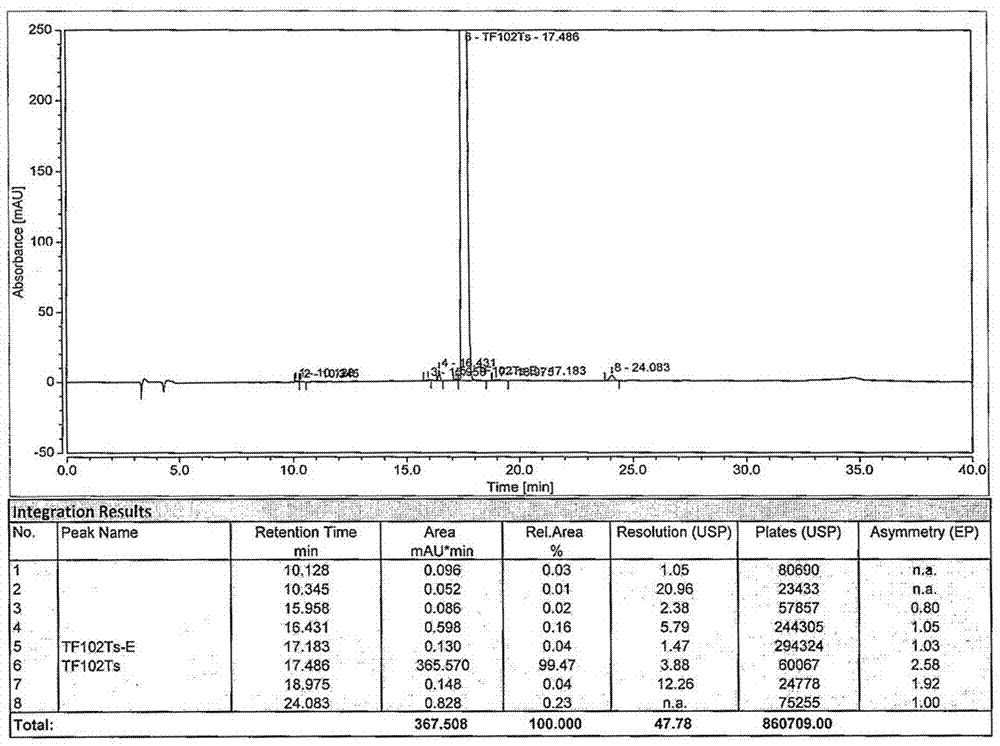
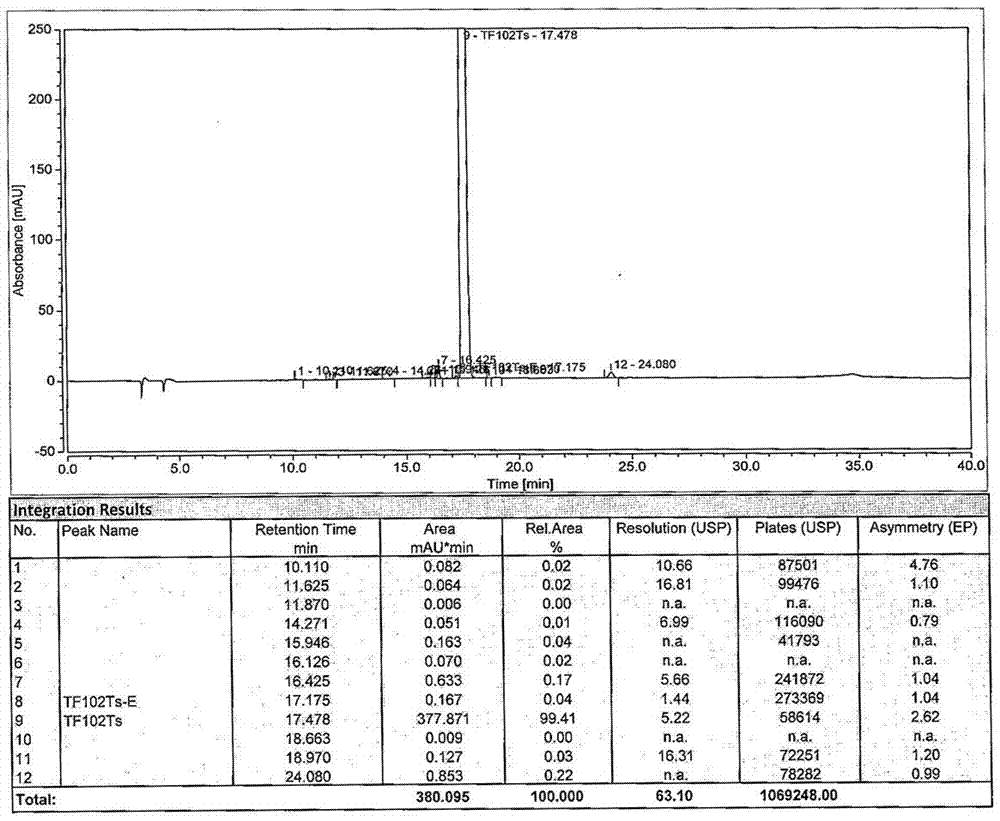
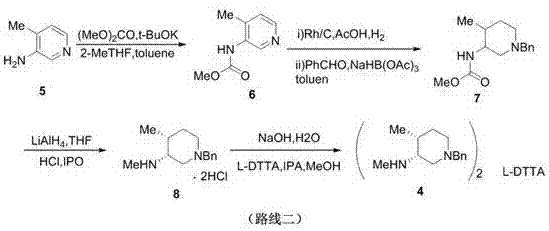
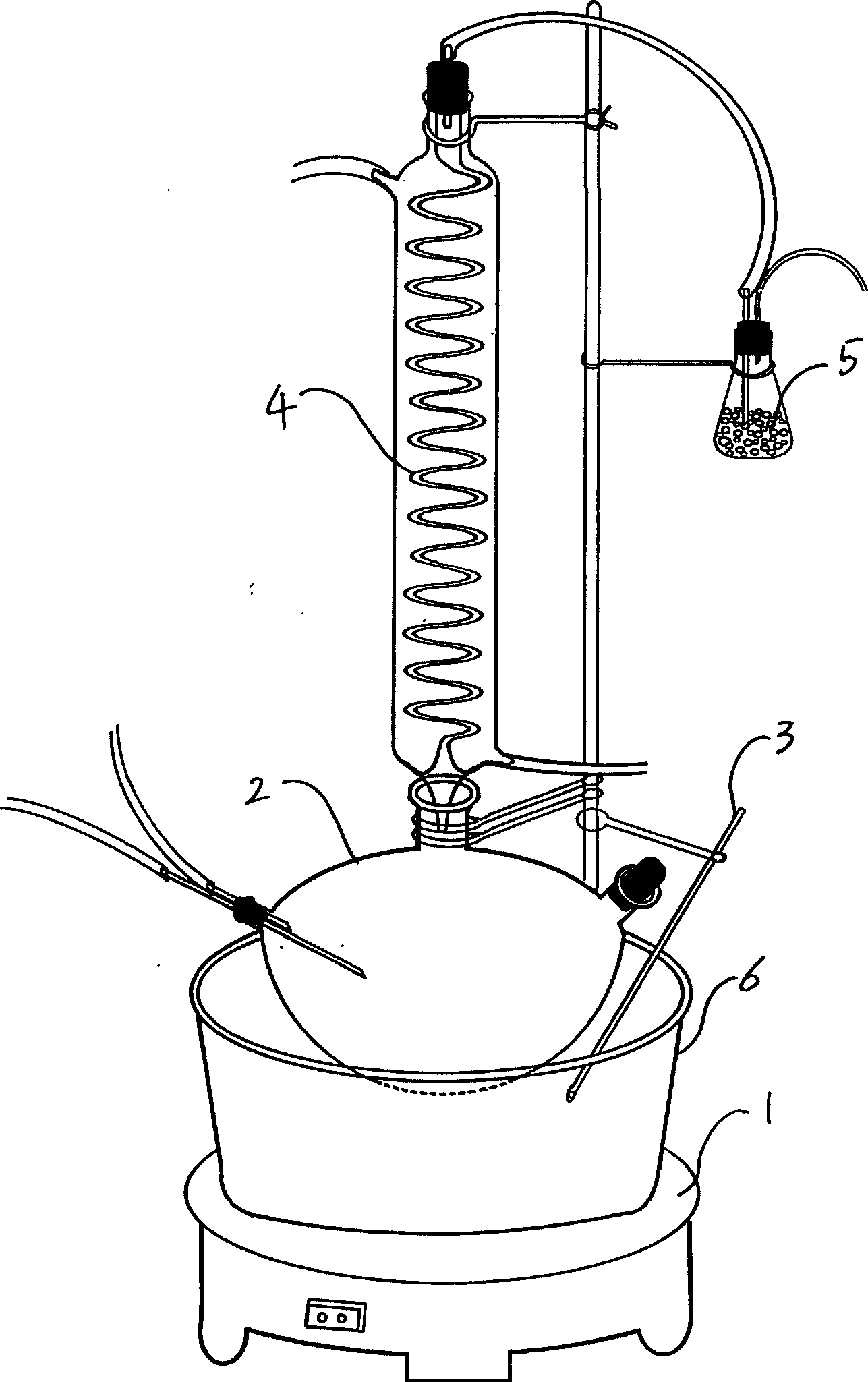
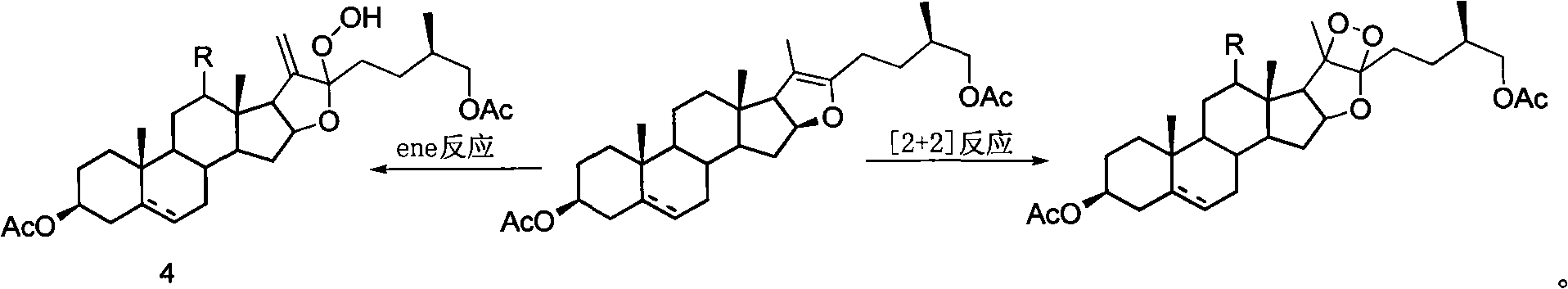
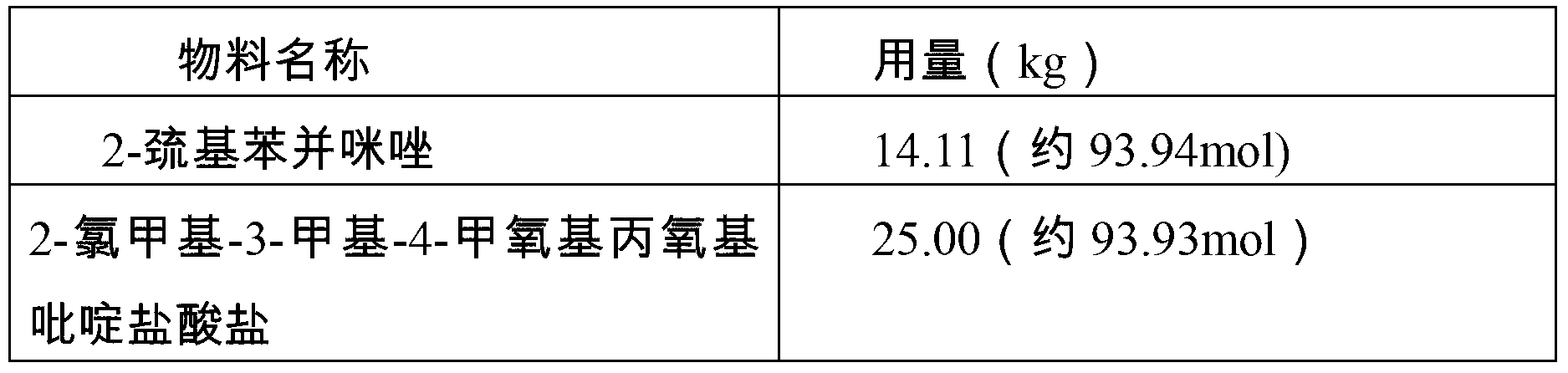
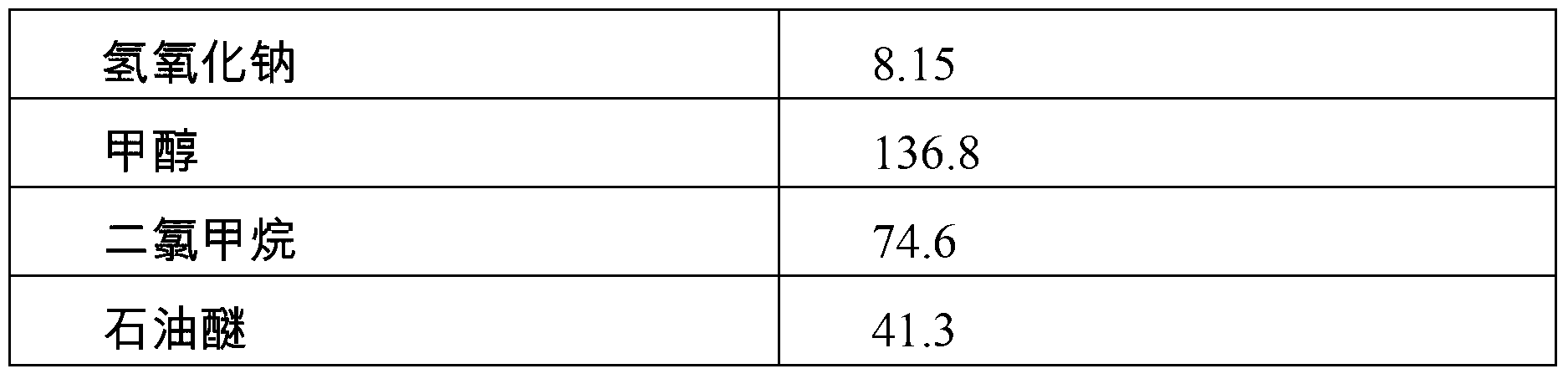
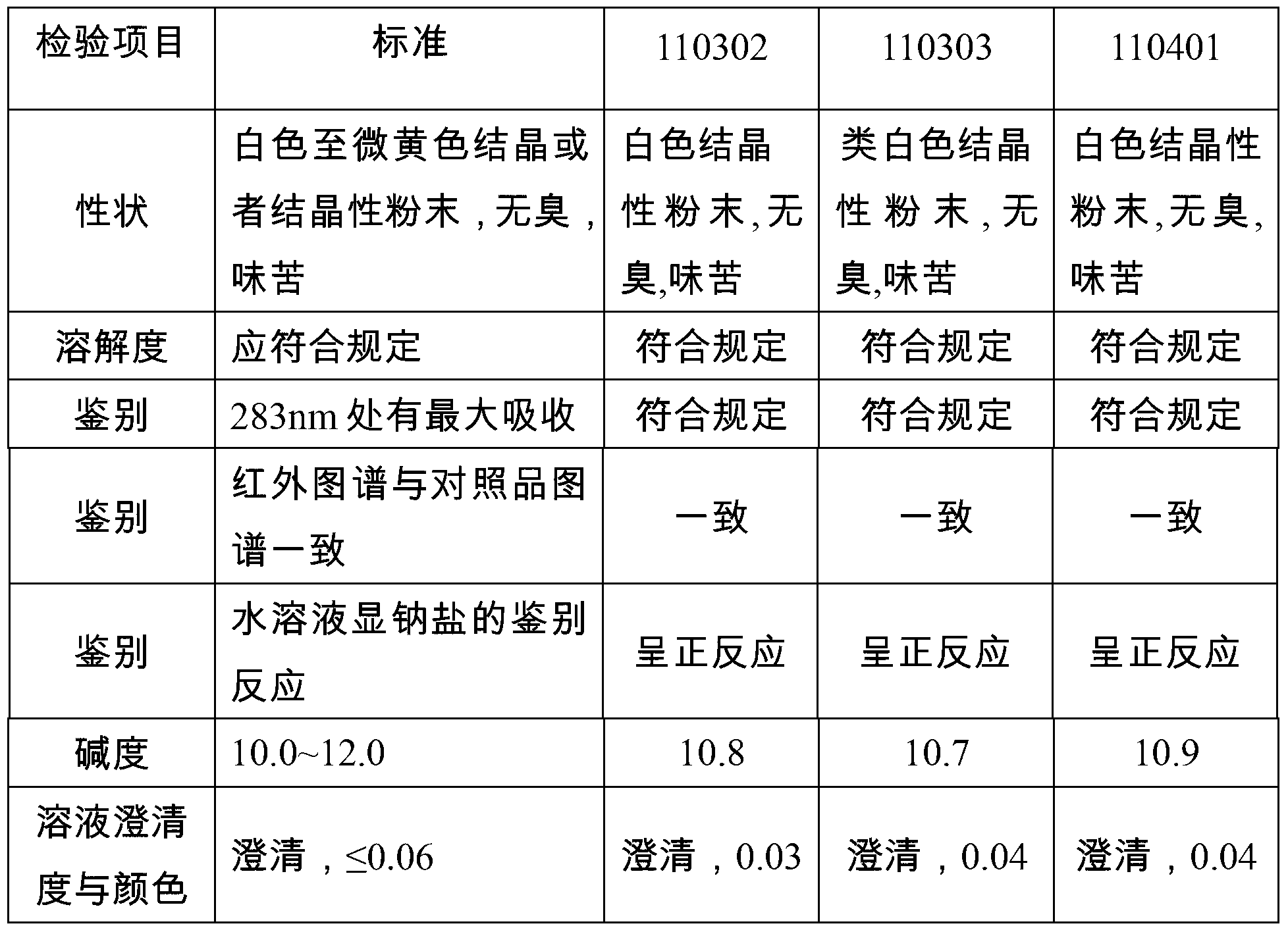
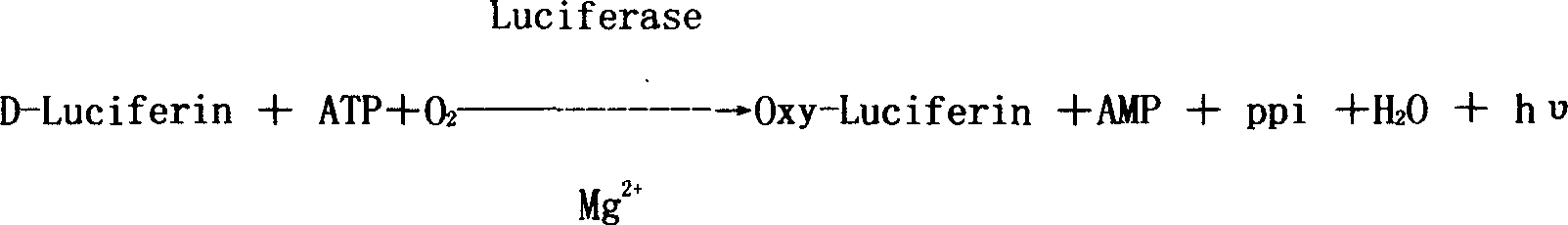Patents
Literature
208 results about "Pyridine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyridine Hydrochloride is the hydrochloride salt of pyridine, a basic six-membered heterocyclic ring. Pyridine is a base structure present in many biologically active compounds like the vitamins niaci n and pyridoxal. Pyridine is used in dehalogenation reactions and can be used as a base in condensation reactions.
Preparation method of benzbromarone
ActiveCN102659727ASimple post-processingDoes not affect the quality of the finished productOrganic chemistryAnisoyl chlorideHalohydrocarbon
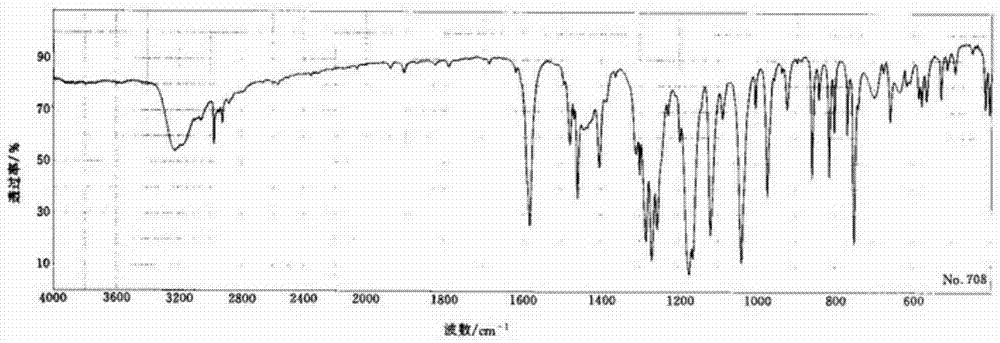
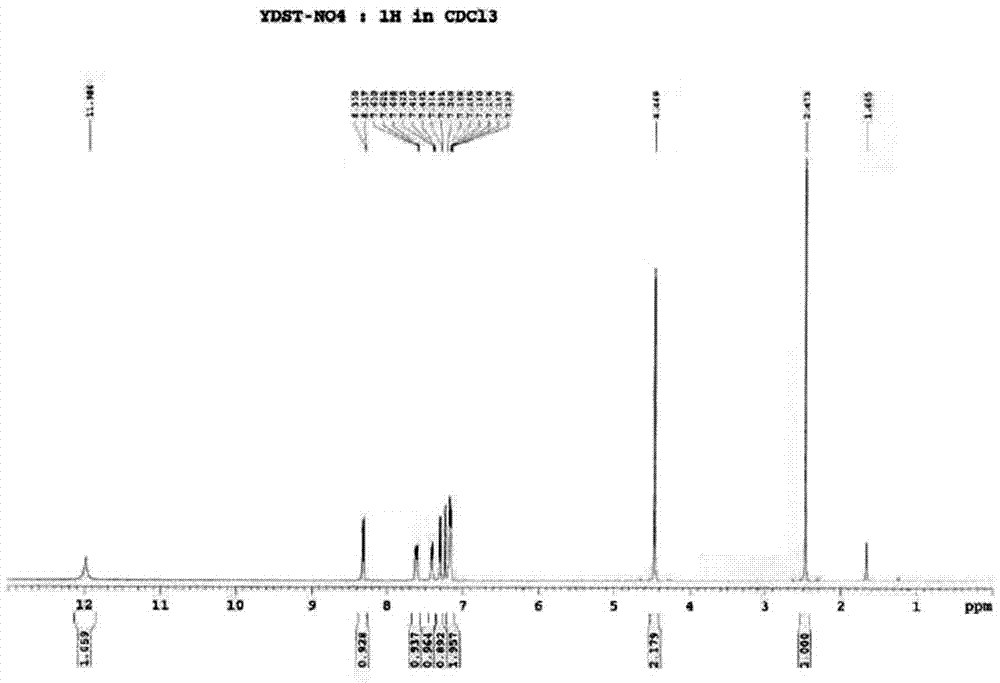
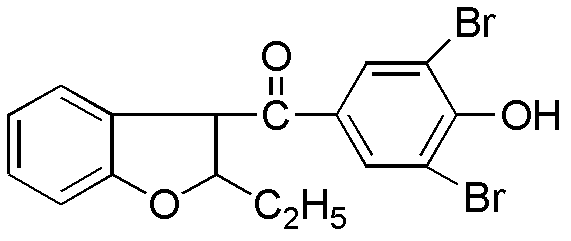
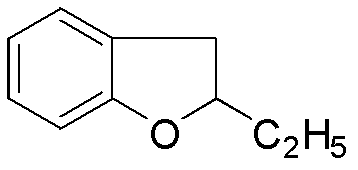
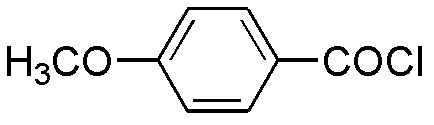
The invention relates to a preparation method of benzbromarone applied to the field of pharmaceutical synthesis, which comprises the following steps: taking 2-ethylbenzofuran and p-anisoyl chloride as starting raw materials, carrying out friedel-crafts acylation under the participation action of a catalyst and prepare 2-ethyl-3-p-methoxyphenyl formyl-benzofuran; carrying out demethylation reaction on the obtained 2-ethyl-3-p-methoxyphenyl formyl-benzofuran and pyridine hydrochloride, removing moisture in a reaction system by using a method that water is contained in toluene and preparing 2-ethyl-3-p-hydroxybenzene formyl-benzofuran; carrying out bromination reaction on the prepared 2-ethyl-3-p-hydroxybenzene formyl-benzofuran and bromide to prepare benzbromarone; and carrying out acidolysis with hydrochloric acid after the 2-ethylbenzofuran is fully reacted with the p-anisoyl chloride and extracting to obtain the 2-ethyl-3-p-methoxyphenyl formyl-benzofuran. The preparation method has the advantages that in the friedel-crafts acylation, methylene dichloride, trichloromethane and other halohydrocarbon are used for replacing carbon disulfide, and the post-processing process is simplified; and in the bromination reaction, bromine which is strong in corrosivity, generates great harm to human bodies and pollutes the environment is changed into the bromide.
Owner:NORTHEAST PHARMA GRP
Pantoprazole sodium and preparation method thereof
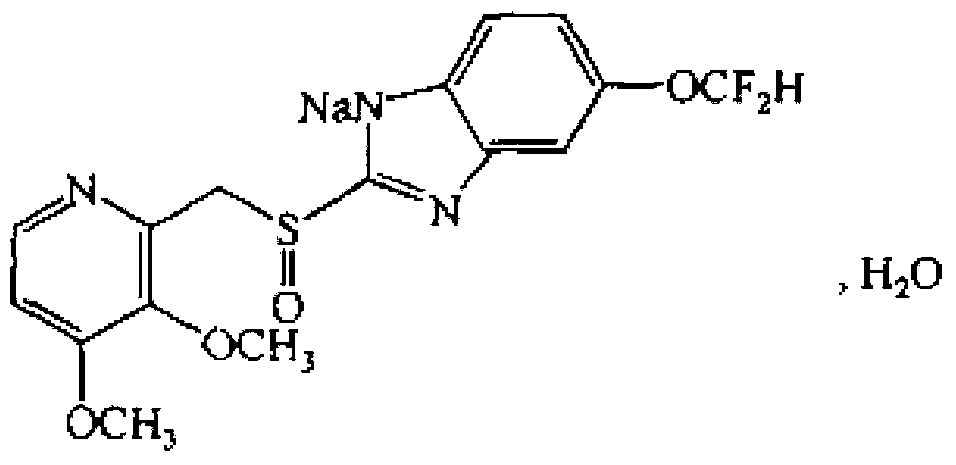
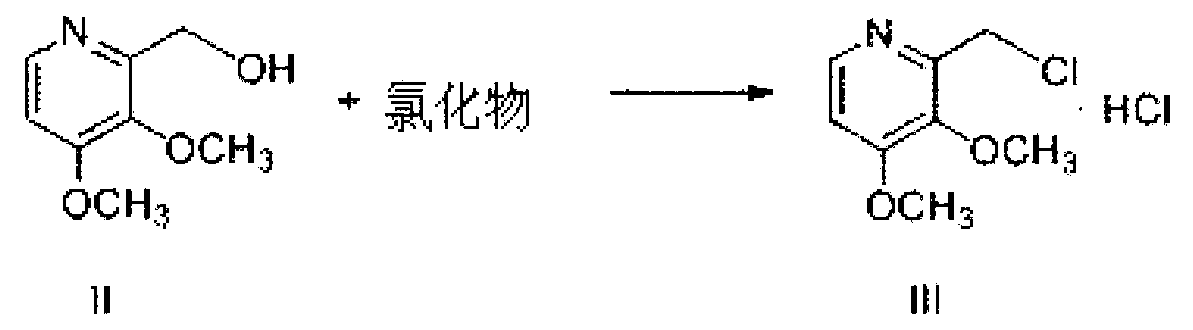
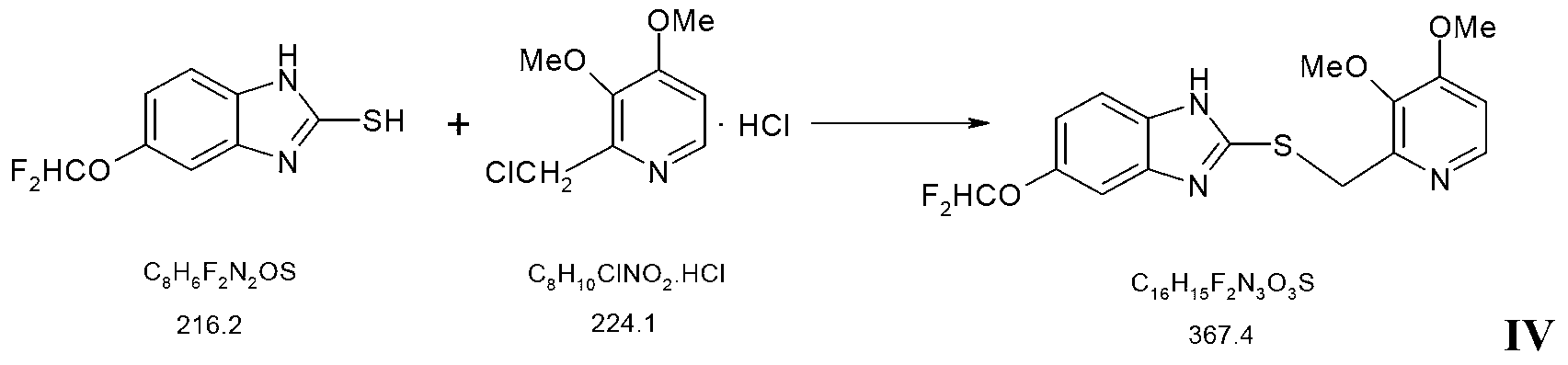
The invention relates to pantoprazole sodium and a preparation method thereof and in particular relates to a method for preparing the pantoprazole sodium. The preparation method comprises the following steps of: (1) with 2-hydroxymethyl-3,4-dimethoxyl pyridine (II) as a starting material, generating 2-chloromethyl-3,4-dimethoxyl pyridine hydrochloride (III) under the action of chlorides; (2) carrying out condensation on the obtained compound (III) and 5-difluoromethoxyl-2-sulfydryl-1H-benzimidazole in the presence of inorganic base to generate 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfenyl-1H-benzimidazole (IV); (3) oxidizing the obtained compound (IV) by using an oxidant to generate 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfinyl-1H-benzimidazole; (4) enabling the 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfinyl-1H-benzimidazole to react with sodium hydroxide to generate a salt, namely the pantoprazole sodium (I); and optionally (5) refining the prepared pantoprazole sodium. According to the preparation method, the prepared pantoprazole sodium product has high purity.
Owner:CHENGDU TIANTAISHAN PHARMA
Supported catalyst, its preparation and use in hydrodesulphurization and olefin-reducing techniques for gasoline
ActiveCN1690171AEfficient separationEasy to separateHydrocarbon oils refiningSodium BentoniteFuel oil
A supported catalyst contains ionic liquor of 10-40 mass percent and carrier of 60-90 mass percent. Said ionic liquor prepared by the reaction of Lewis base and Lewis acid, said Lewis base being amine salt of the general formula NRnH4-nCl, alkyl phosphonate of the general formula PR'xH3-x.HCl, 1, 3 - dial alkyl imidazole hydrochlorate, pyridine hydrochlorate or alkyl substituted pyridine hydrochlorate, in the said general formula, R being the alkyl of C1-C24, n being the integral number of 1-4, R' being the aryl of C6-C8, x being the integral number of 1-3; and said Lewis acid being the halide of aluminium, ferrum, zinc, copper, boron or phosphor, the mole ratio of Lewis acid and Lewis base is 1-5: 1. And the carrier is bergmeal, bentonite, silica dioxide, active carbon or silica-alumina molecular sieve. The catalyst has a perfect effect on the catalytic-cracking of gasoline desulfurising and reducing olefin, and can generate part of fraction of fuel-oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
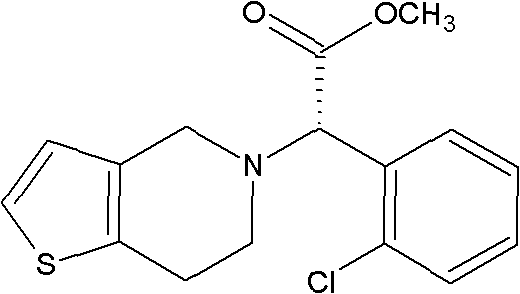
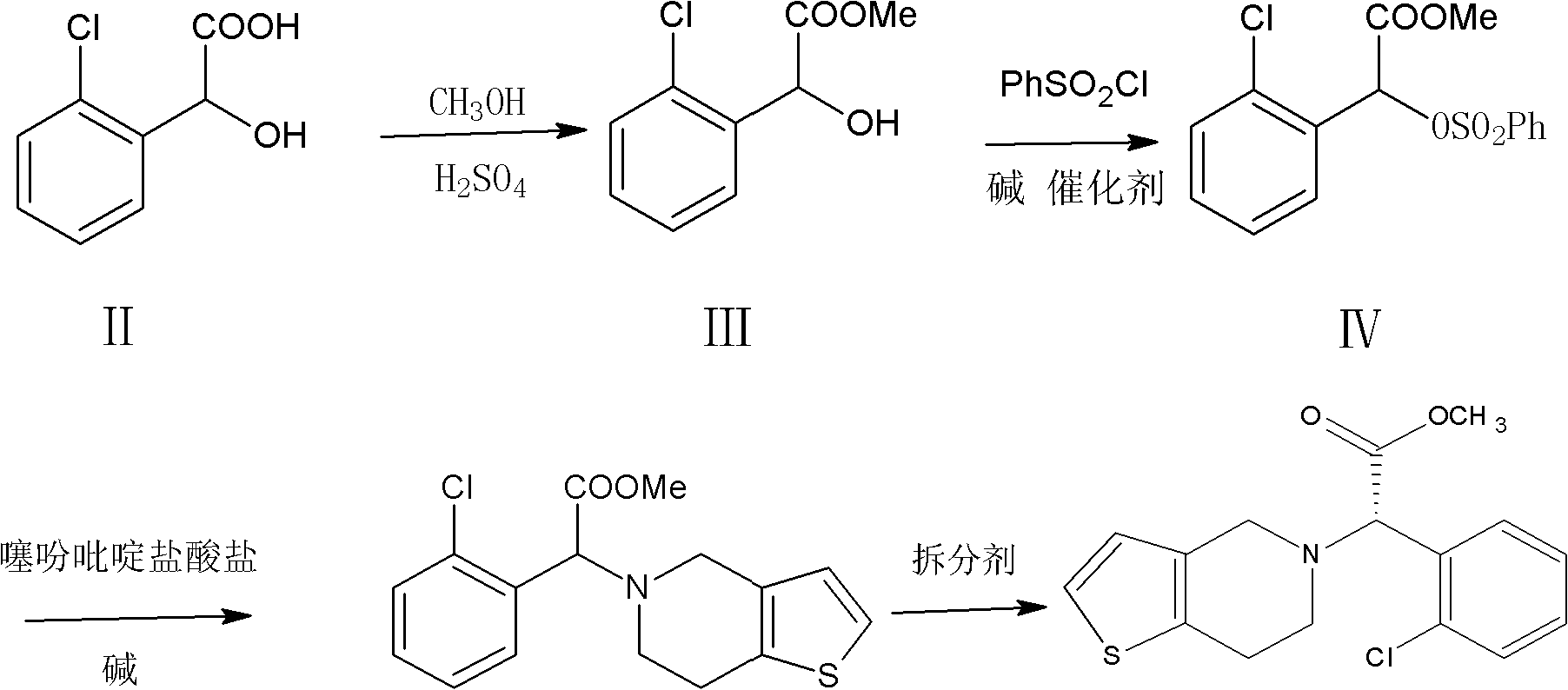
Method for preparing clopidogrel
InactiveCN101845050APromote environmental protectionEliminate the splitting stepOrganic chemistrySulfonyl chlorideMethyl o-chloromandelate
The invention relates to a method for preparing clopidogrel. The conventional synthetic methods have the disadvantages of poor environmental protection, disadvantageous industrial production, low optical purity of final products and high cost. The technical scheme adopted by the invention comprises the following steps of: performing a reaction on a compound, namely, R,S-o-chloromandelic acid and methanol to produce R,S-chloromandelic acid methyl ester; performing the reaction on the R,S-chloromandelic acid methyl ester and benzene sulfonyl chloride under the action of an alkaline catalyst to produce 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate; performing an SN2 substitution reaction on the 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate and 4,5,6,7-tetrahydro-thiophene pyridine hydrochloride under an alkaline condition to produce R,S-clopidogrel free alkali; resolving the R,S-clopidogrel free alkali in resolving solvent by using a resolving agent; and dissociating the resolved R,S-clopidogrel free alkali to prepare the clopidogrel. In a synthetic route of the invention, reaction conditions are temperate, used reaction substrates are environmentally friendly, reaction yield in each step is high, the optical purity of a final product is up to over 99.5 percent, and pollution-free production can be realized.
Owner:SHANGYU JINGXIN PHARMA
Leather care oil
Disclosed is leather care oil. The leather care oil comprises, by mass, 5 to 10 parts of silicone oil, 10 to 20 parts of wax, 15 to 20 parts of turpentine, 8 to 15 parts of lanolin, 0.5 to 0.15 part of mildew preventives, 0.5 to 0.1 part of bactericide, 2 to 6 parts of camphor oil, 2 to 5 parts of lecithin, 0.5 to 1.5 parts of emulsifying agents and 25 to 45 parts of water. The emulsifying agents are fatty alcohol-polyoxyethylene ether or oil-based amino acid sodium, the wax is ba wax or Chinese wax, mildew preventives are methylparaben or ethylparaben, and the bactericide is alkyl pyridine hydrochloride or n-octyl-isothiazolinone. According to the leather care oil, the lanolin is used as a main nourish component for deeply nourishing and moistening leather internal fibers, the lecithin is added for promoting permeation, simultaneously the camphor oil, the mildew preventives and the bactericide are added so as to effectively prevent leather from mildewing and generating worms, and the leather care oil is convenient to use and suitable for various types of leather products.
Owner:SUZHOU GULI BIOTECH
Preparation method of esomeprazole magnesium
InactiveCN103936714AHigh yieldMild reaction conditionsOrganic chemistryEsomeprazole Sodium4-methoxypyridine
The invention discloses a preparation and refinement method of esomeprazole magnesium. The preparation and refinement method comprises the following steps: condensing 2-chloromethyl-3, 5-dimethyl-4-methoxyl pyridine hydrochloride and 2-sulfydryl-5-methoxy-benzimidazole serving as starting materials, carrying out improved sharpless asymmetric oxidation so as to prepare esomeprazole sodium, then carrying out salt displacement so as to prepare esomeprazole magnesium, and finally refining to obtain high-purity esomeprazole magnesium. The preparation method is mild in reaction conditions, simple to operate, good in repeatability and high in yield and facilitates industrial production. The chromatographic purity of esomeprazole magnesium prepared by the method is above 99.8%; the optical purity of esomeprazole magnesium reaches above 99.6%; esomeprazole magnesium is stable in morphology and can meet medicinal requirements.
Owner:北京华禧联合科技发展有限公司
Process for preparing raloxifene hydrochloride
InactiveUS20070100147A1High purityHigh yieldOrganic active ingredientsOrganic chemistryStrong acidsSolvent
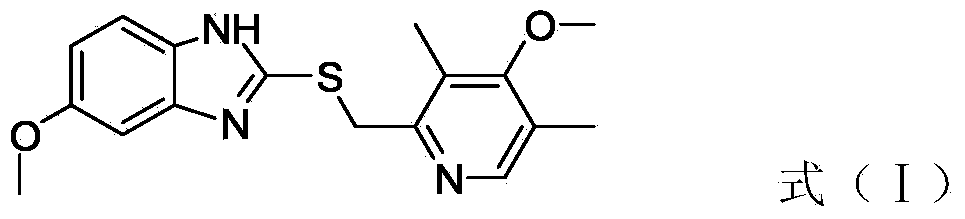
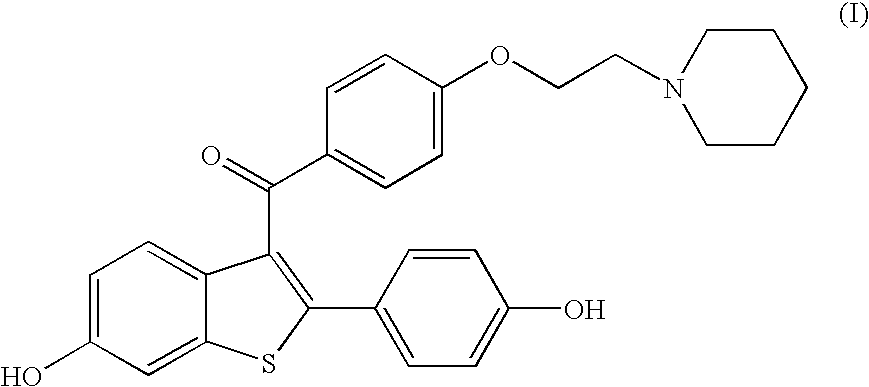
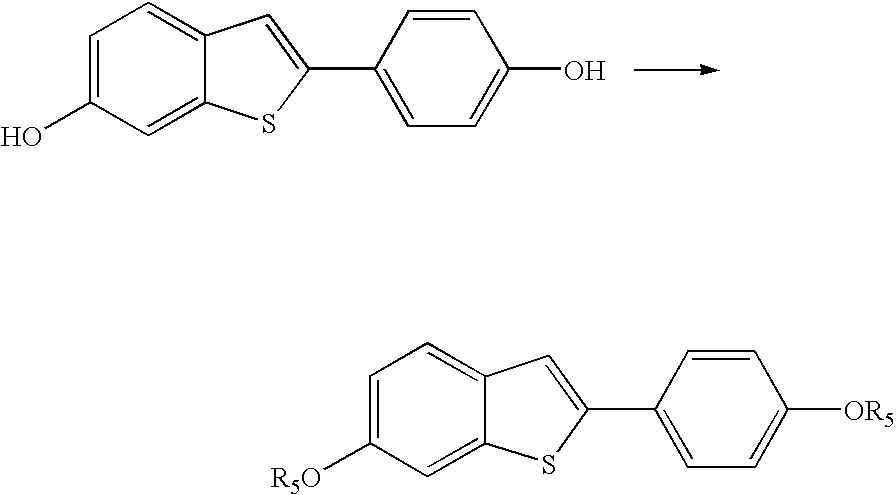
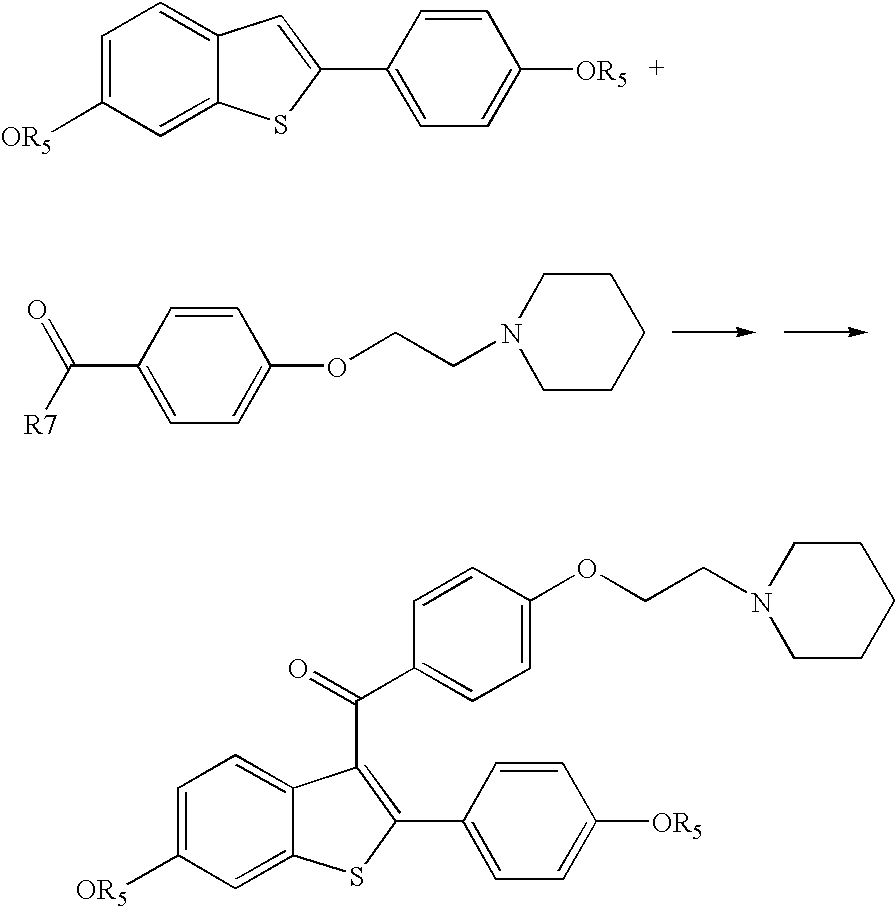
Process for preparing raloxifene hydrochloride with a purity greater than 98% and low aluminium content comprising the following stages a) demethylation of 6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene in pyridine and hydrochloric acid to obtain 6-hydroxy2-(4-hydroxyphenyl)benzo[b]thiophene in pyridine hydrochloride, b) acetylation of 6-hydroxy-2-(4hydroxyphonyl)benzo[b]thiophene with an acetylating agent to obtain the corresponding 6-acetoxy-2-(4 acetoxyphenyl)benzo[b]thiophene, c) acylation of 6-acetoxy-2-(4-acetoxyphonyl)benzo[b]thiophene with 4-(2 piperidinoethoxy)benzoylchloride hydrochloride with aluminium trichloride in halogenated solvent to obtain 6-acetoxy-2-(4acetoxyphenyl)-3-[4-(2 piperidinoethoxy)benzoyl]-benzo[b]thiophene, d) hydrolysis of 6-acetoxy-2-(4-acetoxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyll benzo[b]thiophene according to the following operating conditions: d1) treatment of 6-acetoxy-2-(4-acetoxyphonyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene with alkaline hydroxide in alcohol solvent, d2) acidification of the product obtained in the preceding stage (d1) with a strong acid, to obtain the corresponding raloxifene salt with the strong acid, characterised in that the strong acid used in stage (d2) is concentrated hydrochloric acid.
Owner:ERREGIERRE
Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine
InactiveCN101250192AMild conditionsHigh yieldOrganic chemistryBulk chemical productionExplosive materialTetrahydrothiophene
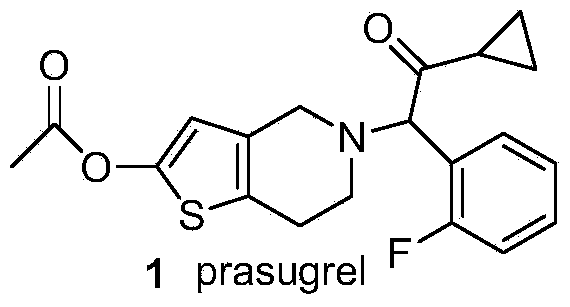
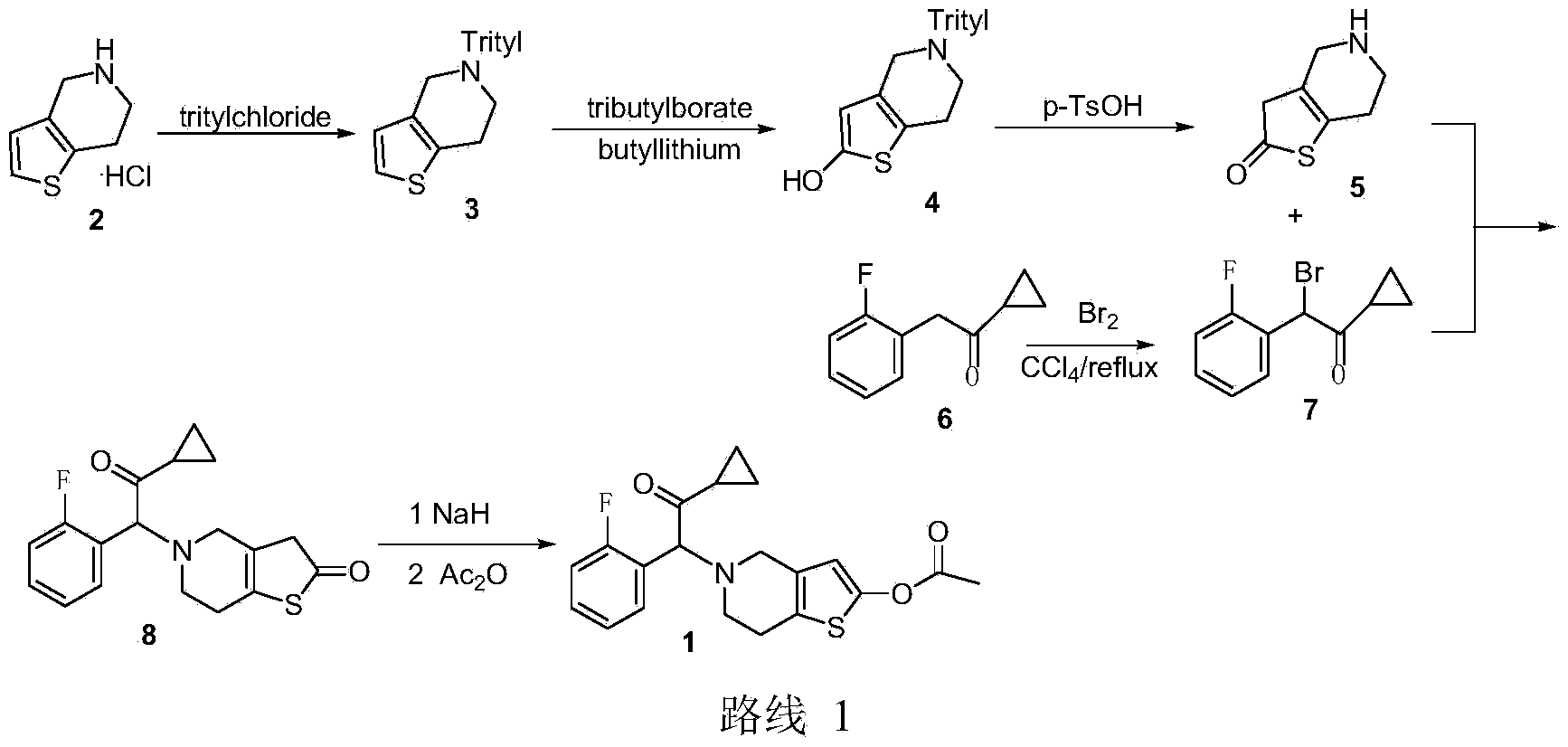
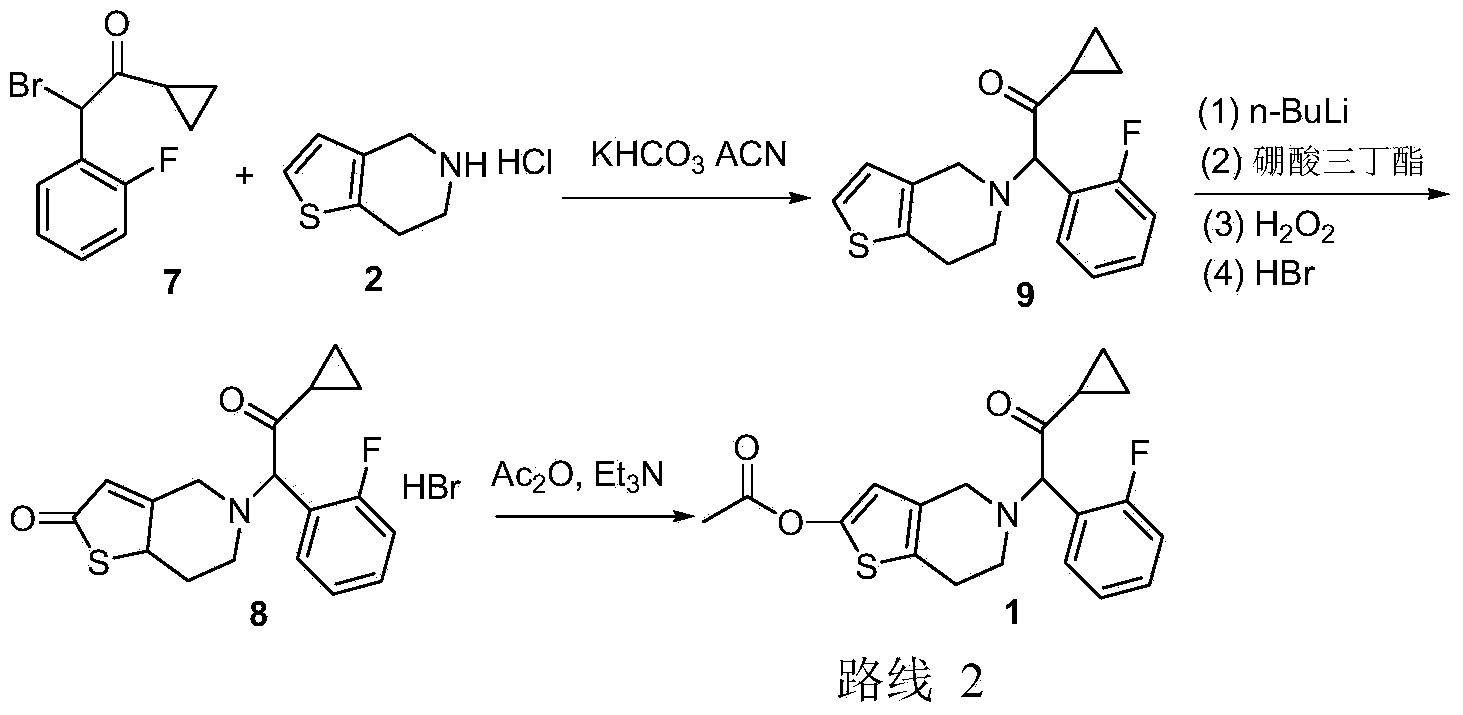
The invention discloses a new synthesis route of 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2, 4, 5, 6, 7, 7a-hexahydrothiophene [3, 2-c] pyridine, which only needs to one step to convert prior 2-methoxy-5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-4, 5, 6, 7-tetrahydrothiophene [3, 2-c] pyridine hydrochloride to target compound, with mild reaction conditions, non low temperature demand, non explosive material, high yield, low cost and high efficiency. The invention is suitable for large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND
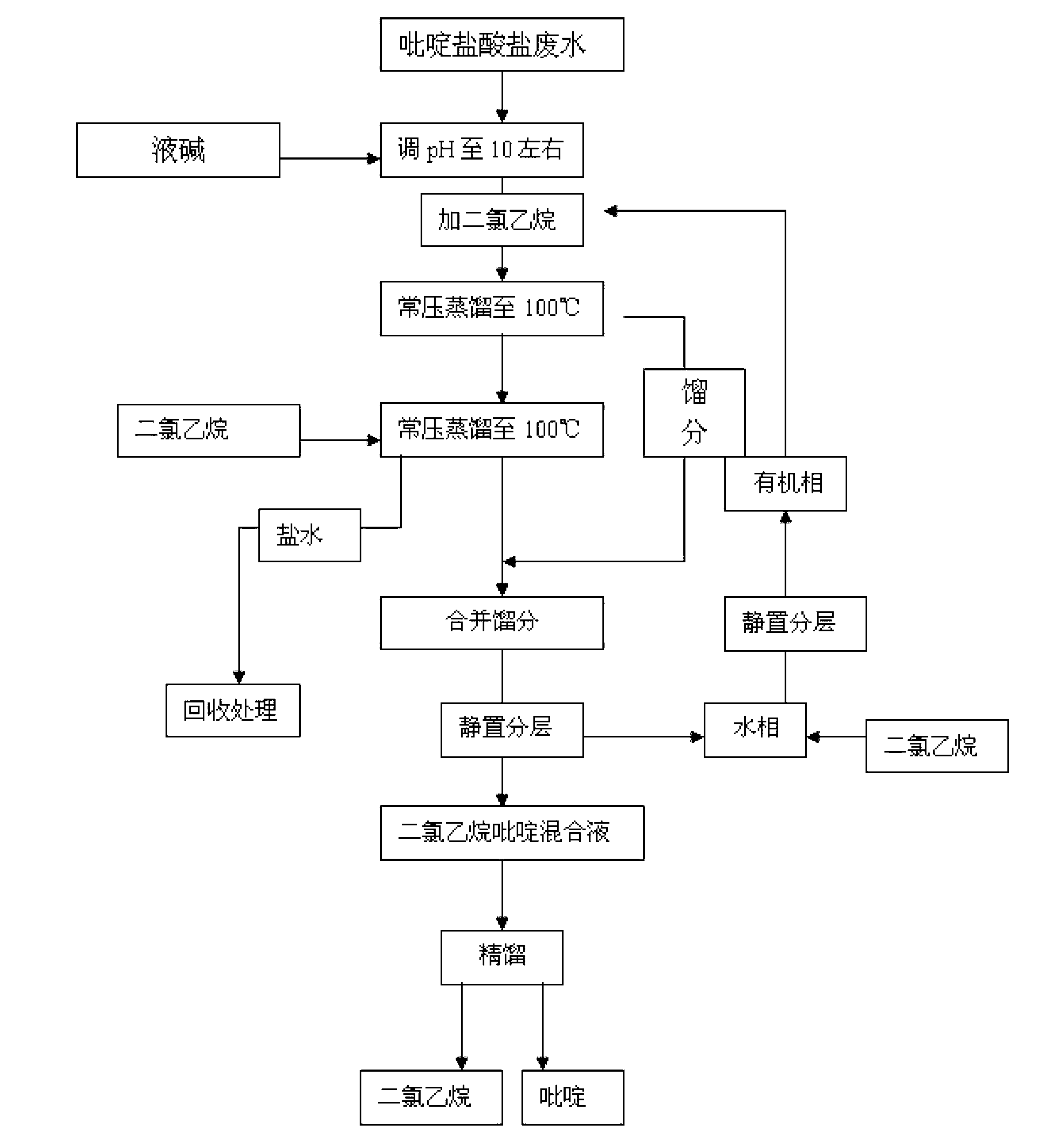
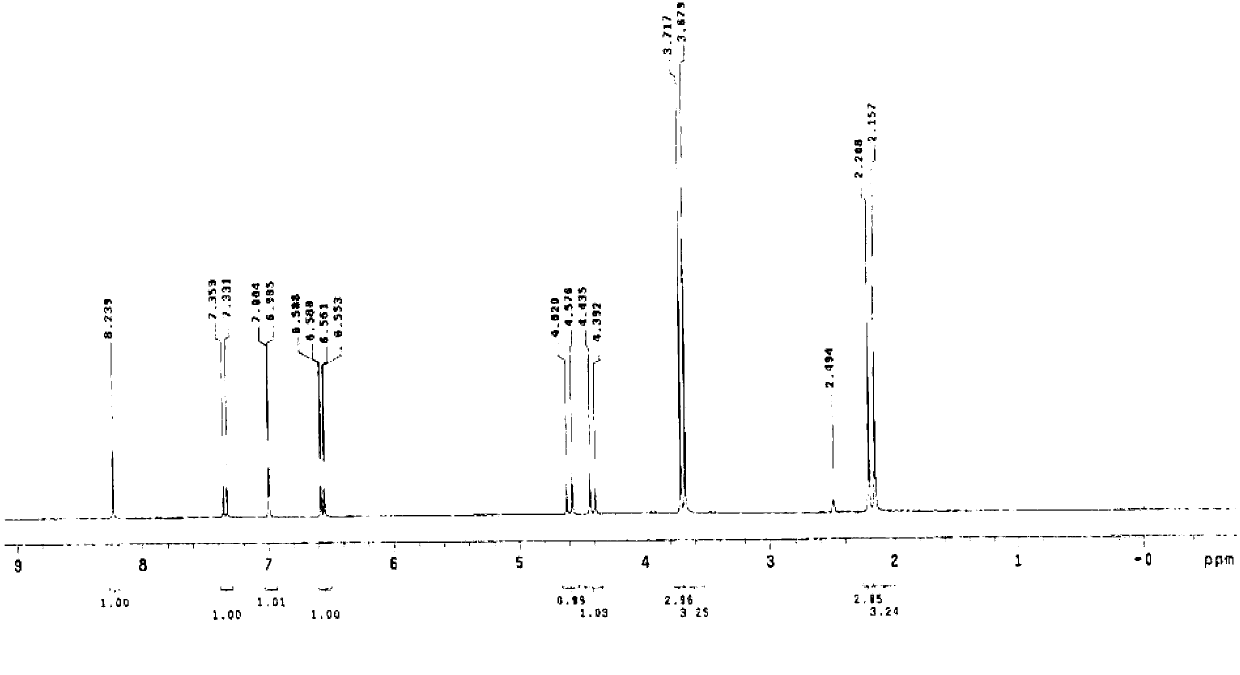
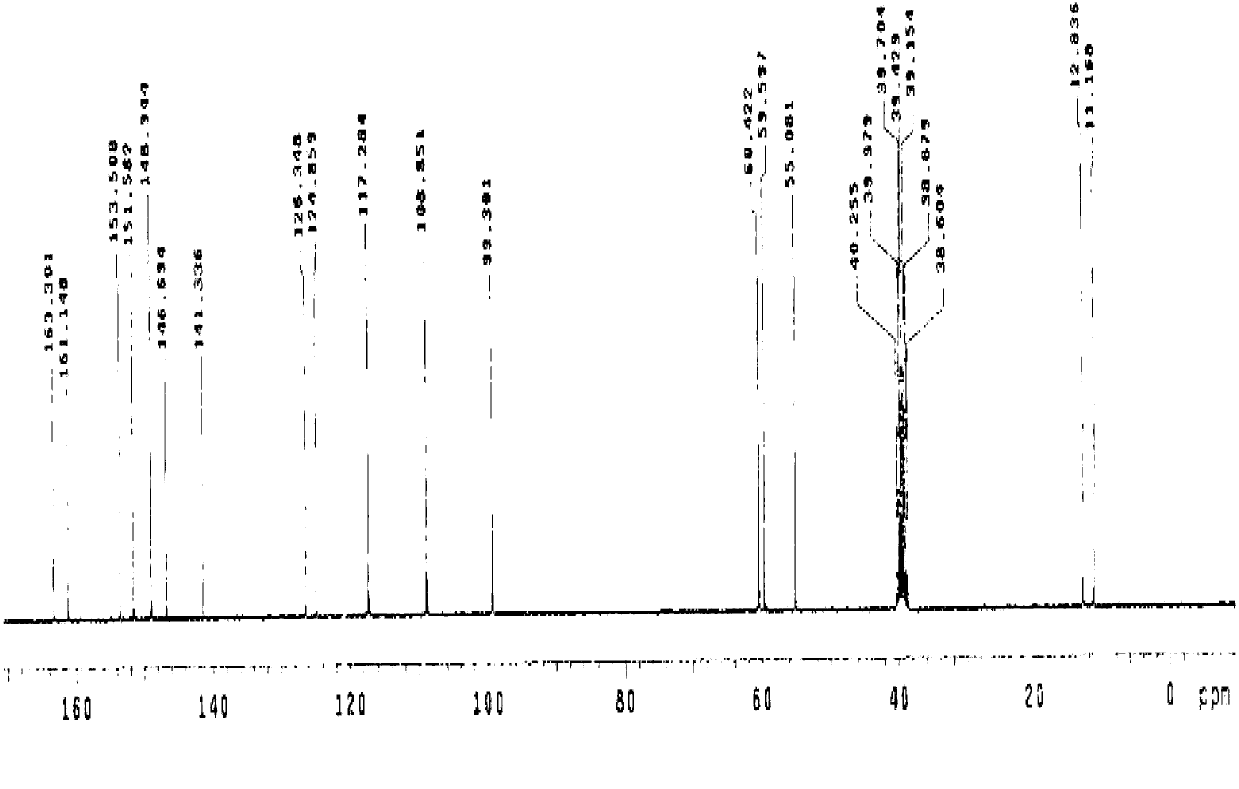
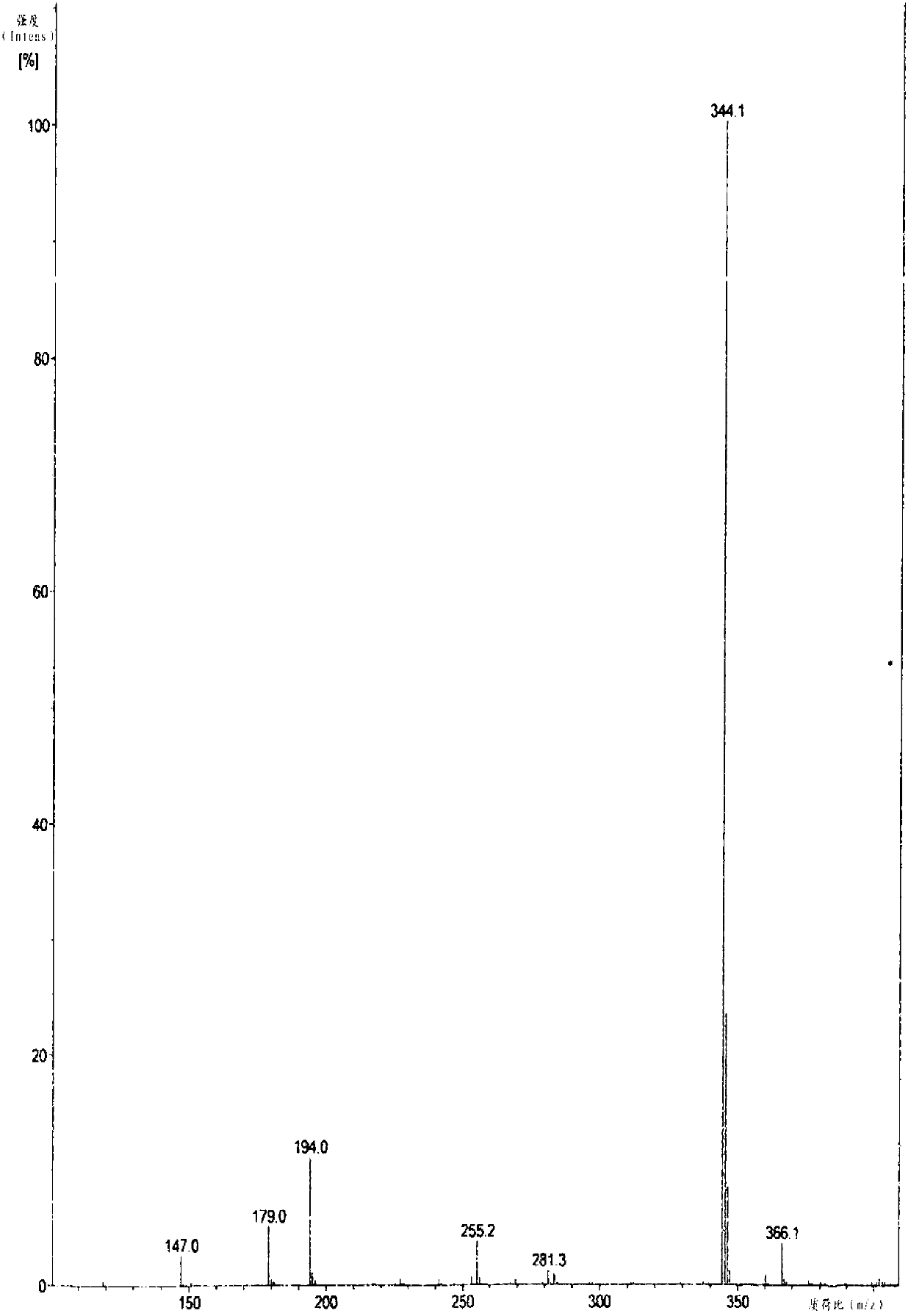
Method for recovering pyridine from pyridine hydrochloride water solution
The invention relates to the field of chemical engineering, particularly a method for recovering pyridine from a pyridine hydrochloride water solution, which comprises the following steps: by using dichloroethane as an extractant, carrying out extraction distillation twice, and rectifying to obtain the pyridine with higher recovery rate (up to 90-95%). The invention can reduce the waste of resources, and does not pollute the environment, thereby reducing the production cost and enhancing the economic benefit.
Owner:HEFEI XINGYU CHEM
Preparation method of rupatadine fumarate
The invention relates to the technical field of pharmaceutical synthesis, and in particular relates to a preparation method of rupatadine fumarate. The preparation method comprises the following steps of: adding 235-237g of 3-chloromethyl-5-pyridine hydrochloride and 465-475ml of tertiary butanol in a reaction container; stirring and heating to 55-65 DEG C; dropping 235-245ml of concentration sulfuric acid, controlling the temperature of the reaction liquid to be 55-65 DEG C and reacting for 8-10 hours; cooling to room temperature, diluting with 234-245ml of water, then adding 345-355ml of methylbenzene, and adjusting pH value of the liquor by stronger ammonia water to 7.8-8.2 to separate out an organic phase; and after water layer extraction, combining the organic phases, washing the organic phase, drying and evaporating to remove the solvent to obtain an oily liquid product. The preparation method of rupatadine fumarate is simple and quick in preparation process, so that the preparation method is suitable for industrialized production. The yield is higher, and the reaction time is effectively shortened. Meanwhile, the refining process is simpler and the product purity is higher.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Preparation method of esomeprazole and preparation method of esomeprazole salt
The invention adopts 2-chloromethyl-4-nitryl-3, 5-dimethyl pyridine hydrochloride and 5-methoxyl-2-mercapto benzimidazole as starting materials to prepare esomeprazole salt by condensation, asymmetric oxidation and methoxidation. A preparation method has the advantages that the repeatability is good, the operation is simple, and the industrial production is easy; and the preparation conditions are moderate, the generation of impurities such as nitric oxide and sulphone is reduced in the preparation process, and the yield and the purity of the esomeprazole salt are increased.
Owner:SHANDONG UNIV +1
Method for pure water phase preparation of rabeprazole sodium
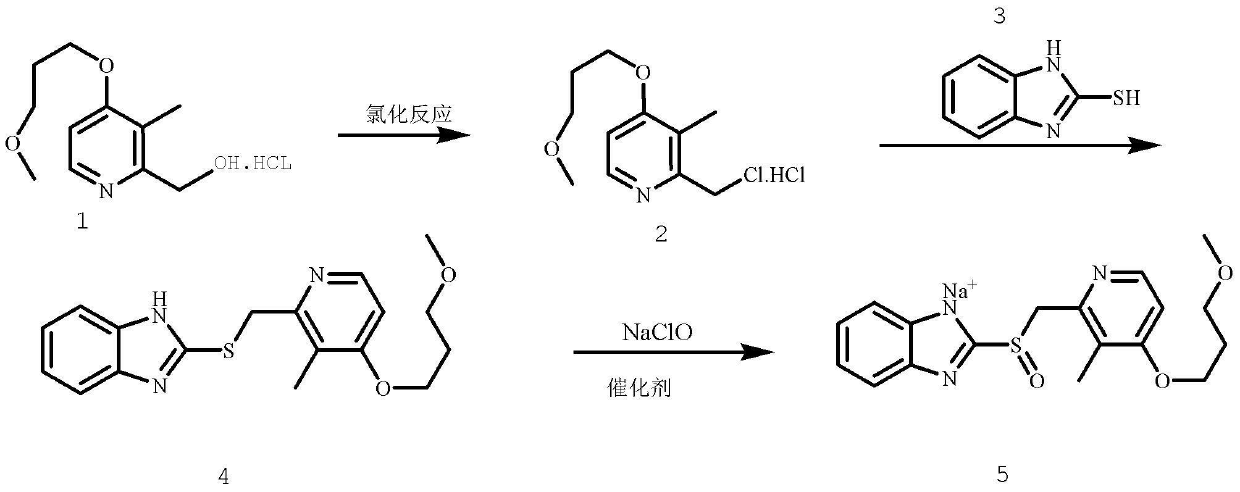
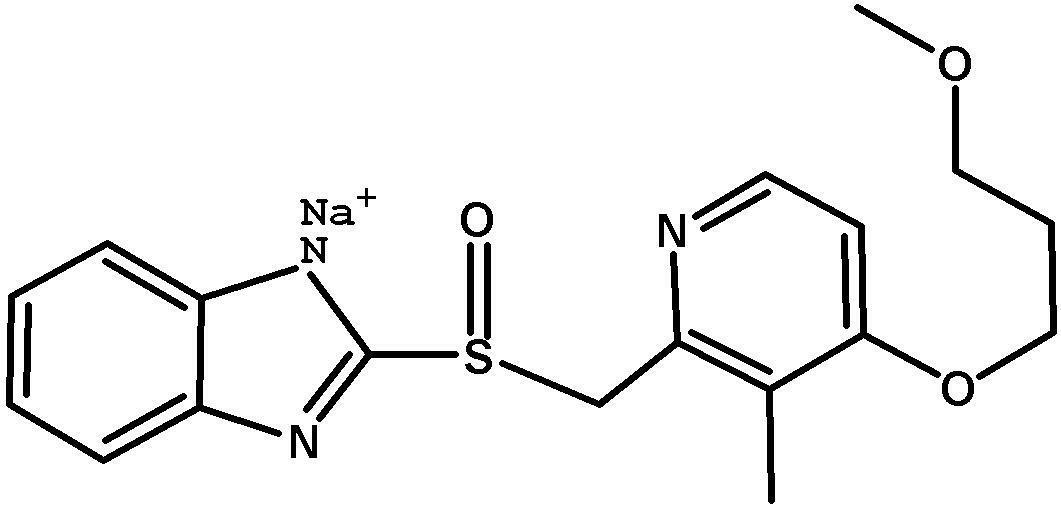
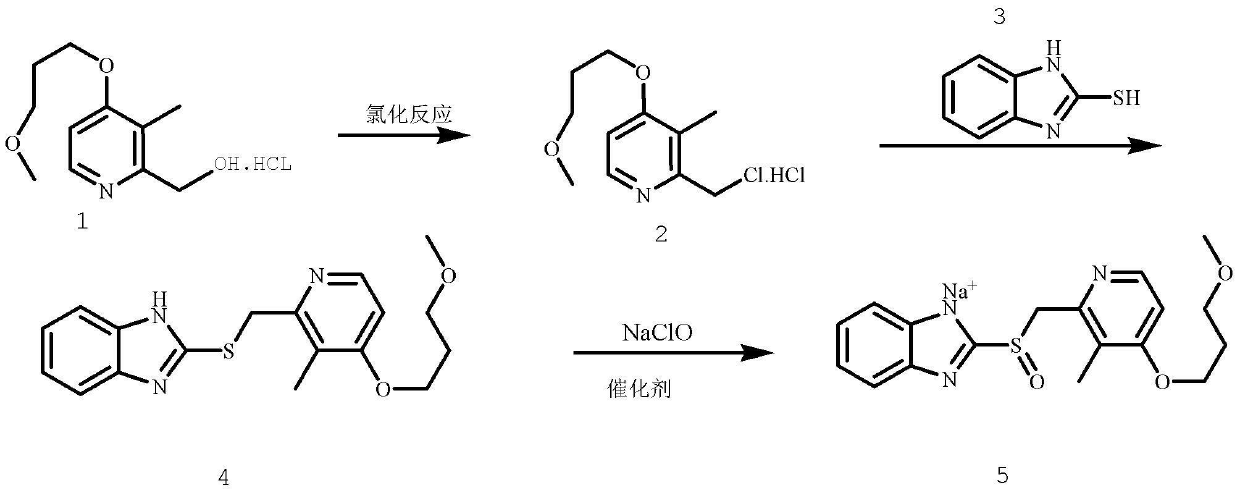
The invention provides a method for pure water phase preparation of rabeprazole sodium and belongs to the technical field of medicines. The method is characterized in that an intermediate, 2-hydroxymethyl-3-methyl-4-(3-methoxyl propoxy) pyridine hydrochloride, of the rabeprazole sodium is adopted as a raw material to be subjected to chlorination reaction, condensation reaction and oxidation reaction, so as to synthesize the rabeprazole sodium through three steps. The method adopts a novel chlorination reaction system and a novel sodium hypochlorite oxidation system, and can achieve a higher chloridization rate and excellently control a sulfur ether intermediate to be oxidized into sulfoxide; the reaction conditions are wild; both the reaction conversion rate and the reaction selectivity are very high; few reaction by-products are produced; the whole process can be carried out under the condition of no solvent; the content of residual solvent is lowered; time-consuming and toilsome vacuum rectification is not needed; the operation is simplified to a great extent; the process flow is short; and suitability for industrial application is achieved.
Owner:DALIAN UNIV OF TECH
Combined collector for feldspar and quartz flotation separation and application method
ActiveCN107185721ASimple compositionGood flotation separation effectFlotationHydrofluoric acidKerosene
The invention discloses a combined collector for feldspar and quartz flotation separation and an application method. The combined collector is prepared from, by weight, 2-5 parts of 1-butyl pyridine hydrochloride, 2-5 parts of sodium dodecyl sulfate and 1-3 parts of kerosene. According to the combined collector for feldspar and quartz flotation separation, the components are simple, and the flotation separation effect is good; by serving as a cationic collector by replacing a common amine reagent, 1-butyl pyridine hydrochloride has the advantages that the adsorption capacity is higher than that of the amine reagent and the requirement on a pH medium is lower than that of the amine reagent, and can act together with an anionic collector sodium dodecyl sulfate to achieve floatation of feldspar minerals in an environment free of hydrofluoric acid; the adverse effects of flotation froth sticking and poor selectivity which are caused by the slime effect are eliminated by adding kerosene according to a certain proportion. The combined collector for feldspar and quartz flotation separation can avoid use of hydrofluoric acid in the feldspar and quartz flotation separation process and has the advantages of being high in operating safety factor and friendly to environment.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
Method for recovering pyridine from waste pyridine hydrochloride and cyclically reutilizing pyridine
InactiveCN102584684AReduce manufacturing costImprove recycling ratesOrganic compound preparationAlkali metal chloridesEthyl ChlorideImpurity
The invention provides a method for recovering pyridine from waste pyridine hydrochloride for producing chloromethyl isopropyl carbonate and cyclically reutilizing the pyridine. The method comprises the following process steps that: the pyridine hydrochloride and water are placed into a container according to a proportion being 1:2 to be stirred to obtain pyridine hydrochloride water solution, the pyridine hydrochloride water solution and two times of pyridine hydrochloride liquid caustic soda are placed into an enamel reaction kettle to be sufficiently stirred for taking reaction to generate pyridine and sodium chloride water solution, the pyridine and sodium chloride water solution are automatically produced in the still state, the upper layer is water-containing pyridine, the lower layer is sodium chloride water solution, the water-containing pyridine at the upper layer is taken out and is subjected to impurity removal through precipitation, rectification elution is adopted for removing water to obtain finished products, and the sodium chloride water solution is conveyed into a concentration pot to be concentrated to obtain sodium chloride finished products. The finished product pyridine can be used as one ingredient to be conveyed into a chloromethyl isopropyl carbonate production process line to be cyclically utilized for producing chloromethyl isopropyl carbonate. The method has the advantages that the resource waste is reduced, the environment cannot be polluted, meanwhile, the production cost of the chloromethyl isopropyl carbonate is also greatly reduced, and the economic benefits of enterprises are improved.
Owner:LIYANG YONGAN FINE CHEM
Preparation method of tofacitinib citrate starting material
InactiveCN107337676AMeet the needs of industrial productionReduce manufacturing costOrganic chemistryPyridiniumSynthesis methods
The invention discloses a synthesis method of a tofacitinib citrate starting material N-((3R, 4R)-4-methyl-1-benzyl-3-piperidyl)-N-methyl-7-tolylsulfonyl-7H-pyrrolo[2,3-D]pyrimidine-4-amine(I). The method comprises the specific steps that 4-methylpyridine is adopted as the starting material to be subjected to nucleophilic substitution with benzyl chloride to obtain 4-methyl-1-benzyl-pyridinium chloride, a reduction reaction is performed under the effect of sodium borohydride, a hydroboration-oxidation reaction is performed, hydroxyl oxidation is performed, two chiral centers are introduced in a reductive amination stereoselectivity mode, splitting is performed through cheap chiral acid (L-DTTA) easy to obtain to obtain an optically pure intermediate body (3R, 4R)-(1-benzyl-4-methyl-piperidine-3-yl)-methyl amine, and finally, the intermediate body and 4-chloropyr are condensed to obtain the tofacitinib citrate starting material. The whole method is easy to implement and low in cost, raw materials are easy to obtain, and aftertreatment is easy. The formula is shown in the description.
Owner:JIANGSU QINGJIANG PHARMA
Deodorant
InactiveCN102872467AEfficient sterilization and deodorization abilityDeodrantsHigh concentrationCopper nitrate
A deodorant is prepared by the following components by mass: 5-10 parts of potassium permanganate, 5-10 parts of NaClO, 15-20 parts of activated carbon powder, 5-10 parts of polyhydric alcohol, 3-8 parts of fungicide, 8-15 parts of nitrate, 5-8 parts of essences and 25-45 parts of water, wherein the polyhydric alcohol is one or several of propylene glycol, glycerol or ethylene glycol; and the nitrate is one or several of silver nitrate, zinc nitrate or copper nitrate. The deodorant is alkyl pyridine hydrochloride or n-octyl-isothiazolinone. The deodorant uses chemical deodorization, adsorption deodorization and covering deodorization in matching mode, is added with high-concentration bacteriacide, has efficient sterilizing and deodorizing effect and is particularly suitable for large treatment plants heavy in stink.
Owner:SUZHOU GULI BIOTECH
Chemical synthetic process of D-fluorescein and device therefor
InactiveCN1754881AEfficient synthesisHigh luminous coefficientOrganic chemistryChemical synthesisFluorescein
The invention relates to chemical synthesis for D-fluorescein. Wherein, using hot demethylate reaction to 2- cyano-6-methoxyl benzothiazole and pyridine hydrochloride with high purity to extract reaction production for column chromatography; finally, taking condensation reaction for demethylate production and D-cysteine to obtain objective product. This invention is simple, needs mild condition and easy to control. The product has yield more than 60% and purity more than 50%, and achieves level of Sigma L-9504 but with low price.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +1
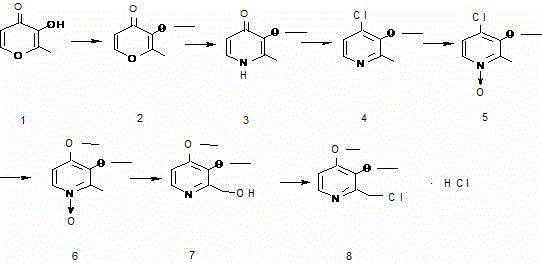
Preparation method of pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride
The invention belongs to the technical field of medicine, and particularly relates to a preparation method of a pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride. The preparation method comprises the following steps: using 3-hydroxyl-2-methyl-4-pyrone as a starting raw material, and then only performing five-step reaction to obtain the pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride. The preparation method reduces the reaction steps, shortens the reaction cycle, improves the working efficiency, and increases the yield coefficient.
Owner:SHOUGUANG FUKANG PHARMA
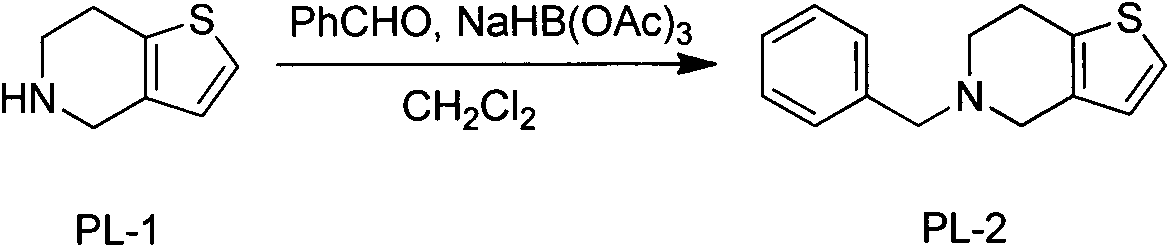
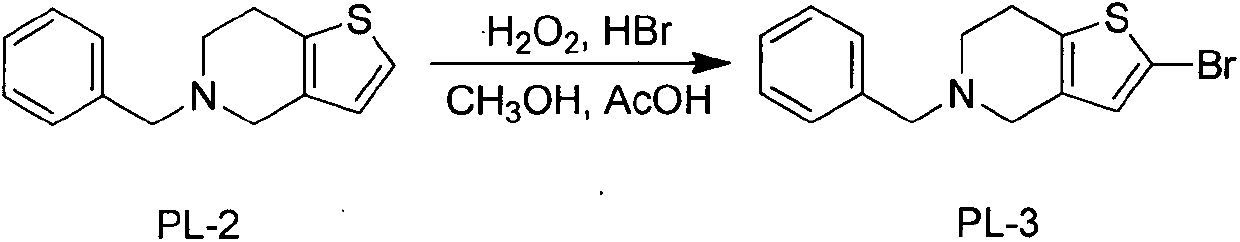
Novel preparation process of prasugrel hydrochloride
ActiveCN103694251AEasy to operateSuitable for industrial scale-up productionOrganic chemistryPrasugrel HydrochlorideSodium triacetoxyborohydride
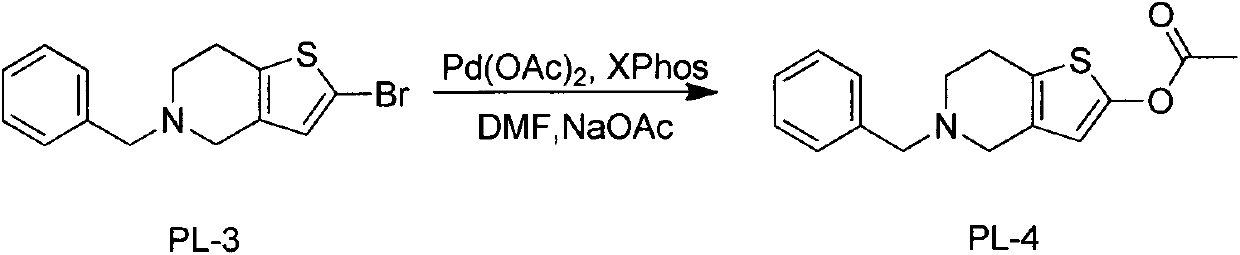
The invention discloses a novel synthesis process of prasugrel. The novel synthesis process comprises the steps: firstly, carrying out benzyl protection by taking 4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine, benzaldehyde and sodium triacetoxyborohydride as starting raw materials to generate 5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine; then, brominating the 5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine by using hydrobromic acid to obtain 2-bromo-5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine; next, catalytically synthesizing 2-acetoxy-5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine by taking palladium acetate as a catalyst and XPhos as a ligand; and finally, carrying out hydrodebenzylation, and coupling the product with alpha-bromo-o-fluorobenzyl cyclopropyl ketone to obtain a target molecule 2-acetoxy-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine hydrochloride, i.e., the prasugrel hydrochloride. The process is simple, convenient and novel in route, easy to obtain raw materials, mild in condition, convenient to operate, high in total yield up to 80-90% and suitable for large-scale production.
Owner:南京恒道医药科技股份有限公司
Preparation method for canrenone
The invention discloses a preparation method for canrenone. According to the preparation method, canrenone is obtained through an etherification reaction and a dehydrogenation reaction, wherein 17 beta-hydroxyl-4-alkene-3-ketone-17 alpha-pregnene-21-carboxylic acid-gamma-lactone is taken as a raw material; under the presence of a catalyst, the etherification reaction is carried out between 17 beta-hydroxyl-4-alkene-3-ketone-17 alpha-pregnene-21-carboxylic acid-gamma-lactone and triethyl orthoformate to generate 17 beta-hydroxyl-3,5-diene-3-ethoxy-17 alpha-pregnene-21-carboxylic acid-gamma-lactone; the catalyst is pyridine hydrobromide or pyridinium hydrochloride; under the presence of an organic solvent, the dehydrogenation reaction is carried out between an etherification reaction product and an oxidant to generate canrenone; the oxidant is tetrachloro-p-benzoquinone, tetrachloro-o-benzoquinone or 2,3-dichloro-5,6-dicyano-p-benzoquinone. Through the adoption of the preparation method, canrenone of which the purity is 99% or higher can be eventually obtained, and the total weight yield can reach 90% or higher. Therefore, the preparation method is suitable for industrialized production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Method for preparing 2,3-dihydro-1H-pyrrolo pyridine hydrochloride
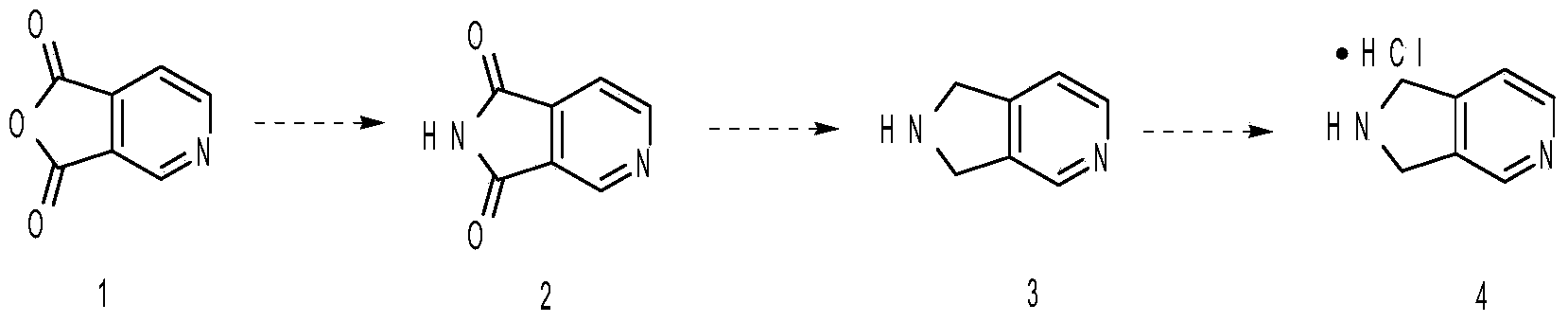
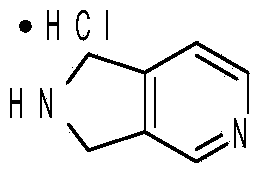
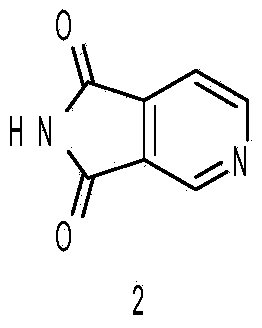
The invention discloses a method for preparing 2,3-dihydro-1H-pyrrolo-[3,4-c] pyridine hydrochloride. A target product is obtained through lactamization, reduction and salification by taking furo[3,4-c] pyridine-1,3-dione as an initial raw material, and the compound is an important medical intermediate.
Owner:湖南华腾制药有限公司
Vitamins carbon steel pickling inhibitor and application thereof
InactiveCN101994123APrevent over erosionAvoid excessive consumptionSurface cleaningVitamin B6 synthesis
The invention discloses a vitamins carbon steel pickling inhibitor used for preventing carbon steel and a product thereof from unnecessary corrosion and acid liquor consumption in the pickling process and application thereof. The inhibitor is one or a composite of vitamins organic compounds of vitamin B1 (chloride3-((4-amino-2-methyl-5-pyrimidyl) methyl) 5-(beta-ethoxyl-4-methyl) onium hydrochloride and vitamins B6 (2-methyl-3-hydroxy-4, 5-dihydroxymethyl pyridine hydrochloride). The application of the pickling inhibitor is shown as follows: a cleaning solution is a diluted hydrochloric acid or a dilute sulphuric acid, and the concentration of the cleaning solution is 0.10-1.0kmol / m<3>; the 0.010kg / m<3>-2.0kg / m<3> of cleaning solution is added; the temperature is controlled to be about 25 DEG C; the carbon steel or the product thereof to be cleaned is added and is immersed for 30min-4 hours. The product of the invention is used for surface cleaning of the carbon steel and the product thereof, can prevent the excessive erosion of metal and the excessive consumption of the acid liquor in the cleaning process and has the outstanding advantages of low use level, high efficiency and strong continuous action capacity.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Flux paste special for unleaded tin-bismuth solder and preparation method thereof
InactiveCN106001998AHigh activityImprove wettabilityWelding/cutting media/materialsSoldering mediaPolyamideSuccinic acid
The invention discloses flux paste special for unleaded tin-bismuth solder. The flux paste special for the unleaded tin-bismuth solder comprises, by weight, 10-20 parts of hydrogenated rosin, 5-10 parts of disproportionated rosin, 10-15 parts of dimer rosin, 5-10 parts of tetrahydrofurfuryl alcohol, 5-10 parts of pentaerythritol, 5-8 parts of butyl cellosolve, 5-8 parts of octyl ether, 5-8 parts of dibasic acid esters, 2-5 parts of phthalic acid dibutyl esters, 2-3 parts of oleyl alcohol polyoxyethylene ether, 3-6 parts of polyamide modified hydrogenated castor oil, 2-4 parts of succinic acid, 1-2 parts of pyridine hydrochloride, 1-2 parts of bromic acid tributylamine, 4-8 parts of DIACID 1550 and 1-3 parts of N,N,N,N'',N''-pentamethyldiethylenetriamine. The flux paste special for the unleaded tin-bismuth solder is good in wettability, the welding defect is lower than that of existing flux paste for the unleaded tin-bismuth solder, organic acid and polyamine decompose and volatilize in the welding process, the amount of residues generated after welding is small, and protective films can be formed on the surfaces of welding points through the used rosin.
Owner:丘以明
Preparation method of pyridine-functionalized chitosan adsorbent
InactiveCN110368911ALow costSpatial structure is stableOther chemical processesWater contaminantsSorbentStrong acids
The invention discloses a preparation method of a pyridine-functionalized chitosan adsorbent. The preparation method includes the following steps that S1, polyethyleneimine with the mass concentrationbeing 5-100g / L, 2-picolyl chloride hydrochloride with the mass concentration being 2.5-100g / L, and sodium carbonate with the mass concentration being 10-100g / L are dissolved in an aqueous solution ofethyl alcohol with the volume concentration being 5-80%. According to the preparation method of the pyridine-functionalized chitosan adsorbent, the pyridine-functionalized chitosan adsorbent can be applied to the fields such as simultaneous removal of heavy metal and antibiotics in livestock and poultry breeding wastewater under a wide pH range, the technical problems such as small adsorption capacity and slow adsorption rate in collaboratively adsorbing the heavy metal and the antibiotics, large resin demand in engineering, long operation time, small adsorption capacity or even no adsorptionunder strong acid conditions and the like are solved, and economic and environmental benefits are remarkable.
Owner:HAINAN NORMAL UNIV
Method for synthesizing pregnenolol acetate and congener thereof
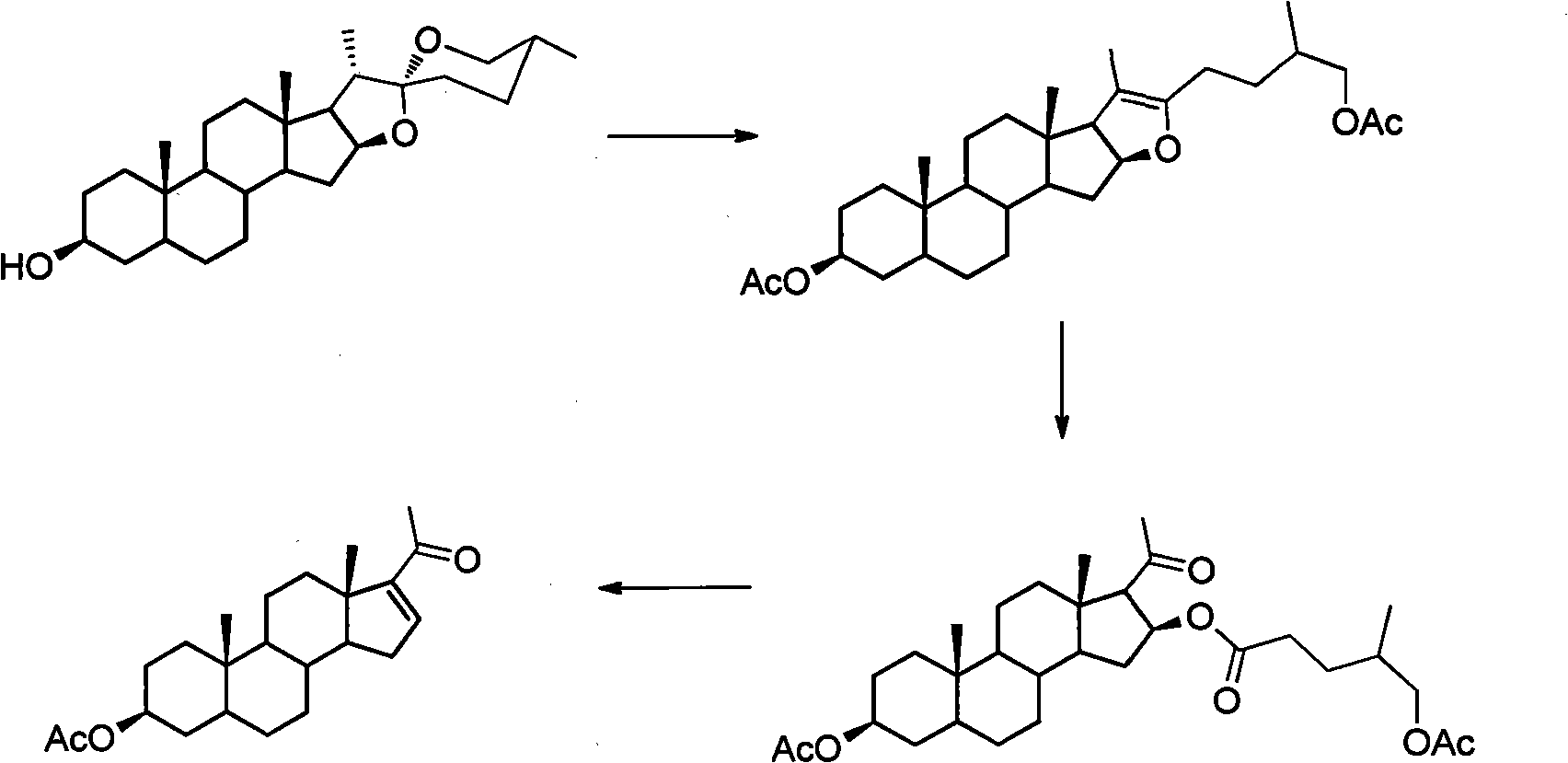
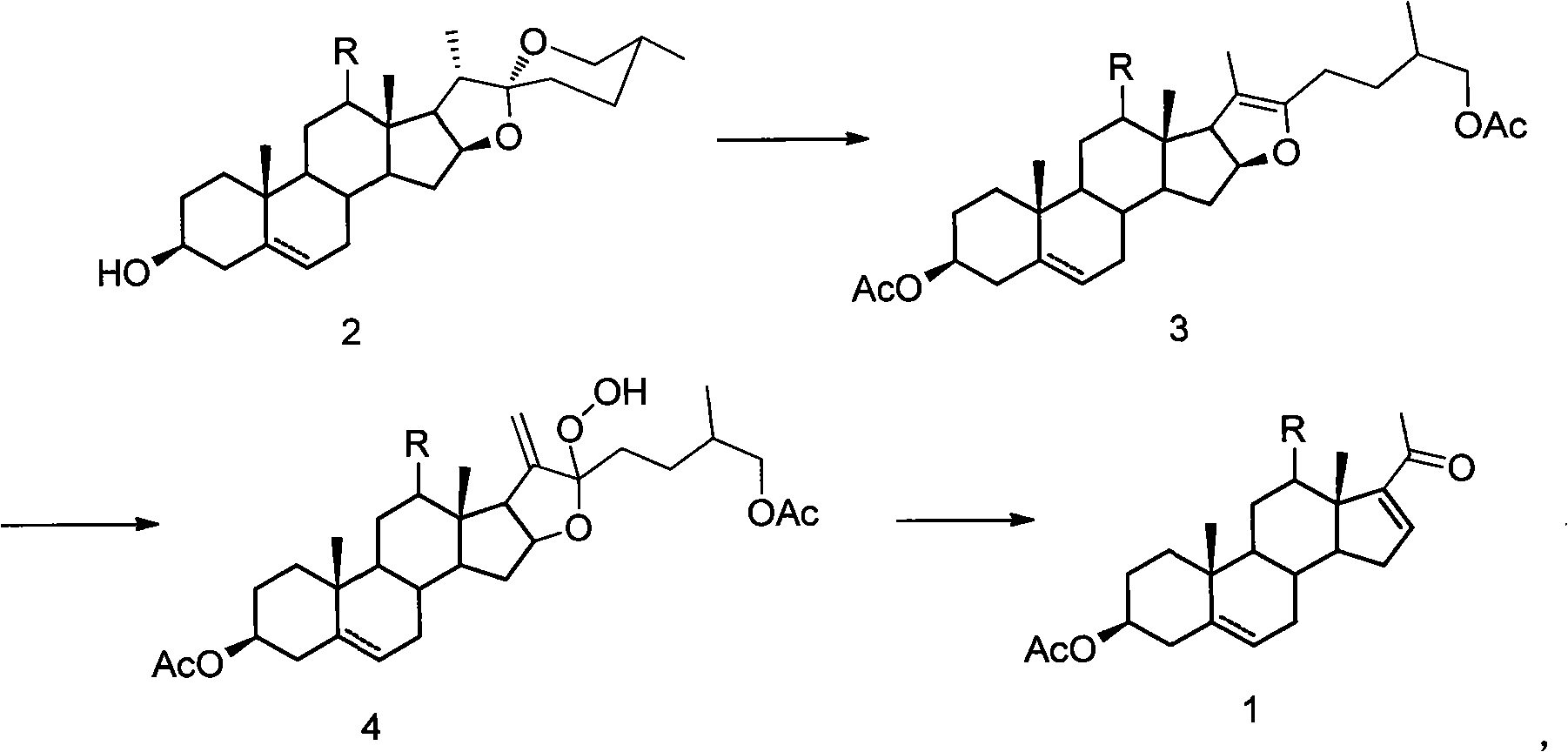
The invention relates to a method for synthesizing a pregnenolol acetate and a congener thereof. In the method, a three-step reaction consisting of cracking, oxygen oxidation and rearrangement and elimination is undergone in a 'one-pot' based on steroid sapogenin to obtain the pregnenolol acetate and the congener thereof. By adopting the method, the problem of environmental pollution caused by the use of a metal compound and high-toxicity pyridine hydrochloride is solved in the reaction, and the problem of the harm of high-toxicity solvents, including acetonitrile, benzene and the like to operating personnel is eliminated. The method has the advantages of simple equipment, easiness and convenience for operation, mild condition and high total yield. A more environmentally-friendly and simpler method is provided for the industrial production of pregnenolone acetate.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method of rabeprazole sodium crystal type compound
ActiveCN103232437AReduce selection costStable in natureOrganic chemistryHigh humiditySodium hydroxide
The invention relates to the field of medicine production and in particular relates to a preparation method of a rabeprazole sodium crystal type compound. The preparation method of the rabeprazole sodium crystal type compound comprises the following steps of: by taking 2-mercapto benzimidazole and 2-chloromethyl-3-methyl-4-methoxy propoxy pyridine hydrochloride as raw materials and carrying out a condensation reaction to prepare rabeprazole thioether, and subsequently carrying out an oxidation reaction via the rabeprazole thioether and carrying out salt forming reaction via rabeprazole and sodium hydroxide to acquire the rabeprazole sodium. According to the preparation method of the rabeprazole sodium crystal type compound, the raw materials are low in cost and easy to purchase; samples are basically invariant in related substances and contents under the condition with high temperature and high humidity; the articles are stable in property and low in overall manufacturing cost; and the preparation method of the rabeprazole sodium crystal type compound has the advantages of strictly controlling the temperatures in the steps, being less in by-products, high in yield and non-toxic, and also has the advantages of being short in production period and further improving the production efficiency.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +3
Cefalonium preparation method
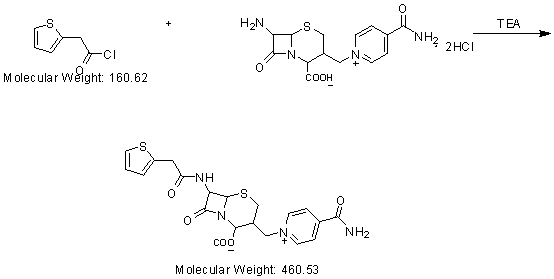
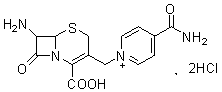
The invention discloses a cefalonium preparation method. The method is characterized by adopting 7-ICAC (1((7-amino-2-hydroxyl-8-oxo-5-thio-1-azabicyclo[4.2.0]octane-2-alkene-3-base)methyl)-4-carbamyl pyridine hydrochloride) and thiopheneacetyl chloride as raw materials, and reacting at the pH of 6-7 so as to obtain the product. The cefalonium provided by the invention is simple in preparation technology, simple to operate, high in yield, high in purity and suitable for industrial production and has a high application value.
Owner:CHONGQING TIAN TECH
Cation exchange membrane and preparation method thereof
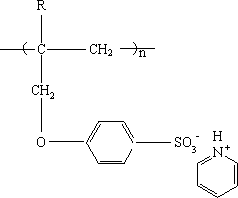
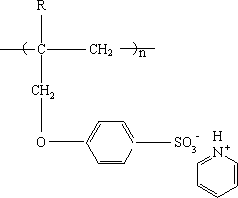
The invention relates to a cation exchange membrane and a preparation method thereof. The cation exchange membrane is characterized in that the membrane has a semi-interpenetrating network structure and uses a microporous membrane as the support material, membrane pores are filled with functional polymer, and the functional polymer has a structure formed by the polymerization of allyloxy pyridinebenzene sulfonate or alkyl allyloxy pyridine benzene sulfonate. The preparation method includes the steps of firstly, performing monomer preparation, to be more specific, allowing sodium 4-hydroxybenzenesulfonate to have reaction with 3-halogenated propylene or 3-halogenated-2-alkyl propylene, and allowing the acquired product to have reaction with pyridine hydrochloride to obtain a monomer solution; secondly, forming the membrane, to be more specific, mixing the monomer solution with a crosslinking agent, an initiator and comonomer to form membrane making liquid, filling the membrane making liquid into the pores of the microporous membrane support material, and heating to perform polymerization reaction to obtain the cation exchange membrane. The cation exchange membrane is applicable toelectrodialysis.
Owner:SHANDONG TIANWEI MEMBRANE TECH
Synthetic method of prasugrel
The invention relates to a synthetic method of prasugrel. 2-bromo-1-cyclopropyl-2-(2-fluorophenyl) acetone and 4,5,6,7-tetrahydro thieno [3,2-C] pyridine hydrochloride are condensed under the action of alkali, so that an intermediate, namely 1-cyclopropyl-2-(6,7-dihydro thieno [3,2-C] pyridine-5(4H)-yl)-2-(2-fluorophenyl) acetone, is obtained, and then oxidation and acylation are carried out, so that prasugrel is obtained. The synthetic method of prasugrel has the advantages that a prasugrel intermediate is synthesized, condensation yield is high, operating steps are simple, protection and deprotection steps in the prior art are eliminated, and cost is greatly saved.
Owner:广东暨大基因药物工程研究中心有限公司
Preparation technology of lansoprazole
The invention provides a preparation technology of lansoprazole. The preparation technology comprises the following steps that 1, a raw material A 2-mercapto benzimidazole is dissolved into methanol in the presence of alkali, a raw material B 2-chloromethyl-3-methyl-4-(2,2,2,-trifluoroethoxyl)pyridine hydrochloride is added for a reaction, filtering is conducted by adding water, obtained precipitates are washed and dried, and then an intermediate C [[[3-methyl-4-(2,2,2,-trifluoroethoxyl)-2-pyridyl]methyl]sulfydryl]-H-benzimidazole is obtained; the intermediate C is dissolved into ethanol, a mixed solution of hydrogen peroxide, a catalyst and ethanol is added for a reaction, filtering is conducted by adding water, obtained precipitates are washed, and then the lansoprazole is obtained. According to the preparation technology, the yield is increased, the cost is reduced, the production cycle is shortened, and the preparation technology is more suitable for industrialized production.
Owner:HENAN KANGDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine](https://images-eureka.patsnap.com/patent_img/2c48673a-3b10-4547-8a60-b0f7c56ce404/s2008100350419c00011.PNG)
![Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine](https://images-eureka.patsnap.com/patent_img/2c48673a-3b10-4547-8a60-b0f7c56ce404/s2008100350419c00012.PNG)
![Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine Method for preparing 5-(alpha-cyclopropyl carbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothiophene [3,2-c] pyridine](https://images-eureka.patsnap.com/patent_img/2c48673a-3b10-4547-8a60-b0f7c56ce404/s2008100350419c00021.PNG)