Novel preparation process of prasugrel hydrochloride
A prasugrel hydrochloride and process technology, which is applied in the field of medicine, can solve problems such as harsh reaction conditions, difficult raw material procurement, and cumbersome routes, and achieve the effects of easy-to-obtain raw materials, simple operation, and economical raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
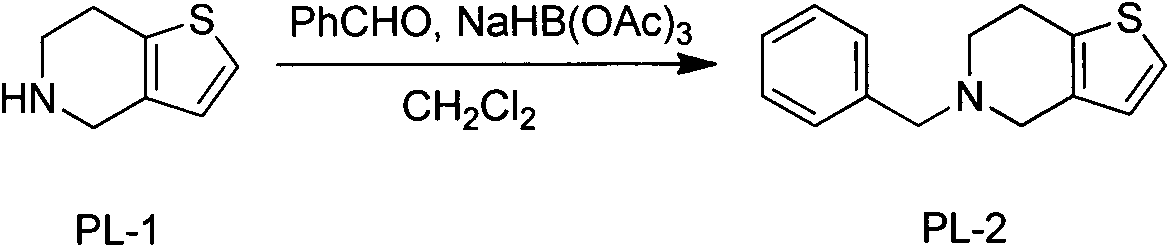
[0019] The preparation of embodiment 15-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0020] Take a 1000mL flask, place it in an ice bath at 0°C, pour 500mL of dichloromethane into it, and add 55.6g of accurately weighed 4,5,6,7-tetrahydrothieno[3,2-c]pyridine in sequence (0.4mol), benzaldehyde 42.4g (0.4mol), glacial acetic acid 2ml, stirred for 30min. Next, 170.0 g (0.8 mol) of sodium triacetoxyborohydride was added, the temperature was slowly raised to room temperature, and stirring was continued for 5 hours. After the reaction is finished, add sodium hydroxide solution to quench and neutralize to weak alkalinity. Continue to stir for 1 h, separate the organic layer, continue to extract the aqueous phase twice with dichloromethane, combine the organic phases, wash the organic phase with saturated sodium chloride, and concentrate the organic phase to obtain 5-benzyl-4,5,6,7-tetra Hydrothieno[3,2-c]pyridine 174g crude product, yield 95%.
Embodiment 2
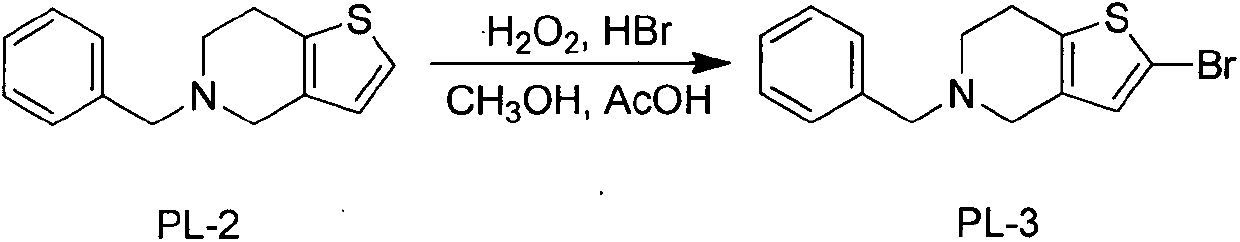
[0021] Synthesis of Example 22-bromo-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0022] Take a clean 5000mL three-necked flask, add 174g (0.76mol) of the compound 5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine crude product obtained in Example 1, and add 500mL of Acetic acid was dissolved, 40% hydrobromic acid 560ml, methanol 500ml, 30% hydrogen peroxide 247.5ml methanol (500ml) solution was added dropwise under ice water cooling, and stirred at room temperature for 3 hours. Add 1100ml of sodium thiosulfate solution dropwise, then add saturated sodium carbonate solution dropwise until the pH is 9, extract with dichloromethane, combine the organic layers, wash with water, dry, and concentrate to dryness to obtain 222.4g of a light yellow solid crude product, which is obtained by recrystallization 210g of 2-bromo-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine, yield 90%.
Embodiment 32
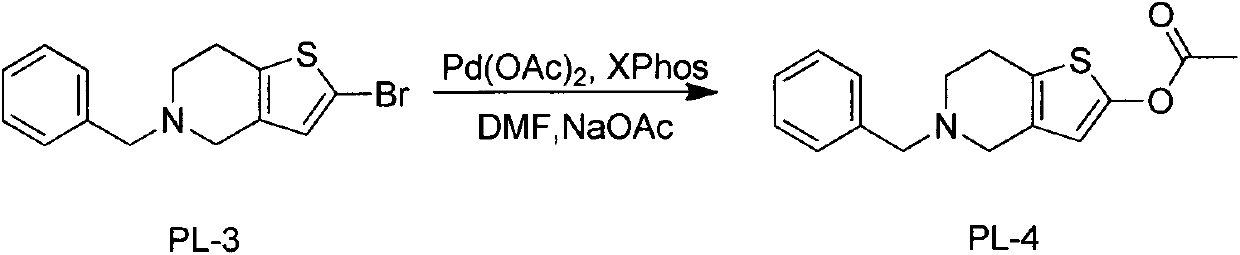
[0023] Example 3 Preparation of 2-acetoxy-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0024] Add 30.8g (0.1mol) of 2-bromo-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine into 180ml of DMF, stir to dissolve, then add acetic acid in sequence under nitrogen protection Palladium 1.23g (0.005mol), 2-dicyclohexylphosphorus-2', 4', 6'-triisopropylbiphenyl (XPhos) 4.76g (0.01mol), sodium acetate 32.8g (0.4mol), reaction The solution was slowly warmed up to 100°C, then stirred for 24 hours, and after the reaction was completed, 500ml of water was slowly added, a large amount of solids precipitated, stirred for 5 hours in an ice bath, filtered, the filter cake was washed with water, and dried to obtain 2-acetoxy-5-benzyl - 25.8 g of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com