Crystal form of quinazoline compound and preparation method thereof
A quinazoline-based, crystal-form technology, applied in the field of drug crystals, can solve the problems of affecting drug efficacy, restricting dissolution and bioavailability, and low solubility, achieving good bioavailability, improving drug efficacy, and low hygroscopicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Weigh about 20 mg of (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-4-(1-piper (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7- Methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide was heated (heating rate was 10°C / min) to about 180°C, then naturally cooled to room temperature (approx. 25°C) to obtain a solid product.
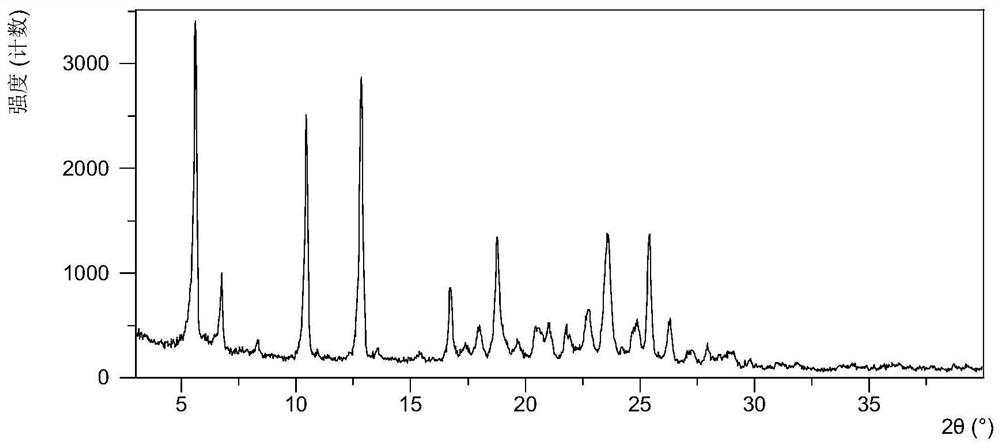
[0083] After testing, the X-ray powder diffraction data of the solid obtained in this embodiment are shown in Table 1, and its XRPD pattern is as follows figure 1 As shown, the results show that the obtained solid product is the crystal form C described in the application.
[0084] Table 1
[0085] Numbering Diffraction angle 2θ(±0.2°) d value Relative Strength% 1 5.62 15.72 100.00 2 6.76 13.09 23.66 3 8.33 10.61 4.71 4 10.46 8.46 73.12 5 12.85 6.89 85.63 6 13.56 6.53 3.45 7 15.40 5.75 2.31 8 16.74 5.30 22.67 9 17.41 5.09 4.91 10 18.04 4.92 10.20...
Embodiment 2
[0087] Weigh about 20 mg of (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-4-(1-piper (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7- Methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide was heated (heating rate: 10°C / min) to 180°C, then naturally cooled to room temperature (about 25°C ) to obtain a solid product, parallel test 20 times, the solid product obtained in 20 times was mixed homogeneously, and sampling was detected.
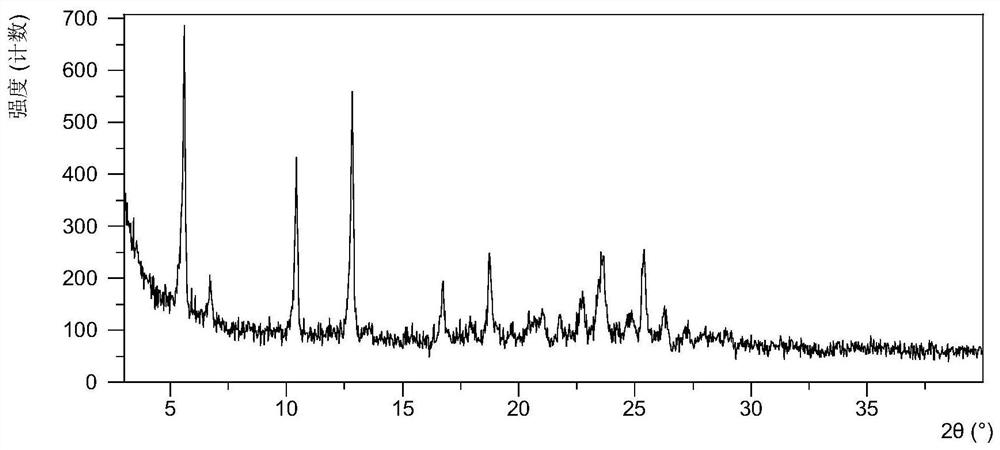
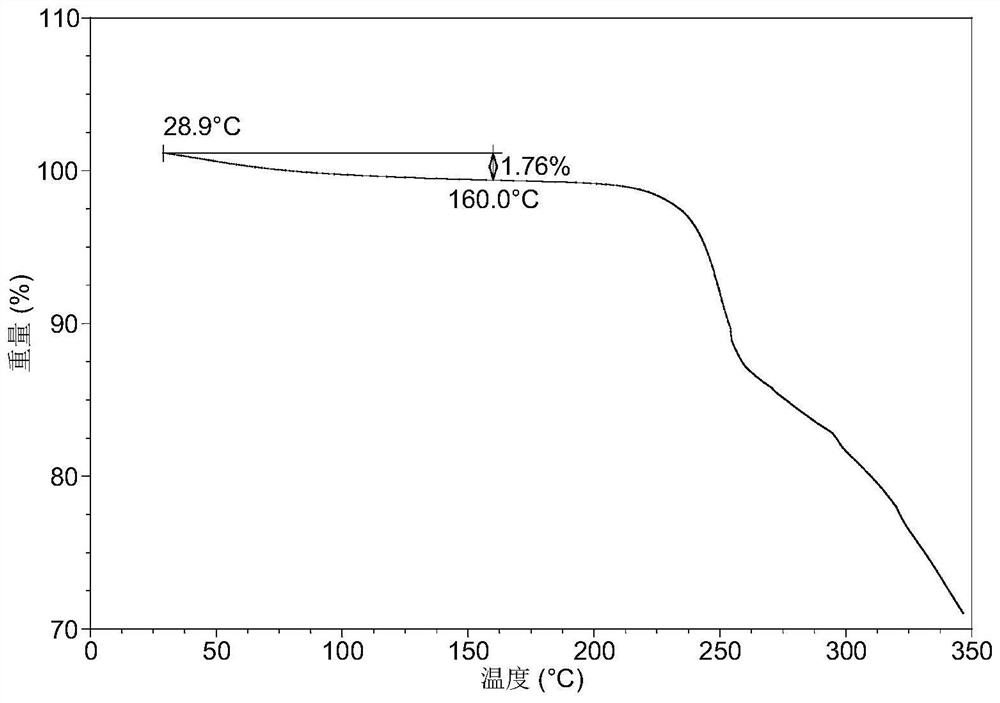
[0088] After testing, the X-ray powder diffraction data of the resulting solid product are shown in Table 2, and its XRPD pattern is as follows figure 2 As shown, the TGA image as image 3 As shown, the DSC image is as Figure 4 As shown, the results show that the obtained solid product is the crystalline form C described in this application, and the TGA data shows that the crystalline form sample loses about 1.8% in weight when heated to 160°C, and there is a single melting endothermic peak at 167°C to 200°C in DSC, It shows...
Embodiment 3
[0092] Weigh about 1 gram of (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-4-(1-piper Pyridyl)-2-butenamide solid is placed in a vacuum drying oven, and (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy Base-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide was heated to 170°C, and then naturally cooled to room temperature (about 25°C) to obtain a solid product, which was sampled for detection.
[0093] After testing, the X-ray powder diffraction data of the resulting solid product are shown in Table 3, and its XRPD pattern is as follows Figure 5 As shown, the results show that the obtained solid product is the crystal form C described in the application, and the crystal form is (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-7-form Oxy-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide anhydrate.
[0094] table 3
[0095] Numbering Diffraction angle 2θ(±0.2°) d value Relative Strength% 1 5.59 15.82 100.00 2 6.72 13.16 15.49 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com