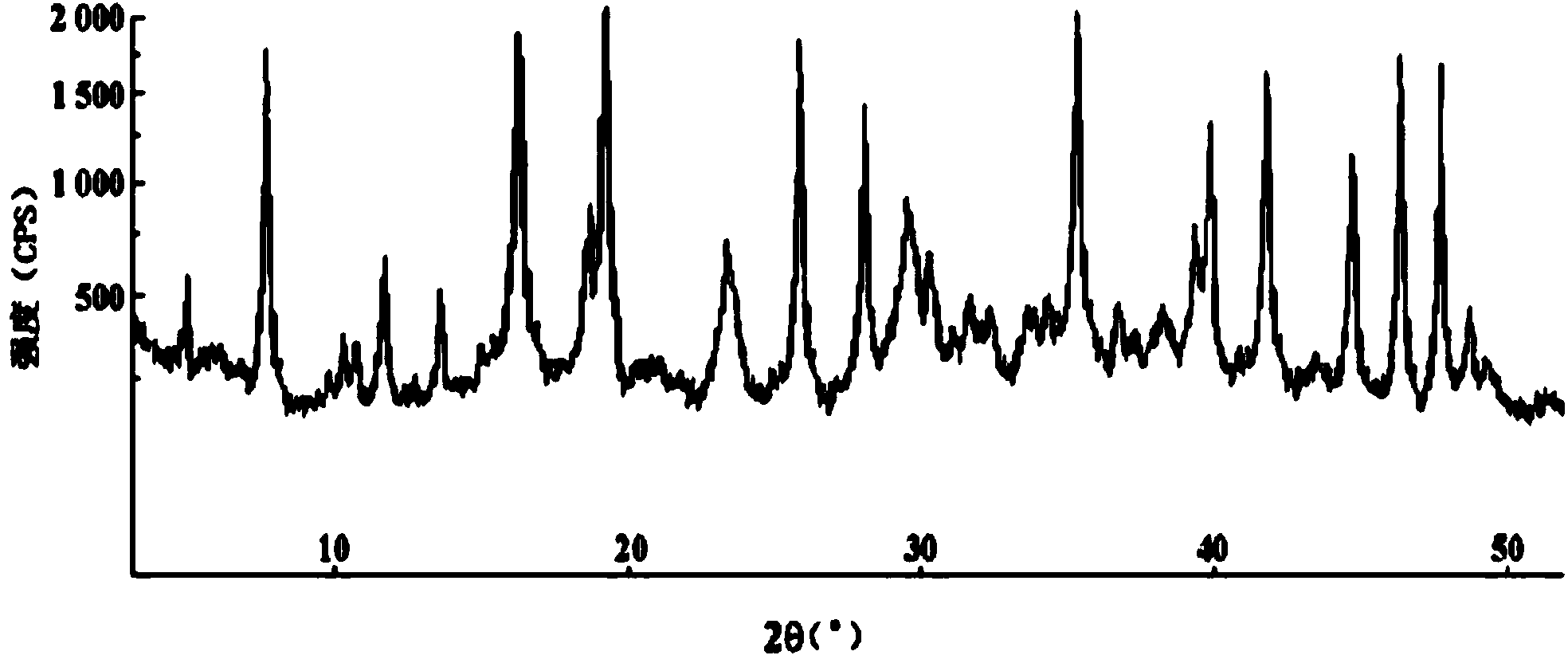
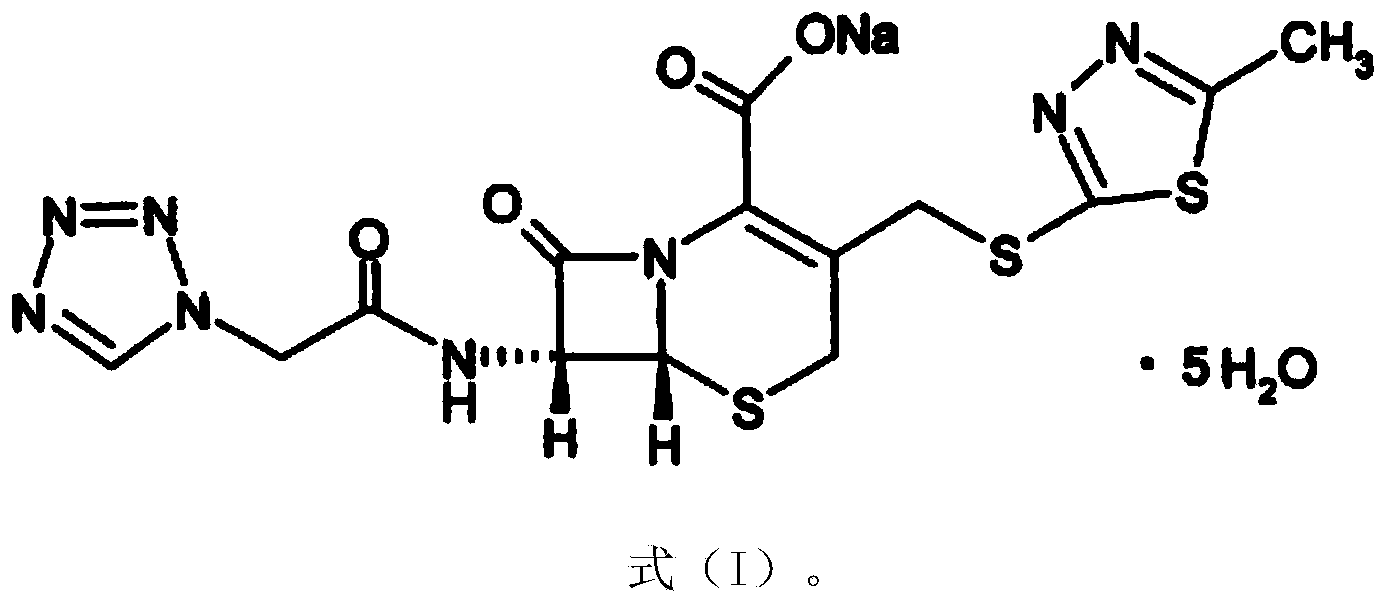
Cefazolin sodium compound and aseptic powder injection thereof
A technology of cefazolin sodium and sterile powder injection, applied in the field of medicine, can solve the problems of cefazolin sodium pentahydrate easily hygroscopic, no solution proposed, long reaction time, etc., and achieves improved hygroscopicity and simple preparation method , the effect of low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] [embodiment 1] the preparation of cefazolin sodium compound
[0036]Get cefazolin sodium bulk drug 50g, add ethanol, wherein the consumption ratio of cefazolin sodium bulk drug and ethanol is 1g: 6ml, heat and reflux until cefazolin sodium dissolves completely, obtain solution; Stop heating then, in gained solution In the horizontal direction of the liquid surface, the applied magnetic field intensity is in the constant magnetic field of 0.4T, and under the condition of the constant magnetic field, the mixed solvent of acetone, chloroform and N, N-dimethylformamide is added dropwise to the solution, wherein The volume ratio of the mixed solvent to ethanol is 3:1, and the volume ratio of acetone, chloroform and N,N-dimethylformamide in the mixed solvent is 2:3:1; After standing still for 6 hours, a white crystalline powder was obtained, which was filtered, the filter cake was washed with ethanol, and vacuum-dried for 2 hours to obtain the cefazolin sodium compound.
[0...
Embodiment 2
[0038] [embodiment 2] the preparation of cefazolin sodium compound
[0039] Get cefazolin sodium bulk drug 50g, add ethanol, wherein the consumption ratio of cefazolin sodium bulk drug and ethanol is 1g: 8ml, heat and reflux until cefazolin sodium dissolves completely, obtain solution; Stop heating then, in the gained solution In the horizontal direction of the liquid surface, the applied magnetic field intensity is in the constant magnetic field of 1.2T, and under the condition of the constant magnetic field, the mixed solvent of acetone, chloroform and N, N-dimethylformamide is added dropwise to the solution, wherein The volume ratio of the mixed solvent to ethanol is 5:1, and the volume ratio of acetone, chloroform and N,N-dimethylformamide in the mixed solvent is 4:5:1; After standing still for 10 hours, a white crystalline powder was obtained, which was filtered, the filter cake was washed with ethanol, and vacuum-dried for 4 hours to obtain the cefazolin sodium compound....
Embodiment 3
[0041] [embodiment 3] the preparation of cefazolin sodium compound
[0042] Get cefazolin sodium bulk drug 50g, add ethanol, wherein the consumption ratio of cefazolin sodium bulk drug and ethanol is 1g: 7ml, heat and reflux until cefazolin sodium dissolves completely, obtain solution; Stop heating then, in the gained solution In the horizontal direction of the liquid surface, the applied magnetic field intensity is in the constant magnetic field of 0.8T, and under the condition of this constant magnetic field, the mixed solvent of acetone, chloroform and N, N-dimethylformamide is added dropwise in the solution, wherein The volume ratio of the mixed solvent to ethanol is 4:1, and the volume ratio of acetone, chloroform and N,N-dimethylformamide in the mixed solvent is 2:5:1; After standing still for 8 hours, the obtained white crystalline powder was filtered, the filter cake was washed with ethanol, and vacuum-dried for 3 hours to obtain the cefazolin sodium compound.
[0043...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com