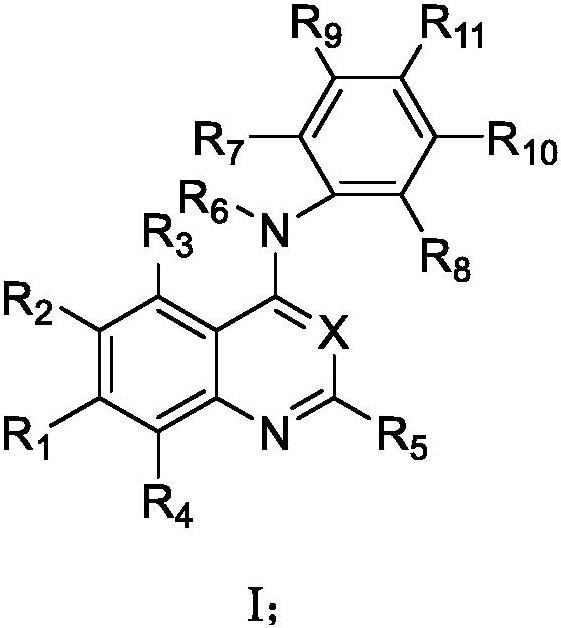
Pharmaceutical composition combining substituted butene amide and mTOR inhibitor as well as application of pharmaceutical composition
A technology of crotene amide and composition, which is applied in the direction of drug combination, antineoplastic drugs, and pharmaceutical formulations, and can solve problems such as lack of effective means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 The pharmacodynamic effect of compound SZMD4-mal administered alone and in combination with rapamycin on EGFR-L858R, EGFR-T790M mutated human lung cancer cell NCL-H1975 subcutaneous tumorigenic model nude mice
[0114] The SZMD4-mal involved in this example is (E)-N-(3-cyano-7-ethoxy-4-(3-ethynylphenylamino)quinolin-6-yl)-4-(di Methylamino)but-2-enamide maleate monohydrate.
[0115] 1. Experimental animals
[0116] Mice, BALB / c-nu / nu nude mice, female, 4-6 weeks old, 17-22g. Breeding environment: SPF grade.
[0117] 2. Test drug
[0118] Drug name: SZMD4-mal; rapamycin (Repamycin).
[0119] Preparation method: SZMD4-mal, under sterile conditions, add sterile deionized water to grind and mix, then add deionized water to the required volume, shake well, prepare once a week and store in a 4°C refrigerator, take it out before use to room temperature, fully vortexed before administration; rapamycin, aseptically ground in an agate mortar with saline containing ...
Embodiment 2
[0149] Example 2 The pharmacodynamic effect of compound SZMD4-mal administered alone and in combination with rapamycin on EGFR and HER2 positive and K-ras mutated human lung cancer cell A549 subcutaneous tumorigenic model nude mice
[0150] The SZMD4-mal involved in this example is (E)N-(3-cyano-7-ethoxy-4-(3-ethynylphenylamino)quinolin-6-yl)-4-(dimethyl (amino)but-2-enamide maleate monohydrate.
[0151] 1. Experimental animals
[0152] Mice, BALB / c-nu / nu nude mice, female, 4-6 weeks old, 17-22g. Breeding environment: SPF grade.
[0153] 2. Test drug
[0154] Drug name: SZMD4-mal; rapamycin (Repamycin).
[0155] Preparation method: SZMD4-mal, under sterile conditions, add sterile deionized water to grind and mix, then add deionized water to the required volume, shake well, prepare once a week and store in a 4°C refrigerator, take it out before use To room temperature, fully vortex and mix before administration; rapamycin, prepared as a solution with physiological saline u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tumor inhibition rate | aaaaa | aaaaa |
| Tumor inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com