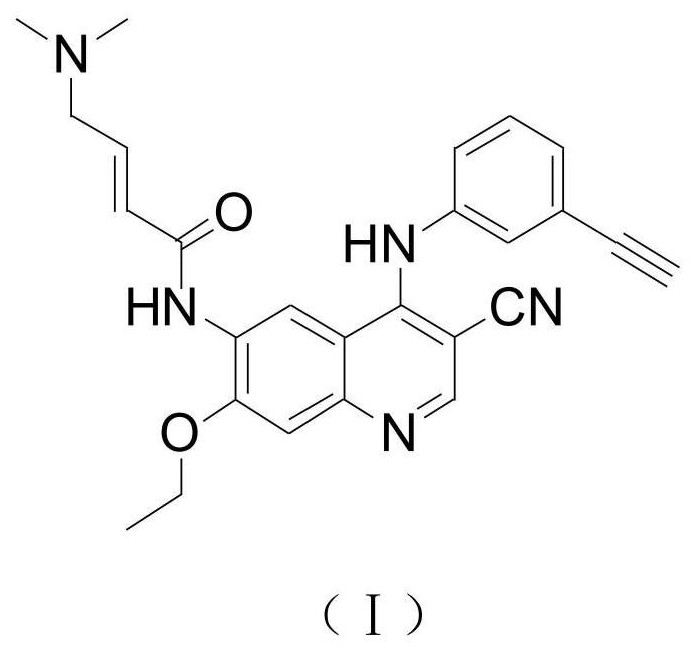
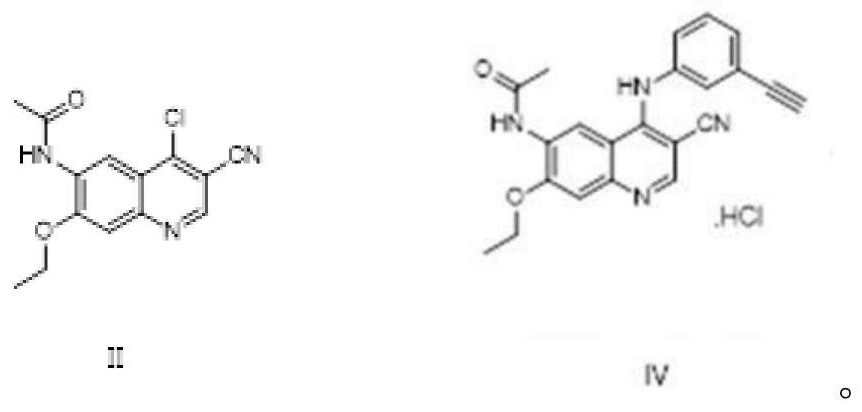
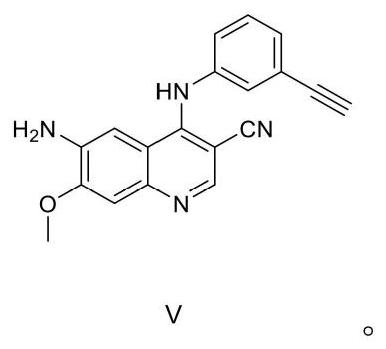
A kind of preparation method of EGFR molecular targeted anti-tumor drug
A cyano- and ethoxy-based technology, which is applied in antineoplastic drugs, drug combinations, organic chemistry, etc., can solve the problems of high impurity content, difficult quality control, and low conversion rate of Compound IV, and achieve easy-to-obtain raw materials and reduce The content of impurities and the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0065] Example 1-1: Preparation of Compound IV
[0066] In the 50L reaction kettle, add 1.155kg N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide, 18.48kg n-propanol, stir evenly; add 0.52kg m-aminophenylacetylene (molar ratio of compound III and compound II is: 1.1:1); be warmed up to 90 ℃~95 ℃, react 3 hours to reach the end point, cool down to 0 ℃~5 ℃, centrifuge, the solid is washed with n-propanol, and dried to obtain the product 1.544kg, in the form of hydrochloride, the yield is 96%, and the HPLC test content is 99.58%.
Embodiment 1-2
[0067] Example 1-2: Preparation of Compound IV
[0068] In the 50L reaction kettle, add 1.500kg N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide, 18.00kg of n-propanol, stir evenly; add 0.789kg m-aminophenylacetylene (The molar ratio of compound III to compound II is 1.3:1); the temperature is raised to 85°C to 90°C, the reaction reaches the end point in 3 hours, the temperature is lowered to 10°C to 15°C, centrifuged, and the solid is washed with n-propanol and dried to obtain the product 2.015kg, the content is 99.46%, and the yield is 96%.
Embodiment 1-3
[0069] Preparation of Example 1-3 Compound IV:
[0070] In the 50L reaction kettle, add 1.00kg N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide, 15.00kg n-propanol, stir evenly; add 0.325kg m-aminophenylacetylene (molar ratio of compound III and compound II is: 0.8:1); be warmed up to boiling reflux, reacted to reach the end point in 2.7 hours, cooled to 0 ℃~10 ℃, centrifuged, and the solid was washed with n-propanol, and dried to obtain 1.336kg of product, The yield is 95%, and the content is 99.53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com