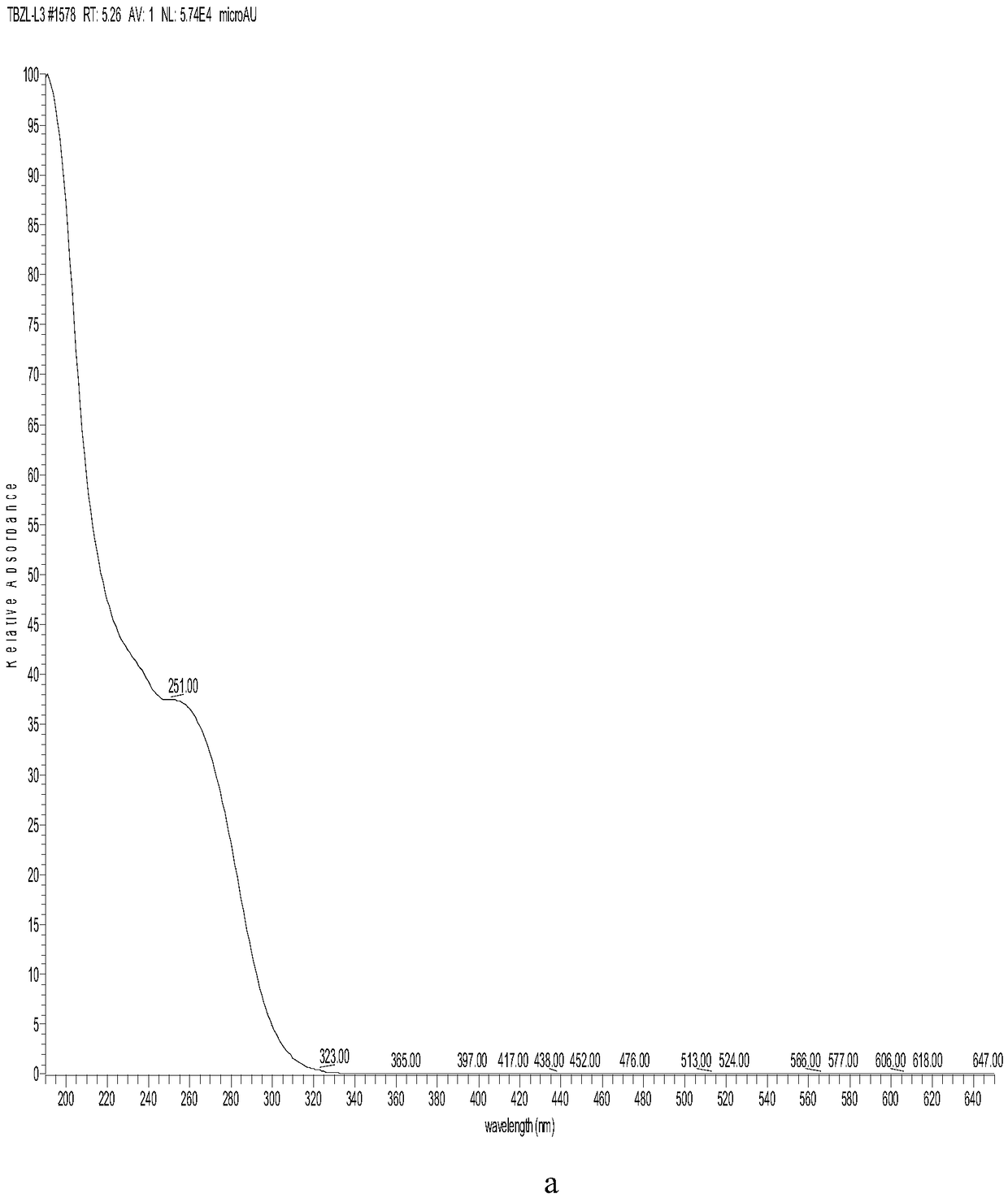
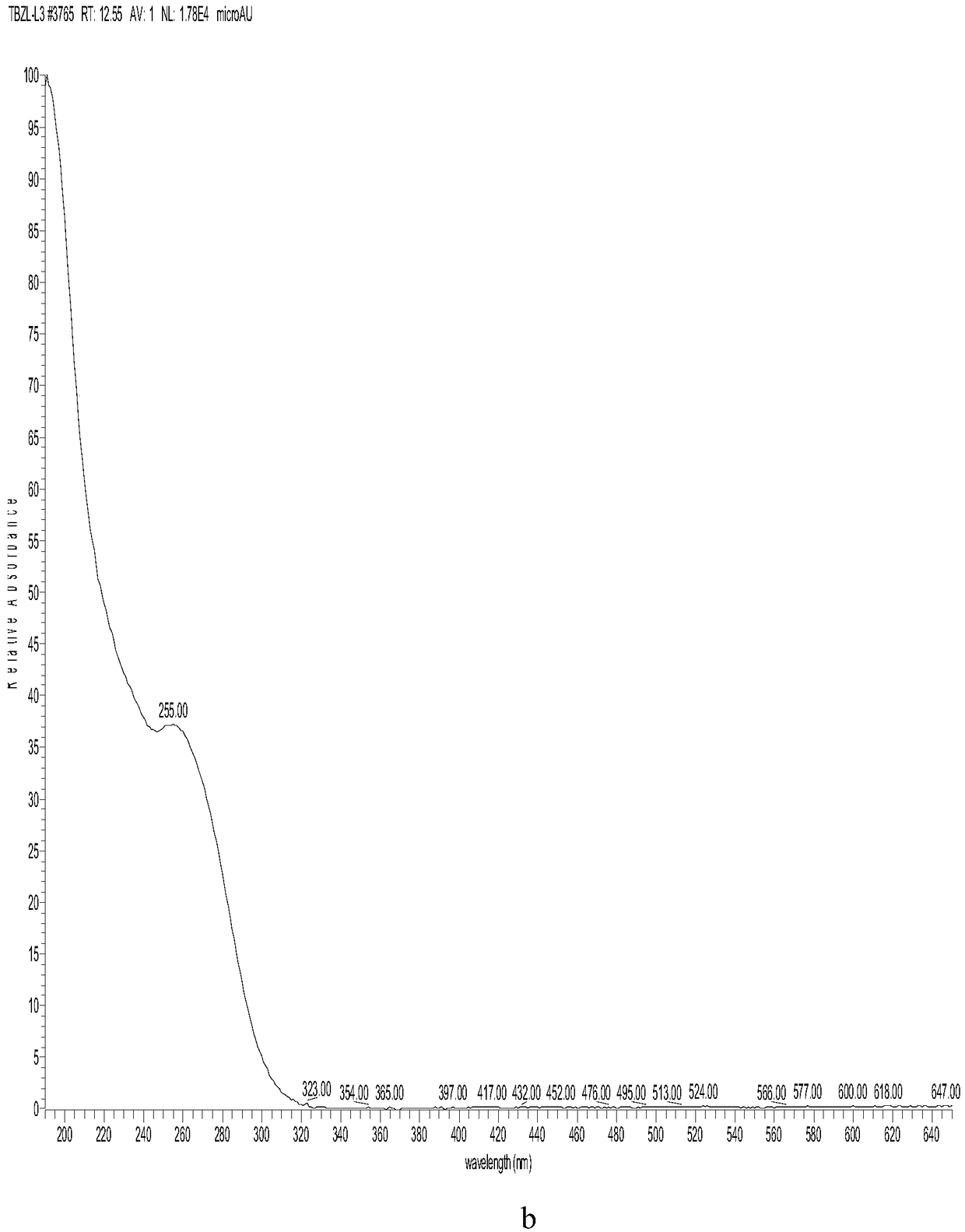
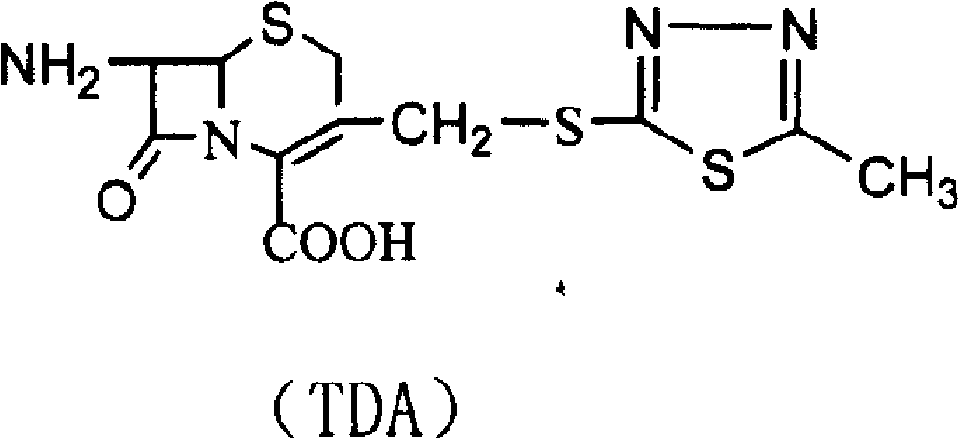
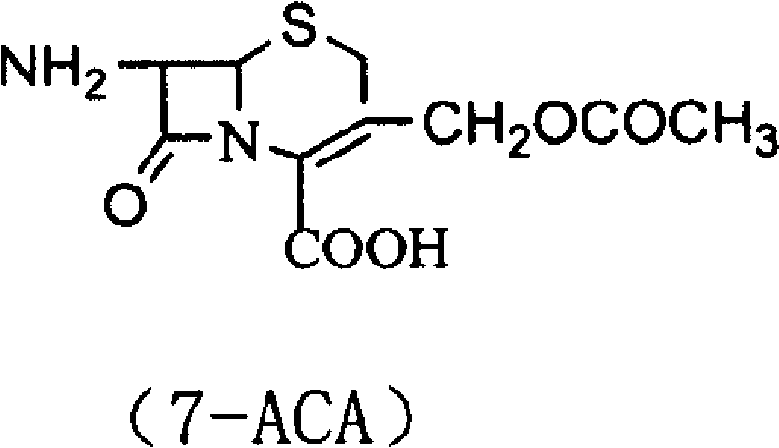
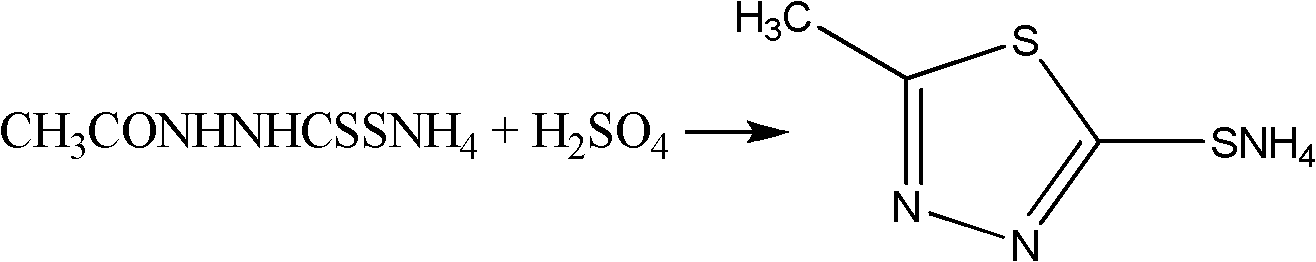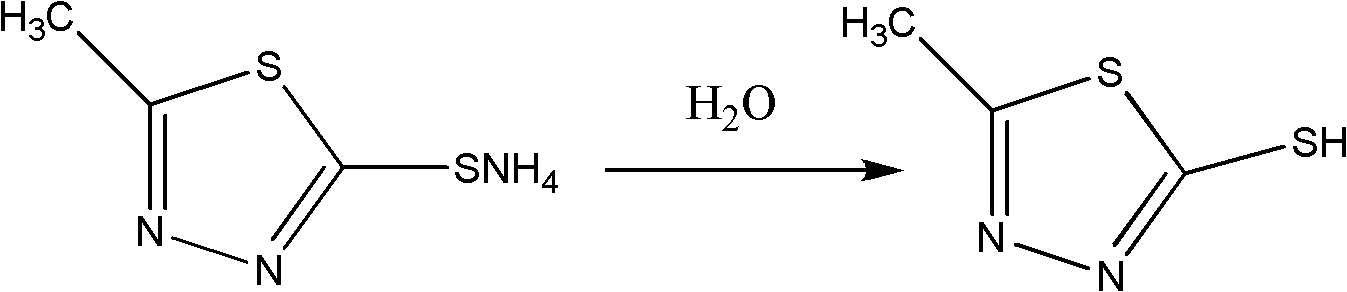Patents
Literature
60 results about "Cefazolin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
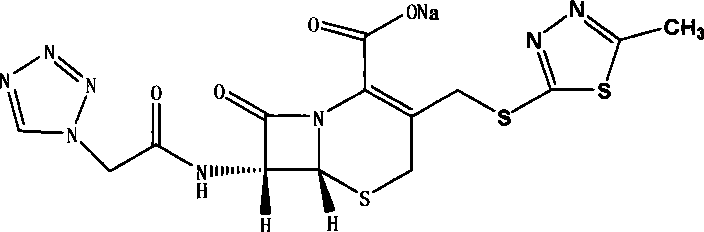
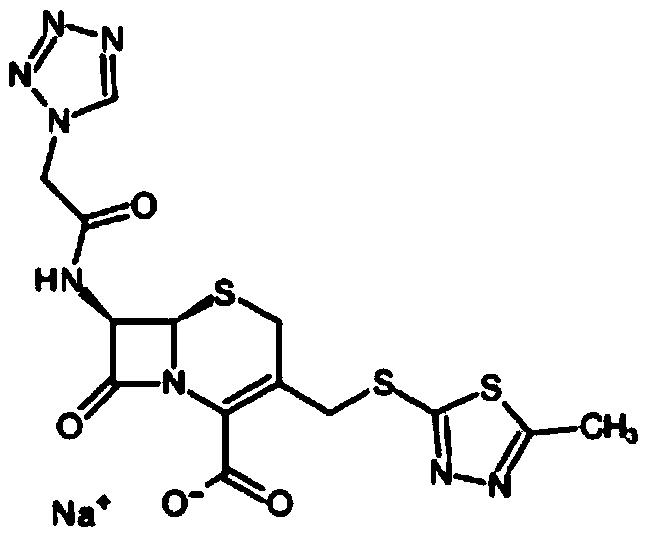
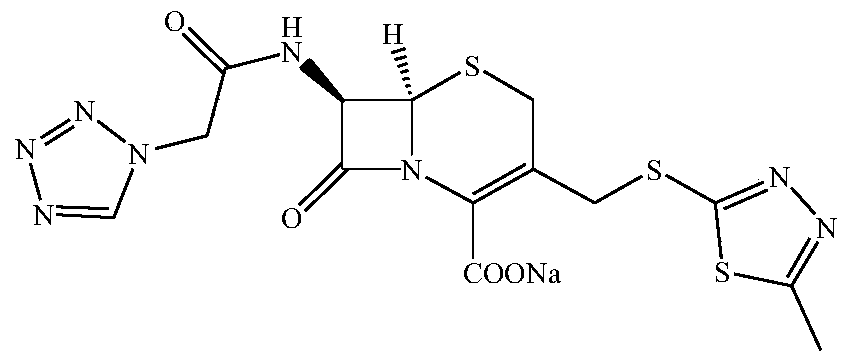
The sodium salt of cefazolin, a beta-lactam antibiotic and first-generation cephalosporin with bactericidal activity. Cefazolin binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity, which results in the weakening of the bacterial cell wall and cell lysis.
Preparation method of cefazolin sodium
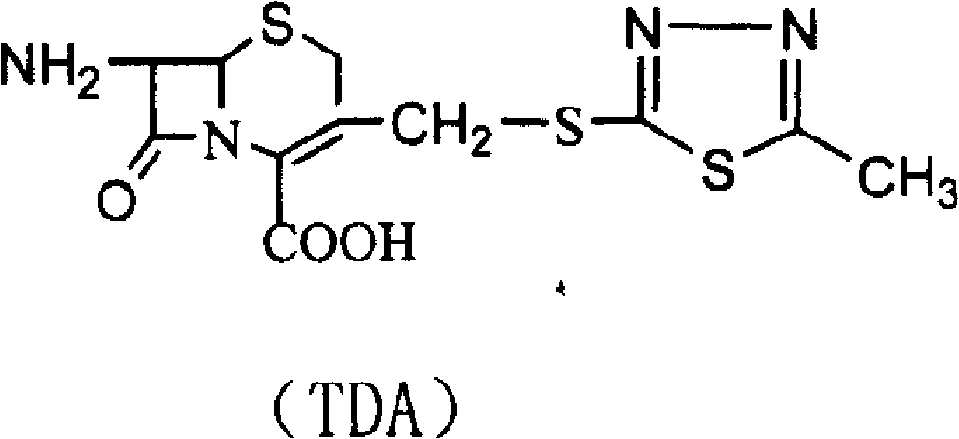
The invention relates to a preparation method of a pharmaceutical compound, in particular relates to a preparation method of cefazolin sodium. The preparation method provided by the invention comprises the following steps: step 1, synthesizing TDA (toluene diamine); step 2, synthesizing cefazolin; and step 3, synthesizing cefazolin sodium.
Owner:哈药集团股份有限公司 +1
Method for preparing cefazolin compounds
ActiveCN102617607ASimple recycling processHigh recovery rateAntibacterial agentsOrganic chemistryCefazolin SodiumMethyl carbonate
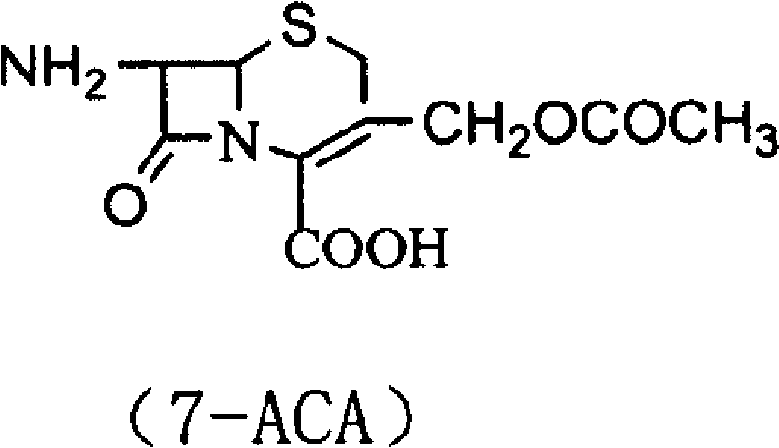
The invention belongs to the field of pharmacy and relates to a method for preparing cefazolin compounds. The method comprises the following steps that: 1, cefazolin sodium imidazo (toluene diamine TDA) is synthetized, thiadiazole and 7-ACA are obtained through reaction, dimethyl carbonate is used as solvents in the reaction, boron trifluoride-dimethyl carbonate is used as catalysts, and reagents used for regulating the pH of the reaction liquid are inorganic alkali after the reaction is completed; 2, anhydride is prepared, and the anhydride is obtained through the reaction between tetrazole acetic acid and pivaloyl chloride, and 3, the cefazolin is synthesized, TDA solution reacts with the anhydride, and the reaction solution is subjected to decoloration and purification through an aluminium oxide column.
Owner:哈药集团股份有限公司 +1
Monohydrate cefazolin sodium special particle and crystallization preparation method thereof
ActiveCN104788472AIncrease master granularityHigh bulk densityAntibacterial agentsOrganic active ingredientsPharmacy medicineCefazolin Sodium
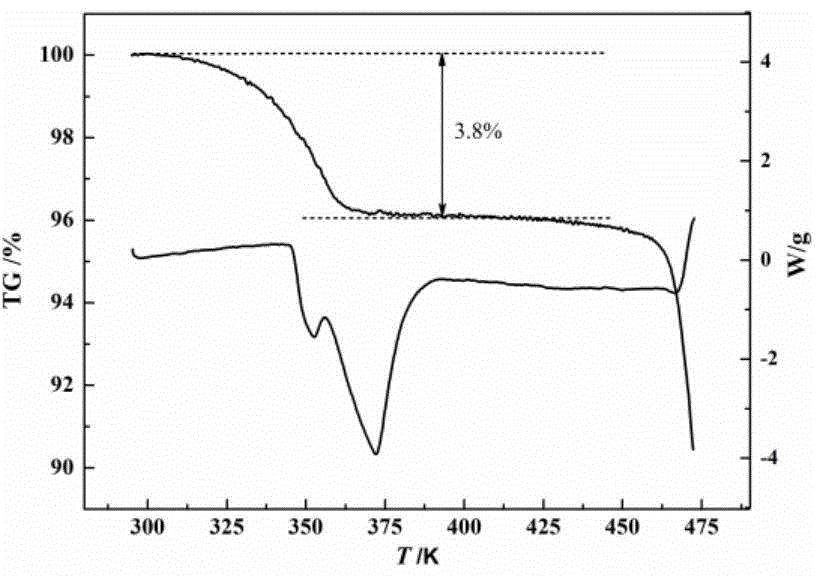
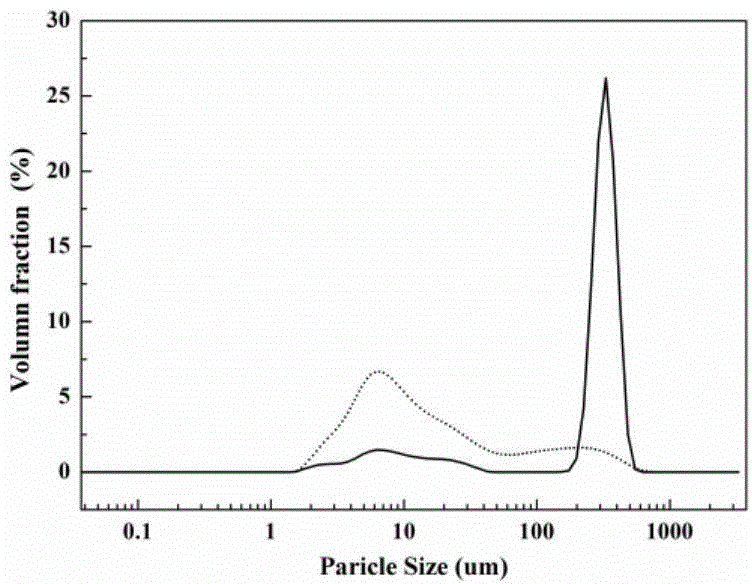
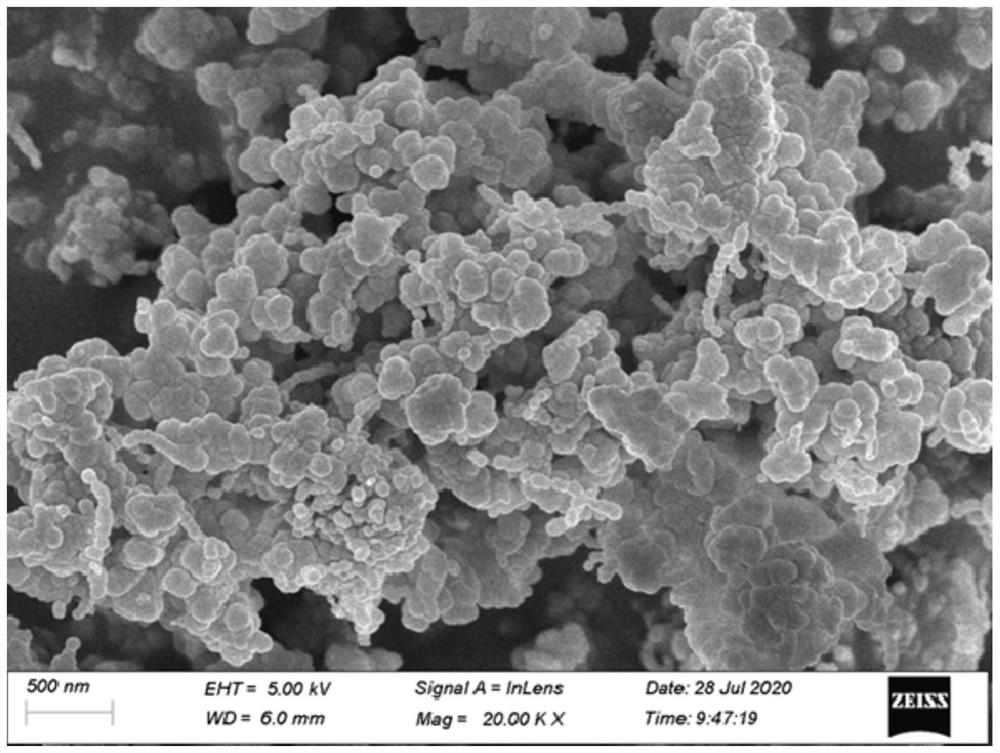
The invention relates to monohydrate cefazolin sodium special particles and a crystallization preparation method thereof. The average particle size of the monohydrate cefazolin sodium special particles is greater than 300mu m, the variable coefficient C.V. of the monohydrate cefazolin sodium special particles is smaller than 0.3, and the bulk density of the monohydrate cefazolin sodium special particles is greater than 0.4g / ml. The crystallization preparation method comprises the following steps: adding cefazolin sodium solid into a solvent system, preparing a solution of which the mass concentration is 0.01-0.12 at 40 DEG C, cooling to be 5-35 DEG C, adding a bridging agent, and stirring at the speed of 100-500rpm so as to separate out crystal; transferring the crystal mush into a filtering system, and drying the filter cakes till constant weight, thereby obtaining the monohydrate cefazolin sodium special crystal. The invention further discloses application of the special particles as an effective medicine component for treating, preventing or delaying various infection diseases caused by sensitive bacteria. The monohydrate cefazolin sodium special particles are simple in process, easy to operate and low in equipment requirement, the process yield is higher than 90.0%, and a spherical medicinal product is relatively excellent in filling property and compression formation property, and is relatively easy in commercial industrial scale production.
Owner:TIANJIN UNIV
Preparation of cefazolin sodium sterilized raw medicine
InactiveCN101463040ALow clarityQuality improvementAntibacterial agentsOrganic active ingredientsCLARITYCefazolin Sodium
The invention provides a preparation method of a cefazolin sodium sterile bulk drug. The preparation method comprises the following steps: suspending cefazolin in deionized water, performing a neutralization reaction with alkali solution containing sodium ions in a hermetic stirring container under depressurized condition, and obtaining the cefazolin sodium sterile bulk drug by rough filtration, sterile filtration and lyophilization of reaction liquid.. The preparation method solves the technical problems of the existing production process that the clarity and the water content of the cefazolin sodium are difficult to be controlled and the contents of related materials are too much higher, improves the product quality and is suitable for large-scale industrialized production.
Owner:YAOPHARMA CO LTD
Novel crystal form of cefazolin sodium and preparation method thereof
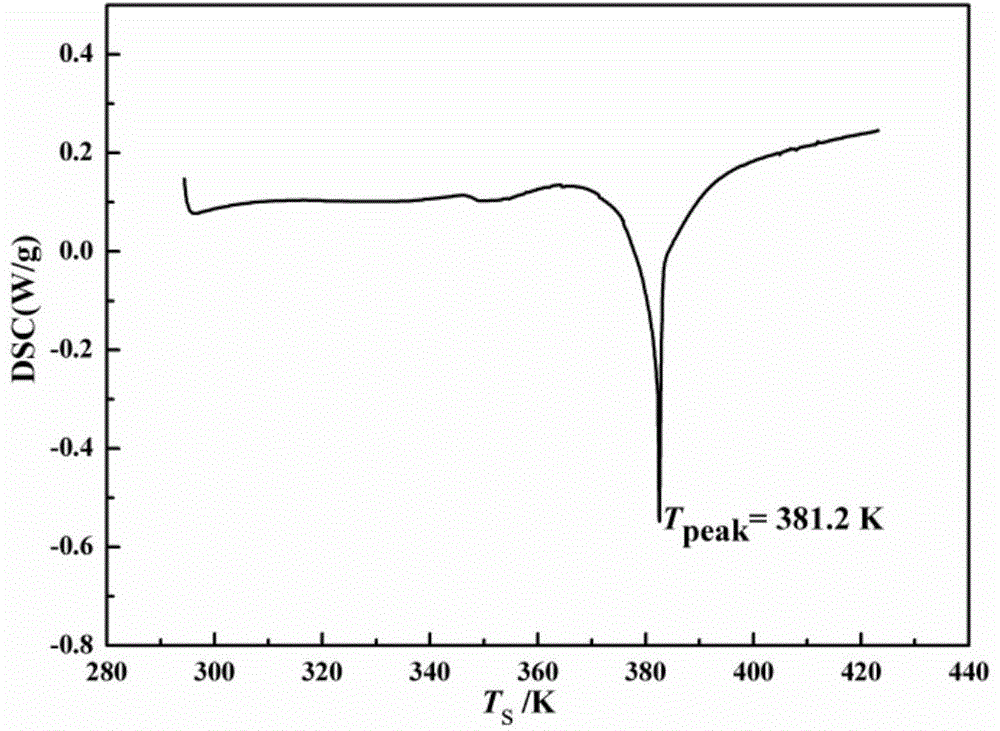
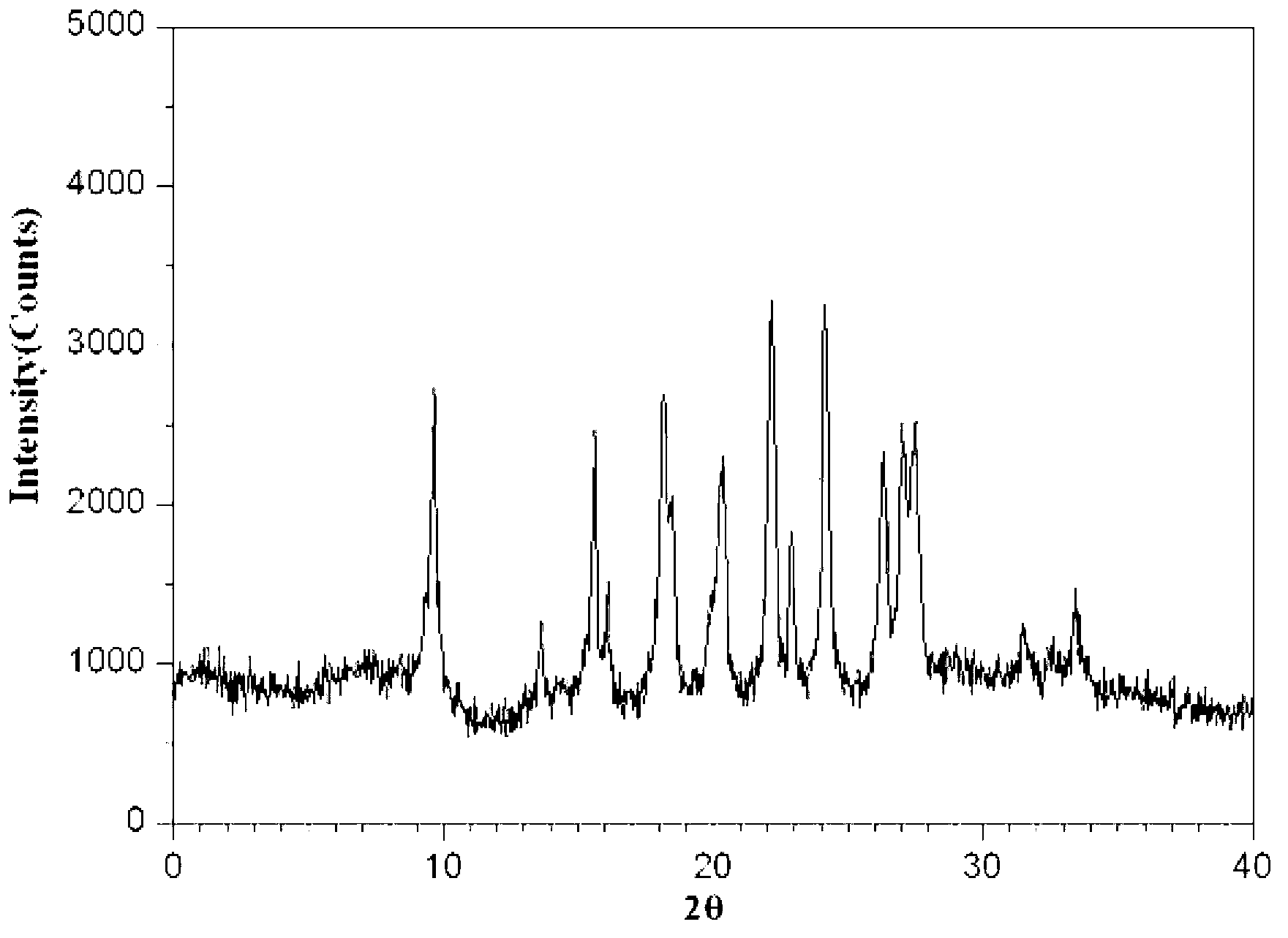
The invention relates to a novel crystal form of cefazolin sodium and a crystallization preparation method thereof. An X-ray powder diffraction pattern is used for defining at the characteristic peaks of a diffraction angle of 2-theta degrees and DSC. The preparation method comprises the following steps: adding a cefazolin sodium solid into a solvent to prepare a suspension of 0.3-0.7g / mL; heating the suspension to 30-45 DEG C under a stirring condition; stirring for 0.5-12 hours at a constant temperature; cooling to 0-20 DEG C, and growing a crystal at a constant temperature for 0.5-2 hours; then filtering to obtain crystal slurry; and drying the filter cake obtained by filtering to obtain a product of the novel crystal form of cefazolin sodium. The novel crystal form has a higher heat-absorption temperature, the product purity, color and shape do not change after the product is stored for 100 days under a normal-temperature and dry condition, and the crystal form has relatively good stability. Compared with already reported crystal forms, the short-bar shaped novel crystal form has the advantages of relatively high dissolving speed and relatively high fluidity and bulk density, and is easy for commercially industrial large-scale implementation.
Owner:SHENYANG SANJIU PHARMA
Double-template molecularly-imprinted solid-phase extraction column and application method
ActiveCN105233809AHigh enrichment efficiencyImprove purification efficiencyIon-exchange process apparatusOther chemical processesAnimal foodCapillary electrophoresis
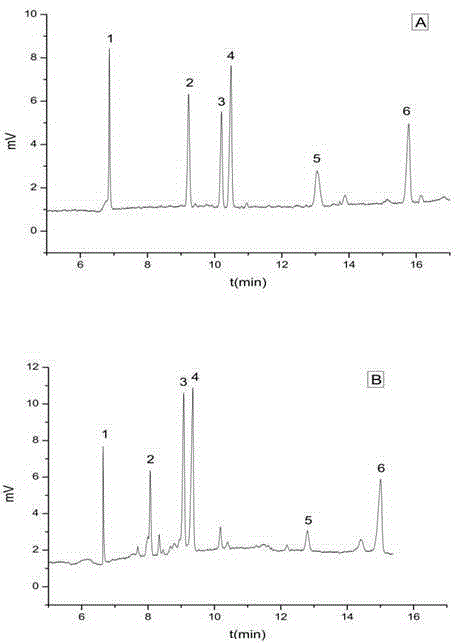
The invention relates to the technical field of solid-phase extraction, specifically to an oxacillin-and-cephalexin double-template molecularly-imprinted solid-phase extraction column and an application method. The extraction column is cooperatively used with a capillary electrophoresis method for selective separation, enrichment and detection of residues of amoxicillin, cephalexin, oxacillin, penicillin G, cefazolin sodium and cefoperazone sodium in animal food.
Owner:JIANGSU UNIV
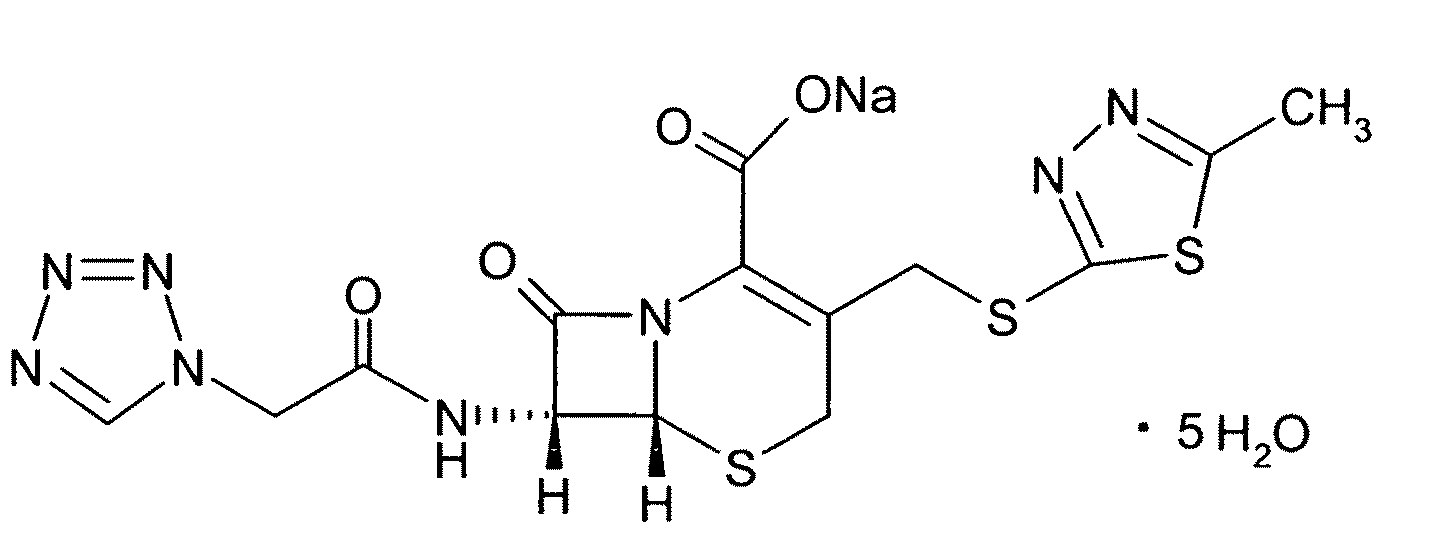
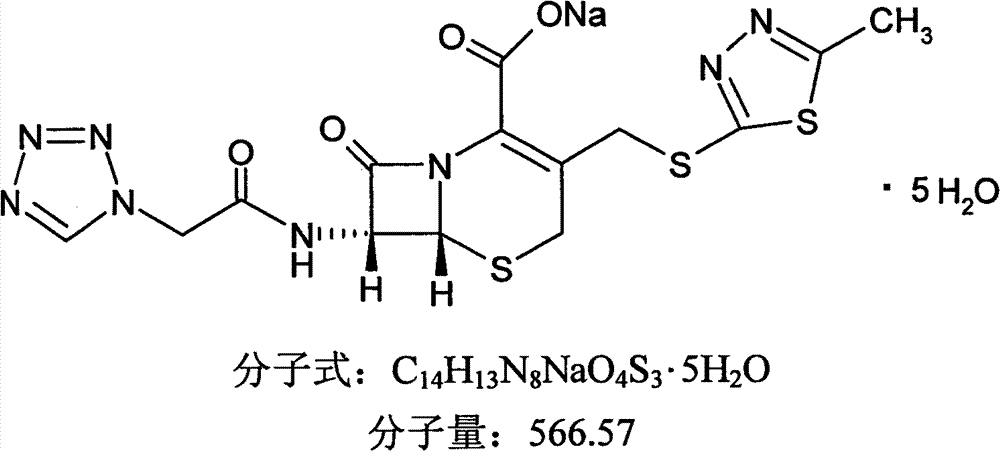
Cefazolin sodium pentahydrate compound and preparation method and medicine composition thereof
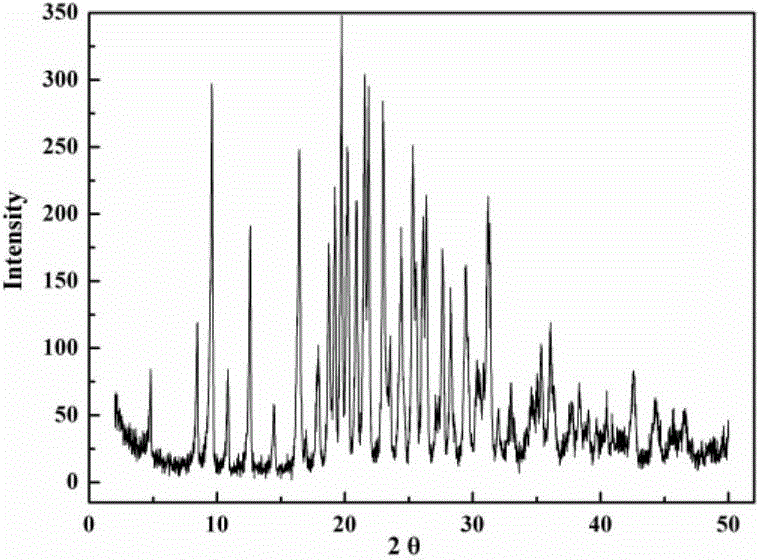
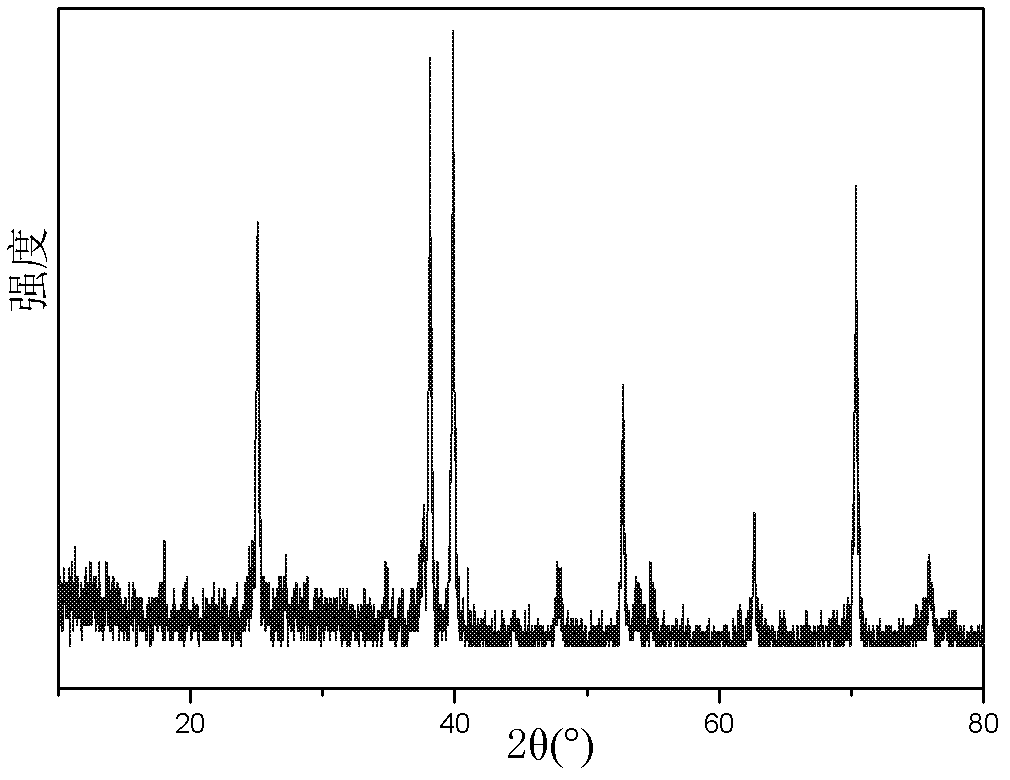
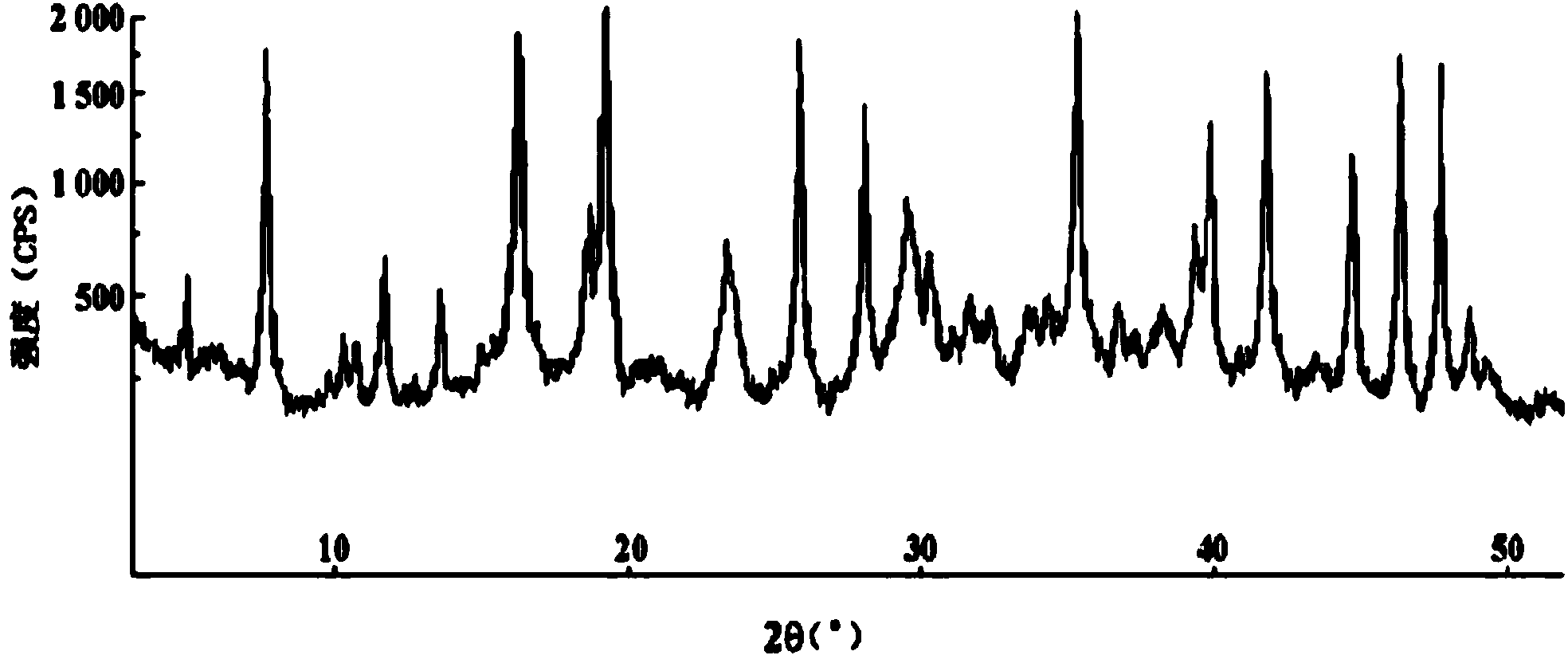
InactiveCN103288854ALow impurity contentGood storage stabilityAntibacterial agentsOrganic active ingredientsCefazolin SodiumPowder diffraction
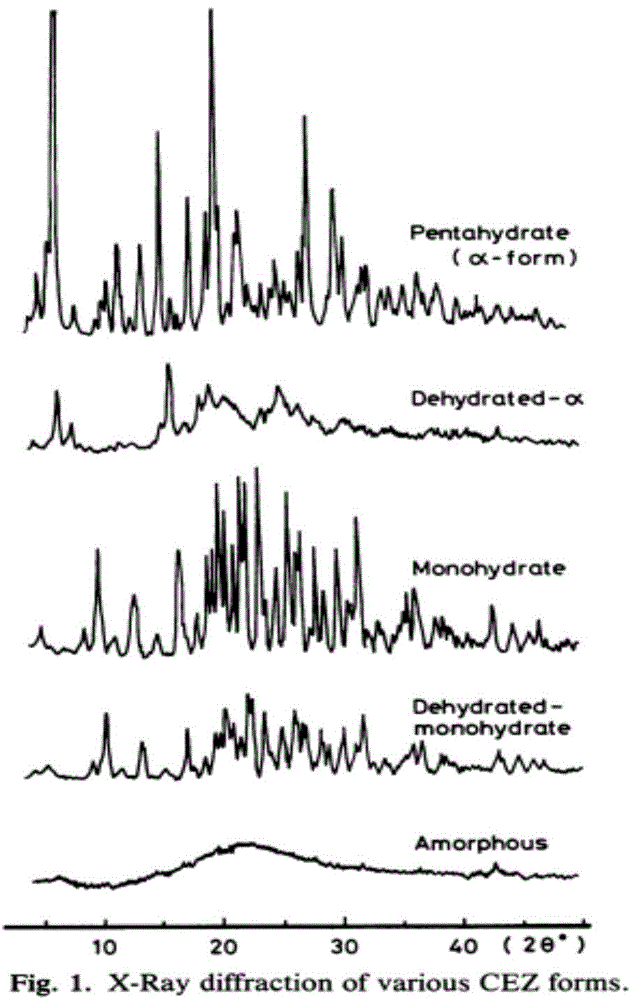
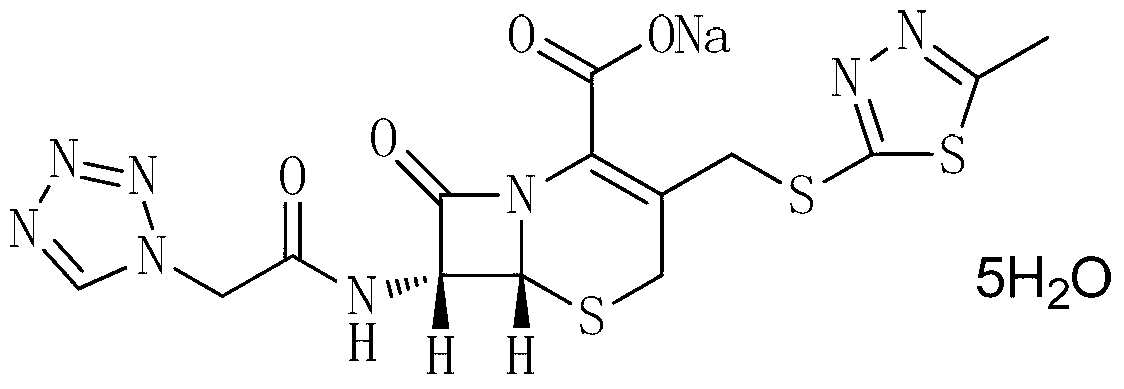
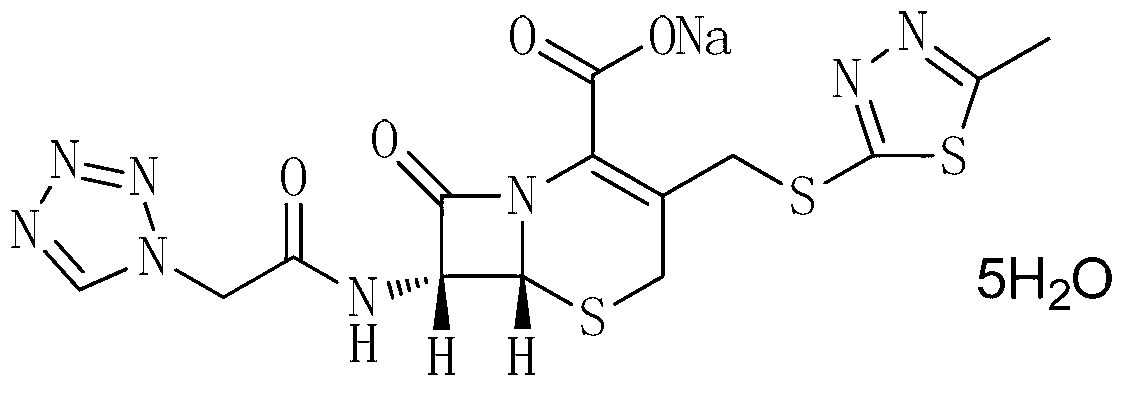
The invention provides a cefazolin sodium pentahydrate compound. The structural formula of the cefazolin sodium pentahydrate compound is shown in the specification, the X-ray powder diffraction pattern of the cefazolin sodium pentahydrate compound measured by means of Cu-Ka ray is shown in Figure 1. The invention also provides a preparation method of the cefazolin sodium pentahydrate compound and a medicine composition containing the cefazolin sodium pentahydrate compound. The preparation of the cefazolin sodium pentahydrate medicine is sterile powder injection. Compared with the prior art, the cefazolin sodium pentahydrate compound and the medicine composition of the cefazolin sodium pentahydrate compound provided by the invention are better in storage stability, and medication safety of a patient is greatly improved.
Owner:四川省惠达药业有限公司
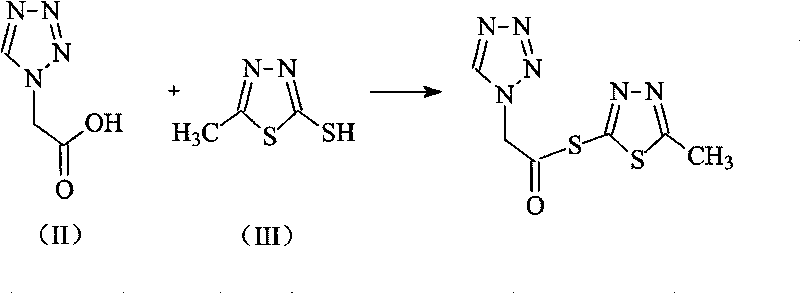
Cefazolin sodium pentahydrate compound of new route
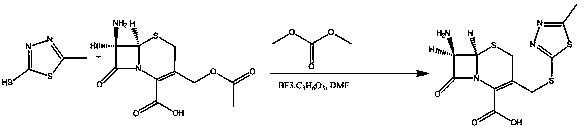
The invention relates to a cefazolin sodium pentahydrate compound of a new route, and particularly provides a method for synthesizing the cefazolin sodium pentahydrate compound. The method comprises the following steps: synthesizing tetrazolyl acetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole ester by using tetrazolyl acetic acid and 2-mercapto-5-methyl-1,3,4-thiadiazole as raw materials; and generating cefazolin sodium through the reaction between the tetrazolyl acetic acid 2-mercapto-5-methyl-1,3,4-thiadiazole ester and 7-aminocephalosporanic acid. Compared with that the method for synthesizing tetrazole acetic acid 2-mercapto-1,3,4-thiadiazole ester in the prior art uses expensive reagents such as trifluoroacetic anhydride, aluminium trimethide or DCC and the like as a condensation agent, the method improves the synthesis method, not only simplifies the operation steps, but also, surprisingly, greatly improves the product yield and the purity, reduces the cost, and lays a foundation for industrialization.
Owner:HAINAN MEIDA PHARMA
Calcium orthophosphate bone cement/PLGA /brizolina natrium compound material and method of preparing the same
InactiveCN101249281AImprove mechanical propertiesSustained releaseProsthesisCefazolin SodiumAntibiotic Y
The invention discloses a calcium phosphate cement virgule PLGA virgule cefazolin sodium composite material and a preparation method thereof. The method uses dry ball milling to mix calcium phosphate cement and polyglycolic-polyactic acid, dissolves antibiotics into deionized water to prepare distiller liquor, mixes the antibiotics and the deionized water according to the set solid-liquid ratio, and forms artificial bone activity material, namely calcium phosphate cement virgule PLGA virgule cefazolin sodium composite. The method solves the problem of low strength of bone cement loading with a large quality of antibiotics effectively, improves the biological compatibility, degradability, and degradation tunability of polyglycolicacid, and acheives the aim of controlling medical release and the growth of the induced bone after polyglycolic acid degradation.
Owner:SHANDONG UNIV
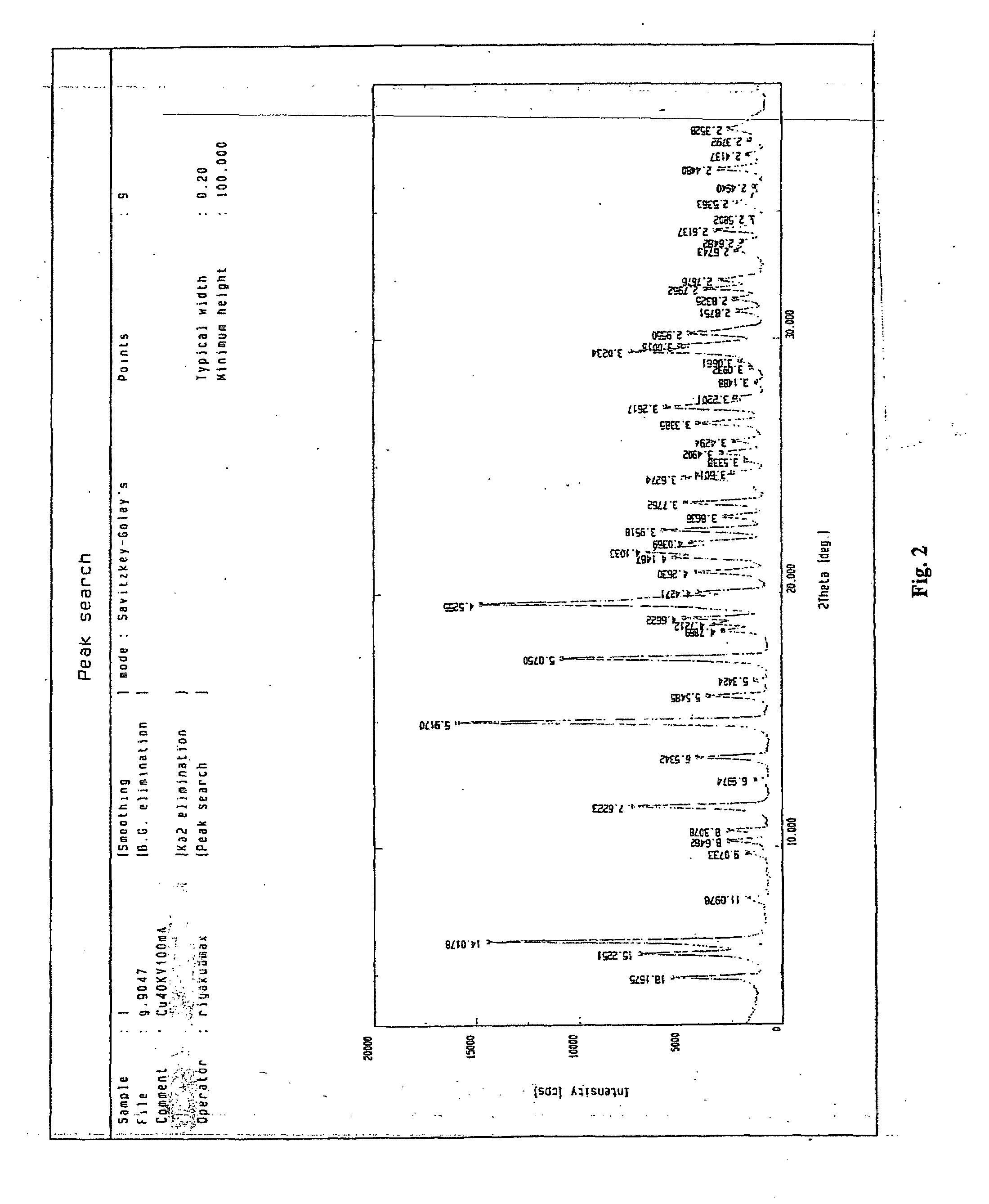
Cefazolin sodium compound prepared according to novel intelligent crystallization technology and preparation of cefazolin sodium compound
InactiveCN106432276ANo residual toxicityNot easy to decompose and destroyAntibacterial agentsOrganic active ingredientsCefazolin SodiumX-ray
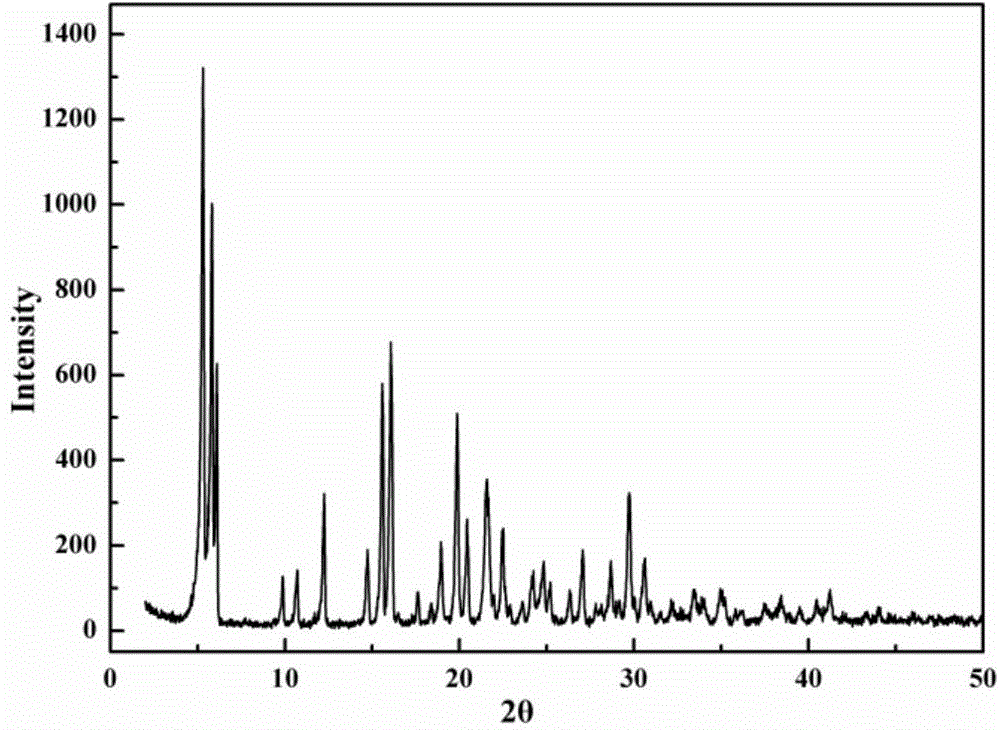
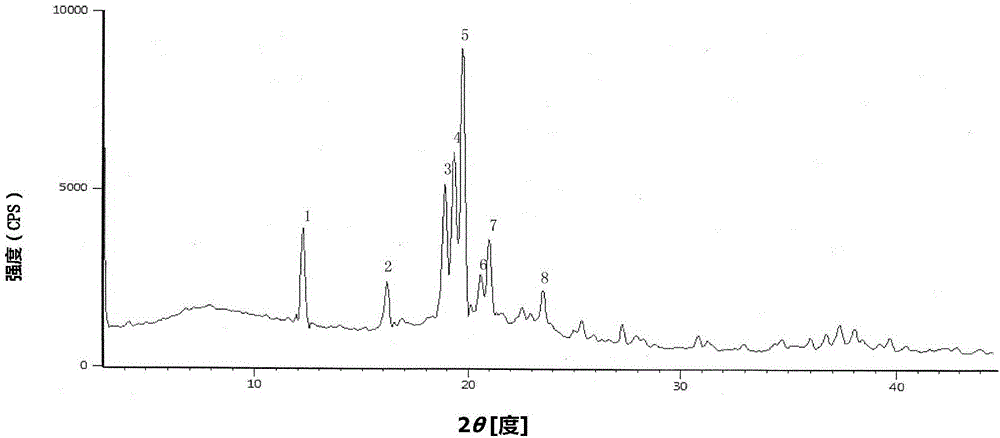
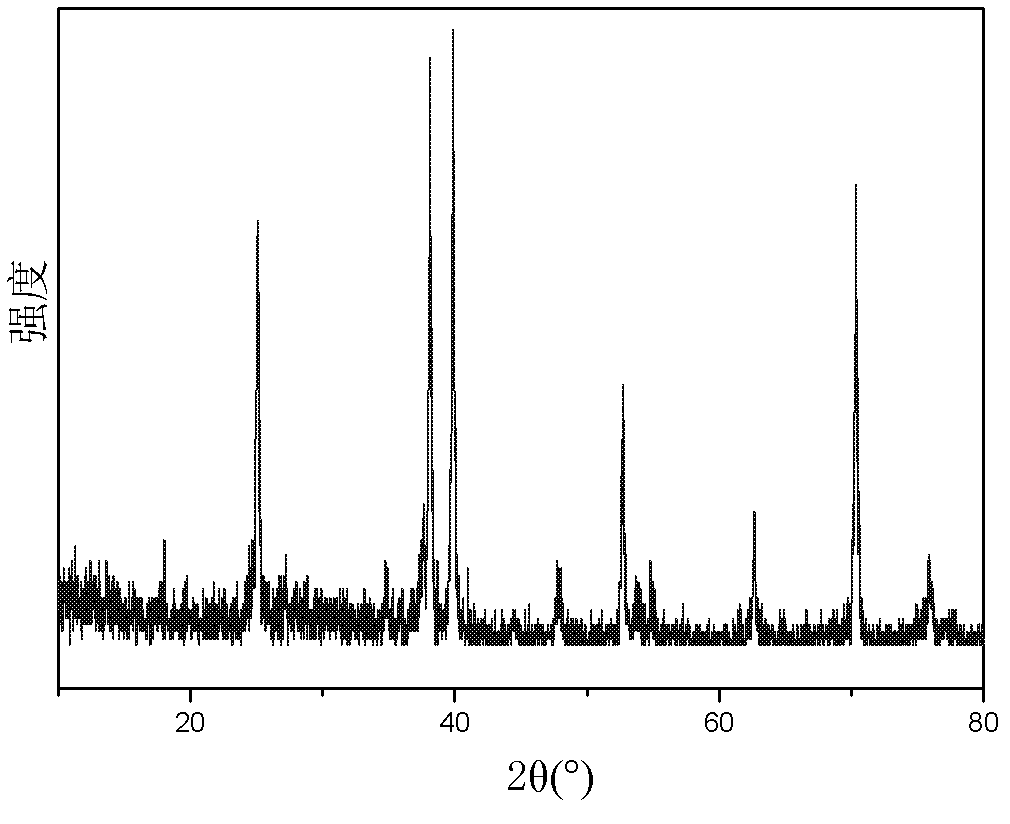
The invention discloses a cefazolin sodium compound prepared according to a novel intelligent crystallization technology and a preparation of the cefazolin sodium compound. 'Research, development and industrialization projects of high-end medical product refined crystallization technologies' won second prize of 2015th National Science and Technology Progress Award, and the novel intelligent crystallization technology is among the high-end medical product refined crystallization technologies. The cefazolin sodium compound is determined through X-ray powder diffraction, and main characteristic peaks represented by diffraction angles 2theta in an atlas are 12.24+-0.2 degrees, 16.23+-0.2 degrees, 18.94+-0.2 degrees, 19.26+-0.2 degrees, 19.80+-0.2 degrees, 20.61+-0.2 degrees, 21.05+-0.2 degrees and 23.57+-0.2degrees. Cefazolin sodium is recrystallized by combining a supercritical fluid extraction technology with a traditional crystallization technology. In a whole crystallization system, the process including extraction, adsorption, crystallization and drying is completed to recrystallize the cefazolin sodium at a specific temperature and at specific pressure under the joint action of supercritical fluid, a solvent, an extraction pond and a crystallizing pond. The preparation technology is high in separation efficiency with few impurities, so that preparation product quality is greatly improved.
Owner:陕西顿斯制药有限公司
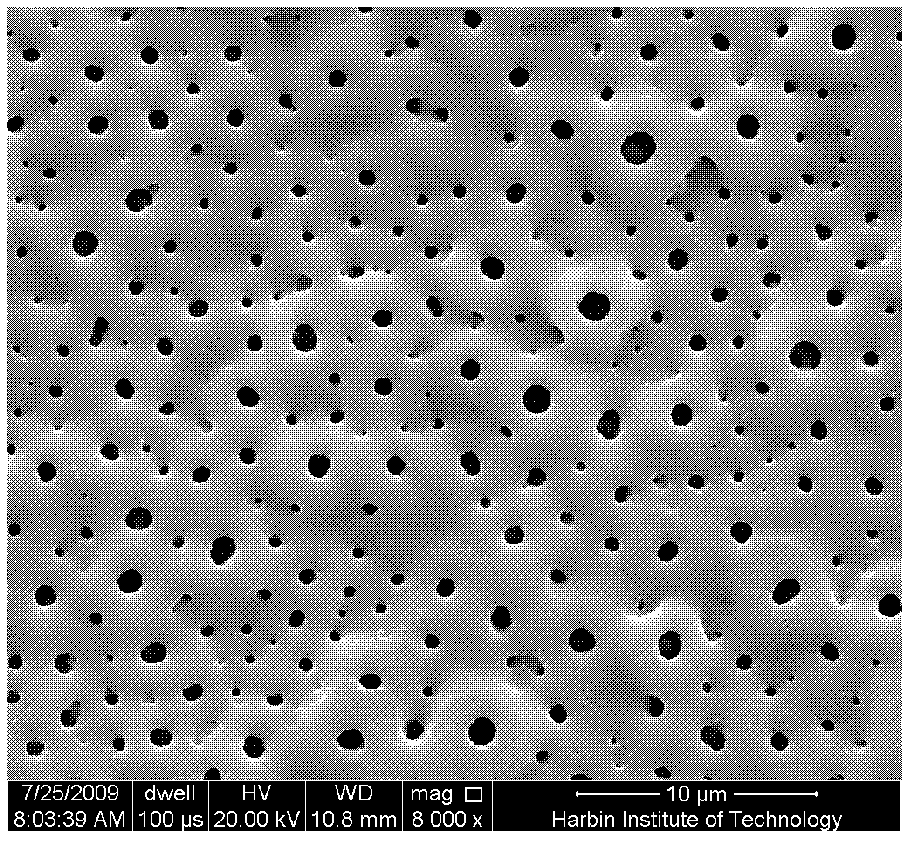
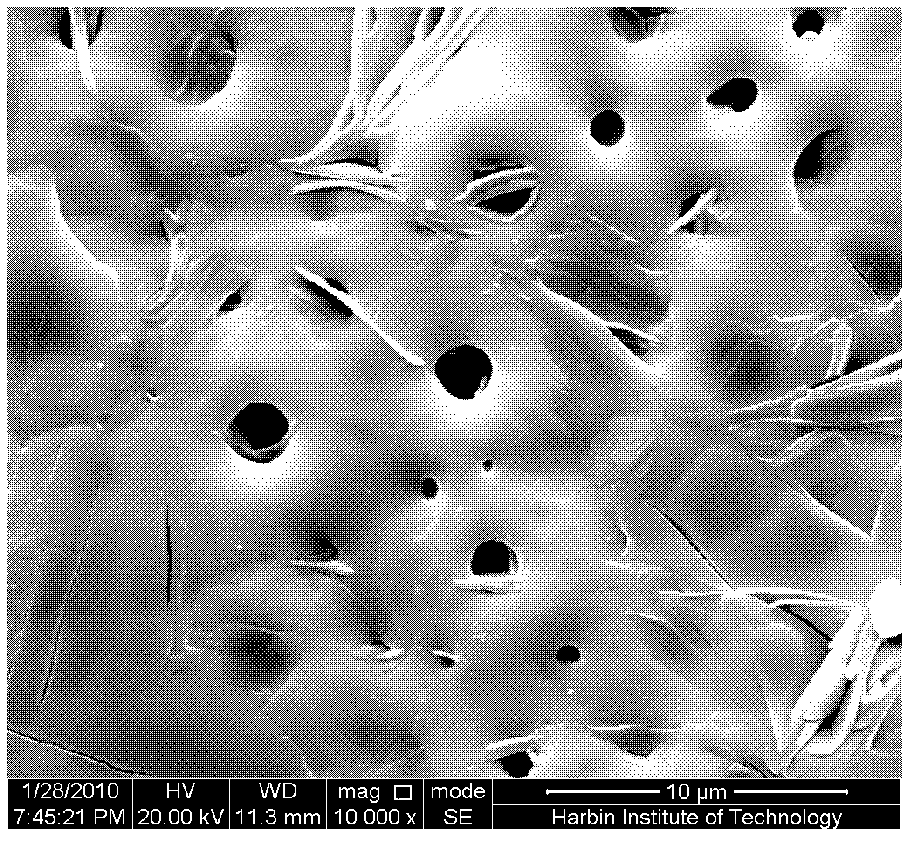
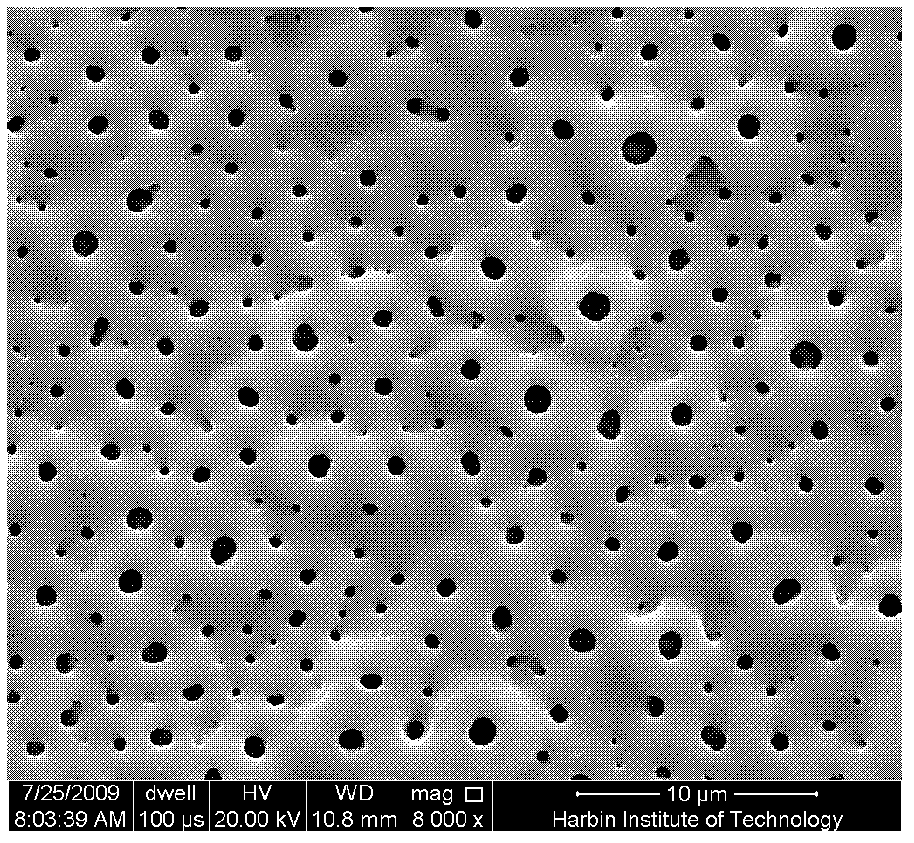
Method for uploading cefazolin sodium medicine film on micro-arc oxidation titanium implant
InactiveCN102526800AImprove matchHigh bonding strengthSurface reaction electrolytic coatingProsthesisMicro arc oxidationPlasma electrolytic oxidation
A method for uploading a cefazolin sodium medicine film on a micro-arc oxidation titanium implant relates to a method for applying an antibiotic medicine film on a medical titanium metal modified coating which serves as a bone substitute. The biological activity of the titanium implant after micro-arc oxidation treatment is greatly improved; and the antibiotic can prevent the wound infection caused by implanting the implant into a body and improve the healing capability of a wound. The method comprises the following steps: putting the titanium implant into a stainless steel tank with alkaline electrolyte; through controlling electrical parameters of the micro-arc oxidation, forming a layer of micro-arc oxidation coating on the titanium surface through breakdown discharge on the titanium surface by using a bipolar pulse power; dipping a titanium substrate after the micro-arc oxidation into an aqueous solution of cefazolin sodium; taking out the micro-arc oxidation titanium substrate soaked in the cefazolin sodium; and putting the titanium substrate into a drying chamber, and drying to obtain micro-arc oxidation titanium implant uploaded with the cefazolin sodium medicine film. By the method, the wound infection caused by implanting the implant into the body can be prevented and the healing of the wound can be promoted.
Owner:HARBIN INST OF TECH
Preparation method of cefazolin sodium for injection
InactiveCN110894197AHigh purityReduce moisture contentOrganic chemistryCefazolin SodiumFreeze-drying
The invention relates to the technical field of aseptic bulk drugs, particularly to a preparation method of cefazolin sodium for injection. The preparation method comprises the following steps: mixingethanol, cefazolin and a sodium salt according to a molar ratio of 30:(0.1-2.0):(0.1-2.0), adding into a reaction container, heating, carrying out stirring reflux, controlling the pH value at 5.0-9.0, carrying out cooling crystallizing in a water bath, adding cefazolin sodium and purified water into the reaction container according to a molar ratio of 1:(10-20), carrying out heating dissolving, adding a solventing-out agent in a dropwise manner, applying a constant magnetic field, performing microporous sterile filtration on the cefazolin sodium solution in a sterile area, and performing freeze drying, crushing and sterile packaging in the sterile area so as to obtain the cefazolin sodium for injection. According to the invention, the method can solve the problems of high water content and easy influence on the purity of cefazolin sodium in the prior art; and through cefazolin sodium water bath crystallization and magnetic field directional arrangement, the prepared cefazolin sodium for injection is low in moisture content and has the significantly improved hygroscopicity.
Owner:上海欣峰制药有限公司
Para-cecropin antibacterial peptide and application thereof
ActiveCN104140458AGrowth inhibitionGood antibacterial effectAntibacterial agentsPeptide/protein ingredientsEscherichia coliBacteroides
The invention discloses a para-cecropin antibacterial peptide and application thereof and belongs to the technical field of molecular biology. The para-cecropin antibacterial peptide consists of 31 amino acids and has the molecular weight of 3833.7Da. The antibacterial peptide can be used for inhibiting the growth of bacteria, such as staphylococcus aureus, avian pasteurella multocida, salmonella gallinarum and avian escherichia coli, has a better bacteriostasis effect compared with the commonly-used antibiotics, such as penicillin, streptomycin, cefazolin sodium and azithromycin, and has stronger bacteriostasis activity compared with the conventional cecropin antibacterial peptides, such as Cecropin B, Cecropin A and Cecropin P1. The antibacterial peptide has the minimum salmonella gallinarum bacteriostasis concentration of 625 micrograms per milliliter, has good thermal stability and has good application prospect in the research and development of antibacterial drugs.
Owner:HENAN UNIV OF SCI & TECH
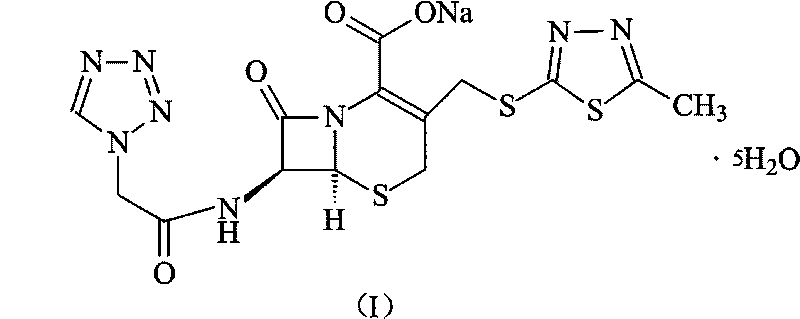
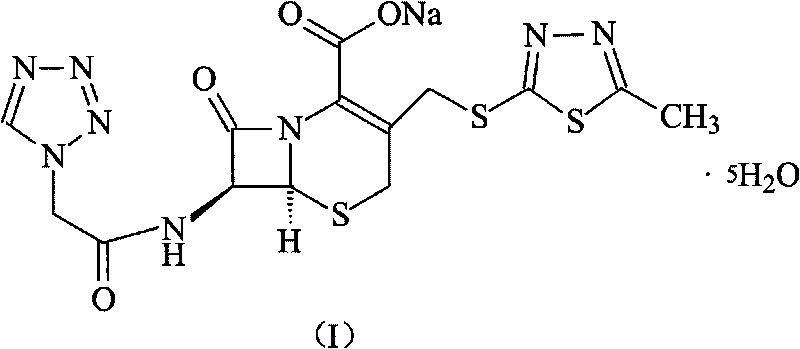
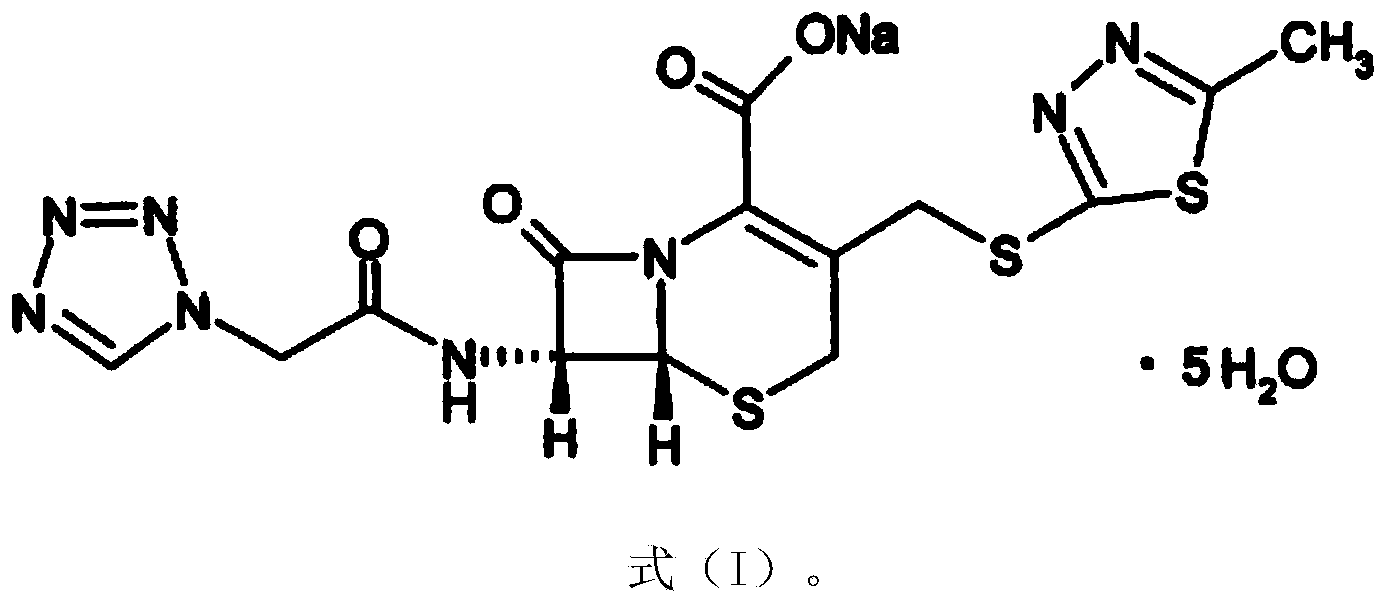
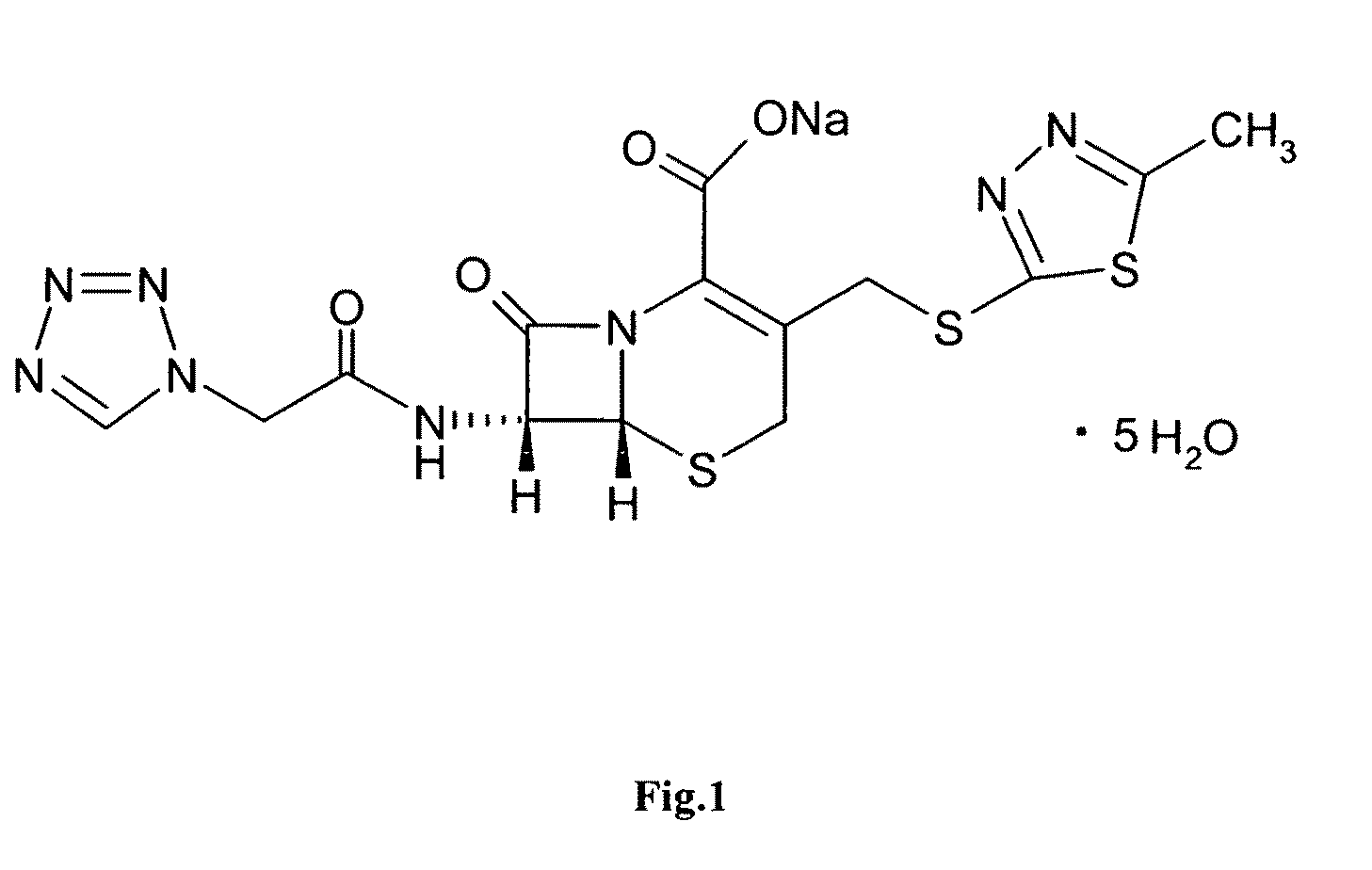
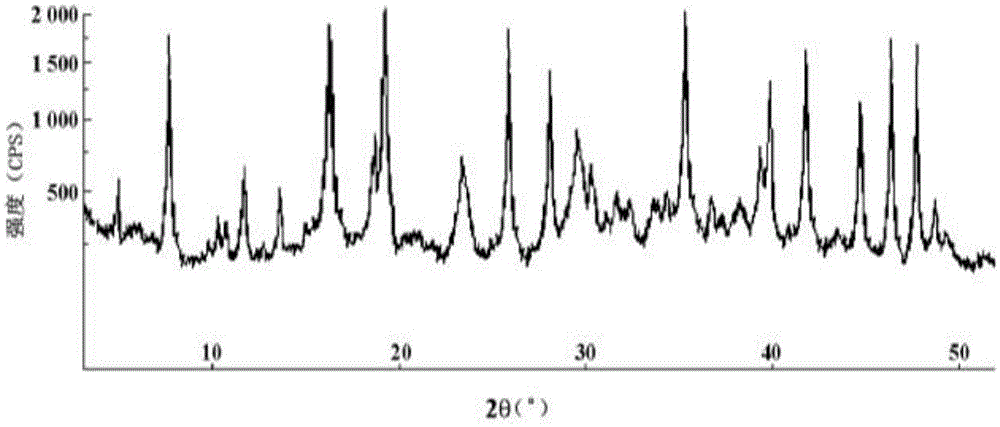
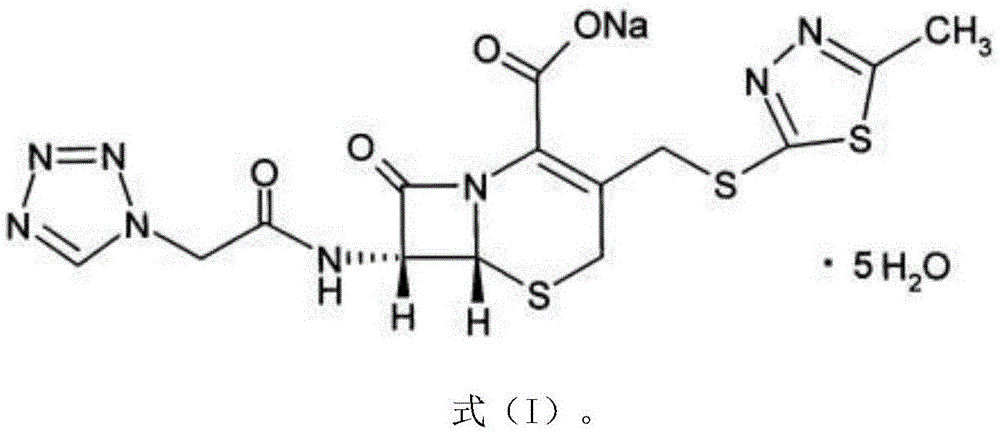
Cefazolin sodium compound and aseptic powder injection thereof
ActiveCN103965215AIncrease humidityExcellent clinical efficacyAntibacterial agentsPowder deliveryState of artClinical efficacy
The invention belongs to the technical field of medicine, and in particular relates to a cefazolin sodium compound and an aseptic powder injection thereof. The structural formula of the cefazolin sodium compound is as shown in Formula (I); the compound is measured by a powder X-ray diffraction measurement method; and the X-ray powder diffraction diagram represented by a diffraction angle of 2 theta + / - 0.2 degrees is as shown in Fig . 1. The cefazolin sodium provided by the invention has a relatively low hygroscopicity; and the clinical effect and the bacteriological effect of the aseptic powder injection prepared from the cefazolin sodium provided by the invention are significantly better than those of the prior art. (the formula is shown in the specification).
Owner:YOUCARE PHARMA GROUP +1
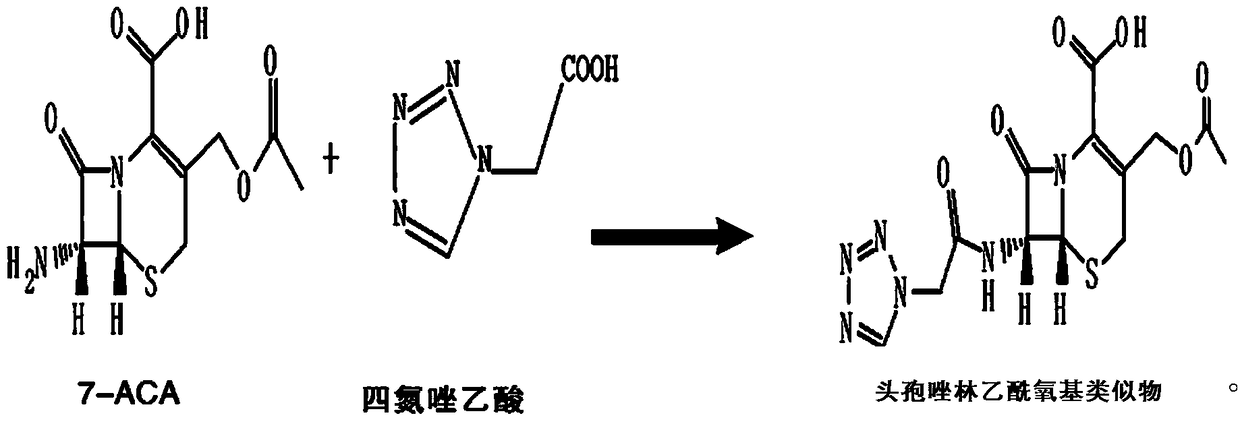
Cefazolin acetoxy analogue preparation method
InactiveCN109293679ASynthetic operation is simple and fastEasy to separate and purifyOrganic chemistryChemical synthesis7-ACA
The invention discloses a cefazolin acetoxy analogue preparation method, wherein the cefazolin acetoxy analogue finished product is prepared by using a 7-ACA solution and a tetrazoleacetic acid mixedanhydride solution as reaction raw materials and carrying out matched control on the raw material ratio, the reaction temperature and the pH value of the system. According to the present invention, the process method for preparing the cefazolin acetoxy analogue by using the chemical synthesis method is provided, such that the cefazolin acetoxy analogue standard substance can be stably and efficiently obtained so as to improve the production quality of cefazolin sodium.
Owner:河北合佳医药科技集团股份有限公司
Cefazolin sodium alkali degraded impurity compounds as well as preparation method and application thereof
The invention discloses three cefazolin sodium alkali degraded impurity compounds and confirms structures of the impurity compounds thereof. The invention also discloses a preparation method of the three impurity compounds. The preparation method can be used for preparing the impurity compounds, the purities of which are greater than 95%. The invention also discloses an application of the impuritycompounds as a cefazolin sodium raw material, an intermediate or a reference substance of a compound preparation, so that the impurity compounds have important meaning in controlling quality of the cefazolin sodium raw material, the intermediate or the compound preparation.
Owner:PI & PI BIOTECH
Method for purifying cefazolin acid
ActiveCN104610282APrevent precipitationAvoid degradationOrganic chemistryPurification methodsCefazolin Sodium
The invention discloses a method for purifying a cefazolin acid, which is implemented by preparing a crude cefazolin acid product with a content of less than 97% into a sodium salt firstly; then, carrying out adsorption on the sodium salt by using a macroporous resin so as to remove impurities; and finally, reducing the sodium salt to an acid, so that the content, individual impurity and color grade of the obtained cefazolin acid satisfy the demands of being directly used for preparing cefazolin sodium by using a freeze-drying method; and the cefazolin acid can be directly used. The method disclosed by the invention overcomes the defects that single purification in existing purification methods can not meet the requirements of use.
Owner:石药集团中诺药业(石家庄)有限公司
Preparation method of cefazolin sodium
Owner:哈药集团股份有限公司 +1
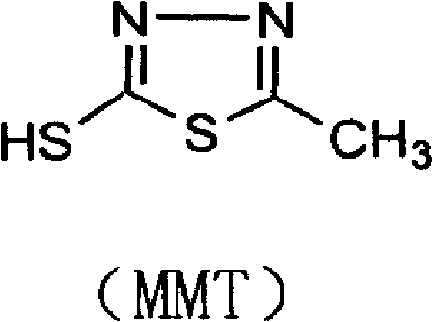
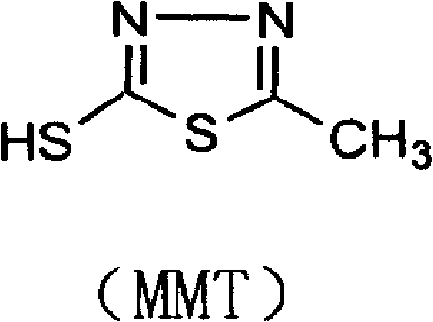
Synthesis method of 2-methyl-5-sulfydryl-1,3,4-thiadiazole
InactiveCN102219758ALow costShorten the production cycleOrganic chemistryThiocarbamateCefazolin Sodium
The invention discloses a synthesis method of 2-methyl-5-sulfydryl-1,3,4-thiadiazole. On the basis of an original process, potassium hydroxide in the original process is replaced with liquid ammonia to synthesize an intermediate N-acetylhydrazine ammonium thiocarbamate (ammonium salt); and the drying is not needed for the intermediate; and ring closure and hydrolysis are further carried out on the intermediate in concentrated sulfuric acid; and the intermediate is purified to obtain a target product. In the invention, because the synthetic process of the key intermediate 2-methyl-5-sulfydryl-1,3,4-thiadiazole of cefazolin sodium is modified, the production period is reduced, the raw material cost is reduced, the operation is simplified, the yield of the product is increased and shortcomings of longer production period and high cost in the original technology are also effectively overcome.
Owner:SHANDONG JINING YUXIN FINE MATERIALS
Method for preparing cefazolin compounds
ActiveCN102617607BSimple recycling processHigh recovery rateAntibacterial agentsOrganic chemistryTetrazoleMethyl carbonate
The invention belongs to the field of pharmacy and relates to a method for preparing cefazolin compounds. The method comprises the following steps that: 1, cefazolin sodium imidazo (toluene diamine TDA) is synthetized, thiadiazole and 7-ACA are obtained through reaction, dimethyl carbonate is used as solvents in the reaction, boron trifluoride-dimethyl carbonate is used as catalysts, and reagents used for regulating the pH of the reaction liquid are inorganic alkali after the reaction is completed; 2, anhydride is prepared, and the anhydride is obtained through the reaction between tetrazole acetic acid and pivaloyl chloride, and 3, the cefazolin is synthesized, TDA solution reacts with the anhydride, and the reaction solution is subjected to decoloration and purification through an aluminium oxide column.
Owner:哈药集团股份有限公司 +1
Preparation method of high-purity cefazolin sodium and medicinal preparation thereof
ActiveCN111548357AEasy to operateImprove product qualityAntibacterial agentsOrganic active ingredientsCefazolin SodiumPhosphate
The invention relates to a preparation method of high-purity cefazolin sodium and a medicinal preparation thereof, which belongs to the technical field of cefazolin sodium preparation, and the methodcomprises the following steps: 1) adding cefazolin acid into a phosphate solution, uniformly stirring, and controlling the temperature; 2) adding sodium carbonate into purified water, controlling thetemperature, stirring and dissolving; 3) slowly adding a sodium carbonate solution into the mixed solution of cefazolin acid, a phosphate solution and purified water, controlling the temperature and pH to dissolve the cefazolin acid to be clear, adding an adsorbent, stirring and filtering, and adjusting the pH; (4) adding ethylenediamine tetraacetic acid disodium and sodium chloride solid, coolingand growing crystals; and (5) filtering a cefazolin sodium crystallization solution, leaching and washing with a solvent, and drying. According to the invention, the method can effectively remove various impurities and polymers in cefazolin acid, the obtained product is high in purity and quality, and the prepared powder injection has higher medicinal safety.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for coating micro-arc oxidation titanium implant with cefazolin sodium and chitosan composite drug membrane
InactiveCN102560596AImprove matchHigh bonding strengthSurface reaction electrolytic coatingProsthesisMicro arc oxidationPlasma electrolytic oxidation
A method for coating micro-arc oxidation titanium implant with cefazolin sodium and chitosan composite drug membrane relates to coating an antibiotic drug membrane having sustained release and degradation actions to a titanium implant surface. According to the method for coating micro-arc oxidation titanium implant with cefazolin sodium and chitosan composite drug membrane, the biological activity of the titanium implant subjected to micro-arc oxidation is greatly improved, the antibiotic drug membrane can avoid causing wound infection when the implant is implanted into a body, and the healing ability is improved. The method includes placing titanium implant in a stainless steel groove containing an alkaline electrolyte, using a bipolar impulsing power source, controlling micro-arc oxidation electrical parameters, enabling the titanium surface to form a micro-arc oxidation coating by a disruptive discharge of the titanium surface; immersing titanium matrix after micro-arc oxidation into a blended solution of cefazolin sodium and chitosan for soaking; taking out the micro-arc oxidation titanium implant which is subjected to sufficient immersion, and drying the implant in a drying box. The method for coating micro-arc oxidation titanium implant with cefazolin sodium and chitosan composite drug membrane is simple in operation, low in cost and good for environment, and can introduce non-toxic substances into the micro-arc oxidation coating and the composite drug membrane.
Owner:HARBIN INST OF TECH
Beta-lactam antibiotic multi-template molecularly imprinted magnetic composite material, preparation method and application thereof
ActiveCN112552547AHigh adsorption selectivityImprove adsorption capacityOther chemical processesCefazolin SodiumAdsorption selectivity
The invention relates to the technical field of adsorption materials, in particular to a beta-lactam antibiotic multi-template molecularly imprinted magnetic composite material, a preparation method and application thereof. The invention discloses a beta-lactam antibiotic multi-template molecularly imprinted magnetic composite material, which has strong adsorption selectivity, can specifically andquickly adsorb cefalexin, cefazolin sodium, penicillin G sodium, oxacillin sodium and amoxicillin, and has large adsorption capacity. The composite material also shows superparamagnetism, solid-liquid separation can be rapidly and effectively realized, and the working efficiency is improved. The composite material can be combined with high performance liquid chromatography to be used for detecting the beta-lactam antibiotics in a complex environment water sample, and the beta-lactam antibiotics in the complex environment water sample can be effectively detected and separated.
Owner:GUANGDONG UNIV OF TECH
Cefazolin Sodium Pentahydrate Crystal and Its Molecular Assembly Preparation Method
The present invention relates to cefazolin sodium pentahydrate crystal and a method for assembly and preparation of the crystal molecule. The cefazolin sodium pentahydrate crystal molecule contains five water molecules, orthorhombic system, space group of C222(1), in which sodium ion is bonded to the cefazolin molecule with a coordinate bond. The method for assembly and preparation of cefazolin sodium pentahydrate crystal molecule are: adding a solvent to a reactor equipped with a jacket, adding cefazolin acid and a sodium salt, heating until the reaction solution is clear, stirring continuously, adjusting pH, upon the completion of the reaction, transferring the liquid into a jacketed crystallizer, adding crystal seeds or nucleating spontaneously, controlling cooling, slowly adding a antisolvent. The particle size of cefazolin sodium pentahydrate crystal according to the present invention is adjustable, and the distribution of particle size is concentrated, the product has good flowability, smooth surface, high crystallinity, good stability, and rapid dissolving rate.
Owner:TIANJIN UNIV +1
Purification method of cefazolin sodium
The invention discloses a purification method of cefazolin sodium, and relates to the technical field of purification of cefazolin sodium. The method comprises the following steps: (1) after cefazolinis synthesized, adding water for hydrolysis, adding dichloromethane into hydrolysate, then adding hydrochloric acid, controlling the pH value to be 1.3-1.6, standing, carrying out phase splitting, discarding a water phase, and collecting a dichloromethane phase; and (2) adding water into the dichloromethane phase, adjusting the pH value to 6.4-6.7 by using a sodium hydroxide solution, standing, carrying out phase splitting, and discarding the dichloromethane phase to obtain a purified cefazolin sodium solution. The method disclosed by the invention is simple and convenient to operate, short in period and less in wastewater discharge, and has the advantage of environmental protection, and the product cefazolin sodium is high in content, less in impurity variety, low in impurity content andsafe to use as medicine.
Owner:石药集团中诺药业(石家庄)有限公司
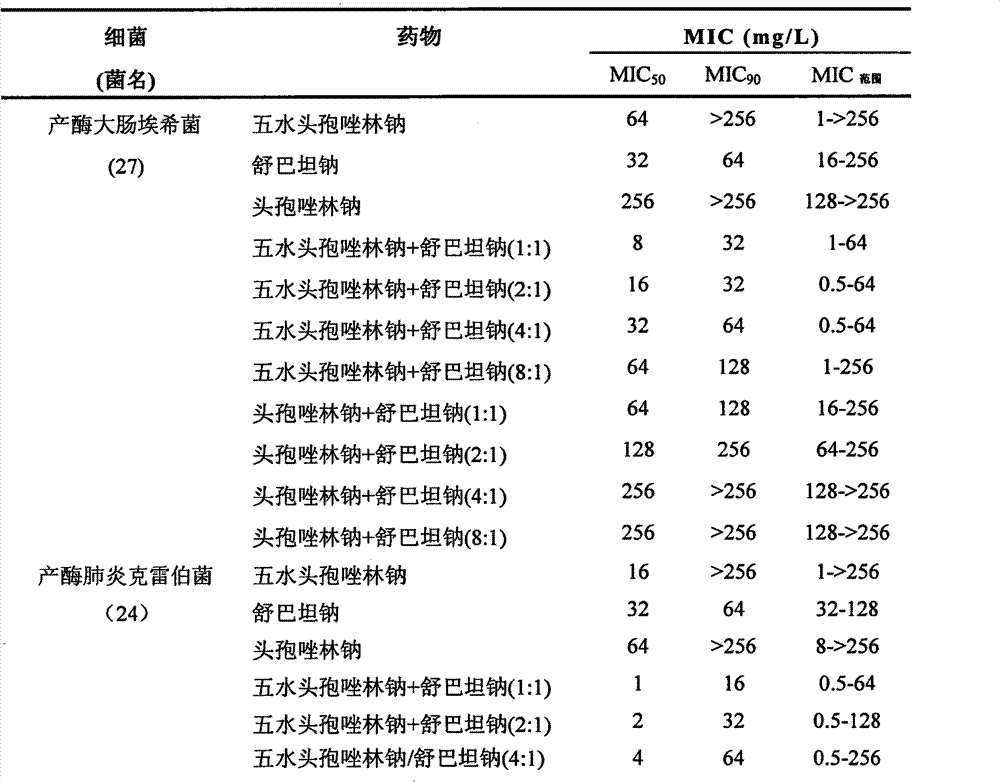
Composition of cefazolin sodium pentahydrate and sulbactam sodium
InactiveCN102813657AImprove antibacterial propertiesDelay drug resistanceAntibacterial agentsHeterocyclic compound active ingredientsCefazolin SodiumSulbactam Sodium
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
A kind of preparation method of original research quality cefazolin sodium and its pharmaceutical preparation
ActiveCN105541870BSmooth responseImprove product qualityPowder deliveryOrganic chemistryResearch qualityFreeze-drying
The invention discloses a preparation method of cefazolin sodium with previous research quality. The preparation method is characterized by comprising the following steps: (1) adding a boron trifluoride-dimethyl carbonate solution into dimethyl carbonate; stirring and adding 2-sulfydryl-5-methyl-1,3,4-thiadiazole and 7-ACA (Acetic Acid) to react; after the reaction is finished, adding dimethyl formamide and dropwise adding hydrochloric acid; adjusting the temperature to 25 to 35 DEG C and reacting for 60 minutes; filtering and washing with acetone; drying in vacuum to obtain a TDA (Toluene Diamine) crude product; (2) preparing mixed anhydride from dichloromethane, tetrazolyl acetic acid, triethylamine and pivaloyl chloride; (3) adding the TDA crude product into a dichloromethane solvent; cooling and dropwise adding tetramethyl guanidine; dropwise adding the mixed anhydride to react, and purifying and refining a crystal through a low-temperature acetonitrile-water extraction process after extraction and crystallization. With the adoption of the preparation method provided by the invention, the moisture content of the product can be reduced and residues of the solvent can be reduced; the increasing of related substances can be effectively reduced, a freeze-drying technology is not used and the production efficiency is improved.
Owner:广东金城金素制药有限公司 +1
A kind of purification method of cefazolin acid
ActiveCN104610282BMeet the requirements of the preparationReduce manufacturing costOrganic chemistryPurification methodsFreeze-drying
The invention discloses a method for purifying a cefazolin acid, which is implemented by preparing a crude cefazolin acid product with a content of less than 97% into a sodium salt firstly; then, carrying out adsorption on the sodium salt by using a macroporous resin so as to remove impurities; and finally, reducing the sodium salt to an acid, so that the content, individual impurity and color grade of the obtained cefazolin acid satisfy the demands of being directly used for preparing cefazolin sodium by using a freeze-drying method; and the cefazolin acid can be directly used. The method disclosed by the invention overcomes the defects that single purification in existing purification methods can not meet the requirements of use.
Owner:石药集团中诺药业(石家庄)有限公司
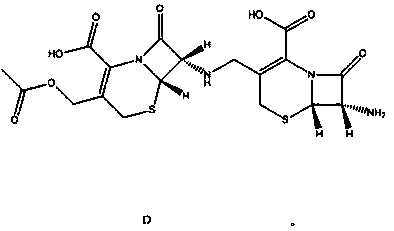
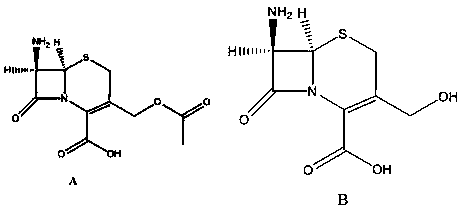
Synthesis method of dimer impurity D produced by cefazolin sodium production
InactiveCN110590814AThe synthesis method is simpleSolve analytical control problemsOrganic chemistryComponent separationCefazolin SodiumSynthesis methods
The invention discloses a dimer impurity D produced by cefazolin sodium, and a synthesis method of the dimer impurity D produced by cefazolin sodium production. The method comprises: carrying out a reaction on an initial raw material A and sodium hydroxide in a solvent ethanol and water, adjusting the pH value with diluted hydrochloric acid, extracting, and distilling to obtain an intermediate B;adding the intermediate B into dichloromethane, adding a Dess-Martin Periodinane reagent, quenching the reaction solution, and filtering to obtain the mother liquor of a compound C so as to be spare;and sequentially adding a compound A and sodium triacetoxyborohydride into dichloromethane, adding the mother liquor of the product C, quenching after completing the reaction, extracting, and purifying by using a chromatographic silica gel column to obtain a dimer impurity D. According to the present invention, the synthesized dimer impurity D can provide impurity control for the reaction.
Owner:TIANJIN LISHENG PHARM CO LTD
A kind of cefazolin sodium compound and its sterile powder injection
ActiveCN103965215BReduce humiditySimple processAntibacterial agentsOrganic active ingredientsClinical efficacyCefazolin Sodium
The invention belongs to the technical field of medicine, and in particular relates to a cefazolin sodium compound and an aseptic powder injection thereof. The structural formula of the cefazolin sodium compound is as shown in Formula (I); the compound is measured by a powder X-ray diffraction measurement method; and the X-ray powder diffraction diagram represented by a diffraction angle of 2 theta + / - 0.2 degrees is as shown in Fig . 1. The cefazolin sodium provided by the invention has a relatively low hygroscopicity; and the clinical effect and the bacteriological effect of the aseptic powder injection prepared from the cefazolin sodium provided by the invention are significantly better than those of the prior art. (the formula is shown in the specification).
Owner:YOUCARE PHARMA GROUP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com