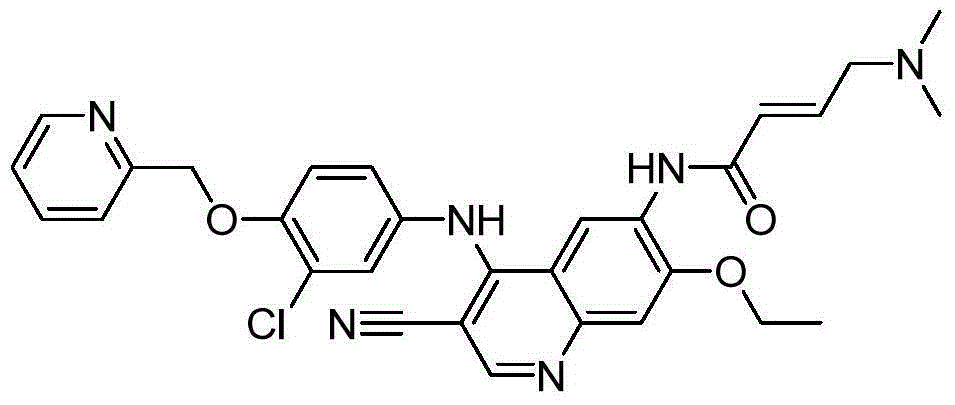
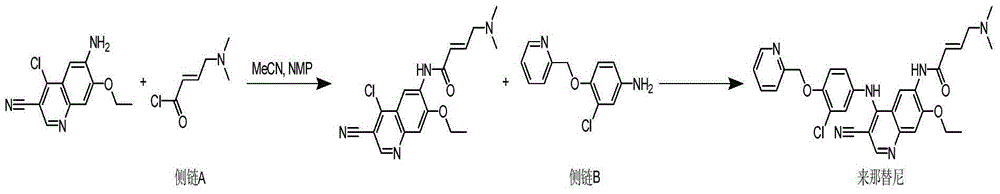
Preparation method of neratinib
A neratinib and ethoxy technology, which is applied in the field of preparation of neratinib, can solve the problems of high temperature and long time reflux, high cost, waste of energy, etc., and achieve improved safety, reduced industrial cost, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
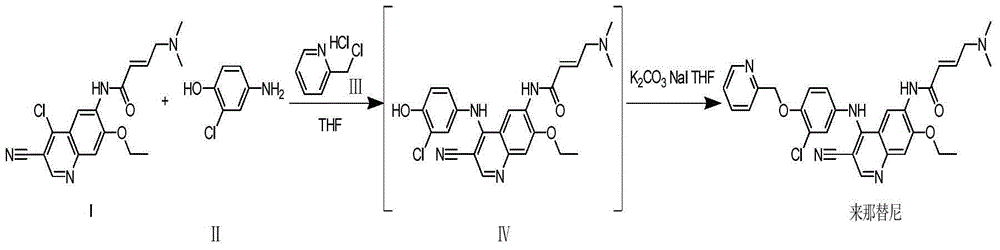
[0034] The present embodiment is the preparation method of Neratinib, which specifically includes the following steps:
[0035] (1) Add 2.5 L of tetrahydrofuran into a three-necked flask, add 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3- Quinolinecarbonitrile (I) (358.82g, 1mol, 1.00eq) and 2-chloro-4-aminophenol (II) (143.57g, 1moL, 1eq) were replaced with nitrogen three times, and the temperature of the reaction system was raised to 65°C. Dichloromethylpyridine hydrochloride (Ⅲ) (237.70g, 1.46moL, 1.05eq) dissolved in 1L tetrahydrofuran was added dropwise for a total of 4 hours. After the addition was completed, the reaction was incubated for 2 hours. HPLC monitored 6-[( E) -4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-quinolinecarbonitrile (I)≤0.3%;
[0036] (2) Cool the reaction system to room temperature, add potassium carbonate (553.48g, 4moL, 4eq) and potassium iodide (16.69g, 0.1moL, 0.1eq), raise the temperature of the reaction system to 60 ° C, ...
Embodiment 2
[0039] The present embodiment is the preparation method of Neratinib, which specifically includes the following steps:
[0040] (1) Add 3.0 L of dimethyl sulfoxide to a three-necked flask, add 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro -3-quinolinecarbonitrile (I) (358.72g, 1mol, 1.00eq) and 2-chloro-4-aminophenol (II) (143.62g, 1moL, 1eq), nitrogen replacement three times, the reaction system was heated to 60 ℃, add dichloromethylpyridine hydrochloride (Ⅲ) (172.23g, 1.05moL, 1.05eq) dissolved in 1L of dimethyl sulfoxide dropwise at a constant speed, add dropwise for a total of 4 hours, and keep warm for 2 hours after the dropwise addition is completed. HPLC monitoring 6-[(E)-4-(dimethylamino)-2-butenamido]-7-ethoxy-4-chloro-3-quinolinecarbonitrile (I)≤0.3%;
[0041] (2) The reaction system was cooled to room temperature, sodium hydroxide (168.91g, 4moL, 4eq) and sodium iodide (14.89g, 0.1moL, 0.1eq) were added, the temperature of the reaction system was raised ...
Embodiment 3
[0044] The difference between the present embodiment and Example 1 lies in the following two points: the dropwise addition time of dichloromethylpyridine hydrochloride (Ⅲ) dissolved in 1L tetrahydrofuran in step (1) is 5 hours; in step (2), the reaction The temperature of the system was raised to 55°C.
[0045] In this example, the yield of neratinib was 80.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com