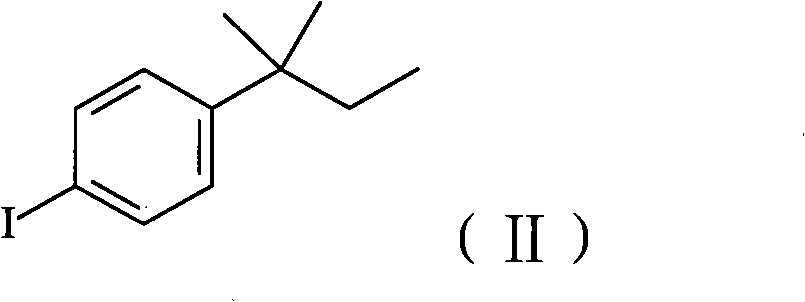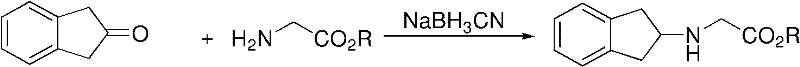Patents
Literature
70 results about "Sodium triacetoxyborohydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na(CH₃COO)₃BH. Like other borohydrides, it is used as a reducing agent in organic synthesis.
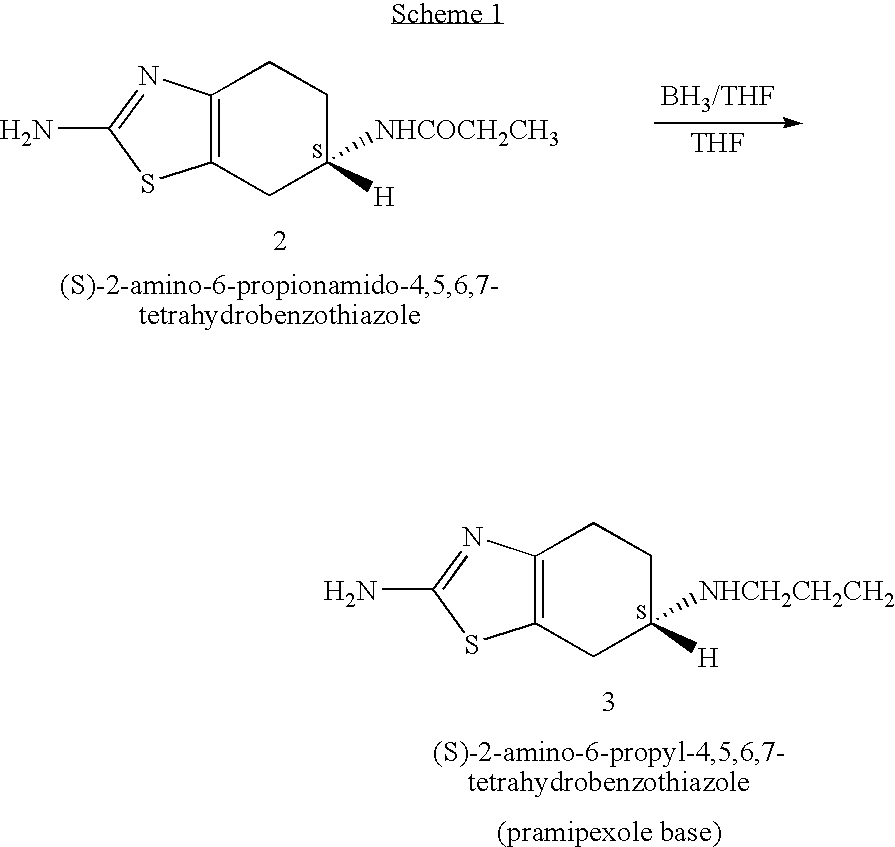
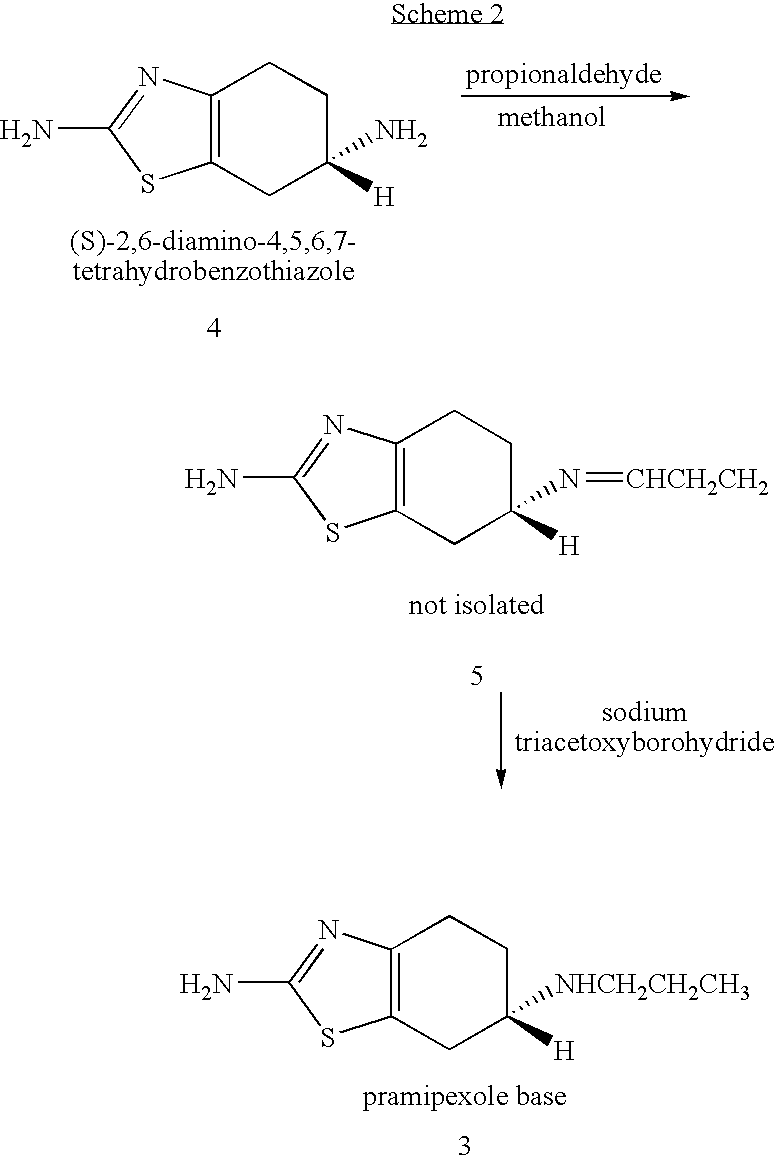
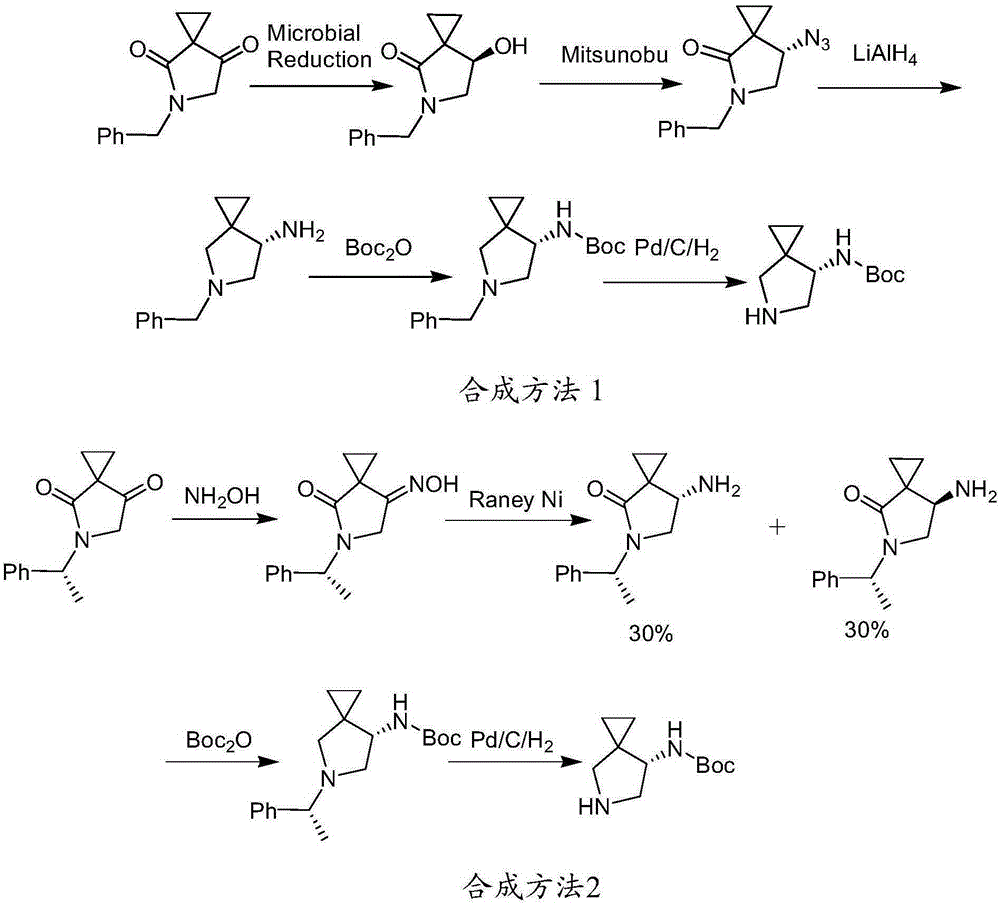
Novel process for preparing pramipexole and its optical isomeric mixture by reduction with sodium triacetoxyborohydride
InactiveUS20060148866A1Reduce usageBiocideOrganic active ingredientsOrganic solventSodium triacetoxyborohydride
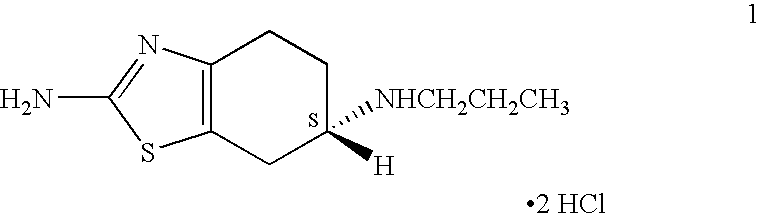
A novel process is provided for producing pramipexole base or its optical isomeric mixture as defined hereinabove i.e. (R,S)-2-amino-6-propyl-4,5,6,7-tetrahydrobenzothiazole avoiding the use of borane tetrahydrofuran complex and using a more convenient reducing agent like sodium triacetoxyborohydride instead. The provided process comprises reacting the starting material (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole or its optical isomeric mixture as defined hereinabove i.e. (R,S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole with propionaldehyde in an organic solvent to obtain the respective enamine, which is subsequently reduced in situ, optionally without isolation, to obtain pramipexole or its optical isomeric mixture as defined hereinabove i.e. (R,S)-2-amino-6-propyl-4,5,6,7-tetrahydrobenzothiazole, and the acid addition salts thereof. The present invention also provides a process for purifying pramipexole dihydrochloride or the dihydrochloride salt of its optical isomeric mixture as defined hereinabove i.e. (R,S)-2-amino-6-propyl-4,5,6,7-tetrahydrobenzothiazole dihydrochloride by re-crystallization from a suitable solvent.
Owner:CHEMAGIS
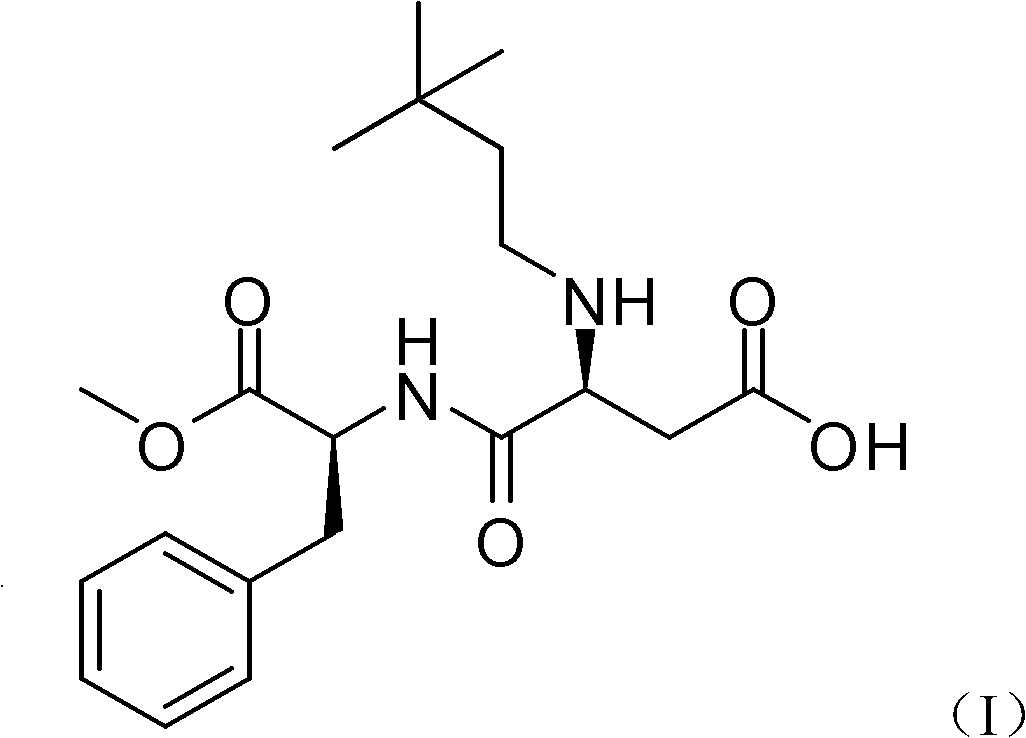
Method for preparing neotame
InactiveCN102167722AHigh purityMild responsePeptide preparation methodsSodium triacetoxyborohydrideNeotame
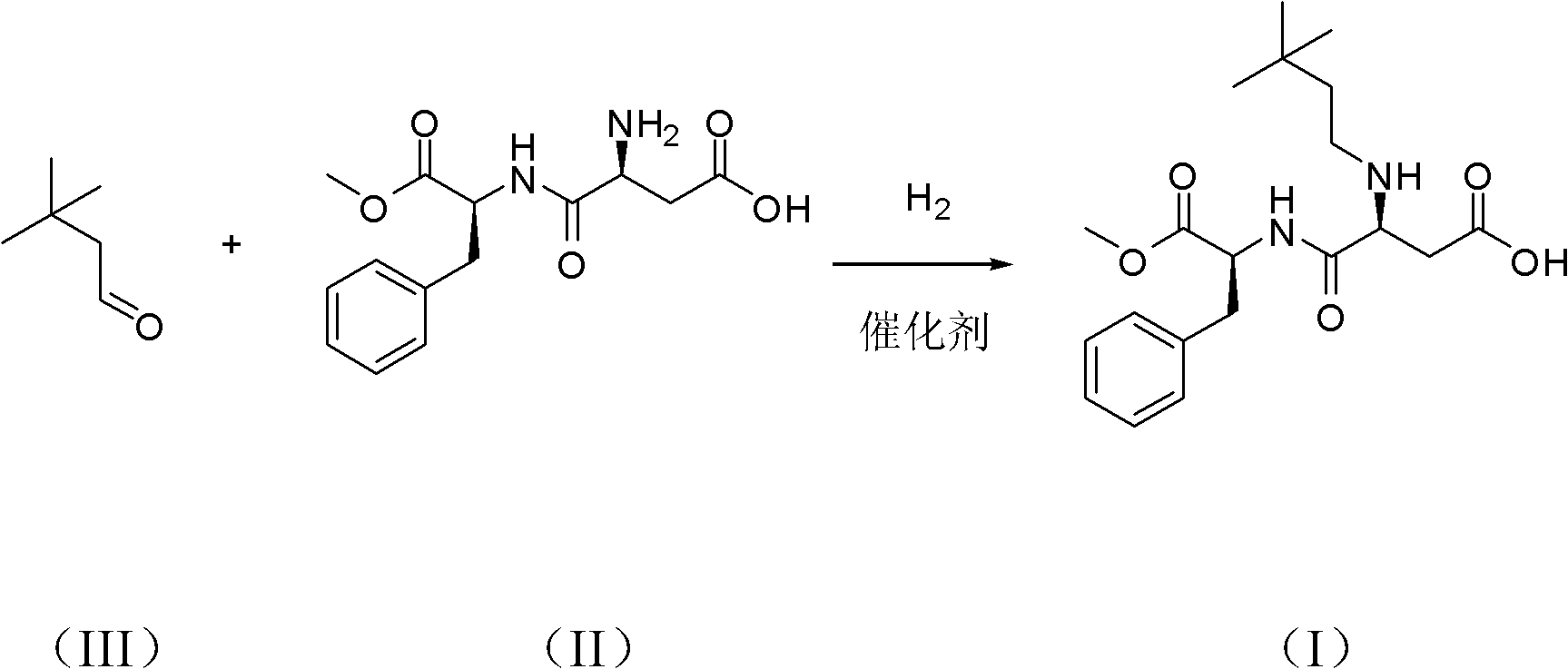
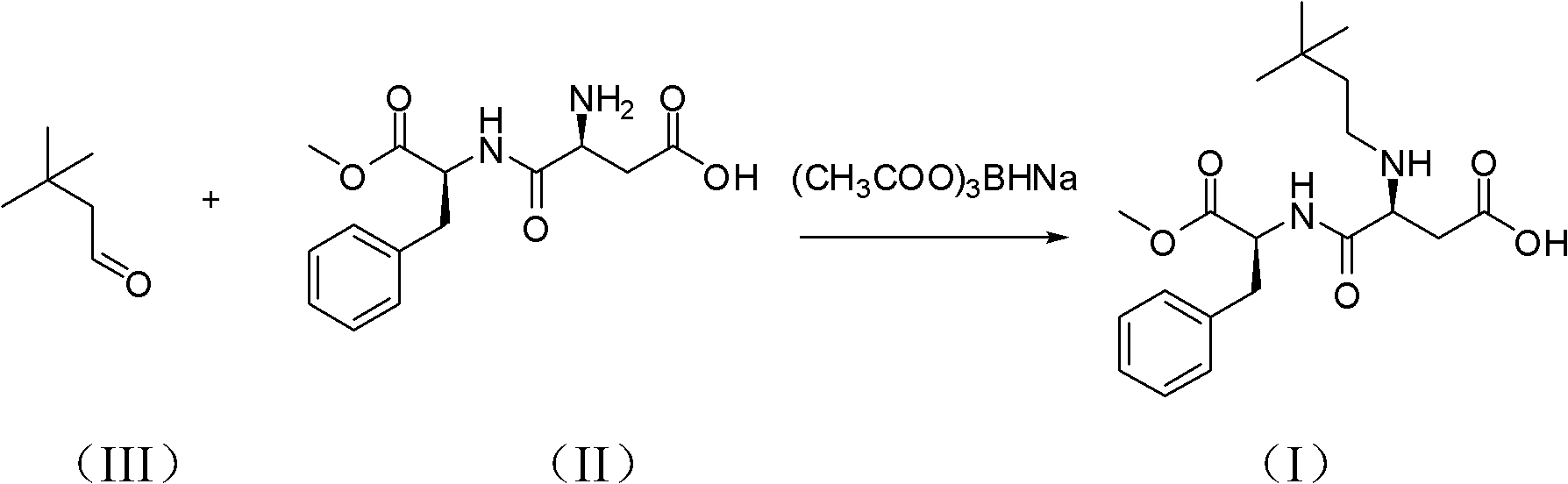
The invention belongs to chemical sciences, and in particular discloses a method for preparing neotame. The method comprises the following steps of: reducing reactants of aspartame and 3,3-dimethyl butyraldehyde to prepare neotame by taking sodium triacetoxyborohydride as a reducing agent; and reducing a reaction solution of the aspartame and the 3,3-dimethyl butyraldehyde, extracting and crystallizing to obtain the neotame. The method for preparing the neotame is easy and convenient to operate, environmental pollution is reduced, and an obtained product has high purity and high yield.
Owner:宁宗超
Ceramic metal material
The invention discloses a ceramic metal material which is composed of following raw materials including 10-15 parts of ferric oxide, 10-15 parts of aluminium oxide, 5-12 parts of nano silicon dioxide, 100-150 parts of kaolin, 80-100 parts of quartz sand, 5-10 parts of zinc oxide, 50-60 parts of zircon, 5-10 parts of silicon nitride, 5-8 parts of aluminium nitride, 20-25 parts of hydroxyapatite, 10-15 parts of paraffin, 5-10 parts of lithium borohydride, 5-10 parts of potassium borohydride and 3-8 parts of sodium triacetoxyborohyride. In the ceramic metal material, the raw materials are reasonably blended. The ceramic metal material is excellent in performance, is good in stability, is high-temperature-resistant and corrosion-resistant, is good in water-proof performance, is high in strength and can be applied in various fields.
Owner:黄惠娟
Method for synthesizing Varenicline intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine
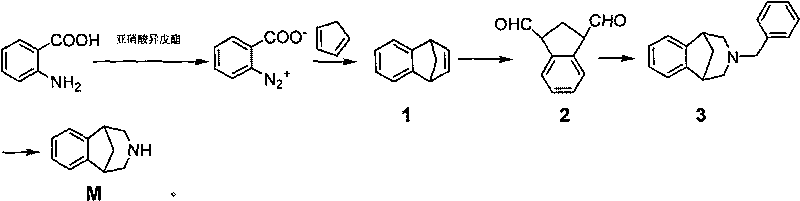
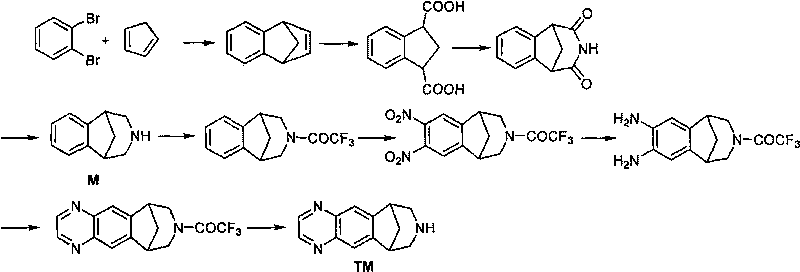
The invention relates to a method for synthesizing Varenicline intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine. The method includes the following steps: under the action of catalyst, mixing amyl nitrite with o-aminobenzoic acid solution to generate diazonium salt, then mixing the diazonium salt with cyclopentadiene, and heating to react to generate compound 1; feeding ozone into the solution of compound 1, after complete conversion, adding reducing agent to generate compound 2, then dripping the compound 2 to the mixed solution of triacetoxy sodium borohydride and benzylamine to generate compound 3 by loop closing; and hydrogenating the compound 3 under the action of palladium and carbon for debenzylation and reduction to obtain M intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine. The method has the advantages of greatly simplifying the method for preparing Varenicline intermediate, being simple in production process and safe in operation, well ensuring no harm to the environment and control on production cost, increasing yield and being capable of becoming a process in great industrial production.
Owner:上海立科化学科技有限公司
Method for preparing amorolfine hydrochloride
ActiveCN102887872AGood reaction selectivityReduce usageOrganic chemistryAcetic acidSodium triacetoxyborohydride
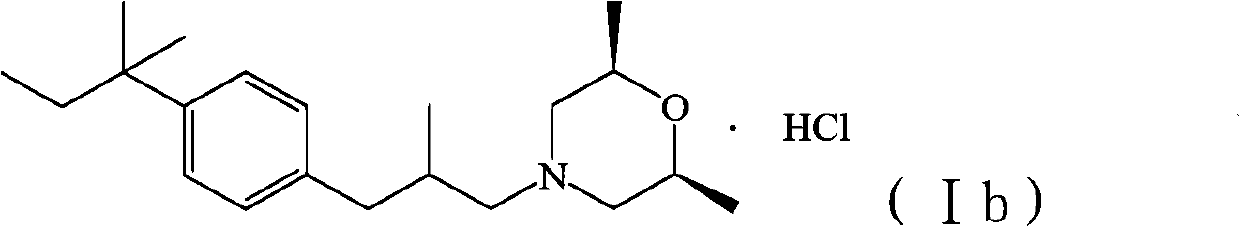
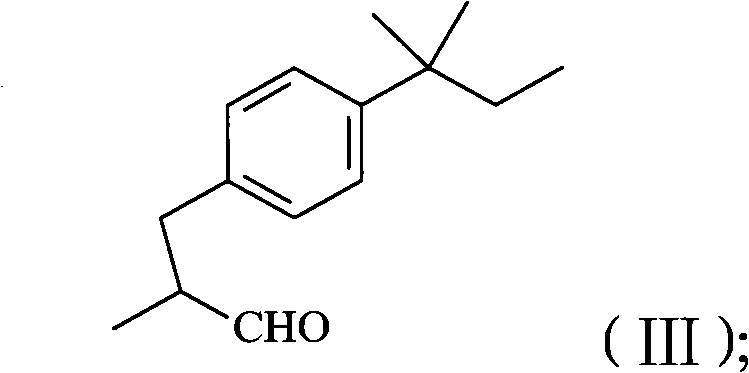
The invention relates to a method for preparing amorolfine hydrochloride. The method comprises the following steps of: reacting 4-iodo-tert-amylbenzene with 2-methylallyl alcohol by taking N-methylpyrrolidone as a solvent in the presence of a palladium catalyst and alkali to obtain 3-tert-pentylphenyl-2-methyl propanal; performing reductive amination reaction of the obtained 3-tert-pentylphenyl-2-methyl propanal and cis-2,6-dimethylmorpholine by taking sodium triacetoxyborohydride as a reducing agent in the presence of glacial acetic acid to obtain amorolfine; and transforming the amorolfine into the amorolfine hydrochloride. The invention has the advantages that the process is reasonable, is beneficial to the environment, health, and safety (EHS), and is suitable for industrialized production, wastewater is easy to biodegrade, the reaction selectivity is high, the high-yield high-purity product is obtained, and the like.
Owner:ZHEJIANG HISOAR PHARMA +1
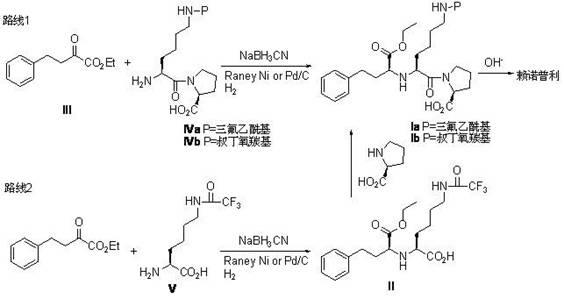
Method for preparing lisinopril intermediate
InactiveCN102617704AAvoid it happening againIncrease contentOrganic compound preparationCarboxylic acid amides preparationSolventLisinopril
The invention relates to a method for preparing a lisinopril intermediate. The method comprises the following steps of: mixing and stirring N-protecting group-L-lysine or N-protecting group-L-lysine-L-proline, sodium triacetoxyborohydride, glacial acetic acid and a reaction solvent uniformly; cooling, dripping a mixed solution of alpha-oxo-phenylbutyrate and the reaction solvent, and continuing to react; reacting at room temperature for 2 to 6 hours; and adding water, extracting, recrystallizing, filtering and drying to obtain the lisinopril intermediate, wherein the sequence of dripping raw materials and the sequence of reaction can be adjusted. According to the method, NaBH3CN is replaced by NaBH(OAc), so that the generation of toxic side products is avoided; and the method is environment-friendly, and the reaction is performed under normal pressure, so that the efficiency and safety are improved, the content of an S isomer in the obtained product is improved, and the cost is reduced.
Owner:JIANGXI DIRUI SYNTHETIC CHEM
Cinacalcet hydrochloride preparation method
InactiveCN103467304AOvercoming technical biases usedOvercoming technical biasAmino compound purification/separationPreparation by reductive alkylationSolventPollution
The present invention discloses a cinacalcet hydrochloride preparation method, which comprises: 1, adopting 3-(trifluoromethyl) phenyl propionaldehyde and R-1-(1-naphthyl)ethylamine as raw materials, and carrying out a condensation reduction reaction in a mixing reaction solvent in the presence of sodium triacetoxyborohydride, 2, extracting the reaction product with water, a salt or an alkali aqueous solution to obtain a cinacalcet base organic phase solution, and 3, carrying out acidification salification on the obtained cinacalcet base organic phase solution with hydrochloric acid to obtain the cinacalcet hydrochloride, wherein one of components in the mixing reaction solvent is water. According to the present invention, the water-containing mixing solvent is adopted as the reaction medium, such that technical defects of solvent use during the sodium triacetoxyborohydride use process are overcome, the harsh condition that the reaction solvent requires a water-free treatment is avoided while reaction selectivity is increased, types of produced impurities and impurity content are substantially reduced, cost is reduced, and environment pollution is reduced.
Owner:NANJING LIFENERGY R & D
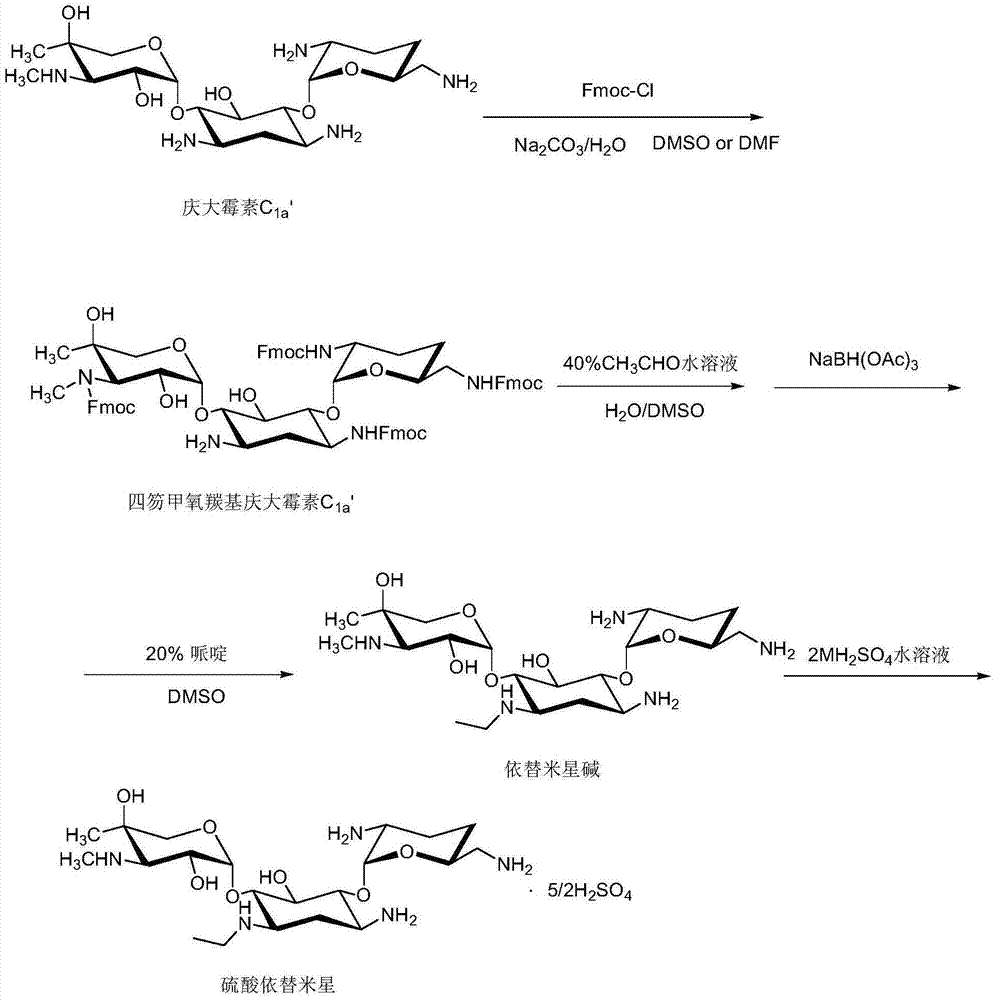
Etimicin sulfate preparation method
ActiveCN104231016ALow impurity contentAcid environment stableSugar derivativesSugar derivatives preparationSodium triacetoxyborohydrideGentamicin C1a
The invention discloses an etimicin sulfate preparation method. The etimicin sulfate preparation method includes the steps of protecting amino groups at 3, 2', 6' and 3' of gentamicin C1a with Fmoc-Cl and performing purification and evaporation to dryness to obtain quadrifluorenyl methoxycarbonyl gentamicin C1a; adding N-ethyl of an acetaldehyde aqueous solution under acidic conditions and performing reduction with sodium triacetoxyborohydride; finally adding a piperidine / DMSO (dimethylsulfoxide) solution for deprotection; performing secondary purification on an reaction solution with weakly acidic cation adsorption resin after performing primary purification on the reaction solution with a macroporous adsorption resin column and adding sulfuric acid to a purified product to obtain etimicin sulfate through reaction. The etimicin sulfate preparation method is high in selectivity and less in side reaction in group protecting, mild in reaction conditions, simple in operation and easy to perform industrial production during the whole reaction process and high in purity and yield of the product. (An equation as shown in the description).
Owner:QILU PHARMA HAINAN +1
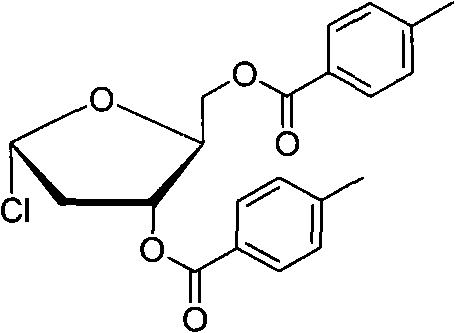
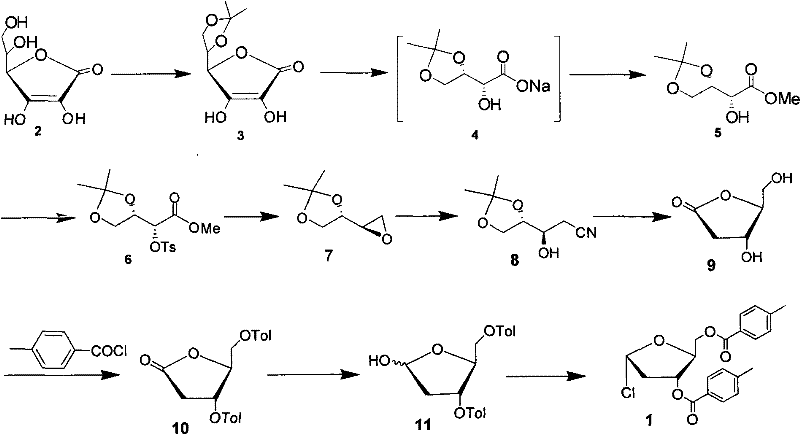
Technology for preparing key intermediate of telbivudine
InactiveCN102477051AReduce pollutionHigh yieldEsterified saccharide compoundsSugar derivativesVitamin CTosylhydrazone
The invention discloses a new technology for preparing a key intermediate Hoffer's chlorosugar of telbivudine. The technology is characterized in that the technology comprises the following steps: 1, obtaining an epoxide by carrying out condensation, oxidation ring-opening, methylation, tosylation, reduction and epoxidation on vitamin C which is massively supplied in China and is treated as an initial raw material; 2, carrying out ring-opening cyaniding on the obtained epoxide; 3, carrying out sulfuric acid hydrolysis and lactonization on the generated cyan butanetriol derivative; and 4, carrying out hydroxy protection with p-toluoyl chloride, reducing with sodium triacetoxyborohydride to obtain a reduction product, and directly chloridizing the reduction product to obtain the target compound Hoffer's chlorosugar without separation, wherein the Hoffer's chlorosugar is 1-choro-3,5-bis-O-p-toluyl-2-deoxy-L-ribose. According to the technology, the total route yield is 42%, and the product purity is greater than 98%.
Owner:FUAN PHARMA LYBON PHARMA TECH
Teneligliptin synthesis method
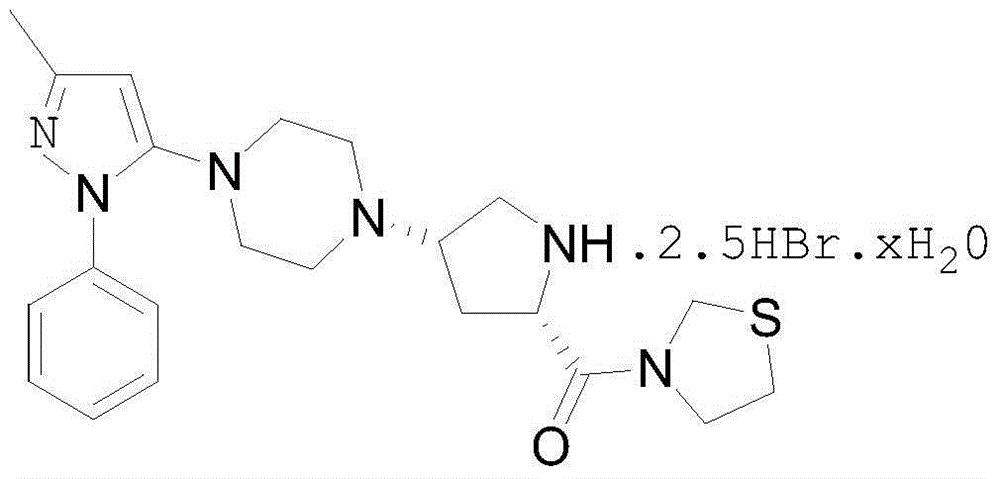
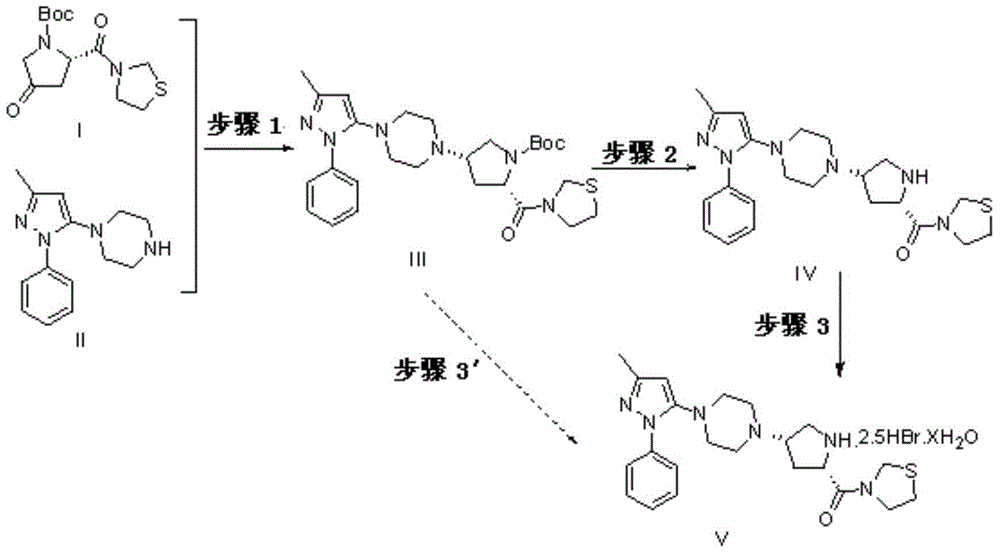
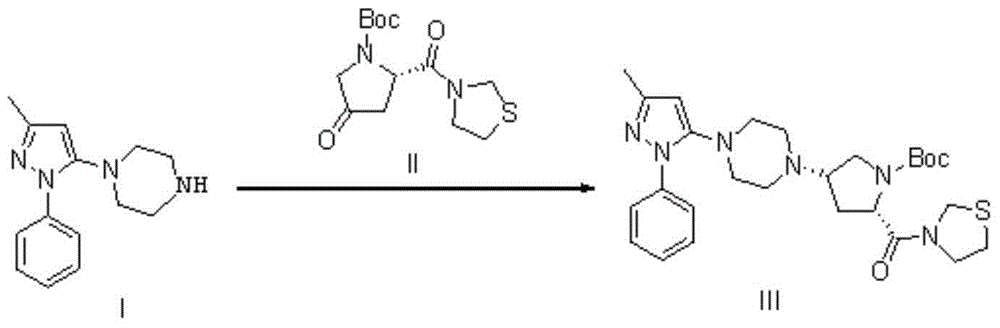
The present invention discloses a Teneligliptin synthesis method. The method comprises the following steps: (1) in the presence of an organic solvent and a catalyst of sodium triacetoxyborohydride, carrying out reduction on 1-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazine (in formula I) and 3-[(2S)-1-(1,1-dimethyl-acetyl carbonyl)-4-oxo-pyrrolidin-2-ylcarbonyl]thiazolidine (in formula II) into an intermediate (in formula III), wherein the solvent is a mixed solution of tetrahydrofuran and toluene; and (2) using sodium tablets to treat the mixed solution of tetrahydrofuran and toluene. According to the Teneligliptin prepared by the method disclosed by the present invention, the operation is simple, the yield is high, and the product purity is high; and the present method disclosed by the invention can remove basic impurities which require column chromatography to remove in the reaction, so that the process steps are simplified, the production cycle is shortened, and the total yield is relatively high; so that the synthesis method provided by the invention is more conducive to industrial production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
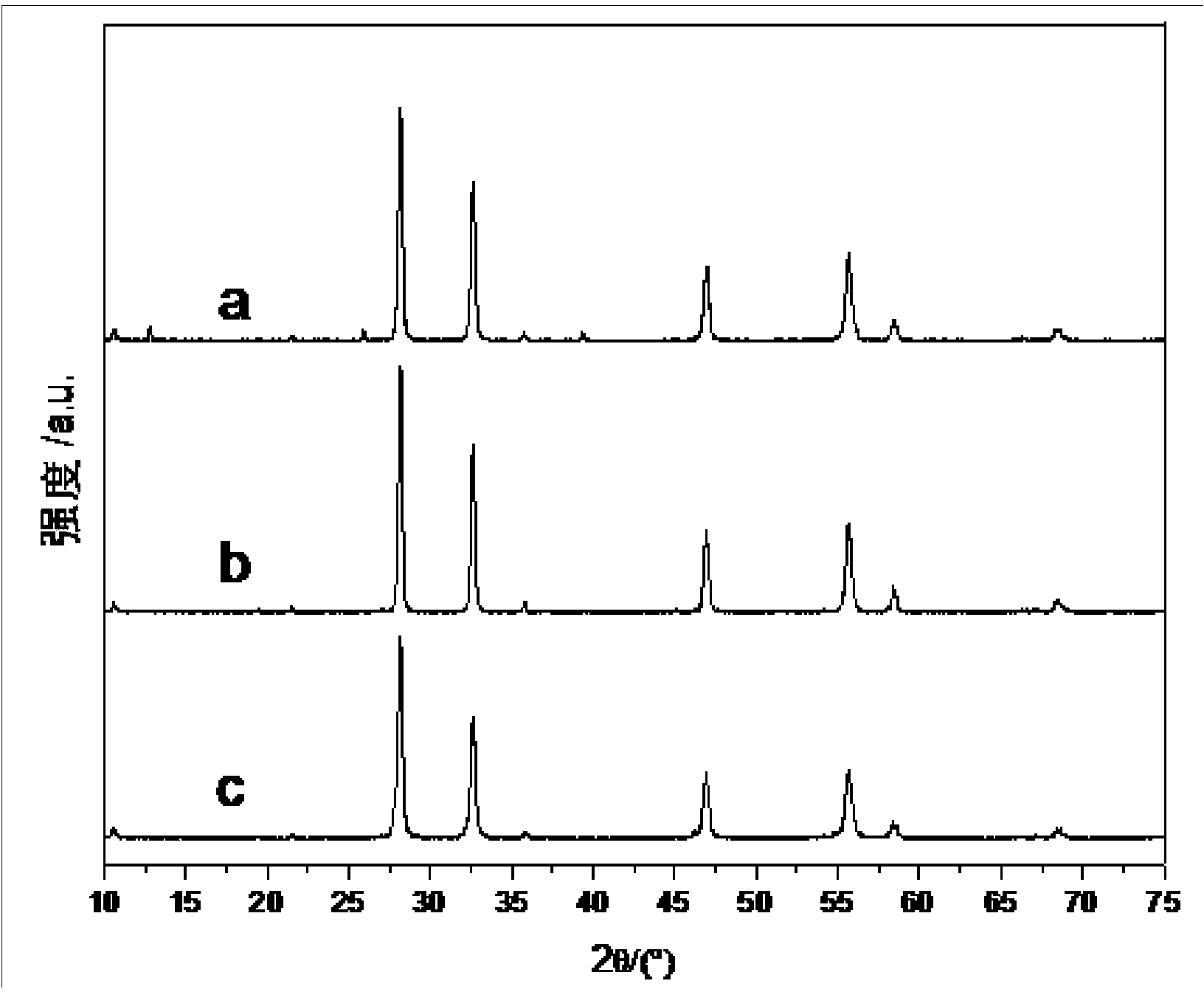
Nanometer bismuth tungstate with hollow square ball structure and preparation method thereof
InactiveCN103950985ANoveltySimple preparation processMaterial nanotechnologyTungsten compoundsTungstatePollution
The invention discloses nanometer bismuth tungstate with a hollow square ball structure and a preparation method thereof. The preparation method is characterized in that a hollow square ball formed by stacked bismuth tungstate nanosheets is prepared by using bismuth nitrate pentahydrate as a bismuth source, sodium tungstate dihydrate as a tungsten source and an STAB (Sodium triacetoxyborohydride)-containing alcohol-water solution as a dispersing agent and utilizing ammonia water and sodium hydroxide under a hydrothermal condition, and the thicknesses of the nanosheets are about 30nm; and the inner diameter of the square ball is about 1.5 micrometers, and the outer diameter of the square ball is about 2 micrometers. The preparation method has the advantages of simplicity, easiness in operation, strong repeatability, environment protection, no toxicity and harmlessness in a preparation process, novel product morphology, high phase purity and the like and has a high application value in terms of pollution treatment, ray absorption and the like.
Owner:SOUTHWEAT UNIV OF SCI & TECH
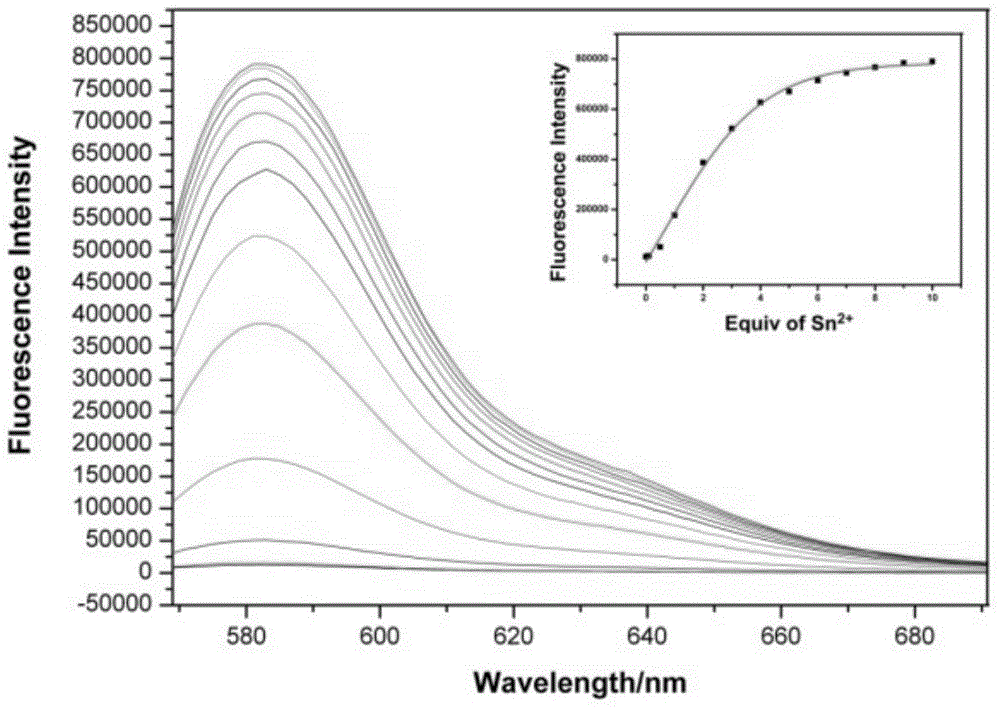
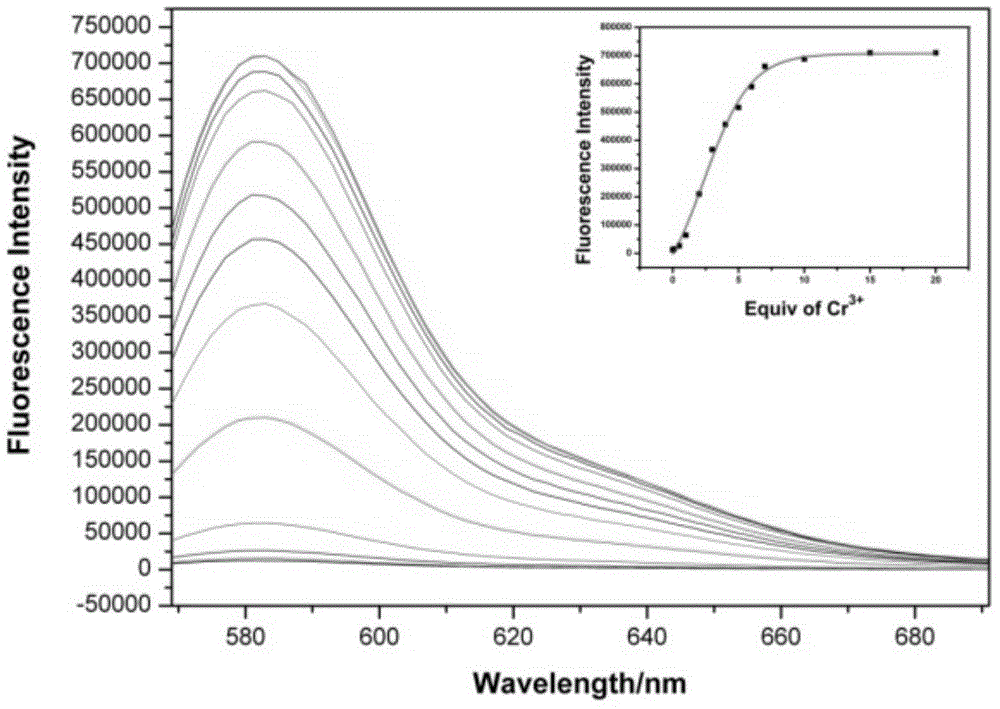
Rhodamine B-based fluorescence sensor and preparation
InactiveCN104447774ASimple structureGood choiceOrganic chemistryFluorescence/phosphorescenceRotary evaporatorTetrahydrofuran
The invention discloses a rhodamine B-based fluorescence sensor and preparation. The preparation comprises the following steps: dissolving 3-(3',6'-bi(diethyl amino)-3-oxaspiro[isoindoline-1,-1,9'-xanthene]-2-yl) propanal (A) in methyl alcohol, adding 6-aminocaproic acid (B), and stirring at room temperature; dissolving sodium triacetoxyborohydride in tetrahydrofuran, adding to a reaction liquid in the step 1, and further carrying out stirring reaction at room temperature; dropwise adding a saturated sodium bicarbonate solution to the reaction liquid, and further stirring; and removing a solvent by virtue of a rotary evaporator, and separating by using a thin layer chromatography, so as to obtain a target product. The rhodamine B-based fluorescence sensor is relatively simple in synthesis step; resources are saved; and nearly all the reported fluorescence probes can only be applied to an organic solvent or an organic water solution, thus the application of the fluorescence sensor in the fields of actual water sample analysis and life is limited.
Owner:NANJING UNIV OF SCI & TECH
Novel preparation process of prasugrel hydrochloride
ActiveCN103694251AEasy to operateSuitable for industrial scale-up productionOrganic chemistryPrasugrel HydrochlorideSodium triacetoxyborohydride
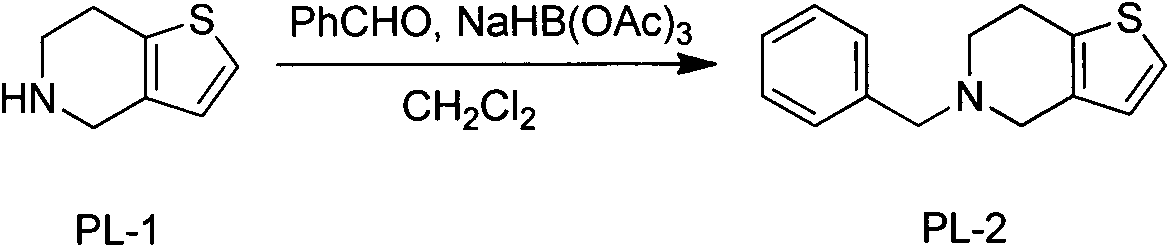
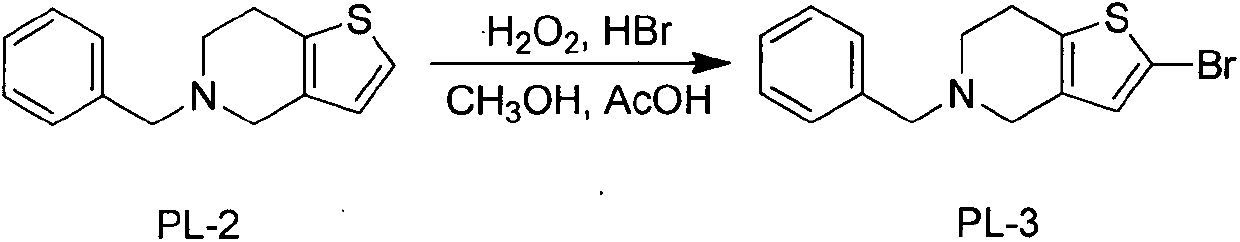
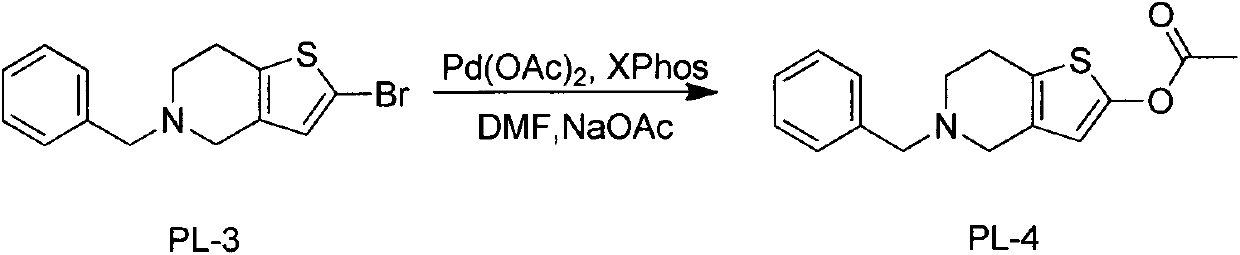
The invention discloses a novel synthesis process of prasugrel. The novel synthesis process comprises the steps: firstly, carrying out benzyl protection by taking 4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine, benzaldehyde and sodium triacetoxyborohydride as starting raw materials to generate 5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine; then, brominating the 5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine by using hydrobromic acid to obtain 2-bromo-5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine; next, catalytically synthesizing 2-acetoxy-5-benzyl-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine by taking palladium acetate as a catalyst and XPhos as a ligand; and finally, carrying out hydrodebenzylation, and coupling the product with alpha-bromo-o-fluorobenzyl cyclopropyl ketone to obtain a target molecule 2-acetoxy-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine hydrochloride, i.e., the prasugrel hydrochloride. The process is simple, convenient and novel in route, easy to obtain raw materials, mild in condition, convenient to operate, high in total yield up to 80-90% and suitable for large-scale production.
Owner:南京恒道医药科技股份有限公司
Ceramic metal material
The invention discloses a ceramic metal material. The raw materials in parts by weight include: 5-10 parts of iron oxide, 10-15 parts of aluminum oxide, 6-10 parts of nano silicon dioxide, 80-120 parts of kaolin, and 80-120 parts of quartz sand 100 parts, 4-8 parts of zinc oxide, 30-50 parts of zircon, 4-8 parts of silicon nitride, 4-6 parts of aluminum nitride, 5-10 parts of molybdenum trioxide, 4-8 parts of magnesium, hydroxyapatite 15-25 parts of stone, 10-14 parts of paraffin, 10-15 parts of titanium, 10-14 parts of lithium borohydride, 4-8 parts of potassium borohydride, 3-5 parts of sodium triacetoxyborohydride, polystyrene particles 30‑40 servings. The composition of the present invention is reasonable, the ratio is coordinated, and the ceramic metal material produced has excellent performance, good stability, excellent high temperature resistance, corrosion resistance and waterproof performance, and can be used in various fields, and the present invention has good wettability, Reduce the fragility of the material and effectively enhance the strength of the material.
Owner:SHENYANG LIGONG UNIV
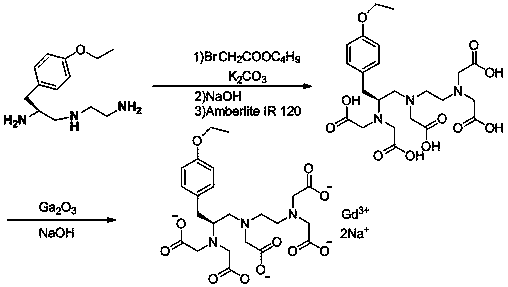
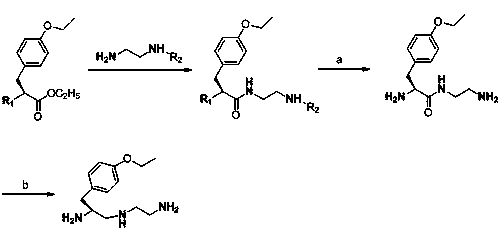
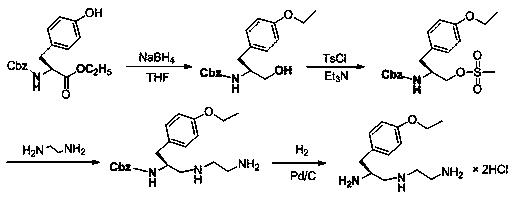
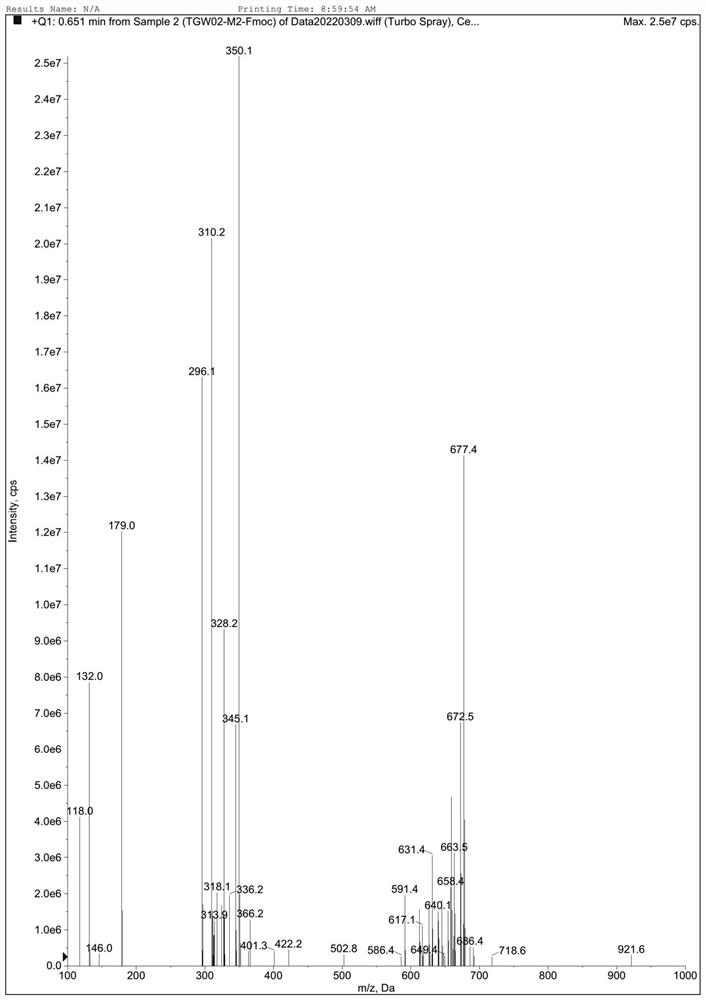
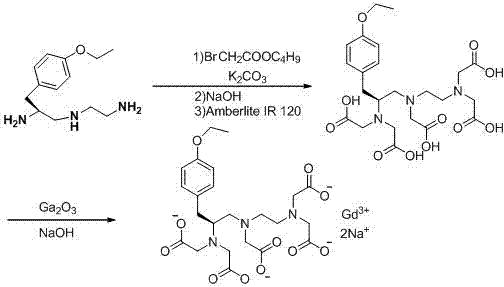
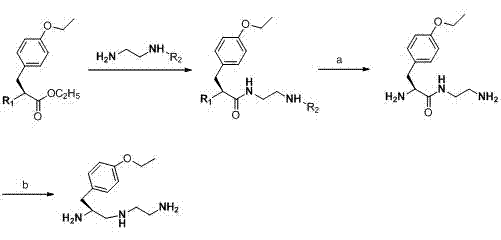
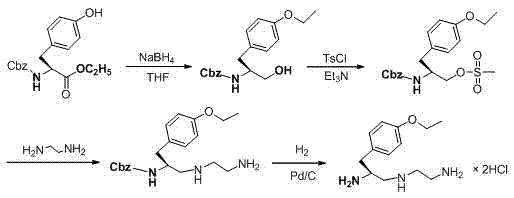
Synthesis method of (S)-1-(4-ethyoxyl benzyl)-3-azapentane-1,5-diamine trihydrochloride
InactiveCN103864630ANovel stepsEasy to purifyOrganic compound preparationBulk chemical productionTyrosineEthyl group
The invention provides a synthesis method of a gadoxetate disodium key precursor-(S-1-(4-ethyoxyl benzyl)-3-azapentane-1,5-diamine trihydrochloride. The synthesis method comprises the following steps: taking O-ethyl-N-Boc-L-tyrosine ethyl ester (a compound 1) as a material; reducing the material by red aluminum to obtain O)-ethyl-N-Boc-L-tyrosine aldehyde (a compound 2); then obtaining 1,5-Boc double-protection (S)-1-(4-ethyoxyl benzyl)-3-azapentane-1,5-diamine (a compound 3) by virtue of amination and reduction with N-Boc-1,2-ethanediamine under catalysis of sodium triacetoxyborohydride; finally, removing Boc protective groups through concentrated hydrochloric acid or a hydrogen chloride gas so as to obtain the (S)-1-(4-ethyoxyl benzyl)-3-azapentane-1,5-diamine trihydrochloride (a compound 4). According to the synthesis method disclosed by the invention, synthesis route steps are novel and concise, dear and dangerous special reagents are not needed, reaction is gentle, a product is easy to purify, after-treatment is convenient, and therefore, the synthetic method is suitable for industrial production.
Owner:FUZHOU UNIV
Preparation method of rafenasin intermediate
PendingCN114573500AReduce pollutionThe reaction conditions are mild and safeOrganic chemistryBulk chemical productionFormic Acid EstersCarbamate
Owner:YANGZHOU ZHONGBAO PHARMA
Synthesis method of (S)-1-(4-ethyoxyl benzyl)-3-azapentane-1,5-diamine trihydrochloride
InactiveCN103864630BNovel stepsEasy to purifyOrganic compound preparationBulk chemical productionTyrosineEthyl group
Owner:FUZHOU UNIV
Preparation method of sitafloxacin hydrate five-membered ring side chain intermediate
The invention relates to a preparation method of a sitafloxacin hydrate five-membered ring side chain intermediate. The preparation method comprises following steps: keto carbonyl groups of a raw material 1 are reacted with sodium cyanoborohydride or sodium triacetoxyborohydride in the presence of ammonium acetate or ammonium chloride; reduction of amide carbonyl groups of an obtained production is realized with lithium aluminum hydride; free amino groups of a reduction product are reacted with di-tert-butyl dicarbonate ester in the presence of an alkali; phenethyl groups of an obtained compound are subjected to reductive destruction with formic acid or a formate in the presence of palladium-carbon so as to obtain the sitafloxacin hydrate intermediate (product 5). Reaction conditions of the preparation method are mild; equipment requirements are low; preparation process is safe; stereoselectivity is excellent; the raw material reagents are cheap and easily available; and production cost is low.
Owner:广州朗启生物科技有限公司
Preparation method of high-molecular-weight cellulose acetate butyrate
ActiveCN112521516AHigh molecular weightSave operating timeChemical recyclingPtru catalystButyric anhydride
The invention discloses a preparation method of high-molecular-weight cellulose acetate butyrate, which comprises the steps of 1) conducting activation, specifically, soaking cellulose powder in acetic acid to obtain activated cellulose powder; 2) conducting esterification, specifically, enabling the activated cellulose powder to react with acetic anhydride and butyric anhydride under the condition of an acid catalyst to obtain an esterification reaction solution; 3) conducting neutralization, specifically, adding a neutralizer into the esterification reaction liquid to neutralize the acid catalyst into salt; and 4) conducting reduction, specifically, adding sodium triacetoxyborohydride into the neutralized esterification reaction solution to partially reduce the ester group into hydroxyl,thereby obtaining the high-molecular-weight cellulose acetate butyrate. The method avoids cellulose acetate butyrate glucose unit breakage and molecular degradation caused by hydrolysis by adding water, and also avoids the problems of difficult separation and high metal content caused by adding a heterogeneous catalyst.
Owner:WANHUA CHEM GRP CO LTD
Method and device for preparing L-selenocystine by using sodium triacetoxyborohydride as reducing agent
ActiveCN111004162AIncrease profitLess side effectsOrganic chemistrySodium triacetoxyborohydrideSodium selenide
The invention belongs to the field of chemical conversion, and particularly discloses a method and a device for preparing selenocystine under mild conditions. The method specifically comprises the following steps: mixing selenium powder, a strong alkaline substance, water and sodium triacetoxyborohydride, adding a 3-chloro-L-alanine aqueous solution, and carrying out a stirring reaction; and afterthe reaction is finished, adding an acid, filtering, adding an alkali into the obtained filtrate to adjust the pH value, and carrying out standing filtering to obtain filter residues, ie., selenocystine. According to the invention, the method has the advantages of few side reactions, high raw material utilization rate, and almost no generation of hydrogen or other undesired reactions; and a sodium diselenide solution is prepared by using sodium triacetoxyborohydride as a reducing agent under a strong alkaline condition, so that the reaction is mild, safe and reliable, the operation is simpleand feasible, the reaction process is mild and controllable, and the method has good application prospect.
Owner:JINAN UNIVERSITY
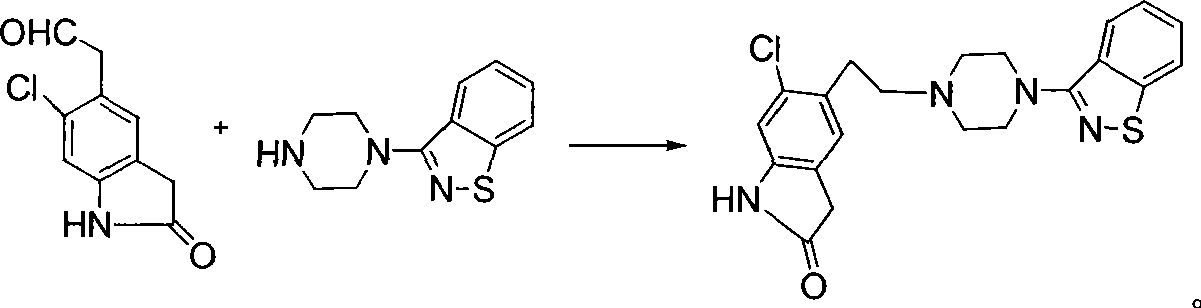
Synthetic method of ziprasidone
The invention provides a new method for synthesizing ziprasidone. The method comprises the following steps: 2,5-dichlorotoluene is taken as a starting material to be condensed with N,N-dimethyldimethoxymethylamine after being nitrified; an obtained intermediate is converted to an acetal compound in the presence of oxalic acid and glycol; and the acetal compound is reacted with ethyl malonate and then is subjected to decarboxylation, reduction and cyclization to form a key intermediate 7; the compound 7 is subjected to deprotection under an acidic condition to from an aldehyde 8; and the aldehyde 8 is reacted with 3-piperazinyl-1,2-benzisothiazole in the presence of sodium triacetoxyborohydride to form the ziprasidone. The invention has the advantages of easy and simple control, readily available raw materials and convenient operation.
Owner:ZHEJIANG HISUN PHARMA CO LTD
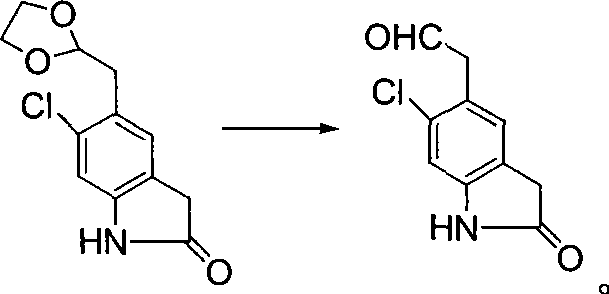
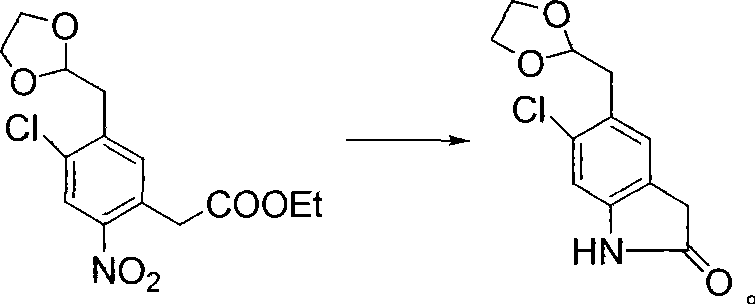
Preparation method of B cell lymphoma factor-2 inhibitor ABT-199
InactiveCN108997333AFew reaction stepsHigh yieldOrganic chemistryAntineoplastic agentsSodium triacetoxyborohydrideTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of a B cell lymphoma factor-2 inhibitor ABT-199, and belongs to the field of pharmaceutical synthesis. The method comprises the following steps: substituting a starting material methyl 4-fluorosalicylate that is compound II with tert-butyl-1-piperazinecarboxylate that is compound III in the presence of a phase transfer catalyst, then de-protecting underthe acidic condition to obtain a compound IV, performing reductive amination on the compound IV and a synthesized key intermediate compound V in the presence of a catalyst (sodium triacetoxyborohydride) to obtain an intermediate VI, substituting the intermediate VI with a raw material VII that is 5-bromo-azaindole, then directly hydrolyzing and acidifying the obtained esterified product to obtainan intermediate VIII, and finally amidating the intermediate VIII and a raw material compound IX to obtain a target product compound I. The raw materials are easy to purchase or synthesize, the synthesis steps are short, the total yield is more than or equal to 40%, the use of expensive or difficult-to-purchase raw materials is avoided, and the method is suitable for industrial production.
Owner:JIANGSU ZHONGBANG PHARMA
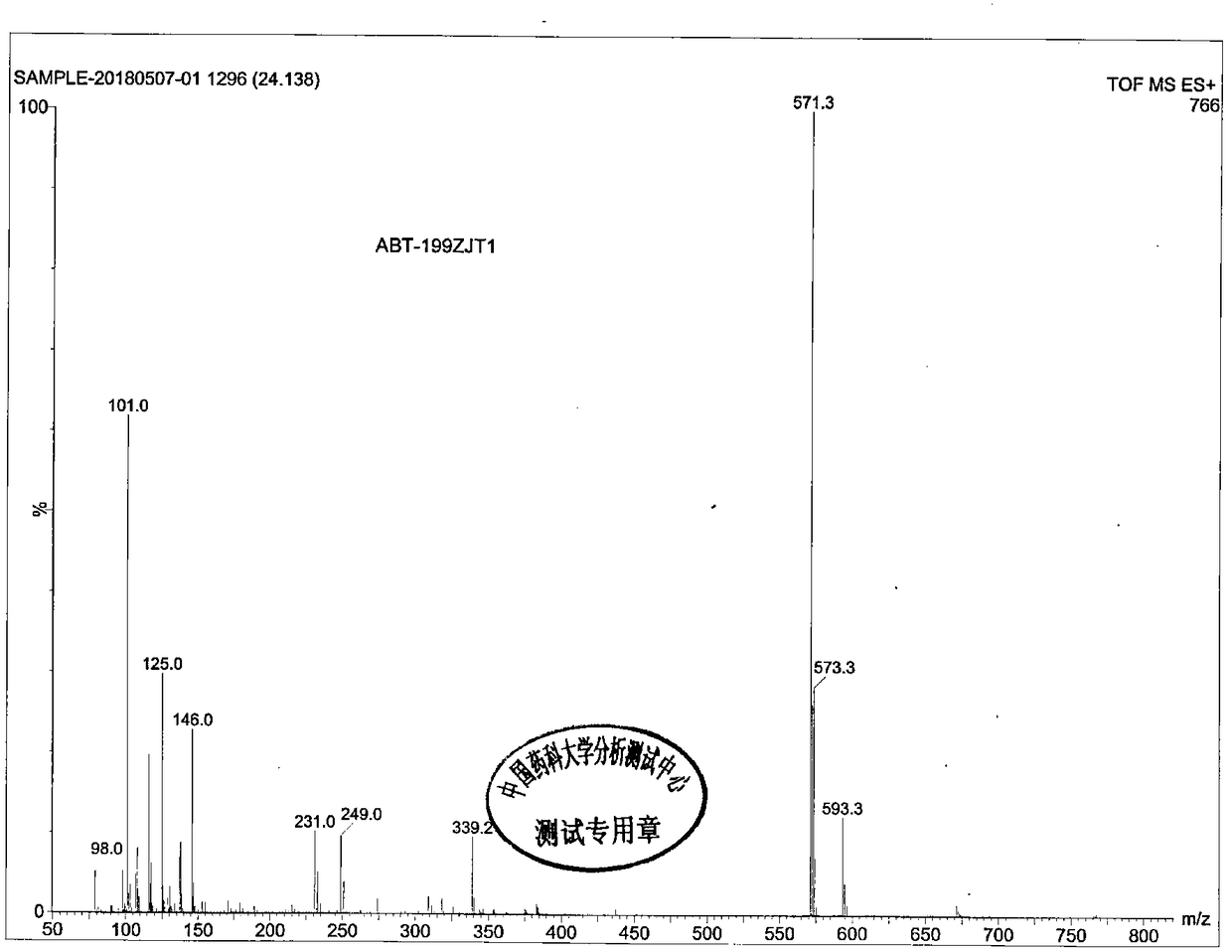
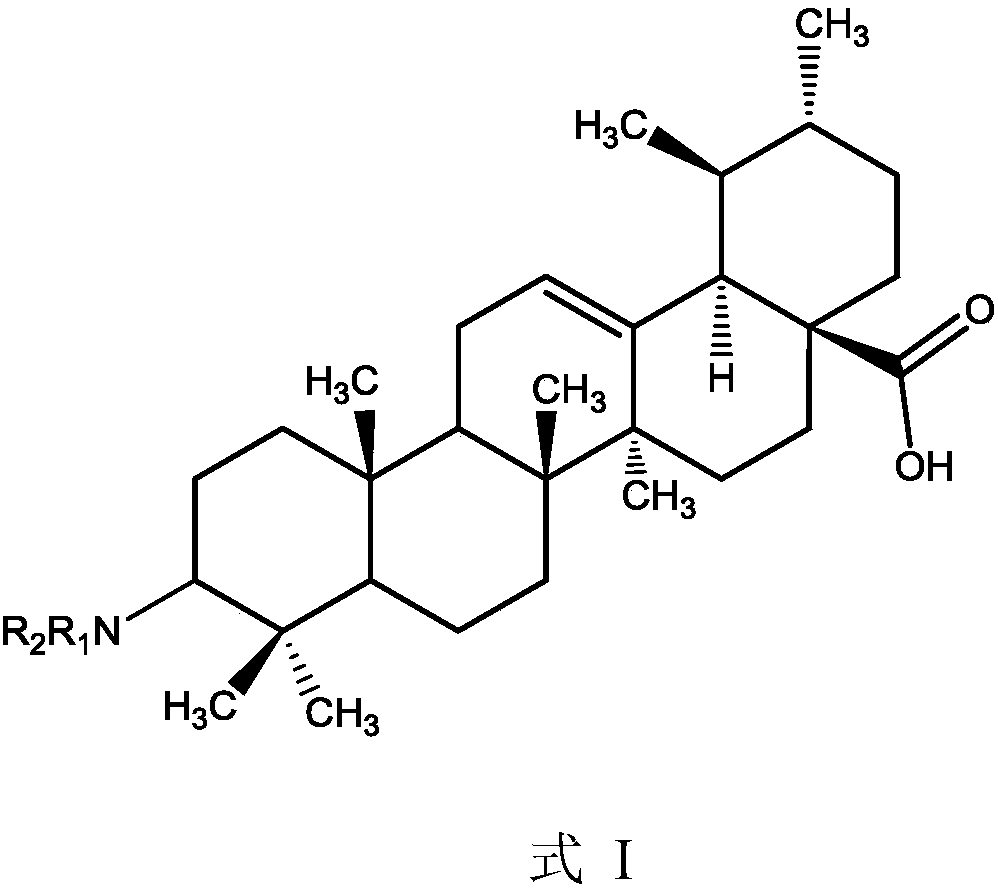
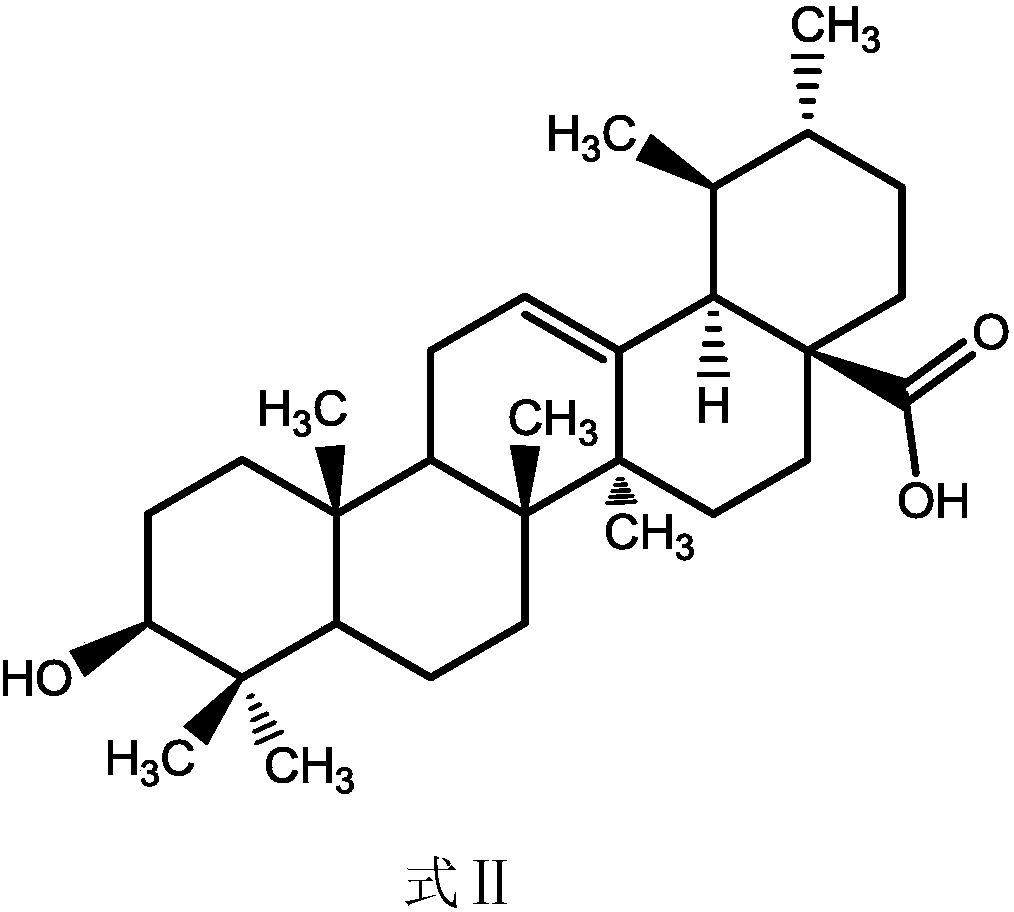
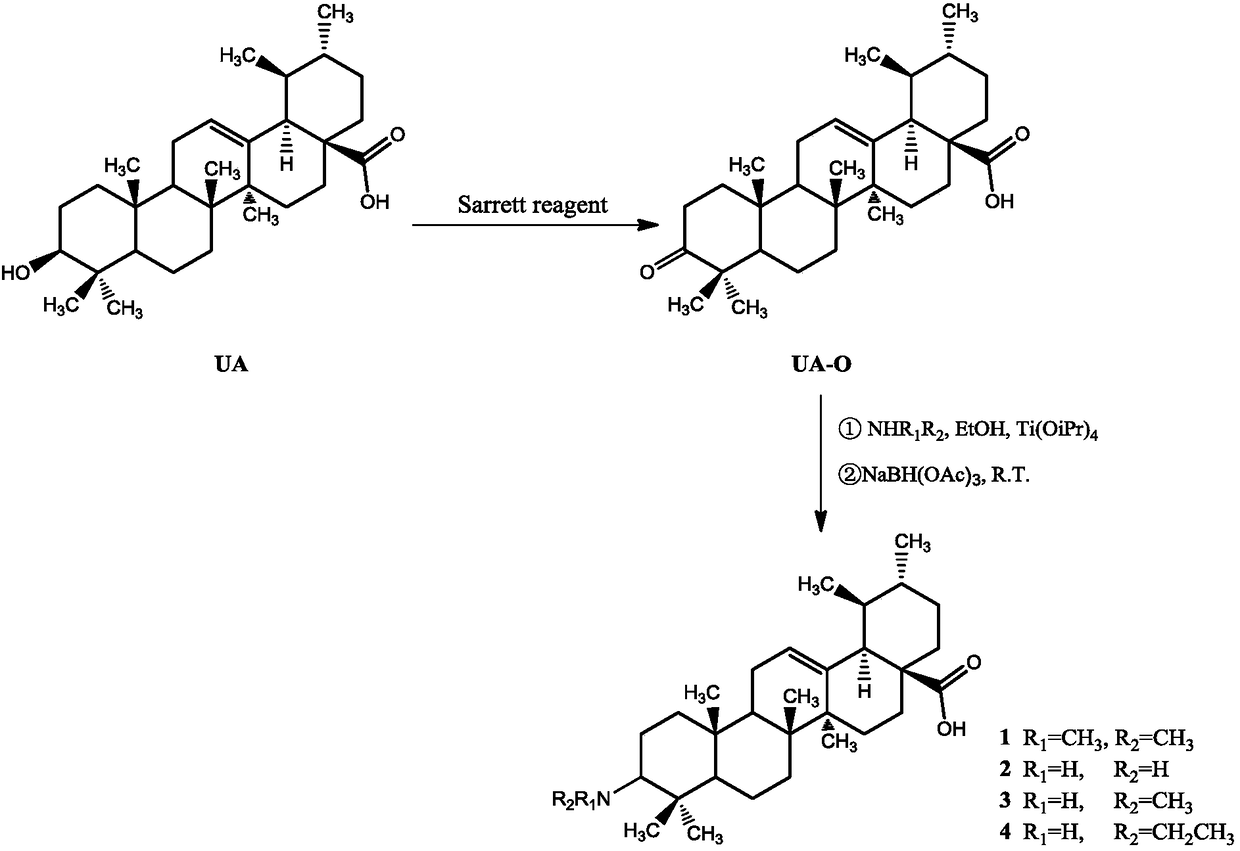
Ursolic acid derivative, preparation method thereof, and application thereof in preparation of drug for treating MRSA infection
ActiveCN108822179AGuaranteed not to be oxidizedOxidation mildAntibacterial agentsSteroidsSolubilitySodium triacetoxyborohydride
The invention discloses an ursolic acid derivative, a preparation method thereof, and an application thereof in the preparation of a drug for treating methicillin-resistant Staphylococcus aureus (MRSA) infection. The preparation method comprises the following steps: oxidizing ursolic acid by a Sarrett reagent to obtain a 3-hydroxy oxidation product of ursolic acid, and carrying out reductive amination on the 3-hydroxy oxidation product of ursolic acid by isopropyl titanate and sodium triacetoxyborohydride in order to prepare the ursolic acid derivative. Anti-MRSA activity test proves that theMIC of the obtained ursolic acid derivative on MRSA test strains is 16-32 [mu]g / mL, the anti-MRSA activity of the ursolic acid derivative is 4-8 times higher than that of the ursolic acid, and the derivative can form a salt together with equimolar hydrochloric acid in order to significantly improve the water solubility. The ursolic acid derivative has a significant anti-MRSA activity and a good development prospect, and can be used to prepare the drug for treating the MRSA infection.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Preparation method and application of one group of pyrazolyl steroid derivative with antitumor activity
InactiveCN106866773AStrong inhibitory activityHigh yieldOrganic active ingredientsSteroidsProgesteronesAmination
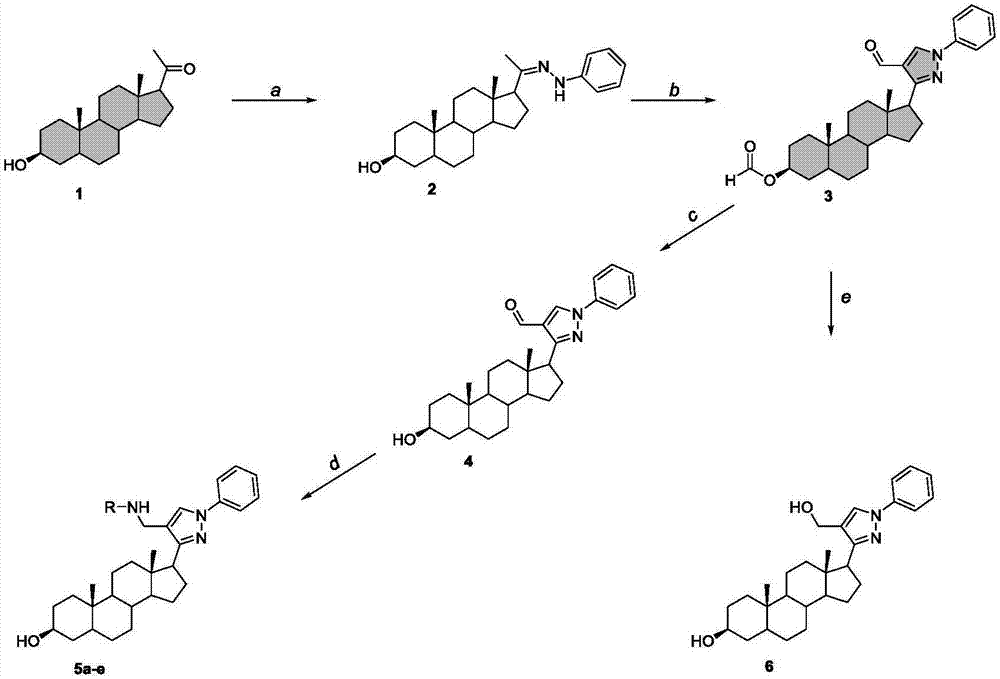
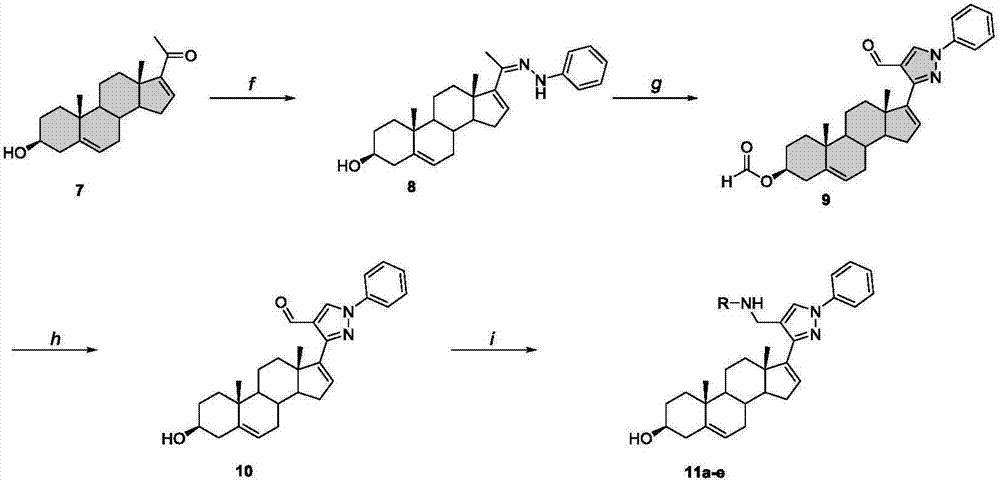
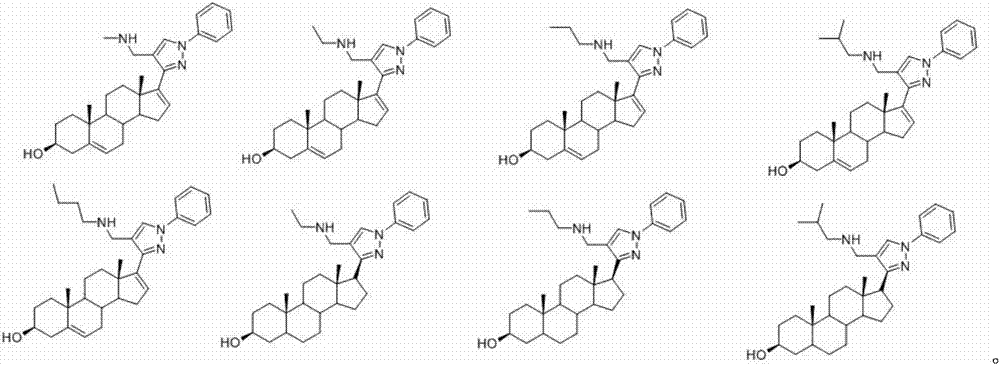
The invention discloses a preparation method and application of one group of pyrazolyl steroid derivative with antitumor activity, which belong to the technical field of medicine synthesis. The preparation method comprises the steps of generating phenylhydrazones 2 and 8 under the catalysis of isopregnenolone 1,5,16-diene progesterone and phenylhydrazine acetic acid; then carrying out ring closing reaction under the catalysis of phosphorus oxychloride to obtain compounds 3 and 9 with pyrazolyl heterocycle; then hydrolyzing to obtain compounds 4 and 10 without formyl groups; carrying out reduction with sodium borohydride to obtain 6; carrying out amination reduction reaction on the intermediate products 4 and 10 and amine under the catalysis of sodium triacetoxy borohydride to obtain 5a-e and 11a-e. The preparation method provided by the invention has the advantages of novel route, high yield and easiness for separation, and the route is an optimal route for preparing the compound.
Owner:NORTHWEST A & F UNIV
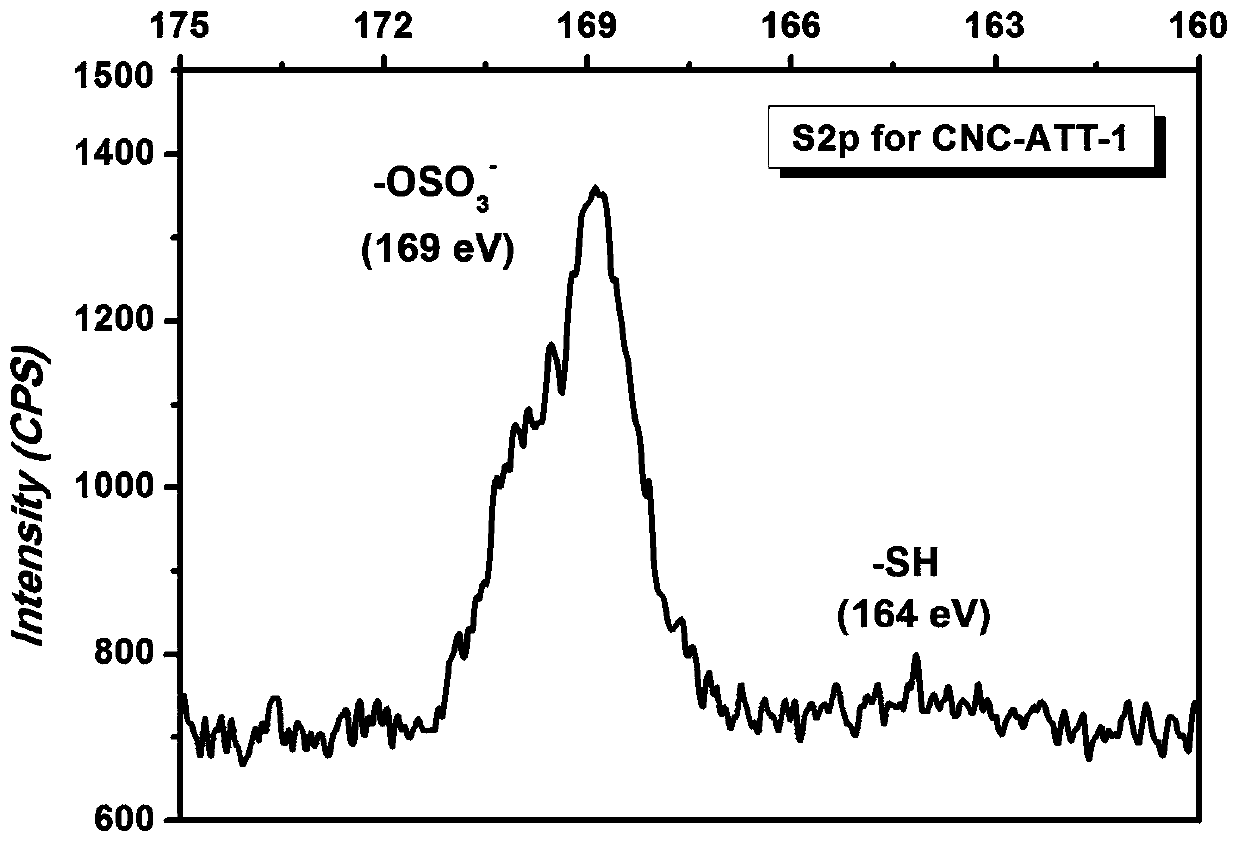
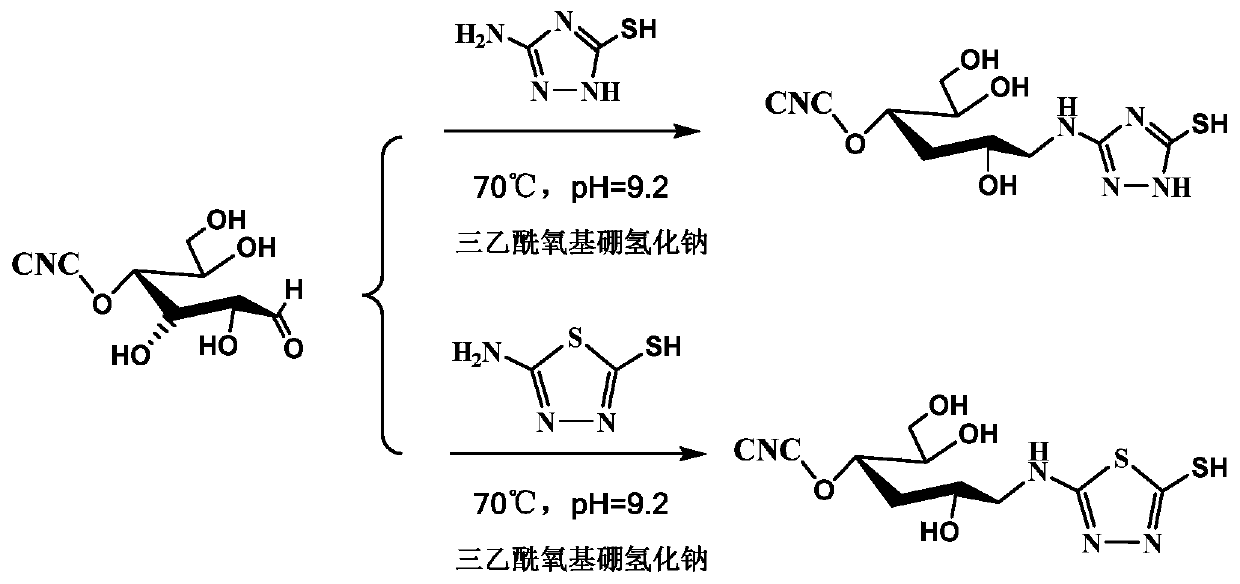
Terminal modification method for improving redispersibility and suspension stability of cellulose nanocrystals
The invention relates to a terminal modification method for improving redispersibility and suspension stability of cellulose nanocrystals. The method comprises the following steps: firstly mixing a cellulose nanocrystal suspension and a pH buffering solution such as a sodium carbonate solution, adding an amino-containing cyclic compound and a reducing agent such as sodium triacetoxyborohydride, and performing an aldehyde amine condensation reaction and a reduction reaction to bond reactive aldehyde groups at the terminal of the cellulose nanocrystals to amino and simultaneously reduce a carbon-nitrogen double bond into a more stable carbon-nitrogen single bond. According to the method provided by the invention, through the above modification, an original rod-like morphology and crystal structure of the cellulose nanocrystals and hydroxyl used as hydrogen bond driving force and a negative charge sulfonate group at the surface of the nanocrystals are retained, and the electrostatic repulsion effect of surface negative charges (the sulfonate group) and the steric hindrance effect of a terminal modified molecule (the cyclic compound) of the cellulose nanocrystals are fully utilized, sothat the redispersibility and stability in an aqueous suspension of the cellulose nanocrystals are efficiently improved.
Owner:WUHAN UNIV OF TECH
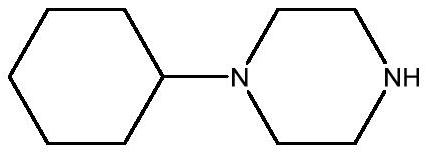
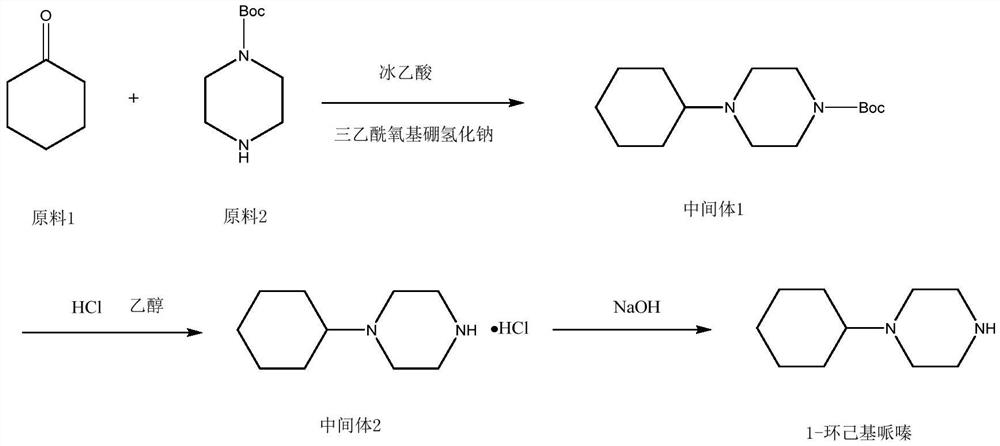
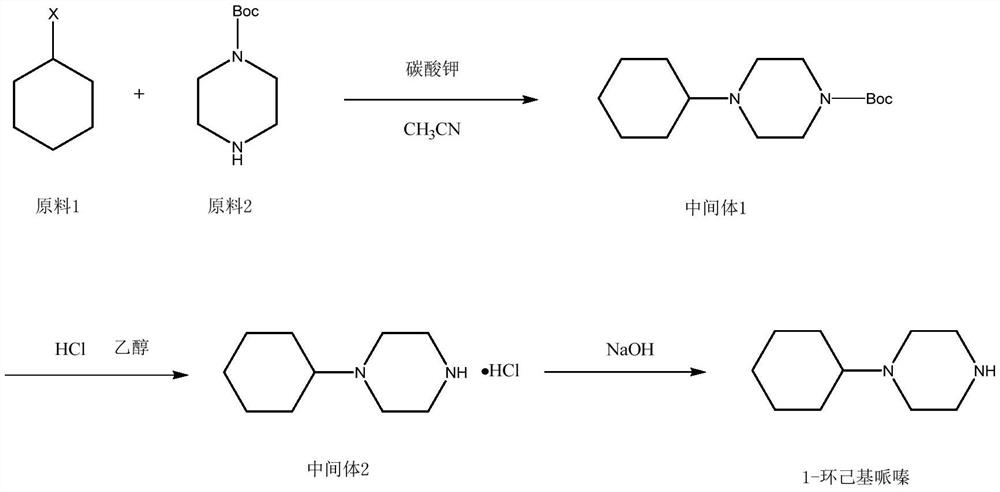
Preparation method of 1-cyclohexylpiperazine
InactiveCN112645901AReduce usageReduce manufacturing costOrganic chemistrySodium triacetoxyborohydrideOrganic solvent
The invention discloses a preparation method of 1-cyclohexylpiperazine. The method comprises the steps of carrying out reflux reaction on cyclohexyl halide, 1-Boc-piperazine and inorganic base in an organic solvent, filtering after the reaction is finished, and concentrating to obtain an intermediate 1; removing Boc from the intermediate 1 under an acidic condition, drying by distillation after the reaction is finished, pulping with isopropanol, and filtering to obtain solid 1-cyclohexyl piperazine hydrochloride; dissolving with water, adding an inorganic base to adjust the pH value to 12 to 14, extracting with an extraction solvent, drying the solvent by distillation to obtain a crude product of 1-cyclohexylpiperazine, and carrying out reduced pressure distillation on the crude product through an oil pump to obtain a pure product of 1-cyclohexylpiperazine. In the synthesis process of the intermediate 1, use of sodium triacetoxyborohydride and sodium hydroxide is avoided, the production cost is greatly saved, meanwhile, only filtering is needed for aftertreatment, the method is very simple, and time and labor are saved.
Owner:SHANDONG BOYUAN PHARM CO LTD
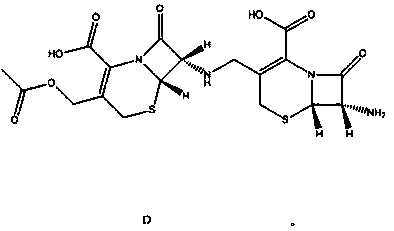
Synthesis method of dimer impurity D produced by cefazolin sodium production
InactiveCN110590814AThe synthesis method is simpleSolve analytical control problemsOrganic chemistryComponent separationCefazolin SodiumSynthesis methods
The invention discloses a dimer impurity D produced by cefazolin sodium, and a synthesis method of the dimer impurity D produced by cefazolin sodium production. The method comprises: carrying out a reaction on an initial raw material A and sodium hydroxide in a solvent ethanol and water, adjusting the pH value with diluted hydrochloric acid, extracting, and distilling to obtain an intermediate B;adding the intermediate B into dichloromethane, adding a Dess-Martin Periodinane reagent, quenching the reaction solution, and filtering to obtain the mother liquor of a compound C so as to be spare;and sequentially adding a compound A and sodium triacetoxyborohydride into dichloromethane, adding the mother liquor of the product C, quenching after completing the reaction, extracting, and purifying by using a chromatographic silica gel column to obtain a dimer impurity D. According to the present invention, the synthesized dimer impurity D can provide impurity control for the reaction.
Owner:TIANJIN LISHENG PHARM CO LTD
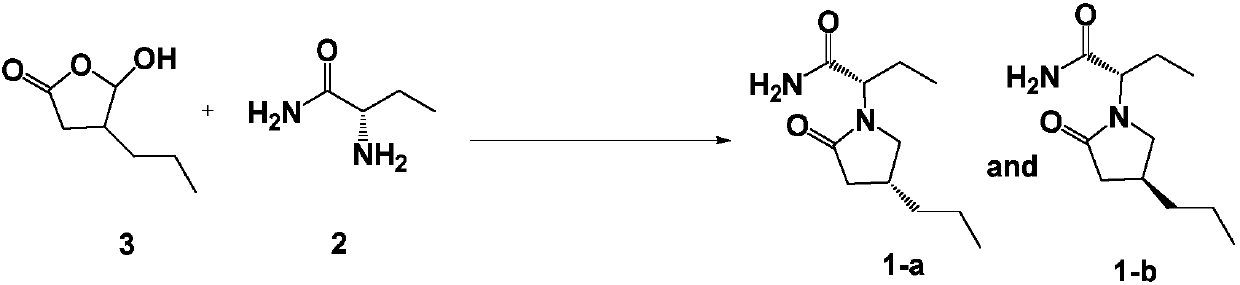
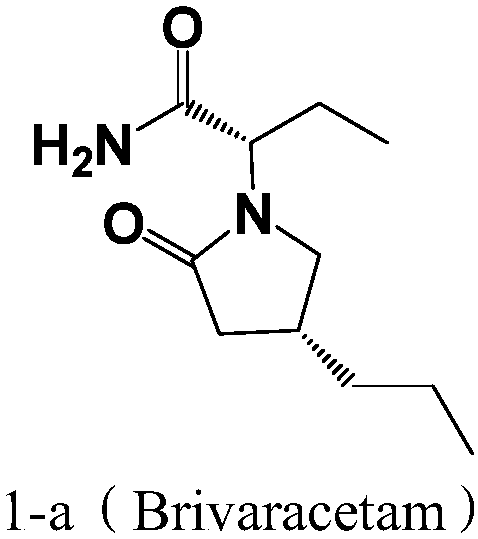
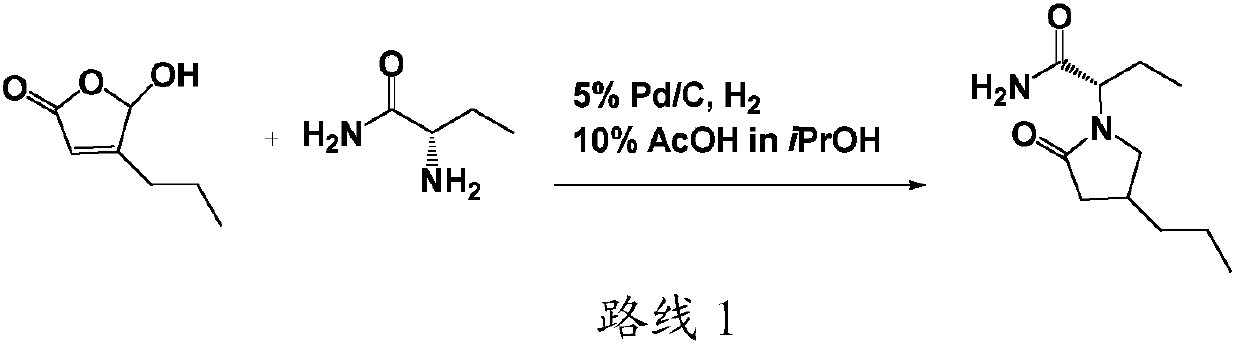
Preparation method of brivaracetam
InactiveCN107793342AReaction steps take less timeShort timeOrganic chemistryPotassium borohydrideSodium triacetoxyborohydride
The invention provides a preparation method of brivaracetam, comprising the steps of (1) aminating, to be specific, dissolving compounds 2 and 3 in solvent 2, and stirring at controlled temperature of10-50 DEG C to allow reacting for 2-3 h, wherein the solvent 2 is one or more of methylbenzene, 1,2-dichloroethane, dichloromethane, trichloromethane and tetrahydrofuran; (2) reducing, to be specific, adding a reducing agent, and allowing reacting at controlled temperature of 10-50 DEG C for 1-4 h, wherein the reducing agent is one or more of sodium borohydride, potassium borohydride and sodium triacetoxyborohydride; (3) performing lactamization, to be specific, allowing reacting at controlled temperature of 20-100 DEG C for 2-5 h, wherein the steps (1) to (3) are performed in inert gas environment. The intermediate compounds of brivaracetam used herein are low in price, and the preparation method has mild reaction conditions and is suitable for industrial large-scale production.
Owner:LIVZON NEW NORTH RIVER PHARMA
Metal ceramic material
A metal ceramic material is prepared from the following raw materials in parts by weight: 6-13 parts of iron oxide, 6-14 parts of hydroxyapatite, 4-10 parts of lithium borohydride, 3-10 parts of sodium triacetoxyborohydride, 1-4 parts of Fe, 2-5 parts of Mn, 3-10 parts of rutile type titanium dioxide, 3-8 parts of quartz, 2.5-9 parts of a razor clam king shell powder, 1-3 parts of boron glass, 4.2-6 parts of calcium hydrogen phosphate, 3-6 parts of agate, 3.5-10 parts of borax decahydrate, 5-8 parts of nano ferrum dioxide, 1-4 parts of magnesium oxide, and 6-13 parts of barium hydroxide. The metal ceramic material has the beneficial effects that the metal ceramic material has the characteristics of small density, high hardness and quite good high-temperature stability, and improves the service life of ceramics.
Owner:青岛海蓝海洋复合功能材料科技有限公司
Preparation method of N-(2- indanyl) amino acid alkyl ester
ActiveCN102391140AAvoid it happening againOrganic compound preparationAmino-carboxyl compound preparationGlycineSodium triacetoxyborohydride
The invention provides a preparation method for N-(2- indanyl) glycine alkyl ester. An N-(2- indanyl) glycine alkyl ester crude product is obtained through the reaction of glycine alkyl ester and 2-indenone, and the reducer used in the reaction of the glycine alkyl ester and 2-indenone is triacetyl oxygroup sodium boro-hydride; and the molar ratio of 2-indenone and triacetyl oxygroup sodium boro-hydride is 1 to 0.79 to 1.59. By adopting the method, NaBH (OAc) 3 is used for preventing the use of NaBH3CN, the production of toxic substance is avoided, and the yield of the prepared N-(2- indanyl) glycine alkyl ester can reach 77.2 percent.
Owner:广东暨大基因药物工程研究中心有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com