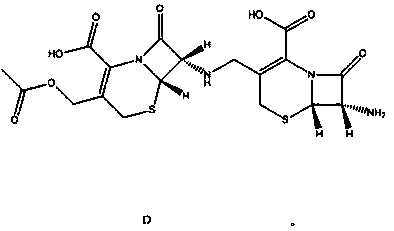
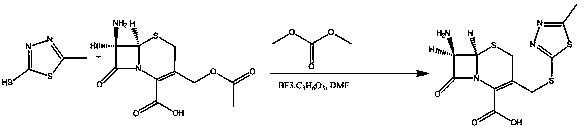
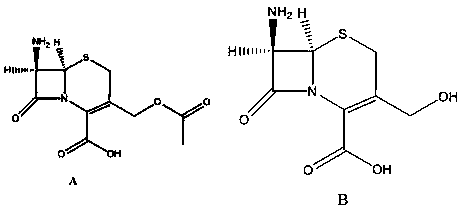
Synthesis method of dimer impurity D produced by cefazolin sodium production
A technology of cefazolin sodium and a synthetic method, which is applied in the field of synthesis of dimer impurities, can solve problems such as no very effective synthetic methods, and achieve the effect of solving the problem of impurity analysis and control and a simple synthetic method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of synthetic method of the dimeric impurity D that produces cefazolin sodium comprises the following steps:
[0037] 1) Add 50.00 g (0.183 mol) of compound A, 250 ml of ethanol, and 125 ml of water to a 1000 ml three-necked flask equipped with a thermometer and mechanical stirring, and slowly add 18.30 g (0.457 mol) of hydrogen hydroxide at a temperature of 30°C Sodium, keep warm at 30 ℃, keep warm for 12 hours, monitor the reaction by TLC, after the reaction, distill off the ethanol in the reaction solution, adjust the pH ≈ 7 with 5% hydrochloric acid aqueous solution, extract with ethyl acetate, and distill off the acetic acid under reduced pressure Ethyl ester was used to obtain 34.50 g of compound B with a yield of 82% and a content of 98.2%.
[0038] 2) Add 34.50 g (0.15 mol) of compound B and 1000 ml of dry dichloromethane into a 2000 ml three-neck flask equipped with a thermometer and mechanical stirring under nitrogen protection, and slowly add 66.80 g (0...
Embodiment 2
[0042] 1) Add 50.00 g (0.183 mol) of compound A, 250 ml of ethanol, and 125 ml of water to a 1000 ml three-necked flask equipped with a thermometer and mechanical stirring, and slowly add 18.30 g (0.457 mol) of hydrogen hydroxide at a temperature of 30°C Sodium, keep warm at 30 ℃, keep warm for 12 hours, monitor the reaction by TLC, after the reaction, distill off the ethanol in the reaction solution, adjust the pH ≈ 7 with 5% hydrochloric acid aqueous solution, extract with ethyl acetate, and distill off the acetic acid under reduced pressure Ethyl ester obtained 34.50 g of compound B, the yield was 82%, and the content was 98.2%
[0043] 2) Add 34.50 g (0.15 mol) of compound B and 1000 ml of dry dichloromethane into a 2000 ml three-necked flask equipped with a thermometer and mechanical stirring under nitrogen protection, and reduce the reaction temperature to 0 °C with an ice-water bath, slowly Add 66.80g (0.158mol) Dess.Martin reagent, keep warm for 1 hour, TLC monitors th...
Embodiment 3
[0047] Agilent 1200 high-performance liquid chromatography, octadecylsilane bonded silica gel as a semi-preparative column (10mm × 250mm, 10μm) as a filler, 5mmol / L ammonium acetate solution-methanol (the volume ratio of the two is 700-900 :300-100) as the mobile phase, take an appropriate amount of sample, dissolve it in water, and prepare the test solution for impurities of 10-200mg / ml. The injection volume is 100μl, the flow rate is 1-3ml / min, the detection wavelength is 246nm, and the column temperature is 30℃ . Under this chromatographic condition, the peak of cefazolin sodium is about 20 minutes, and the peak of impurities is about 15 minutes.
[0048] Get this impurity monomer proper amount, be prepared as the solution of 0.5mg / ml with diluent, measure by cefazolin sodium related substance inspection method, calculate the purity of main peak with area normalization method and be 99.2%. No peak was found at 15 minutes. For further verification, the test solution was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com