Composition of amino-cephalosporanic acid and arginine
A technology of cephalosporanic acid and composition, applied in the field of pharmaceutical preparation, can solve the problems of poor stability, water insolubility, poor crystal form and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 Preparation of cefazolin acid arginine composition:
[0018] Take 35.8 kg of sterile cefazolin acid and 14.2 kg of sterile arginine in a sterile environment, put them in a mixer and mix them evenly, then discharge and pack in aluminum barrels. Then it is transferred to the aseptic preparation subpackaging workshop, and is subpackaged into antibiotic glass bottles according to the specifications to obtain the finished preparation.
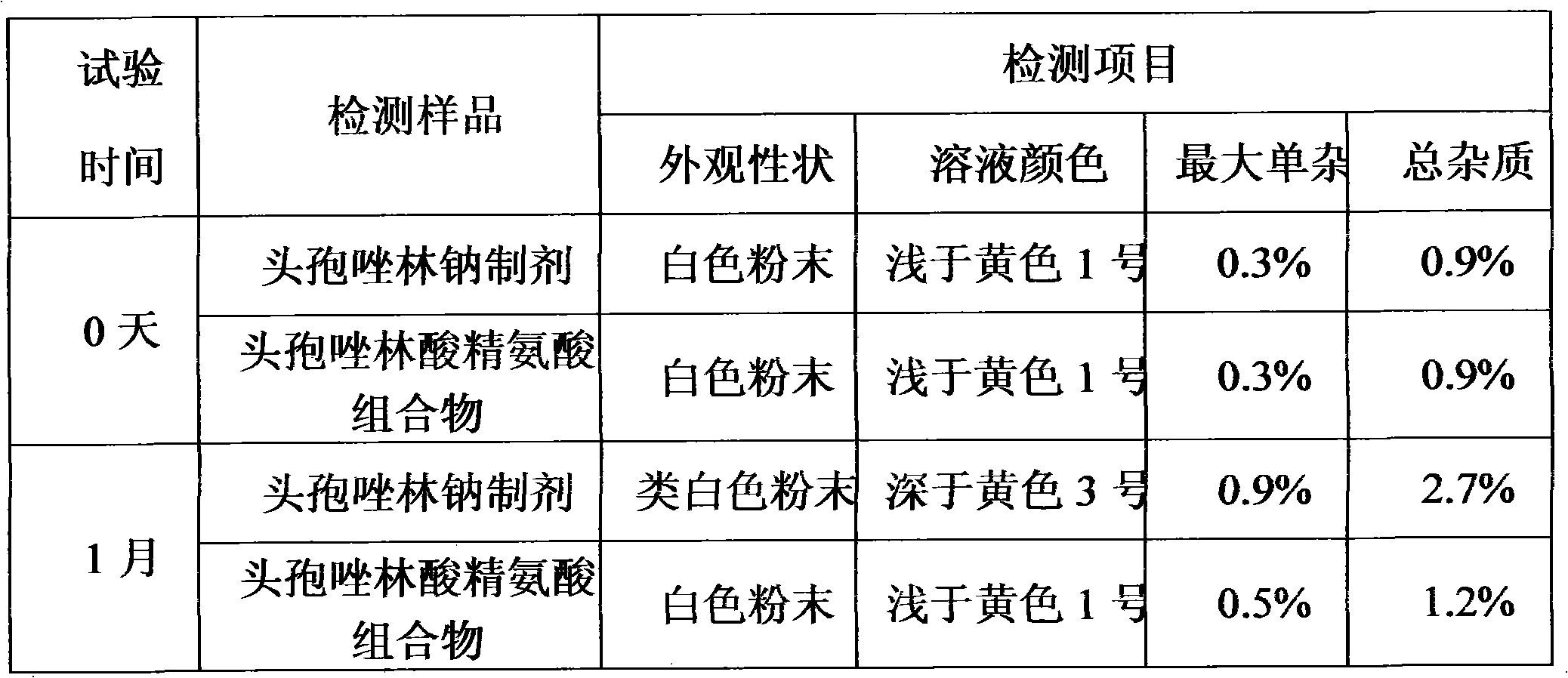
[0019] The cefazolin acid arginine preparation of embodiment 1 and commercially available cefazolin sodium preparation carry out accelerated test (40 ℃ of temperature, in humidity 75% constant temperature and humidity box), according to 2005 edition " Chinese Pharmacopoeia " cefazolin for injection Method detection under the sodium item, the detection results after 1 month of the test are as follows:
[0020]
[0021] The results show that: the cefazolin acid arginine preparation of the present invention is more stable than t...
Embodiment 2
[0022] Example 2 Preparation of cefuroxime arginine composition:
[0023] Take 35.5 kg of sterile cefuroxime acid and 14.5 kg of sterile arginine in a sterile environment, put them in a mixer and mix them evenly, then discharge and pack in aluminum barrels. Then it is transferred to the aseptic preparation subpackaging workshop, and is subpackaged into antibiotic glass bottles according to the specifications to obtain the finished preparation.
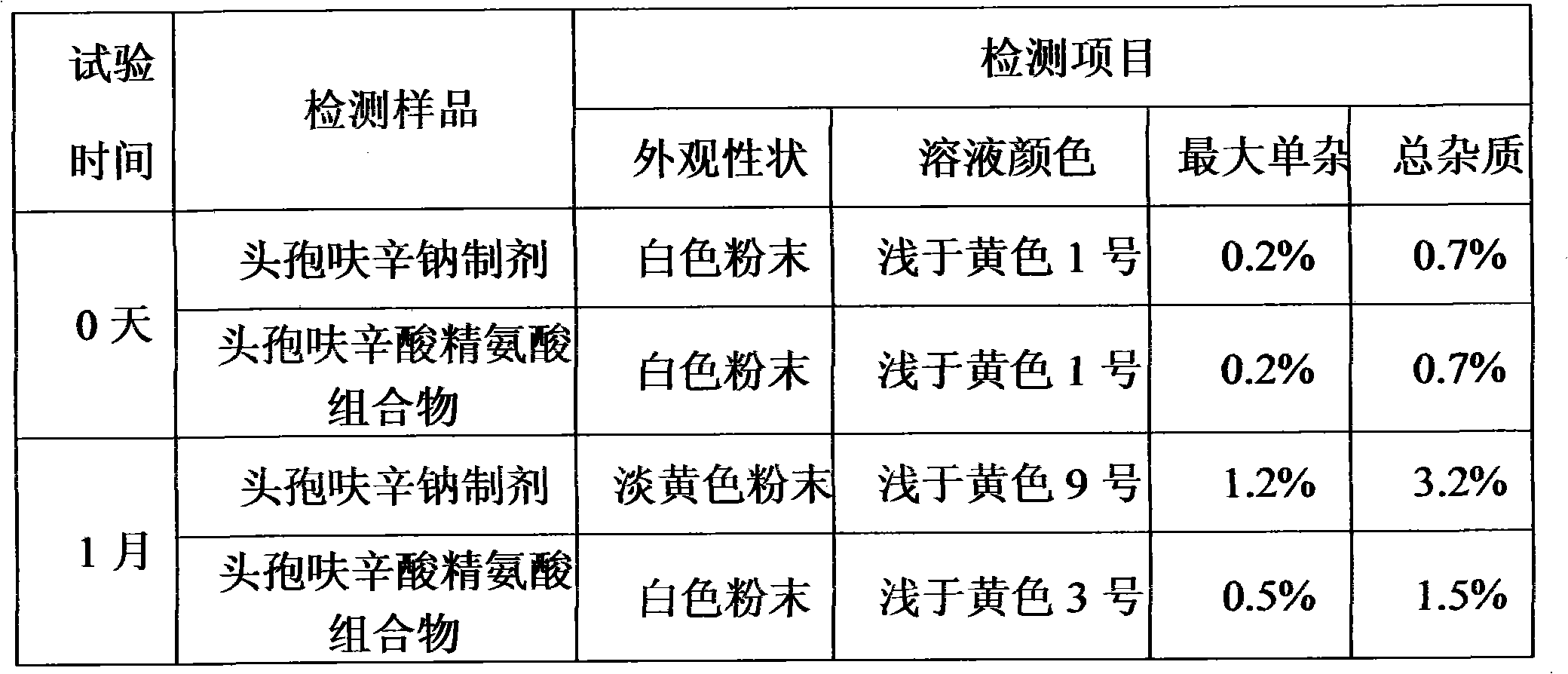
[0024] The cefuroxime arginine preparation of embodiment 2, carry out accelerated test with commercially available cefuroxime sodium preparation (40 ℃ of temperature, in humidity 75% constant temperature and humidity chamber), according to 2005 edition " Chinese Pharmacopoeia " cefuroxime for injection Method detection under the sodium item, the detection results after 1 month of the test are as follows:
[0025]
[0026] The results show that: the cefuroxime arginine preparation of the present invention is more stable than the cef...
Embodiment 3
[0027] Embodiment 3 Preparation of cefamandole acid arginine composition:
[0028] Under the sterile environment, take 36.7kg of sterile cefamandole acid and 13.3kg of sterile arginine, mix them evenly in a mixer, discharge, and pack in aluminum barrels. Then it is transferred to the aseptic preparation subpackaging workshop, and is subpackaged into antibiotic glass bottles according to the specifications to obtain the finished preparation.
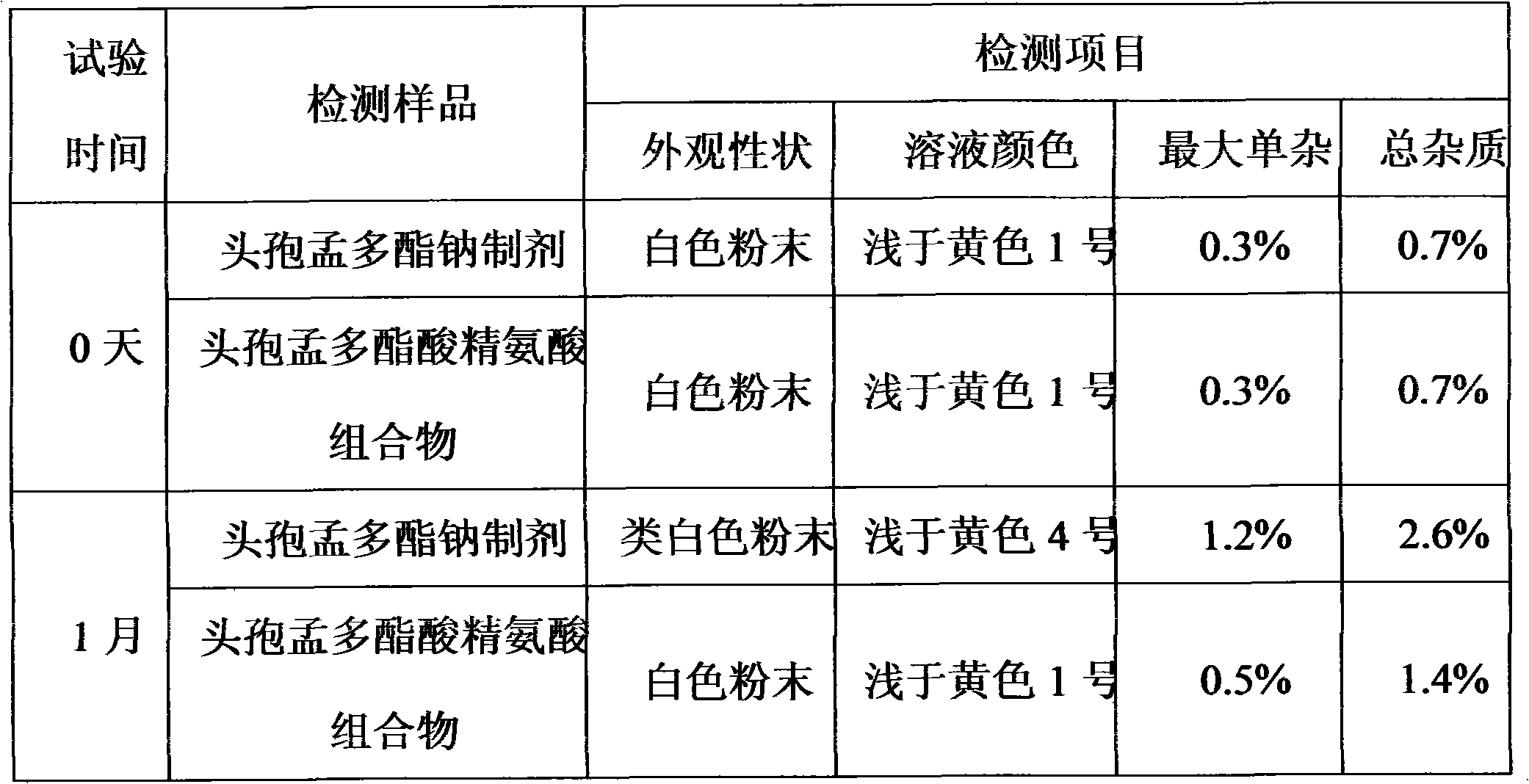
[0029] The cefamandole acid arginine preparation of embodiment 3, carry out accelerated test (40 ℃ of temperature, in humidity 75% constant temperature and humidity box) with commercially available cefamandole sodium, press cefamandole sodium quality standard for injection The following method is tested, and the test results after 1 month of testing are as follows:
[0030]
[0031] The result shows: cefamandole acid arginine preparation of the present invention is more stable than cefamandole sodium preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com