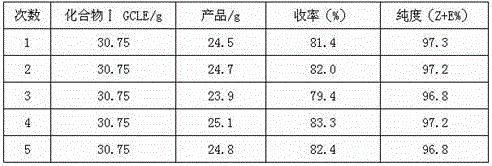
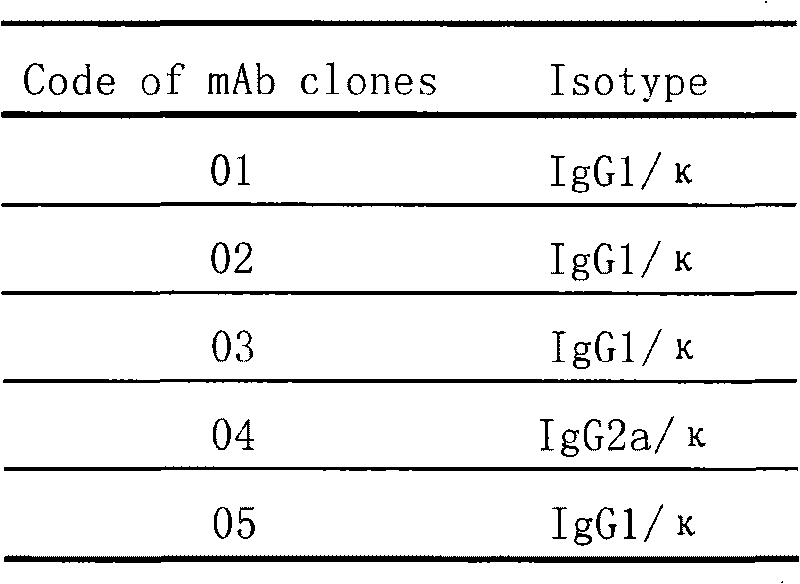
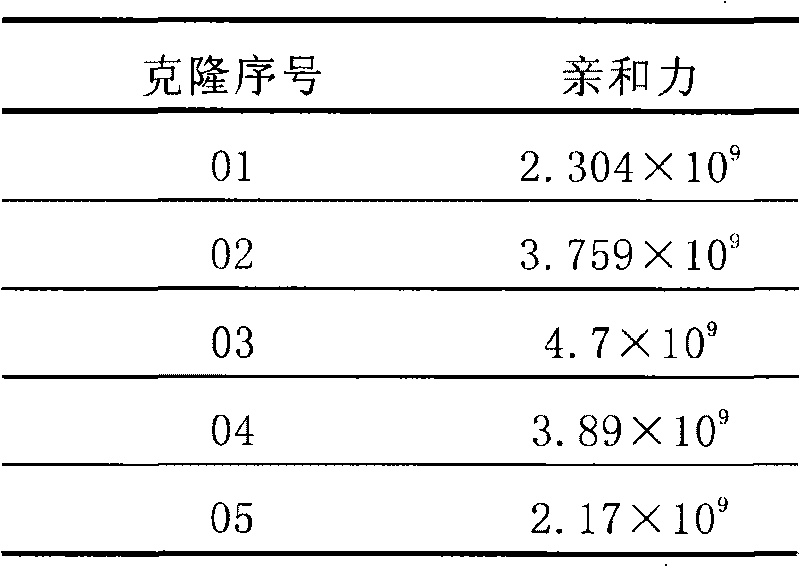
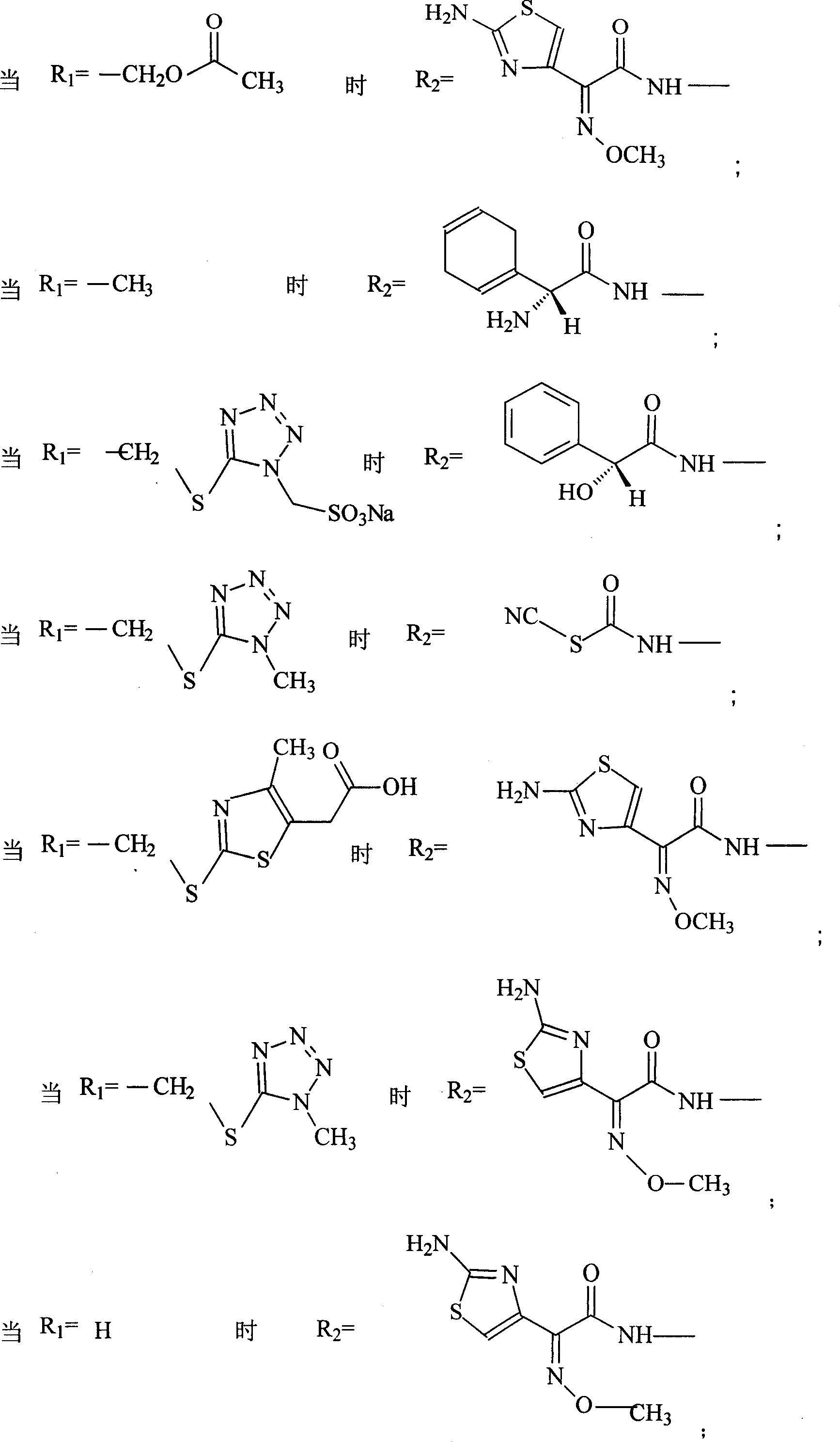
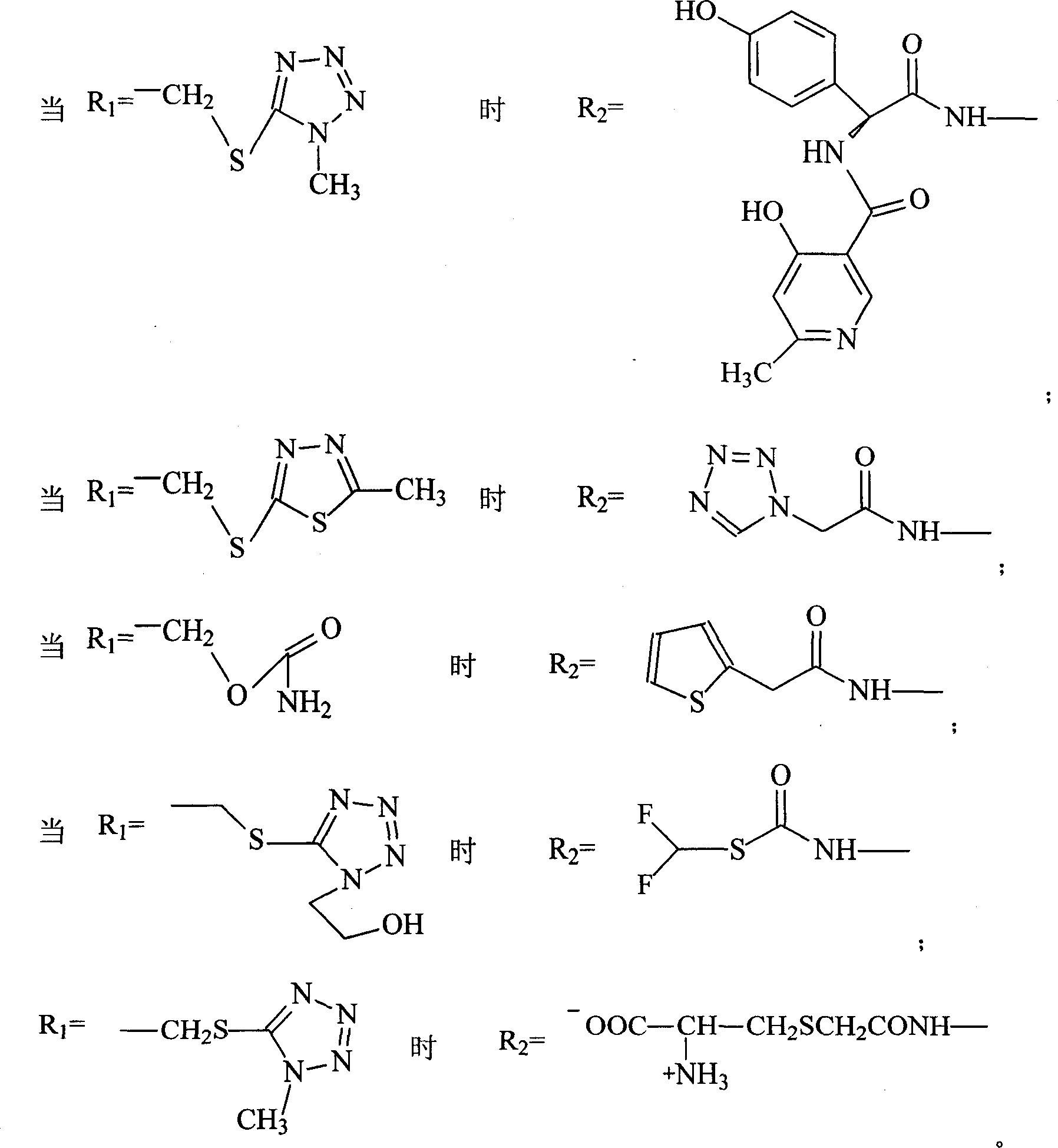
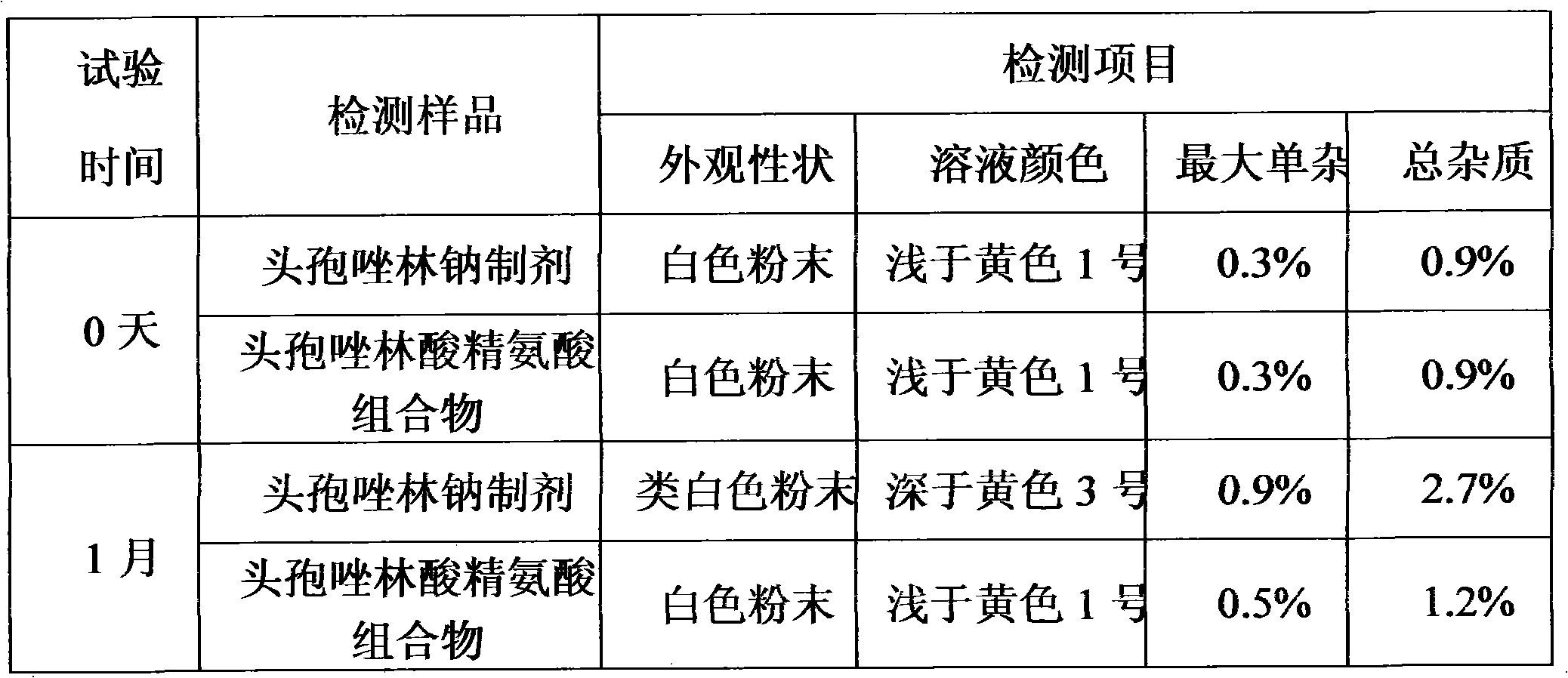
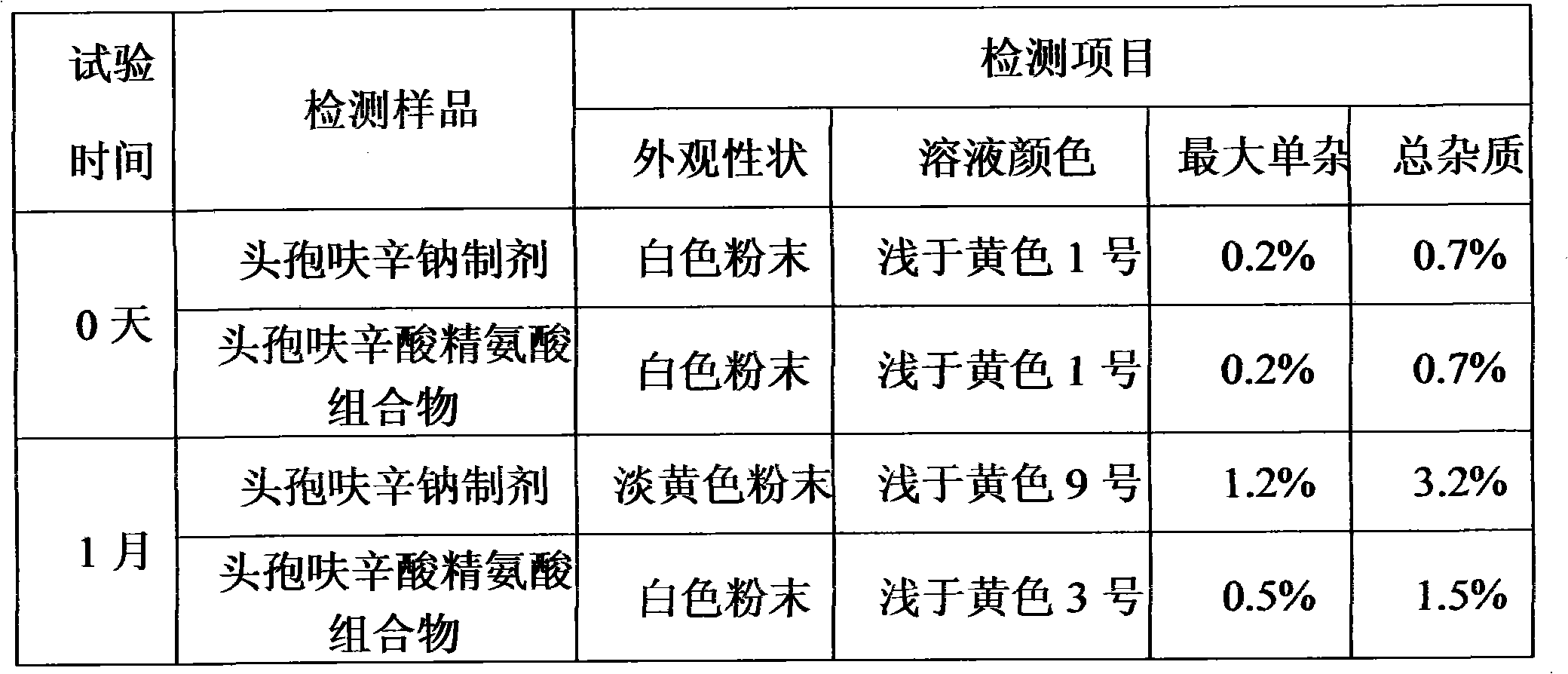
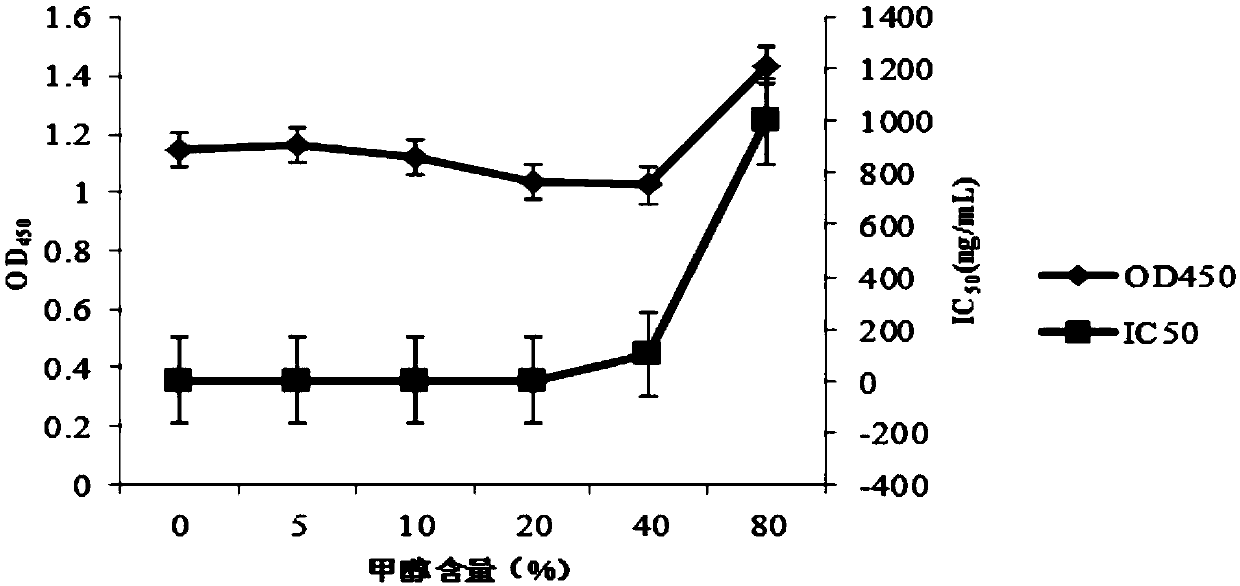
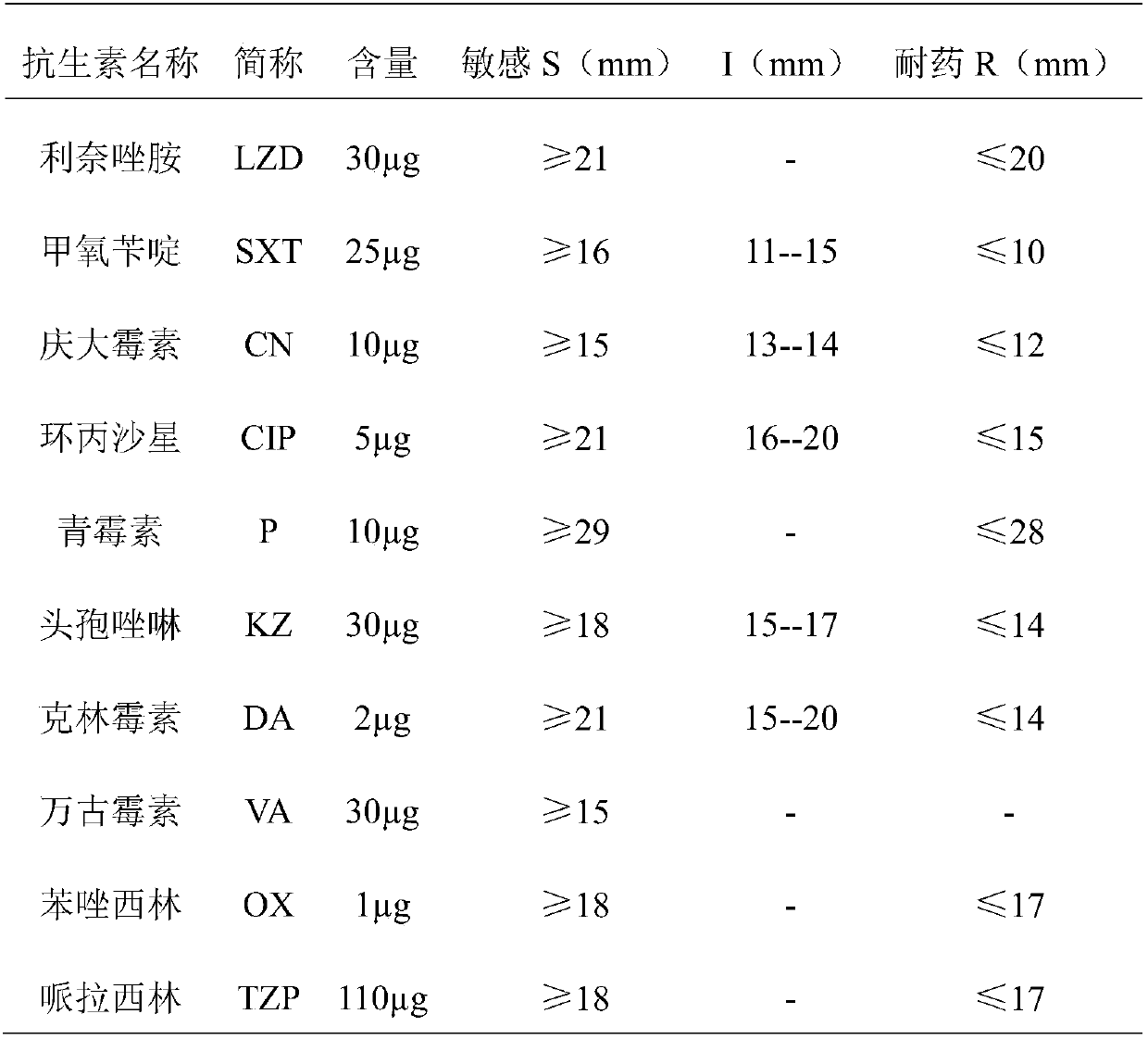
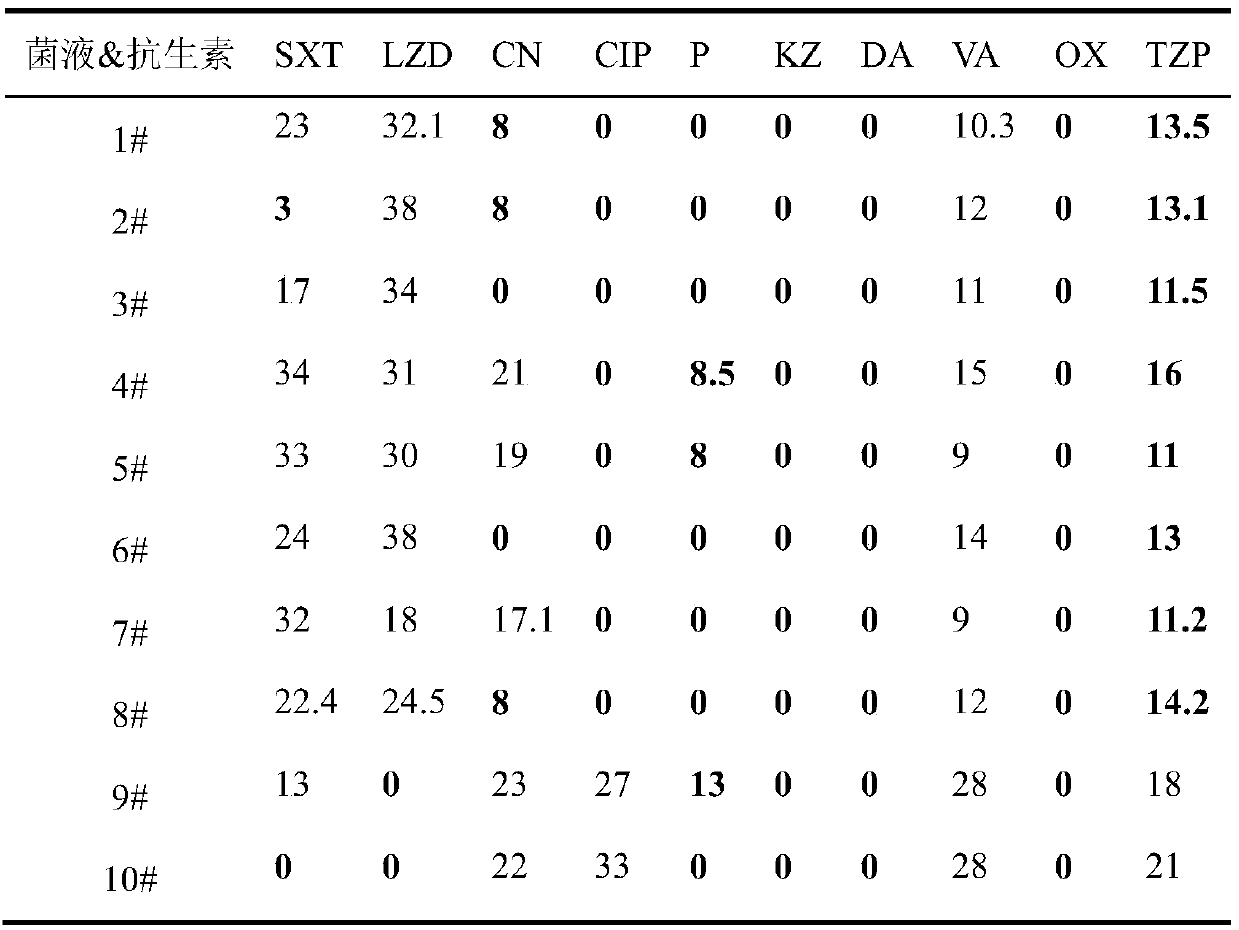
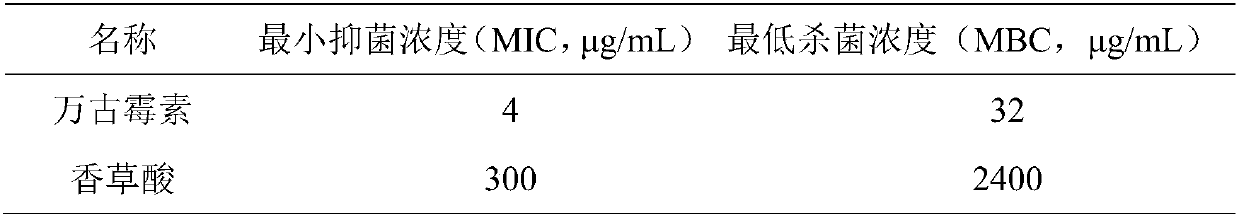
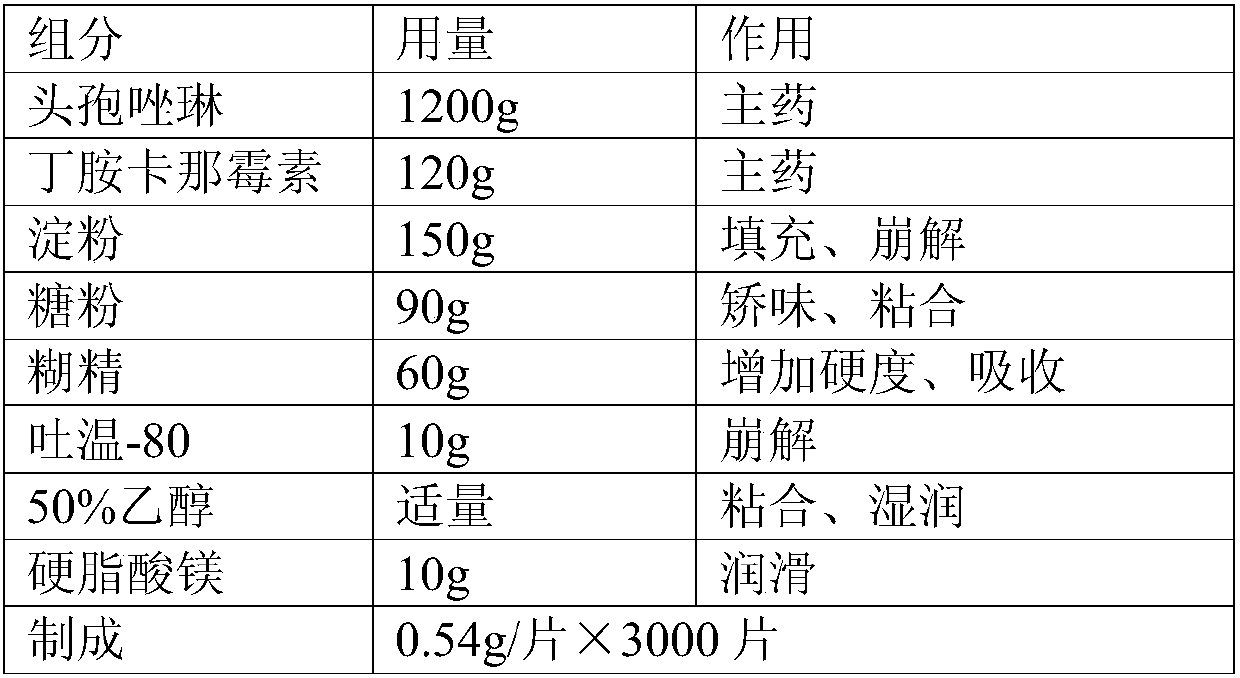
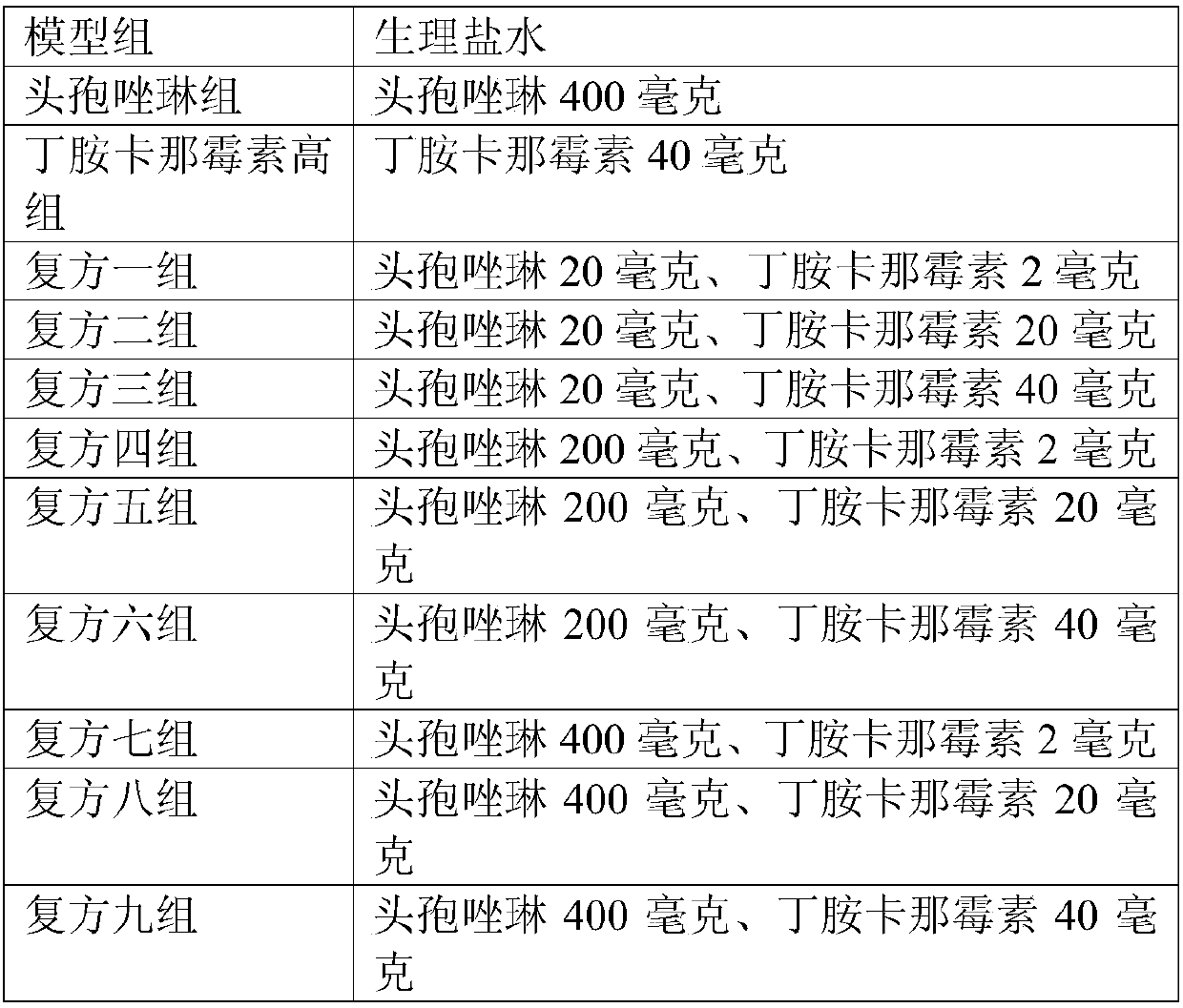
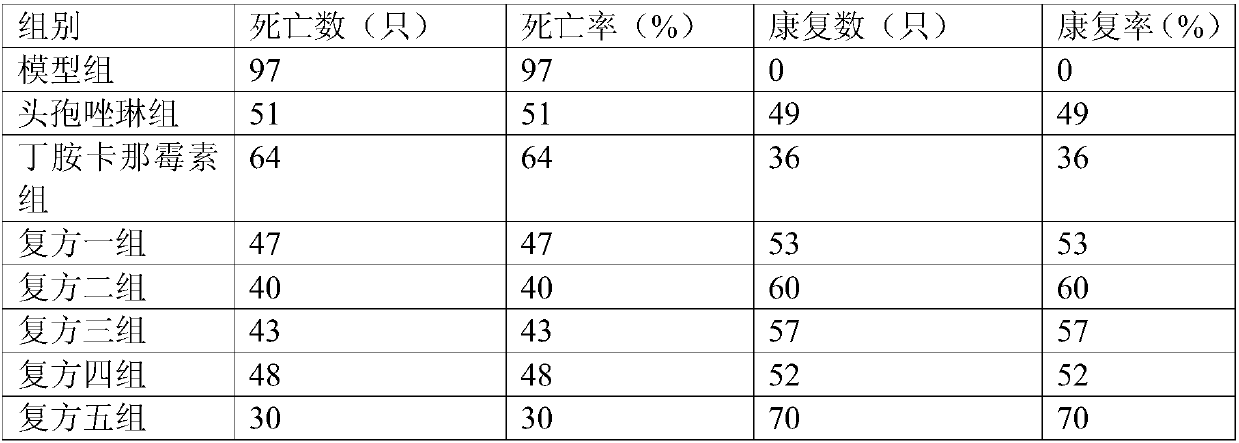
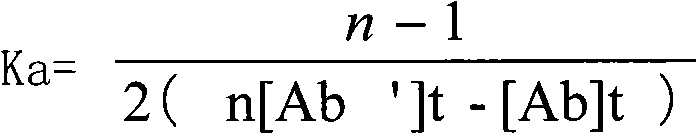Patents
Literature
44 results about "Cefazolin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cefazolin is an antibiotic used to treat a wide variety of bacterial infections. It may also be used before and during certain surgeries to help prevent infection.
Feruloyl esterase and preparing method and application thereof
ActiveCN109652392AIncrease enzyme activityHigh hydrolytic activityBacteriaHydrolasesEscherichia coliCefazolin
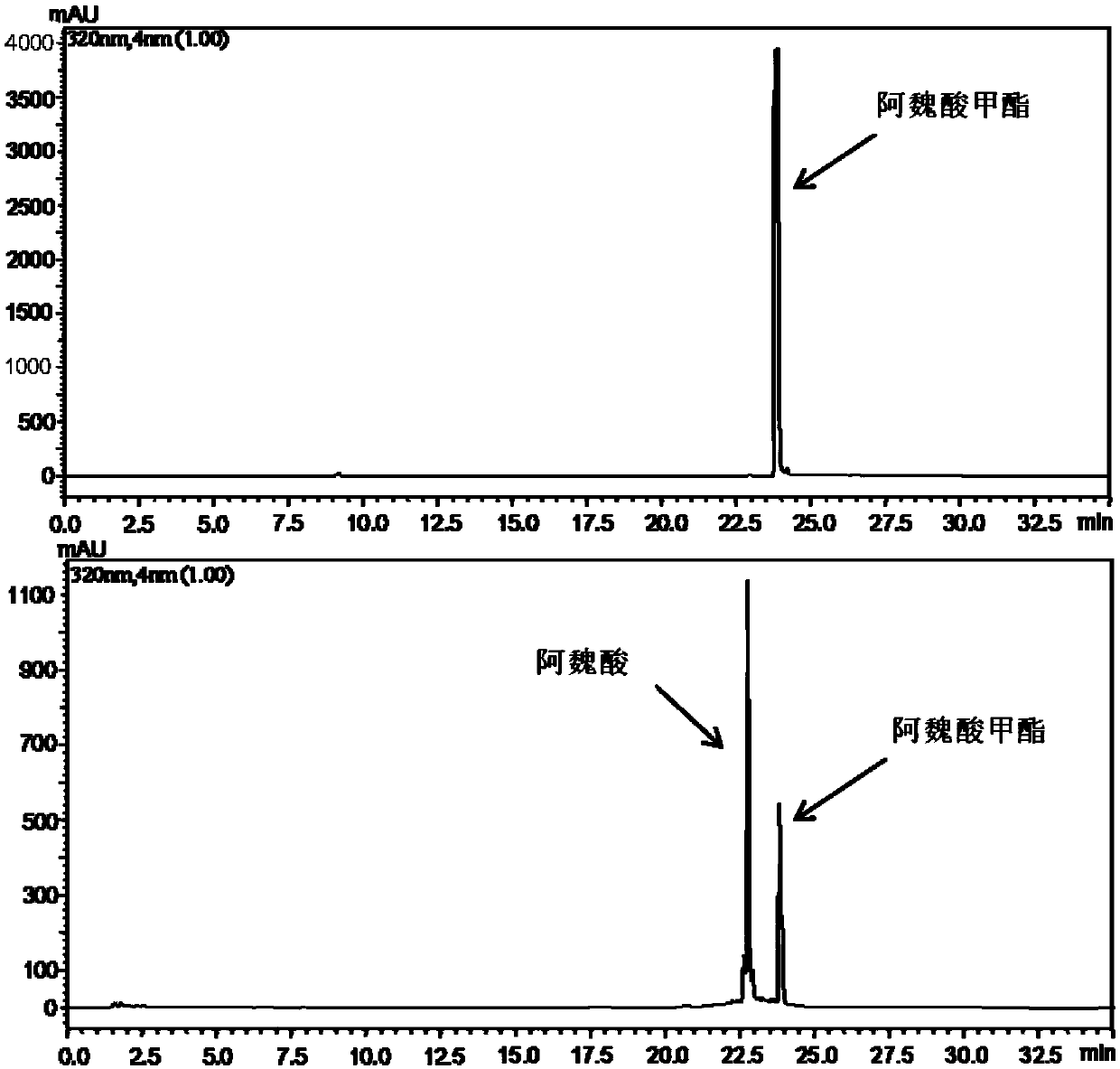
The invention provides feruloyl esterase and a preparing method and application thereof. A feruloyl esterase gene coming from a soil macro gene library have the nucleotide sequence and amino acid sequence shown in SEQ ID NO.1 and SEQ ID NO.2. The gene contains a tetrapeptide SXXK sequence motif which is rarely seen, and after the esterase gene is inserted into plasmid pET28a(+), the gene is transformed into escherichia coli BL21(DE3) to achieve heterogeneous expression. The molecular weight of purified recombinase (DLFae4) is 38.3 kDa. Besides, it is put forward for the first time that novel feruloyl esterase can hydrolyze penbritin, penicillin, cefazolin and other lactam antibiotics. As is shown by site-directed mutagenesis experiments, a catalysis triplet of DLFae4 is composed of serine(S11), histidine (H74) and aspartic acid (D302), and the mutation of any of serine (S11), histidine (H74) and aspartic acid (D302) can cause loss of the catalysis capability of DLFae4. DLFae4 has a high hydrolytic activity on methyl ferulate and has good heat stability. In the presence of cellulase, DLFae4 can obviously increase the amount of ferulic acid released from destarched wheat bran. Due to peculiar activities and enzymatic characteristics of novel feruloyl esterase, novel feruloyl esterase can be applied to feed, paper making, food, pharmacy and other fields.
Owner:NANJING AGRICULTURAL UNIVERSITY
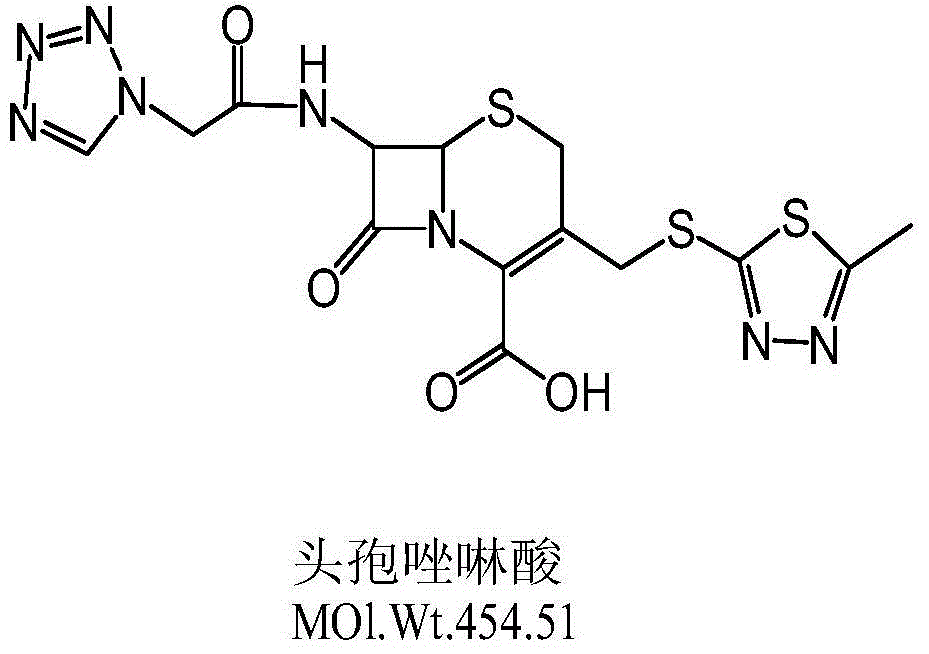
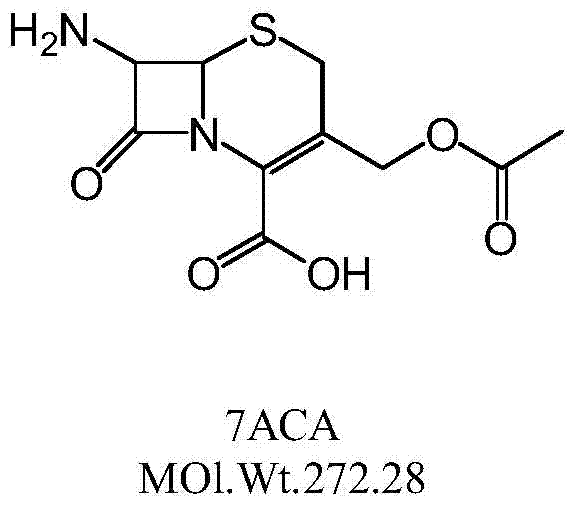
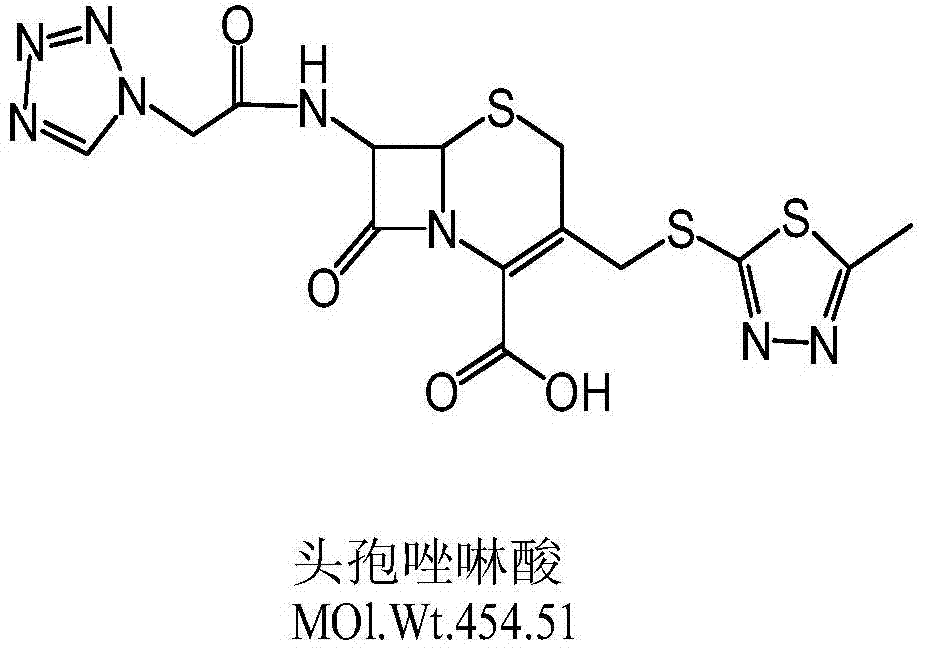
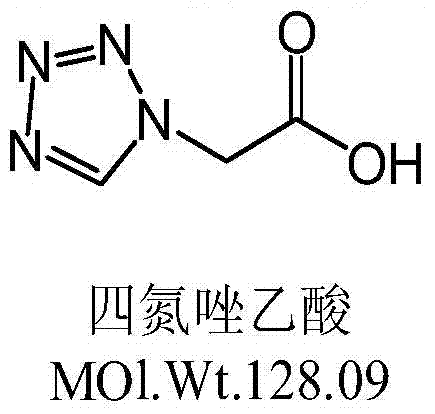
Synthetic method of cefazolin acid
The invention relates to a synthetic method of cefazolin acid. According to the synthetic method, 7-aminocephalosporanic acid is taken as a raw material, and is reacted with 1H-tetrazole-1-acetic acid in a solvent so as to obtain an intermediate; the intermediate is reacted with 5-mercapto-1-methyltetrazole under base catalysis at solution states without crystallization, washing, and drying so as to obtain cefazolin acid. One-pot method is adopted; the synthetic method is simple; solvent application amount is low; environmental pollution is low; product yield is high; cost is low; and the synthetic method is suitable for industrialized production.
Owner:QILU ANTIBIOTICS PHARMA
Drug composition containing cefazolin and beta-lactamase inhibitor
InactiveCN1424039AAddressing drug resistanceHigh activityAntibacterial agentsHeterocyclic compound active ingredientsCefazolinAntibacterial action
An antibacterial composite medicine contains ancef or its salt and beta-lactamase inhibitor (tazobactam or clavulanic acid) in Wt ratio of (1-20):(1-5). Its advnatage is high synergistic antibacterial action.
Owner:YOUCARE PHARMA GROUP +1
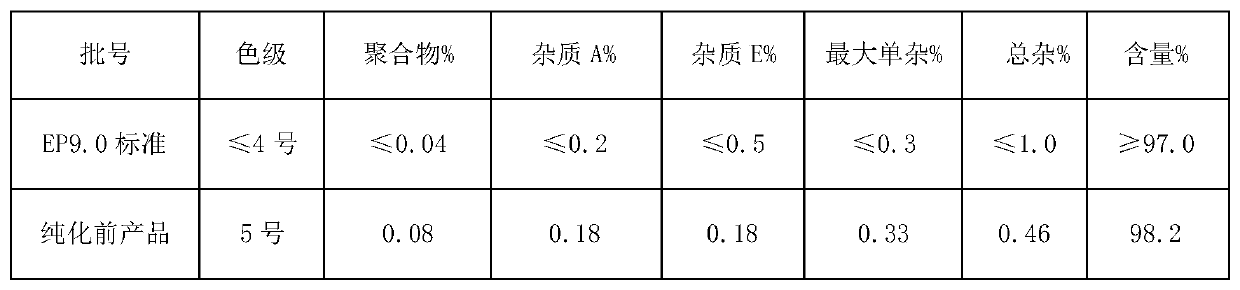
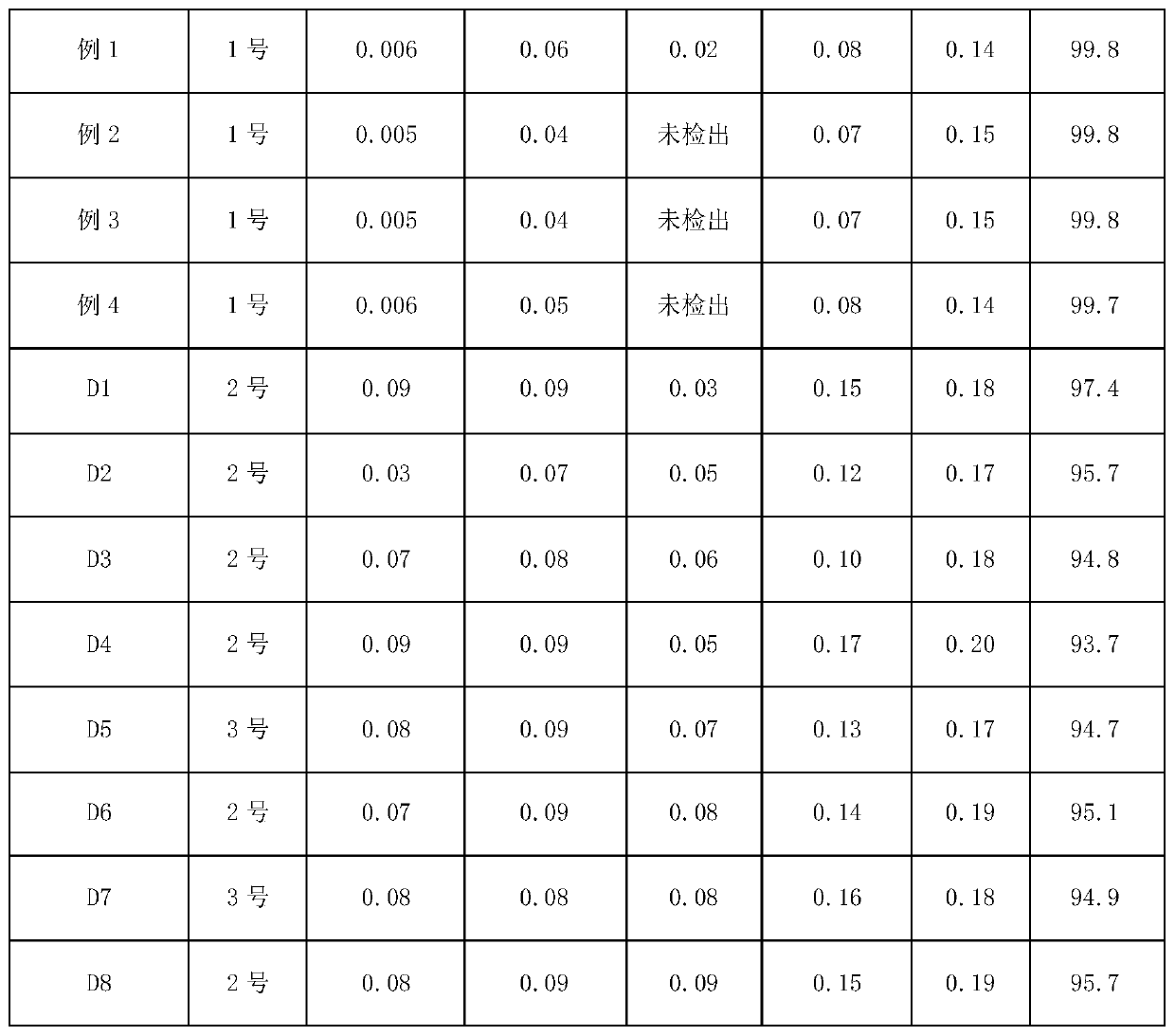
Purification method of cefazolin acid
ActiveCN109748926AQuality improvementEasy to operateOrganic chemistryPurification methodsOrganic solvent
The invention discloses a purification method of cefazolin acid, and belongs to the technical field of medicines. The purification method of the cefazolin acid comprises the following steps: A, addingraw materials into purified water, controlling the temperature, adding alkali liquor to adjust the pH of a system, and stirring until the solution is clear; B, adding an organic solvent and stirring,standing for phase separation, and retaining a water phase; C, adding acetone and a buffer solution into the water phase, controlling the temperature, dropwise adding an acid solution to regulate thepH value of the system, adding a seed crystal, and carrying out primary crystal growing; D, adding purified water, dropwise adding an acid solution to adjust pH, cooling and secondarily growing crystals; and E, filtering to obtain a filter cake, washing the filter cake at first, drying the filter cake under vacuum and discharging to obtain the purified cefazolin acid. The purified cefazolin acidobtained by the purification method not only meets the requirement of preparation of the cefazolin acid by a subsequent freeze-drying method, but also can be applied to preparation of high-content cefazolin acid standard substances.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for preparing 7-phenylacetyl amino-3-allyl-4-cefazolin acid p-methoxybenzyl acetate
The invention discloses a method for preparing 7-phenylacetyl amino-3-allyl-4-cefazolin acid p-methoxybenzyl acetate, and relates to the technical field of preparation of cephalosporin medical intermediates. The method comprises the following steps that 1, a compound I, sodium bromide, potassium iodide and triphenylphosphine react in a mixed solution of dichloromethane and water, and a compound III is obtained; 2, after the reaction in the step 1 is finished, a water layer in the step 1 is separated out, alkali liquor is dropwise added into an organic layer in the step 1 for reacting, and a compound IV is obtained; 3, after the reaction in the step 2 is finished, a water layer in the step 2 is separated and recycled and circularly used for the reaction in the step 1, and the addition of sodium bromide and potassium iodide is reduced; a solvent and acetaldehyde are added into an organic layer in the step 2 for a Wittig reaction, and a compound V is prepared. According to the method, after the alkali liquor is added to generate the compound IV, the water phase can be used for circulated to be applied to the next reaction, use of sodium bromide and potassium iodide is saved, and the production cost is reduced.
Owner:湖北凌晟药业股份有限公司
Mouse monoclonal antibody cell strain for resisting amoxicillin and ampicillin
The invention relates to a mouse monoclonal antibody cell strain for resisting both amoxicillin and ampicillin, belonging to the technical field of B cell hybridoma. The mouse monoclonal antibody cell strain for resisting the amoxicillin and the ampicillin has the preservation number of CGMCC NO.2674. The monoclonal antibody aiming at the amoxicillin has 100 percent of cross reaction with the ampicillin which also belongs to the penicillin antibiotics, and has no cross reaction with cefazolin of cephalosporin antibiotics. The invention can be used for rapidly detecting the residual condition of the amoxicillin and the ampicillin in animal-sourced food, and can be further used for developing an immunity detection product rapidly and accurately detecting the amoxicillin and the ampicillin in the animal-sourced food.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
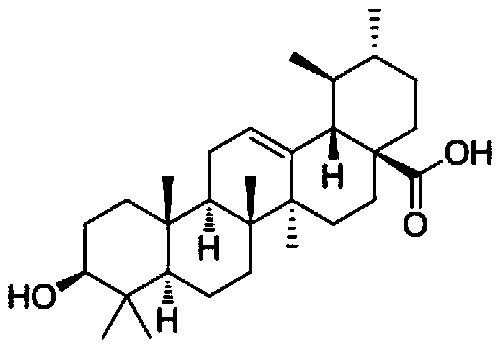
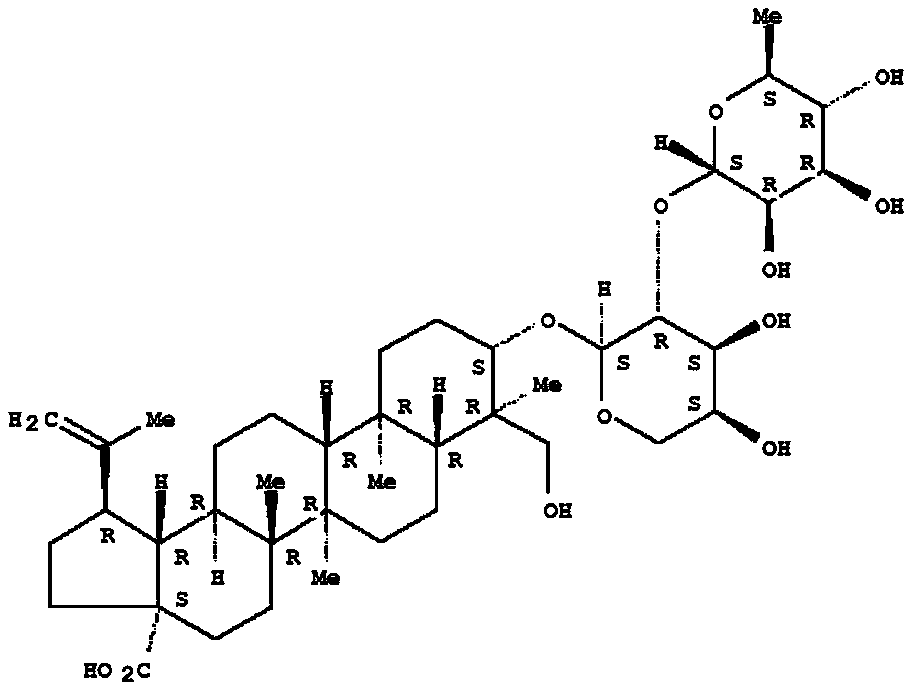
Application of ursolic acid in inhibiting growth of multi-drug-resistance enterobacter cloacae
InactiveCN110279701AMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsOrganic active ingredientsInfections problemsCase fatality rate
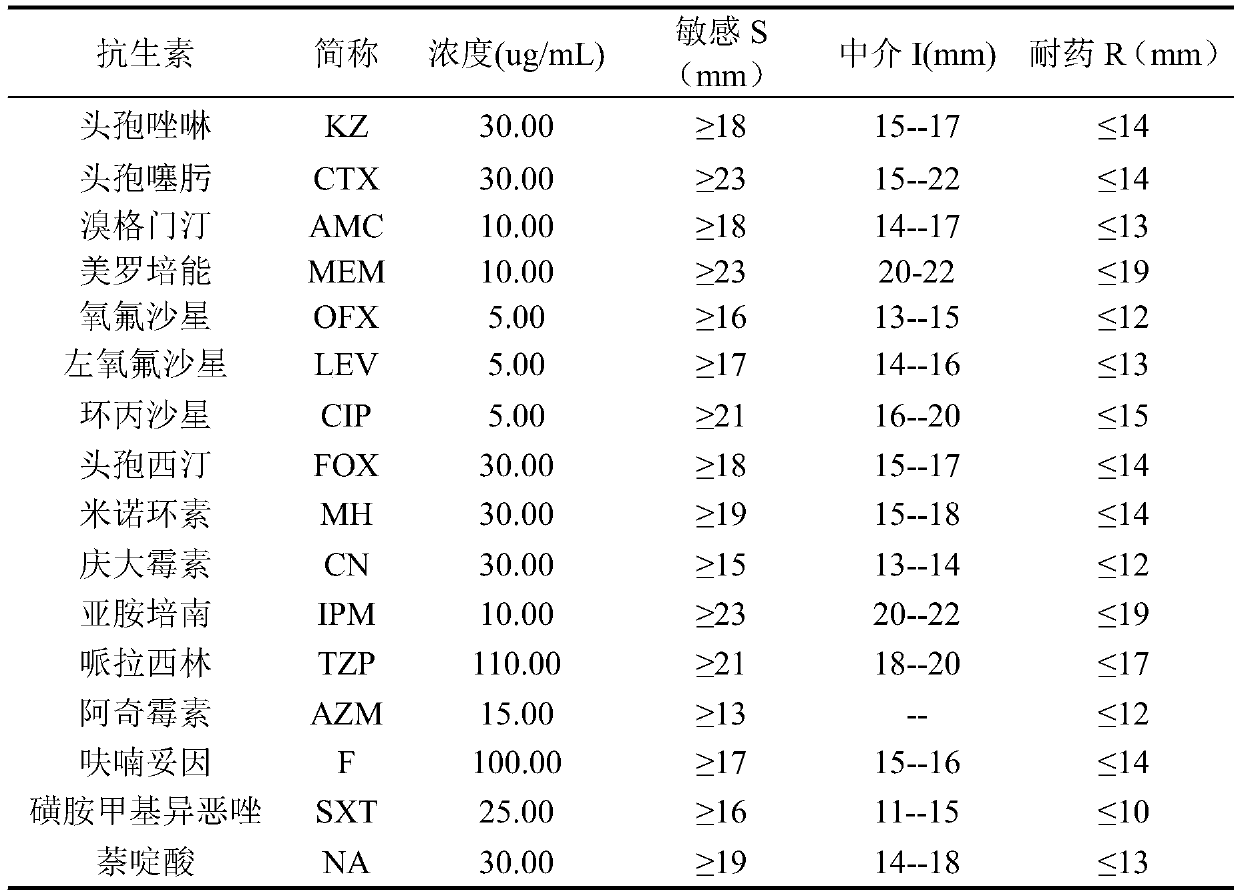
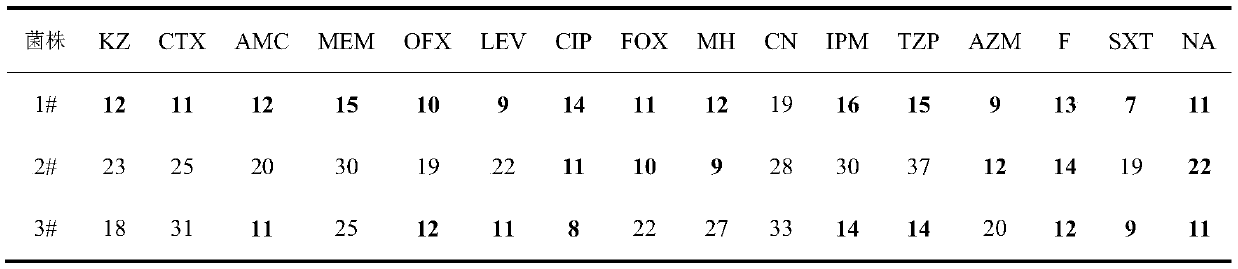
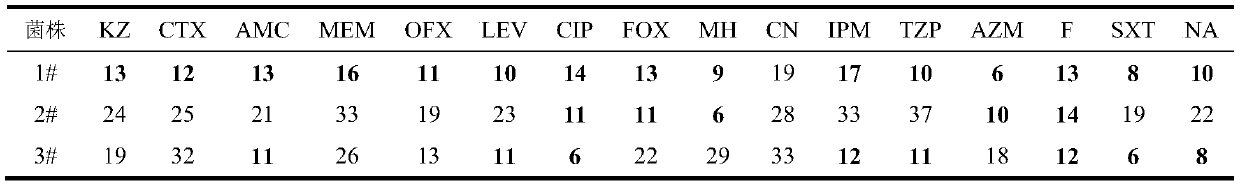
The invention discloses application of ursolic acid in inhibiting growth of multi-drug-resistance enterobacter cloacae. According to a good in-vitro killing function of ursolic acid upon anthropogenic multi-drug-resistance enterobacter cloacae which resists to cefazolin, cefotaxime, aupmentn, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocycline, imipenem, piperacillin, azithromycin and macrodantin, growth of multi-drug-resistance enterobacter cloacae can be inhibited, the lowest sterilization concentration is 0.6mg / mL, and the lowest antibacterial concentration is 0.3mg / mL. The invention discloses an inhibition function of the ursolic acid upon the multi-drug-resistance enterobacter cloacae, and the ursolic acid is capable of effectively alleviating or solving the drug-resistance infection problem of the multi-drug-resistance enterobacter cloacae and reducing the case fatality rate, provides new ideas for inhibiting anthropogenic multi-drug-resistance enterobacter cloacae, and has great practical significances.
Owner:SHAANXI UNIV OF SCI & TECH
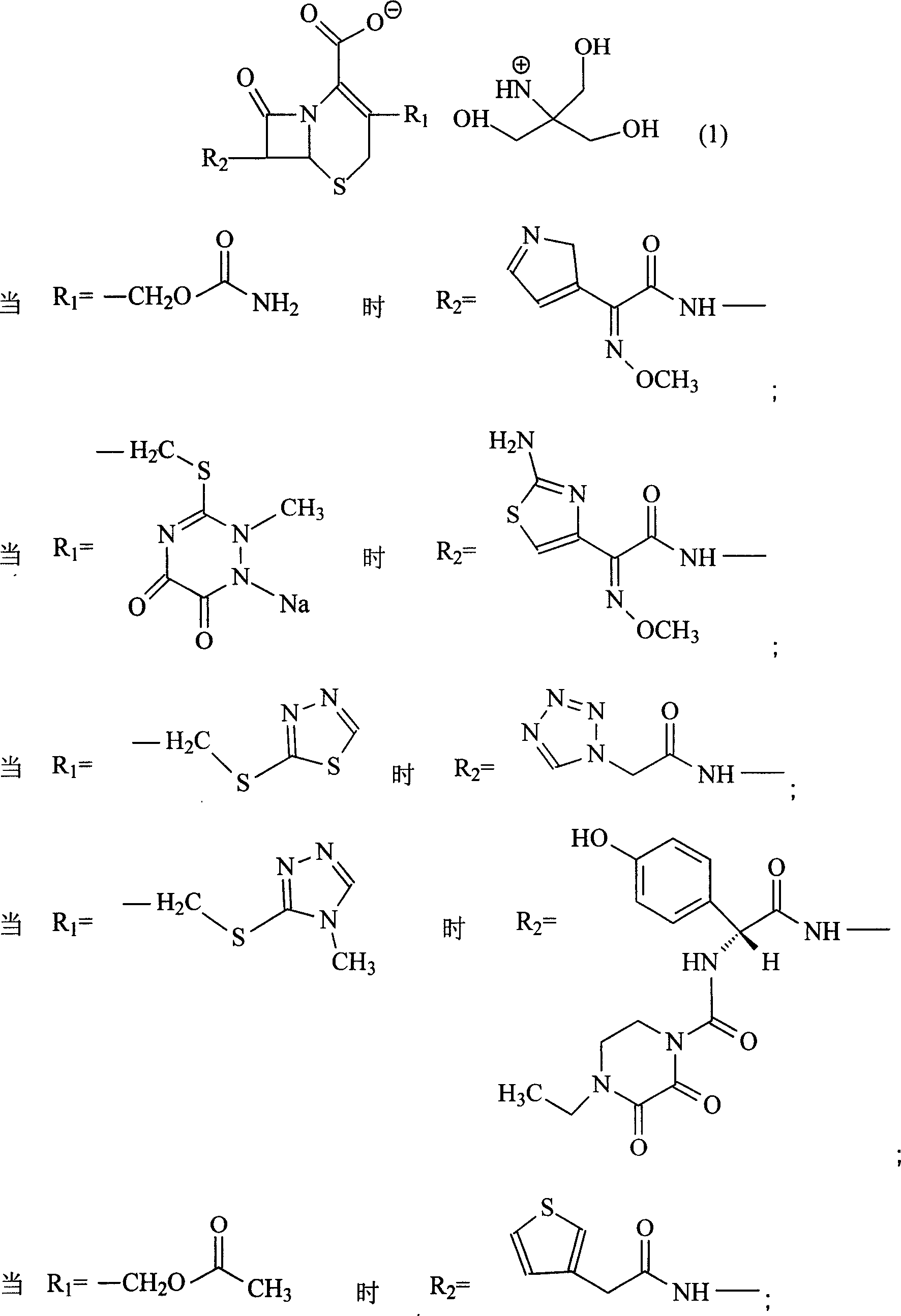
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
Composition of amino-cephalosporanic acid and arginine
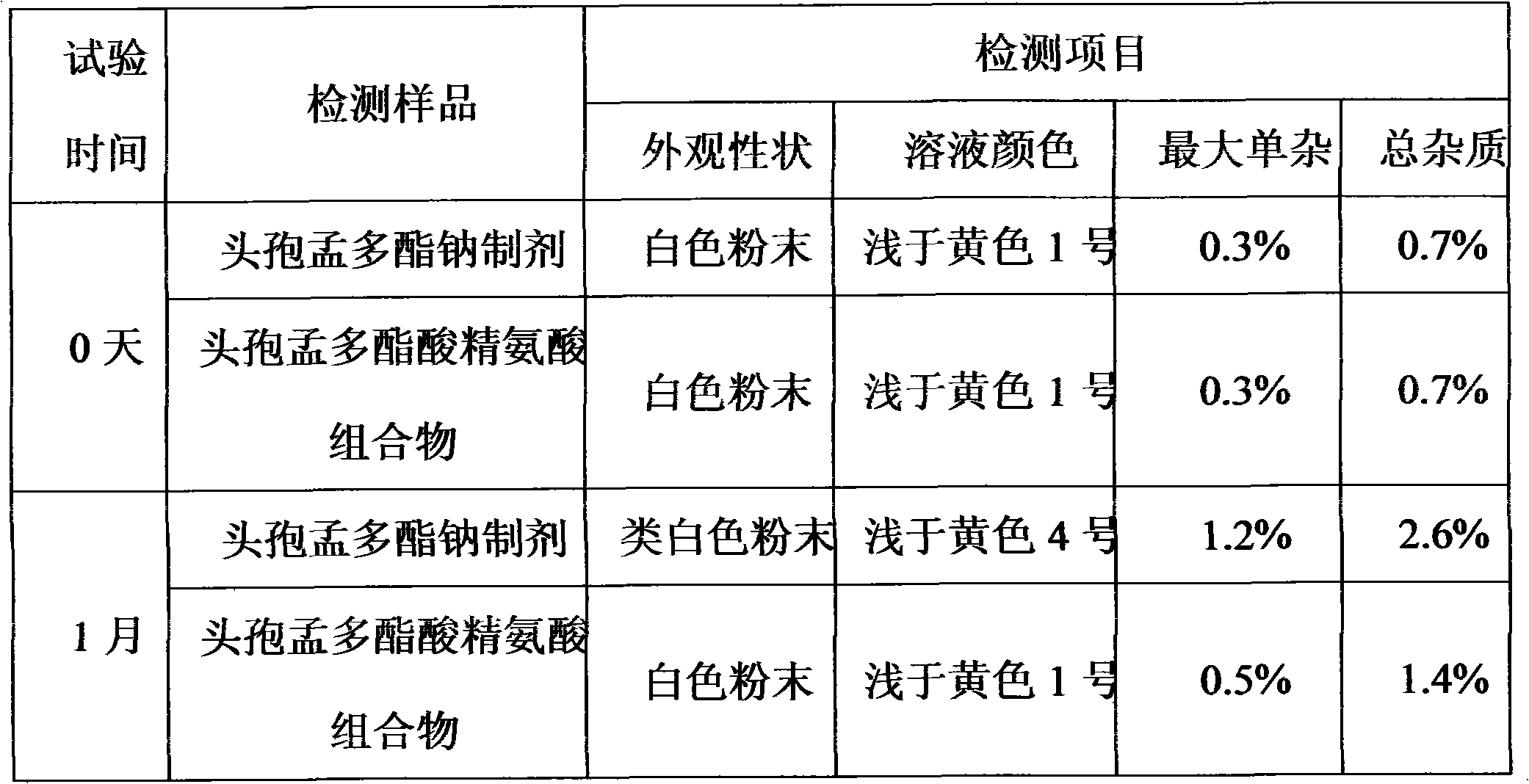
The invention relates to a composition of amino-cephalosporanic acid and arginine, which is characterized in that amino-cephalosporanic acid in the composition comprises cefazolin acid, cefuroxime acid, cefamandole nanfate nitrosate, cefonicid acid and cefotetan acid, and one of the above amino-cephalosporanic acids is combined with arginine to obtain the composition. The invention comprises the following steps: in gnotobasis, scaling asepsis arginine and above amino-cephalosporanic acid which have suitable granularity and specific gravity according to ratio of recipe to serve as asepsis bulkdrugs; pouring the drugs into a mixer to operate according to the standard powder mixing operating instruction; after evenly mixed, discharging and slit charging. The composition of the invention solves the problems that sodium salt agent widely applied in cephems drugs specifically has poor crystal form, solution color is easily out of limits, no bacteria exist or pyrogen is not qualified, wateris hard to control, stability is poor and the like. The invention is predicted to have important clinic application value.
Owner:CHANGSHA KINGDAY BIO PHARMA TECH
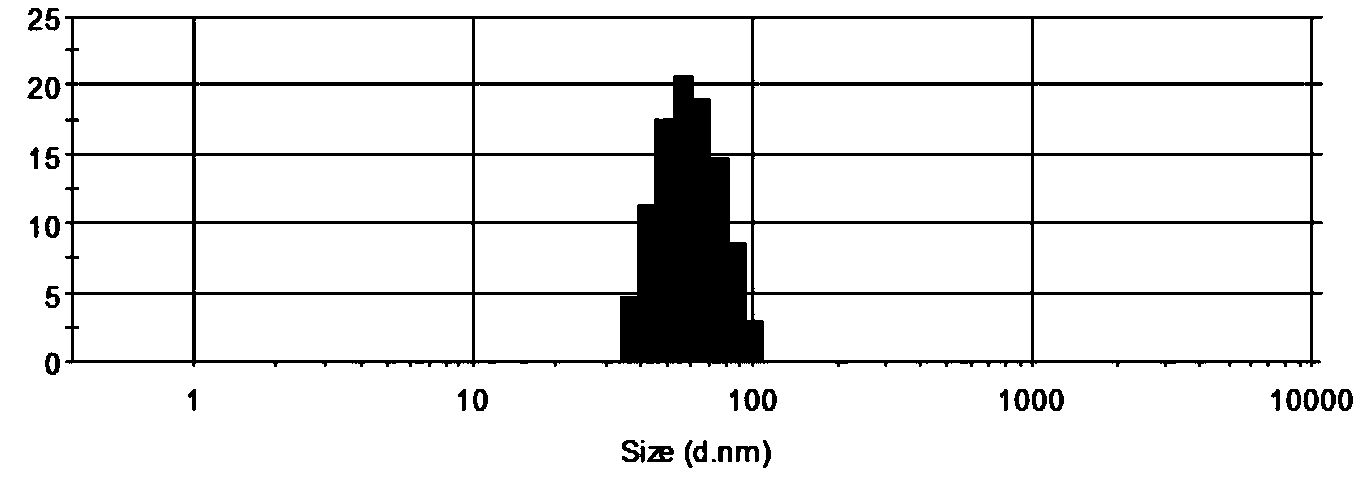
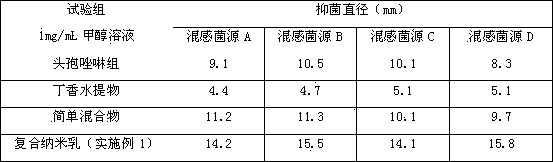
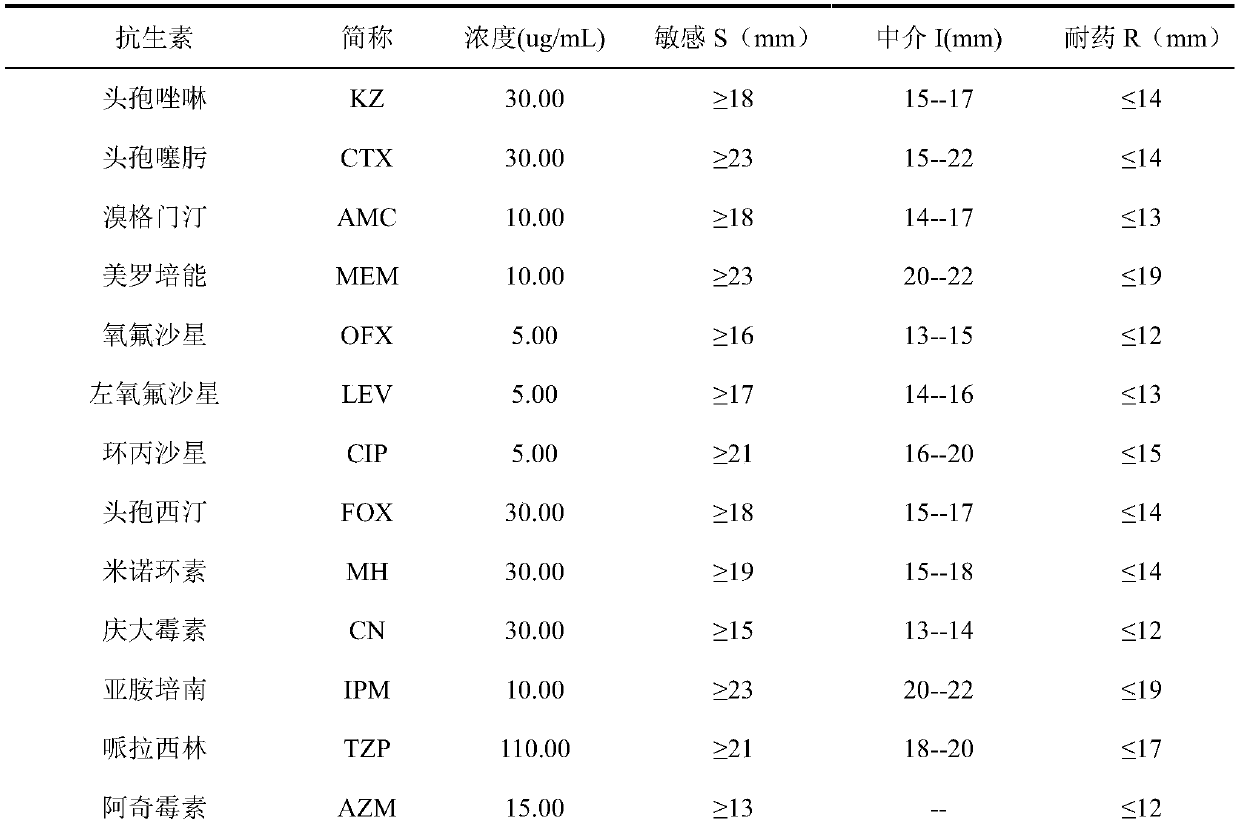
Oil-in-water compound antimicrobial cefazolin nanoemulsion medicine
InactiveCN104352433AImprove solubilityGuaranteed efficacyAntibacterial agentsOrganic active ingredientsCefazolinDistilled water
An oil-in-water compound antimicrobial cefazolin nanoemulsion medicine is a nanoemulsion medicament with cefazolin and an aqueous clove extract as effective components, and comprises the following components in parts by weight: 1-15 parts of cefazolin, 10-30 parts of a surfactant, 0-25 parts of a co-surfactant, 1-10 parts of aqueous clove extract, 1-20 parts of oil and 20-70 parts of distilled water. By compounding the cefazolin and the aqueous clove extract, the oil-in-water compound antimicrobial cefazolin nanoemulsion medicine is good in medicinal effect and good in antimicrobial effect; a preparation method is very simple; the oil-in-water compound antimicrobial cefazolin nanoemulsion medicine is easier to popularize.
Owner:HENAN SOAR VETERINARY PHARMA
Application of citral in inhibiting growth of multi-drug resistant enterobacter cloacae
ActiveCN110279679AAvoid drug resistanceMitigate or resolve drug-resistant infectionsAntibacterial agentsAldehyde active ingredientsNalidixic acidCefotaxime
The invention discloses an application of citral in inhibiting growth of multi-drug resistant enterobacter cloacae. The citral can inhibit growth of multi-drug resistant enterobacter cloacae as the citral has relatively good in vitro killing action to multi-drug resistant enterobacter cloacae resisting cefazolin, cefotaxime, augmentin, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocyline, imipenem, piperacillin, azithromycin, macrodantin, sulfamethoxazole and nalidixic acid. The minimum bactericidal concentration is 1.6 mg / mL and the minimal inhibitory concentration is 1.0 mg / mL. The invention provides inhibiting action of citral to multi-drug resistant enterobacter cloacae and the citral has wide application value in the field of medicine.
Owner:SHAANXI UNIV OF SCI & TECH
Staphylococcus drug resistance inducing method
PendingCN109355241AReduce processing difficultySolve the difficulty of operationMicroorganism based processesMicrobiology processesMicro Broth Dilution MethodCefazolin
The invention discloses a staphylococcus drug resistance inducing method. The staphylococcus drug resistance inducing method comprises (1) selection of staphylococcus strains, (2) screening of antibiotics, (3) solution preparation of standard antibiotics, (4) measurement of minimal inhibitory concentration through a microdilution broth method, (5) passage induction, and (6) measurement of drug resistance of every generation of staphylococcus to cefazolin. According to the staphylococcus drug resistance inducing method, instruments and equipment required by experimental operation can be reduced, and subsequent material processing difficulty can be greatly reduced. Therefore, the staphylococcus drug resistance inducing method can well solve the problems on operation difficulty and costs, andmeanwhile improve the induction efficiency, accuracy and success rate. Compared with other methods, the staphylococcus drug resistance inducing method is more convenient to operate and easier to grasp.
Owner:FOSHAN UNIVERSITY
Preparation method of 3-methylol cefazolin
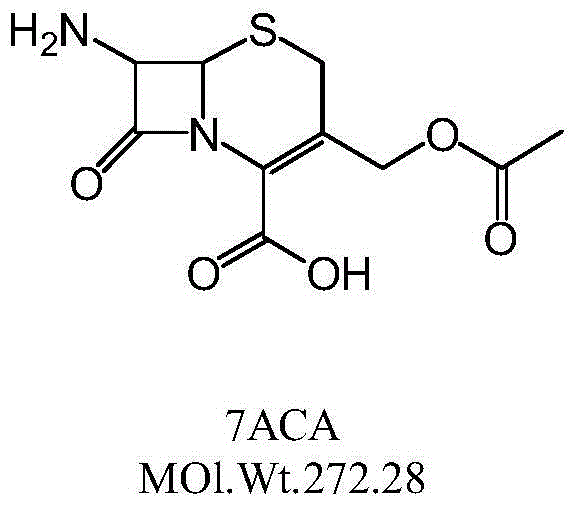
The invention discloses a preparation method of 3-methylol cefazolin. The preparation method comprises the following steps of dropwise adding a 7-ACA solution to a 1H-tetrazole-1-acetic acid anhydridemixing solution, performing a condensation reaction, after the reaction, adjusting pH phase splitting, then adding purified water, performing phase splitting, combining water phases, then adding an organic solvent for phase splitting, and performing concentration to obtain a primary purified water phase and a secondary purified water phase; and after adjusting pH of the secondary purified water phase, adding cephalosporin-C deacetylase, then adding a dispersing agent to the filtered water phase, then reducing the temperature, performing filtration after crystal formation, performing washing,and performing drying until the moisture is smaller than 1.0%. According to the preparation method disclosed by the invention, a traditional strong basic hydrolysis technology is substituted, waste water discharge can be reduced by 80%, the cephalosporin-C deacetylase after enzymolysis can be in cyclic utilization, solvent remains are smaller than 5%, the concentration weight reduction ratio is 2%-5%, the influence of various solvents on the enzymatic activity of the cephalosporin-C deacetylase is thoroughly eliminated, the consumption of enzymes and the activity of the enzymes being utilizedonce again are guaranteed, the cost is reduced, the reaction condition is mild, the conversion rate of products is as high as 90% or above, the purity of the products is not less than 98%, and subsequent structure elucidation and pharmacological research are facilitated.
Owner:HARBIN HEJIA PHARMA CO LTD
Plaster for treating skin stubborn dermatitis
InactiveCN110559259ALess medicationQuick resultsAntimycoticsHydroxy compound active ingredientsSyphilisCefazolin
The invention relates to a plaster for treating skin stubborn dermatitis. The plaster is prepared from the following raw materials: medical vaseline, liquid paraffin, lanolin, dried alum, chlortrimeton, dexamethasone, borneol, white pepper and cefazolin. The invention has the advantages as follows: the plaster can treat various skin stubborn dermatitis diseases, such as tinea manuum and beriberi,neurodermatitis, eczema, psoriasis, tinea versicolor, decrustation of hands and feet, verruca vulgaris, impetigo, yellow-water sores, dermatitis rhus, acute universal eczema, herpes simplex, chilblain, syphilis, chancre, nucha carbunde, scldhed, snake-head furuncle, snake venom, herpes zoster, pustule, scabies, universal eczema, pityriasis simplex, rotten feet, fungal infection of the hand, innominate inflanunatory of unknown origin, burn due to hot liquid or fire, incised wound, incised wound and genital herpes in sexually transmitted diseases, has efficacy of killing parasites to relieve itching, removing necrotic tissue to promote tissue regeneration, detoxifying and dispelling cold and dispelling wind and eliminating dampness, and is less in dosage, quick in effectiveness and convenient to use.
Owner:郭小兵
Methods and drug compositions for treating lyme disease
InactiveUS20190117630A1Antibacterial agentsHeterocyclic compound active ingredientsCefotaximeAzlocillin
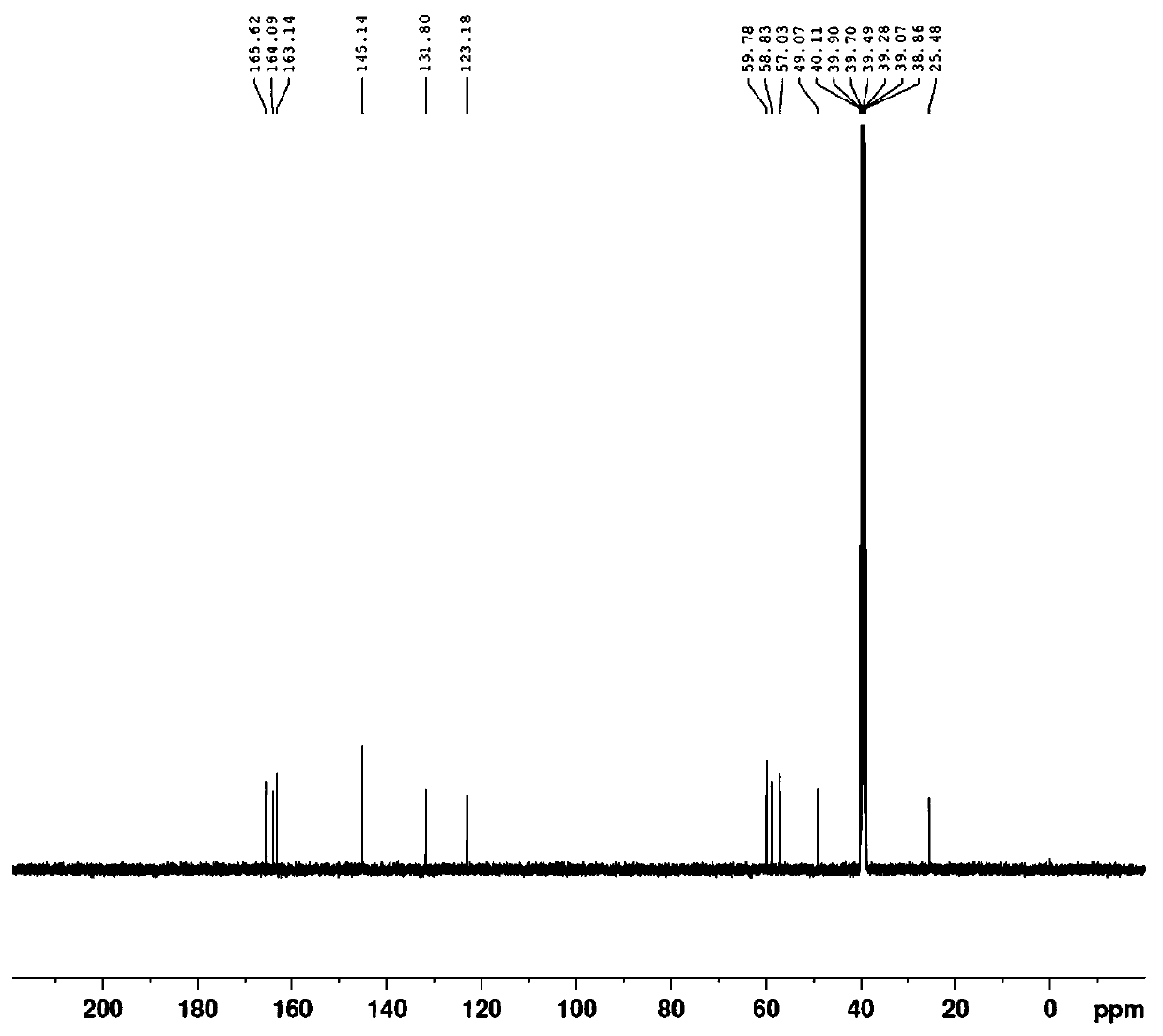
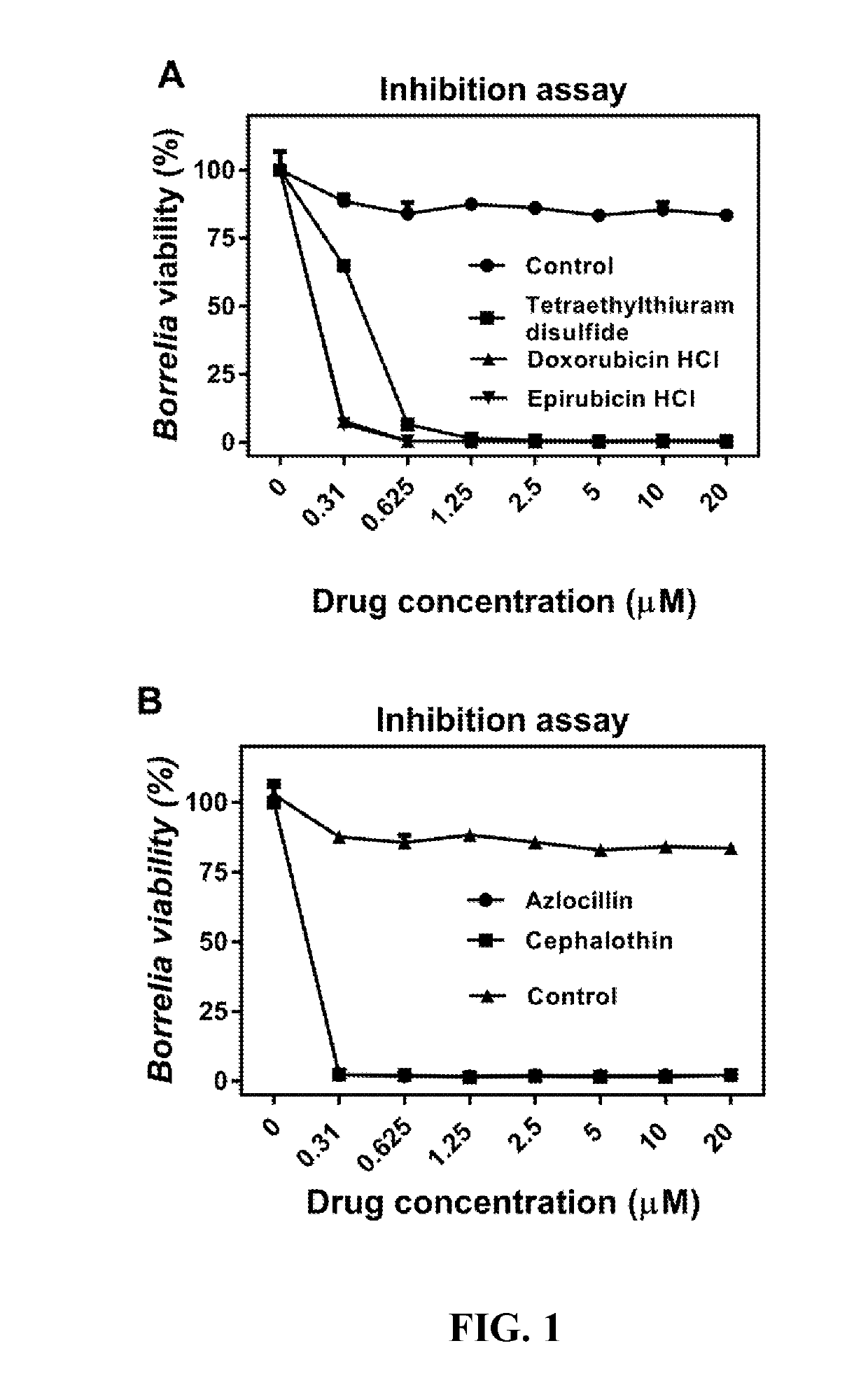
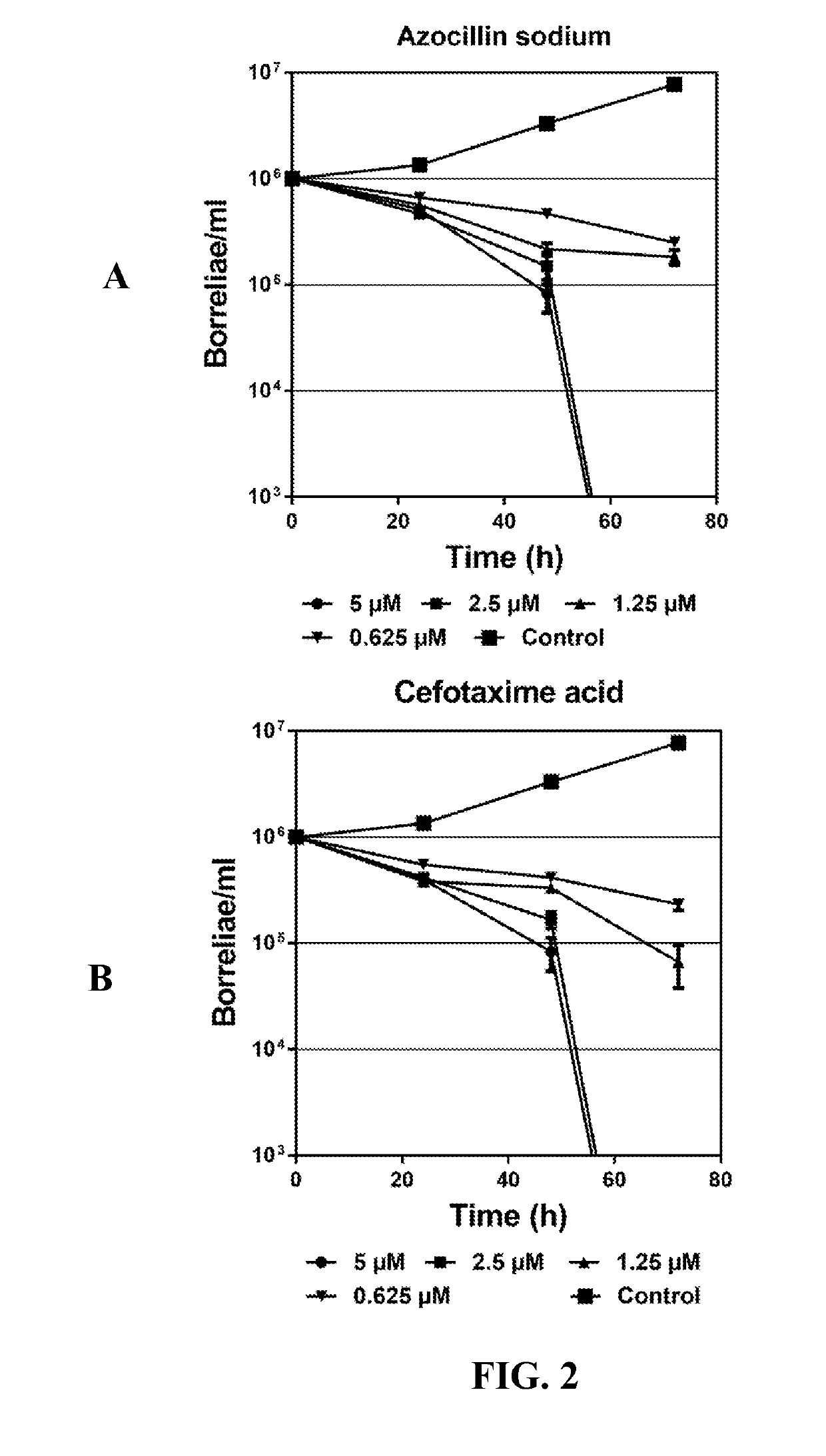
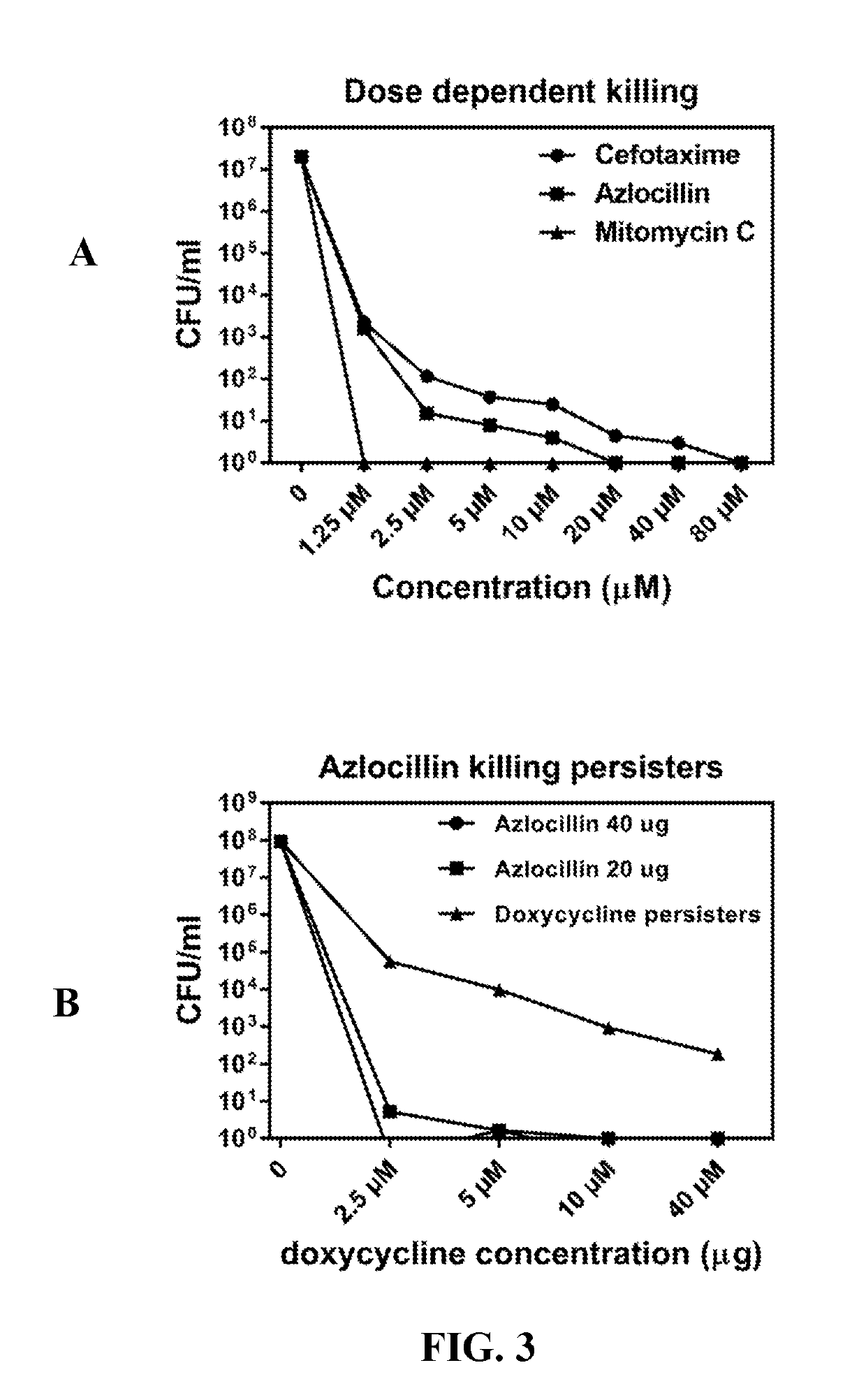
Disclosed herein are methods and drug compositions for treating Lyme disease and post-treatment Lyme disease syndrome (PTLDS) or chronic Lyme disease (CLD). In one embodiment, a method of treating a subject with Lyme disease involves administering an effective amount of a therapeutic agent selected from the group consisting of tetraethylthiuram disulfide, doxorubicin, epirubicin, azlocillin, cephalothin, josamycin, cefotaxime, cefazolin, erythromycin, calcimycin, gramicidin, cefdinir, gambogic acid, ceftazidime, ticarcillin, valinomycin, moxifloxacin, linezolid, idarubicin, tosufloxacin, loratadine, ceftriaxone, and combinations thereof, and pharmaceutical salts, hydrates, and solvates thereof.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Preparation method of cefamedin acid tris-intermediate
The invention relates to a preparation method of a cefamedin acid tris-intermediate. According to the preparation method, by the adoption of a concentrated sulfuric acid catalysis method, the dangerousness of the reaction process is lowered, and the safety and smoothness of a reaction are guaranteed; through optimized hydrolysis reaction operation, the product processing technology is simplified while the reaction yield is significantly increased and the product quality is significantly improved. Thus, the preparation method is suitable for large-scale industrial production. The obtained cefamedin acid tris-intermediate is high in content and purity and can be directly used for preparing high-quality cefazolin antibacterial medicine as a production raw material.
Owner:HARBIN HEJIA PHARMA CO LTD
Mouse monoclonal antibody cell strain for resisting amoxicillin and ampicillin
The invention relates to a mouse monoclonal antibody cell strain for resisting both amoxicillin and ampicillin, belonging to the technical field of B cell hybridoma. The mouse monoclonal antibody cell strain for resisting the amoxicillin and the ampicillin has the preservation number of CGMCC NO.2674. The monoclonal antibody aiming at the amoxicillin has 100 percent of cross reaction with the ampicillin which also belongs to the penicillin antibiotics, and has no cross reaction with cefazolin of cephalosporin antibiotics. The invention can be used for rapidly detecting the residual condition of the amoxicillin and the ampicillin in animal-sourced food, and can be further used for developing an immunity detection product rapidly and accurately detecting the amoxicillin and the ampicillin in the animal-sourced food.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
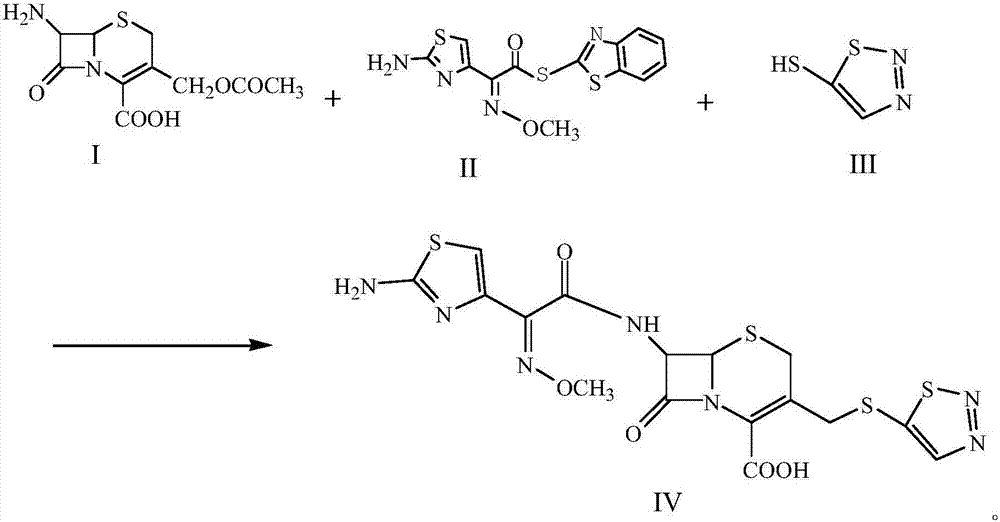
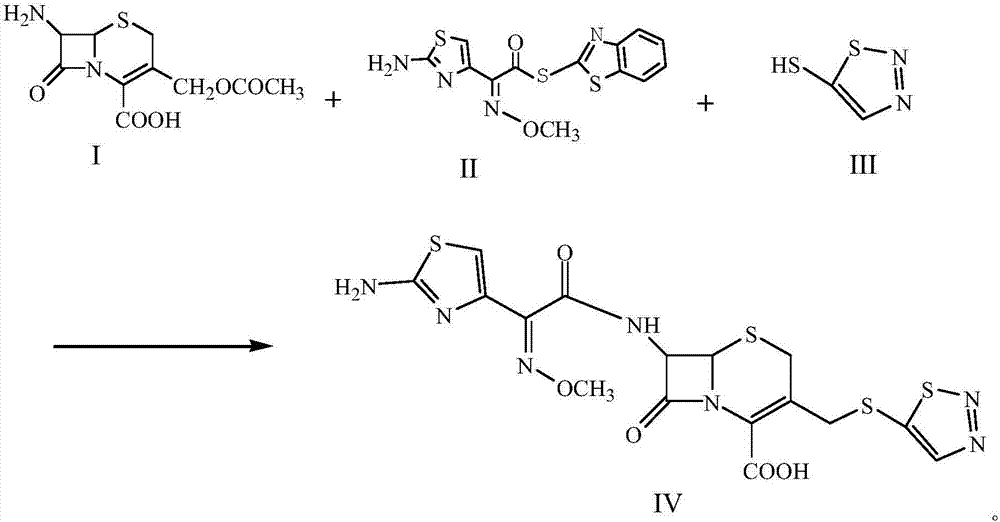
A kind of preparation technology of cefotazone
InactiveCN105646538BReactant exposureFully contacted reactantsOrganic chemistryCefazolinEthyl acetate
The invention discloses a process for preparing cefuzonam. The process includes steps of (1), weighing 7-aminocephalosporanic acid (7-ACA, a chemical compound I) and AE-mica ester (AEMA, a chemical compound II), mixing the 7-aminocephalosporanic acid and the AE-mica ester with each other to obtain a mixture, then uniformly grinding the mixture, adding a chemical compound III and triethylamine into the mixture, stirring the chemical compound III, the triethylamine and the mixture so allow solid phases and liquid phases to be in sufficient contact with one another, adding polyethylene glycol 400 into the solid phases and the liquid phases and stirring the polyethylene glycol 400, the solid phases and the liquid phases for 2-5 minutes; (2), carrying out 450W microwave reaction for 2-3 minutes, carrying out 800W microwave reaction for 2-3 minutes and carrying out 1000W microwave reaction for 3-5 minutes; (2), washing reaction residues by the aid of cold water at the temperature of 0-5 DEG C after reaction is completed, dissolving remaining solid in ethyl acetate, adding petroleum ester at the temperature of 0-5 DEG C into the remaining solid, dissolving out white solid and drying the white solid to obtain a chemical compound IV. The process has the advantages that the microwave one-pot reaction is carried out, so that various reactants are in sufficient contact with one another by the aid of the polyethylene glycol 400 which is a surfactant under the alkaline actions of the triethylamine, the process is easy and convenient to implement and short in reaction time, and the high-purity cefuzonam can be obtained in a high-yield manner.
Owner:上海博速医药科技有限公司
Application of pulsatilla saponin A3 in inhibiting growth of multi-drug-resistant Providencia rettgeri
Owner:SHAANXI UNIV OF SCI & TECH
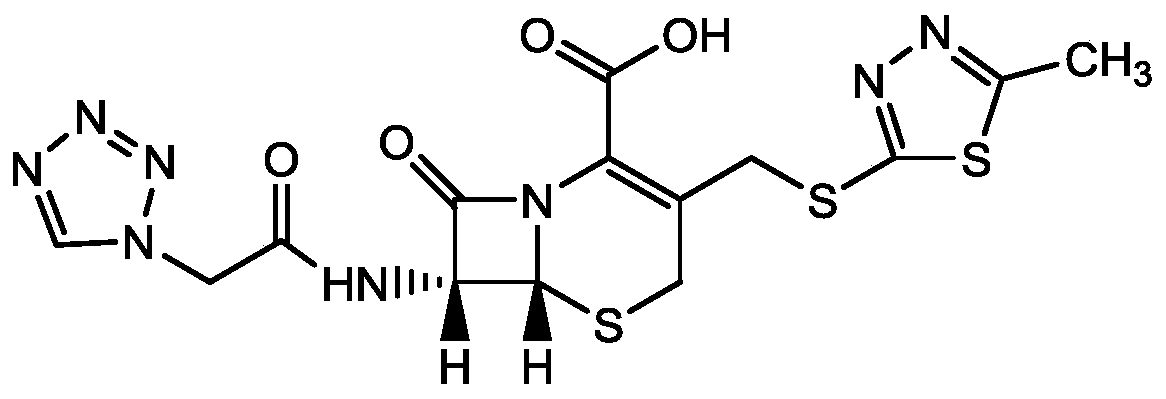
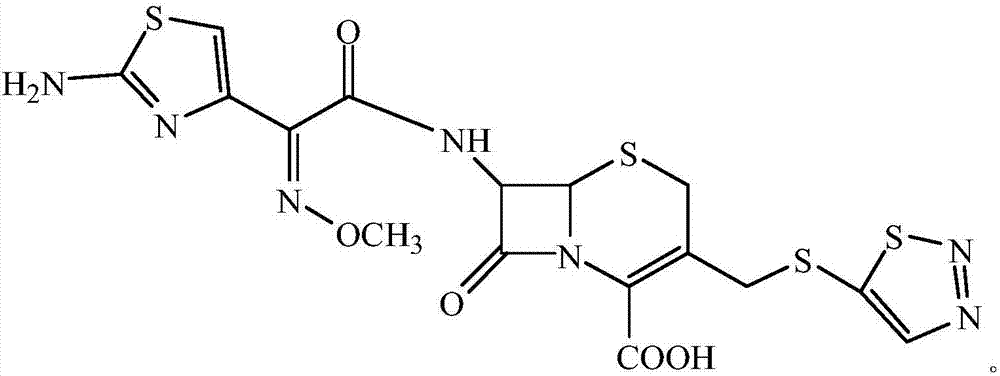
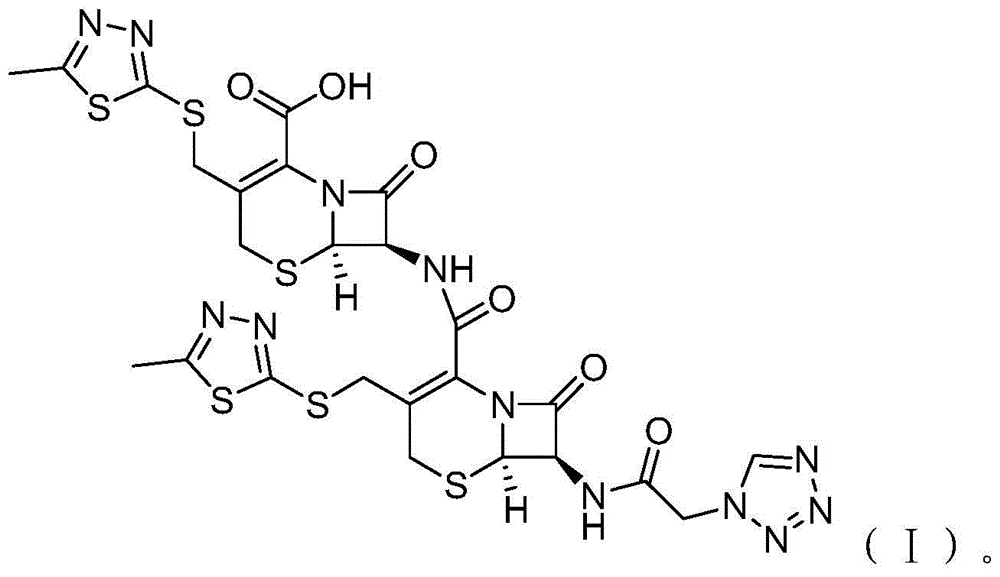
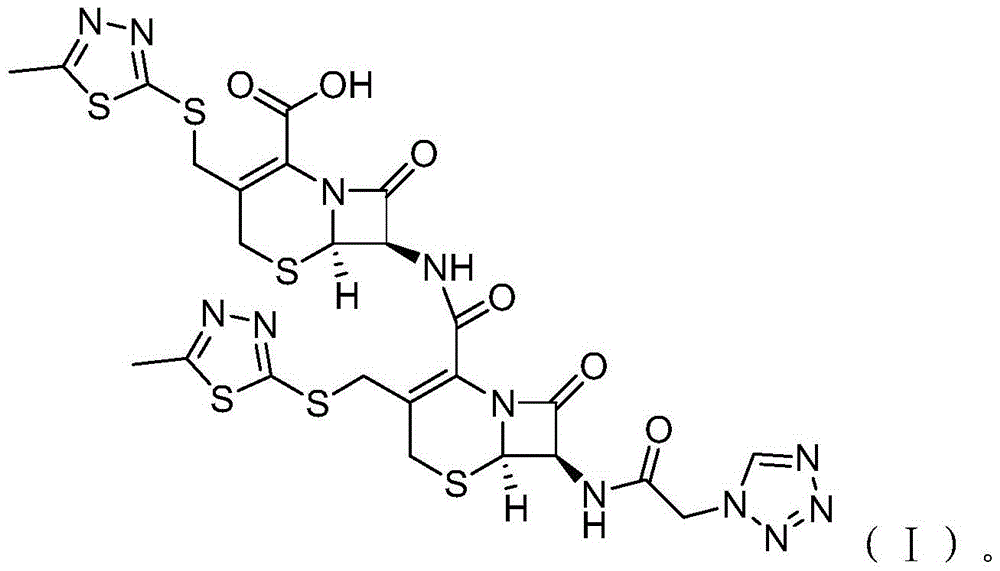
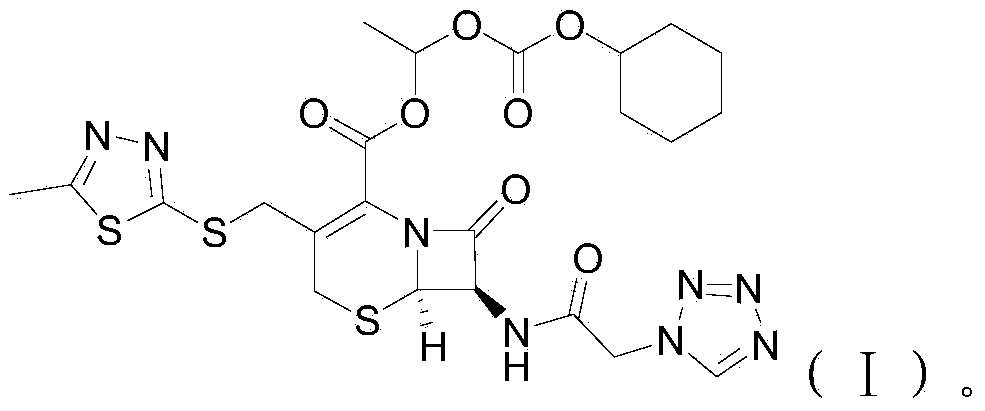
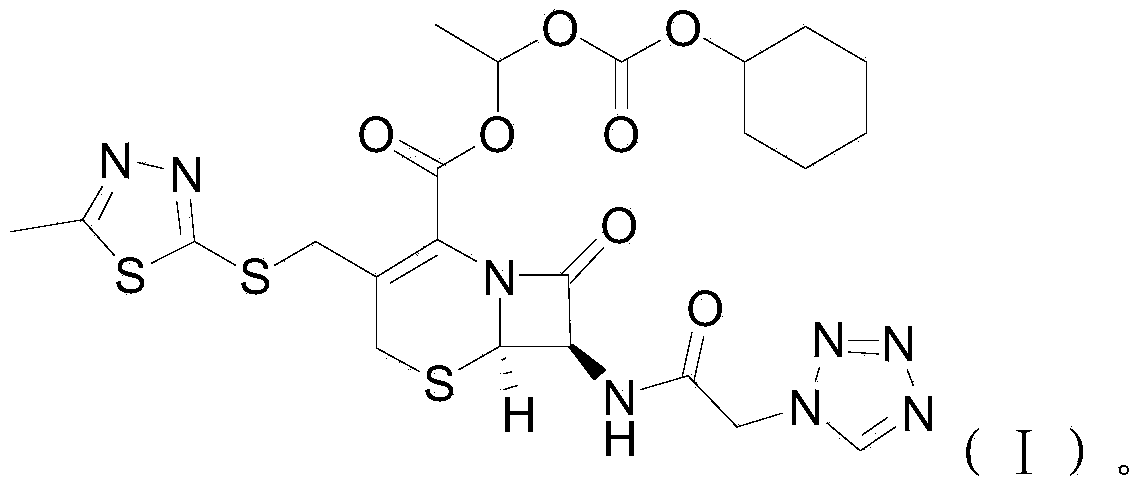
Cefazolin derivative and its preparation method, oral antibiotic preparation
ActiveCN103755728BChange in water solubilityPharmaceutically activeAntibacterial agentsOrganic chemistryCefazolinAntibiotic Y
The invention discloses a cefazolin derivative and a preparation method thereof as well as an oral antibiotic preparation. The molecular structural formula of the cefazolin derivative is (I) shown in description. The preparation method comprises the following steps: preparing a low-temperature reactant mixed solution of a cefazolin mother nucleus and cefazolin acid and carrying out condensation reaction on the low-temperature reactant mixed solution through heating. The oral antibiotic preparation contains the cefazolin derivative with the structural formula (I). The cefazolin derivative disclosed by the invention has the activity of a cefazolin medicine, has a suitable lipid-water partition coefficient and is suitable for gastro-intestinal tract dosing. The preparation method has easily-controlled reaction conditions, a simple process and a high product yield. The oral antibiotic preparation has a good efficacy, is convenient and safe to use, and can effectively reduce the occurrence rate of abusing antibiotics; meanwhile, the range of selecting the oral antibiotic preparation in China is expanded.
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
Application of luteolin in inhibiting growth of multi-drug resistant enterobacter cloacae
PendingCN111000841AGrowth inhibitionDefinite inhibitory effectAntibacterial agentsOrganic active ingredientsNalidixic acidCefotaxime
The invention discloses application of luteolin in inhibiting the growth of multi-drug resistant enterobacter cloacae. Luteolin has a good in-vitro killing effect on multi-drug resistant enterobactercloacae with resistance to cefazolin, cefotaxime, augmentin, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocycline, imipenem, piperacillin, azithromycin, furantoin, sulfamethoxazole and nalidixic acid, can inhibit the growth of the multi-drug resistant enterobacter cloacae, and has a minimum bactericidal concentration of 0.5mg / mL and a minimum inhibitory concentration of 0.3mg / mL. The invention provides the inhibition effect of luteolin on the multi-drug resistant enterobacter cloacae, and has wide application value in the fields of medicine and the like.
Owner:SHAANXI UNIV OF SCI & TECH
An indirect competition ELISA kit for detecting cephalosporin antibiotics in food of animal origin and its application
ActiveCN107014993BIncreased cross-reactivityIncreased sensitivityMaterial analysisElisa kitCefotaxime
The invention discloses an indirect-competitive ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting cephalosporin antibiotics in animal derived foods and application of the kit. The kit comprises an ELISA plate coated with a coating antigen, a cephalosporin antibiotic standard substance, a cephalosporin antibiotic general antibody, an enzyme labeled secondary antibody, a dilution buffer, a washing buffer, a substrate developing solution and a stop solution, wherein the cephalosporin antibiotic general antibody is capable of specifically identifying cefalexin, cefradine, cefadroxil, cefoperazone, cefazolin or cefotaxime and cannot identify penicillin sodium. According to the cephalosporin antibiotic general antibody prepared in the invention, the general antibody and most of cephalosporin antibiotics have high cross reaction rates, while hardly have any cross reaction for the analogue penicillin sodium containing a beta-lactam ring; and therefore, the general antibody has high specificity. The kit prepared by the general antibody has the advantages of high sensitivity, capability of detecting many types of drugs, low cost, simple operation and short detection time.
Owner:HEBEI AGRICULTURAL UNIV.
Application of vanillic acid in inhibiting growth of multi-drug-resistance staphylococcus aureus
PendingCN109646428AMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsOrganic active ingredientsPenicillinCefazolin
The invention discloses application of vanillic acid in inhibiting growth of multi-drug-resistance staphylococcus aureus. The inhibition effect of the vanillic acid on humanized multi-drug-resistancestaphylococcus aureus is put forward based on the fact that the vanillic acid has a good in-vitro killing effect on humanized multi-drug-resistance staphylococcus aureus which is resistant to linezolid, trimethoprim, gentamicin, ciprofloxacin, penicillin, cefazolin, clindamycin, vancomycin, oxacillin and piperacillin, and can inhibit the growth of multi-drug-resistance staphylococcus aureus, wherein the minimum bactericidal concentration is 2.4 mg / mL, and the minimum inhibitory concentration is 0.3 mg / mL. By means of the vanillic acid, drug-resistance infection of multi-drug-resistance staphylococcus aureus can be effectively relieved or solved, the mortality is reduced, a new idea is put forward for inhibition of humanized multi-drug-resistance staphylococcus aureus, and the vanillic acidhas important practical significance.
Owner:SHAANXI UNIV OF SCI & TECH
Pharmaceutical compound preparation for treating staphylococcal pneumonia
InactiveCN107753487AEasy to takeEasy to useAntibacterial agentsOrganic active ingredientsAmikacinTreatment effect
The invention discloses a pharmaceutical compound preparation for treating staphylococcal pneumonia. The pharmaceutical compound preparation for treating staphylococcal pneumonia is prepared from thefollowing components in parts by weight: 10 to 300 parts of cefazolin, 10 to 120 parts of amikacin and 10 to 1,600 parts of a medicine carrier, wherein the cefazolin and the amikacin which are used asmedicine compositions are mixed with the medicine carrier to form the compound preparation. The invention provides the cefazolin and amikacin pharmaceutical compound preparation for treating the staphylococcal pneumonia, which is convenient to take and safe to use; the pharmaceutical compound preparation is outstanding in therapeutic effect, reduces complications to an extremely large extent, causes fewer sequel, generally has no side effects, and can help a patient to get well quickly.
Owner:CHENGDU LINGXI SHANGPIN TECH CO LTD
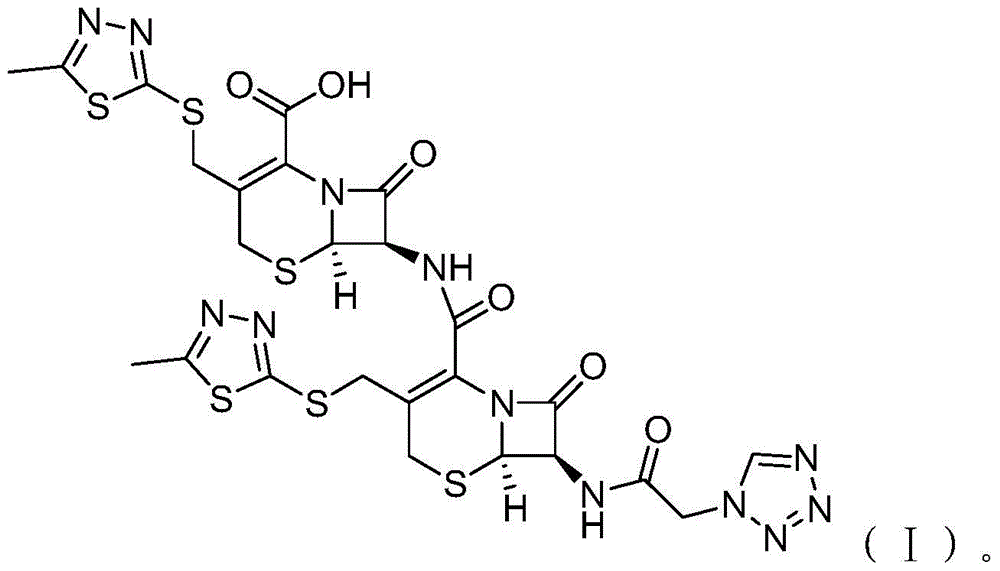
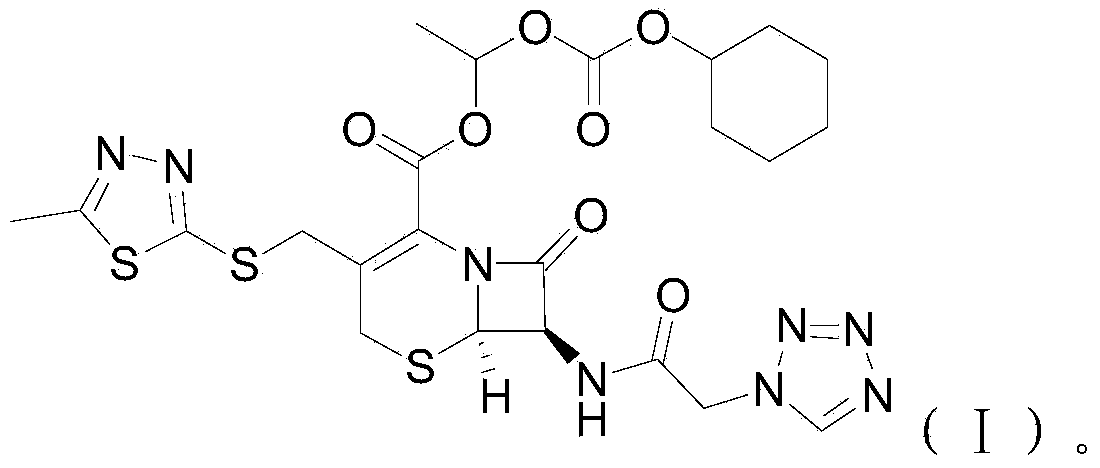
Cefazolin ester and preparation method thereof as well as oral antibiotic preparation
ActiveCN103739618APharmaceutically activeChange in water solubilityAntibacterial agentsOrganic active ingredientsCefazolinAntibiotic Y
The invention discloses a cefazolin ester and a preparation method thereof as well as an oral antibiotic preparation. The molecular structural formula of the cefazolin ester is as shown in (I) in descriptions. The preparation method of the cefazolin ester comprises preparation of cefazolin ester acid-base salt and esterification reaction of cefazolin ester acid-base salt and carbonic acid-1-iodine ethyl ester ochxl cyclohexyl ester. The oral antibiotic preparation contains the cefazolin ester, the structural formula of which is as shown in (I). The cefazolin ester has the activity of cefazolin medicines and proper lipid / water partition coefficients and is suitable for drug administration of gastrointestinal tracts. The preparation method has the advantages that reaction conditions are easy to control, the process is simple, and the product yield is high. The oral antibiotic preparation has a good medicine effect and is safe and convenient to use; the occurrence rate of antibiotic misuse can be effectively reduced; and meanwhile, the selective range of oral antibiotics at home is expanded.
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
A kind of synthetic method of cefazolin acid
The invention relates to a synthetic method of cefazolin acid. According to the synthetic method, 7-aminocephalosporanic acid is taken as a raw material, and is reacted with 1H-tetrazole-1-acetic acid in a solvent so as to obtain an intermediate; the intermediate is reacted with 5-mercapto-1-methyltetrazole under base catalysis at solution states without crystallization, washing, and drying so as to obtain cefazolin acid. One-pot method is adopted; the synthetic method is simple; solvent application amount is low; environmental pollution is low; product yield is high; cost is low; and the synthetic method is suitable for industrialized production.
Owner:QILU ANTIBIOTICS PHARMA
Application of citral in inhibition of growth of multi-drug resistant providencia rettgeri
InactiveCN110812346AMitigate or resolve drug-resistant infectionsReduce fatalityAntibacterial agentsAldehyde active ingredientsErtapenemCefazolin
The invention discloses application of citral in inhibition of growth of multi-drug resistant providencia rettgeri. The citral has better in-vitro killing effects on human-derived providencia rettgeriresisting ampicillin, ampicillin / sulbactam, cefazolin, cefotetan, ceftriaxone, ceftazidime, tobramycin, tazobactam and piperacillin, ciprofloxacin, levofloxacin, amikacin, cotrimoxazole, cefepime, ertapenem, imipenem, aztreonam and gentamicin, can inhibit growth of resistant providencia rettgeri, and has the minimum bactericidal concentration of 0.5 mg / mL and the minimum inhibitory concentrationof 0.25 mg / mL; and the inhibition function of the plant extract citral on the multi-drug resistant providencia rettgeri can relieve or solve the problem of increase of drug resistance of the multi-drug resistant providencia rettgeri and provide a theoretical basis for clinical antibacterial treatment and research and development of novel drugs.
Owner:SHAANXI UNIV OF SCI & TECH
Composite medicine comprising cefazolin
InactiveCN103751123AGood treatment effectPyrogenic reaction noAntibacterial agentsOrganic active ingredientsVitamin CMedicine
The invention provides a composite medicine comprising cefazolin, which is prepared from the following active ingredients: cefazolin, vitamin C, propylene glycol and lidocaine at a fraction ratio of (63-130):(6-12):(12-40):(15-36). The invention also provides a preparation method of the composite medicine. The composite medicine provided by the invention is superior to the prior art in safety, stability and therapeutic effect, and the preparation method is energy-saving and environment-friendly.
Owner:邓学峰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com