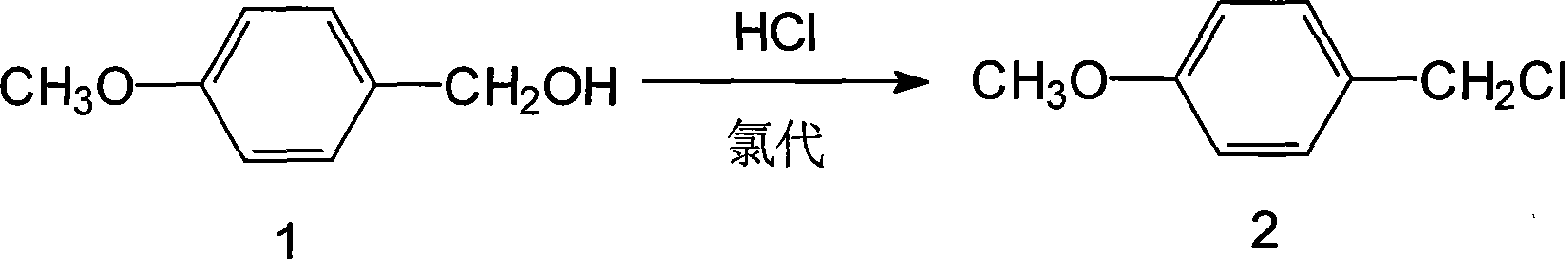
Patents
Literature
93 results about "Cephalosporanic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A family of organic compounds that are composed of a dihydrothiazine ring and a beta-lactam ring.
Method for preparing cefalexin
The invention discloses a method for preparing cefalexin. The method comprises the steps of charging a D-2-phenylglycine ester derivative and 7-ADCA (amino desacetoxy cephalosporanic acid) in a molar ratio of (1.15-1.6):1, and adding a catalyst penicillin acylase with amount of 1-2 times of amount of the 7-ADCA for acylation reaction, wherein the coded gene sequence of the penicillin acylase is shown as SEQIDNO:3. The method effectively overcomes reverse reaction during enzyme condensation reaction, so as to greatly reduce the using amount of side chains, avoid the phenomenon that more impurities are generated when high side chains are consumed, and solve the problem that the objective product is difficult to purify; and besides, the 7-ADCA conversion rate can be greatly improved to be up to more than 98%, the product quality is further improved and the production cost is reduced.
Owner:NORTH CHINA PHARMA COMPANY
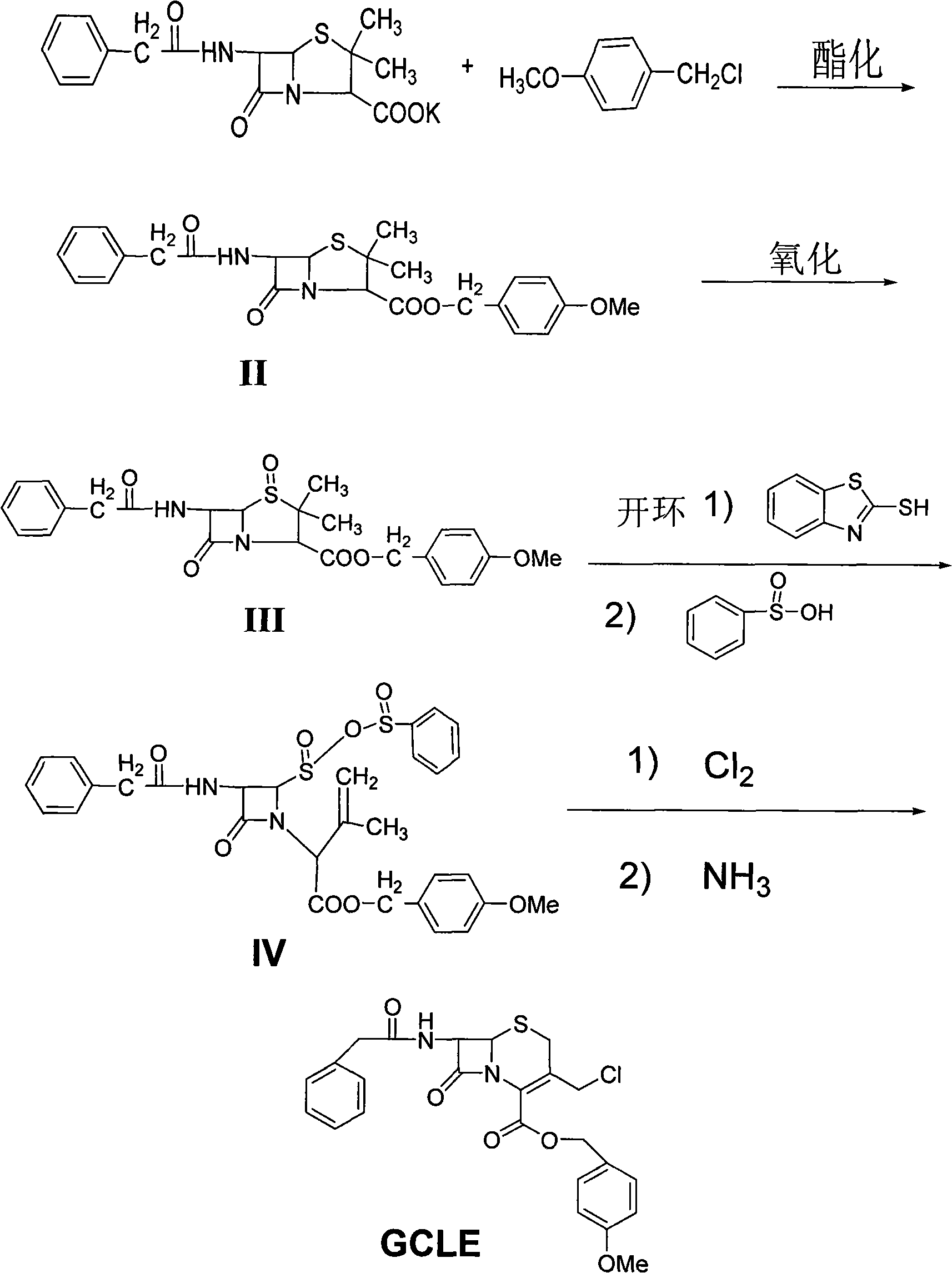
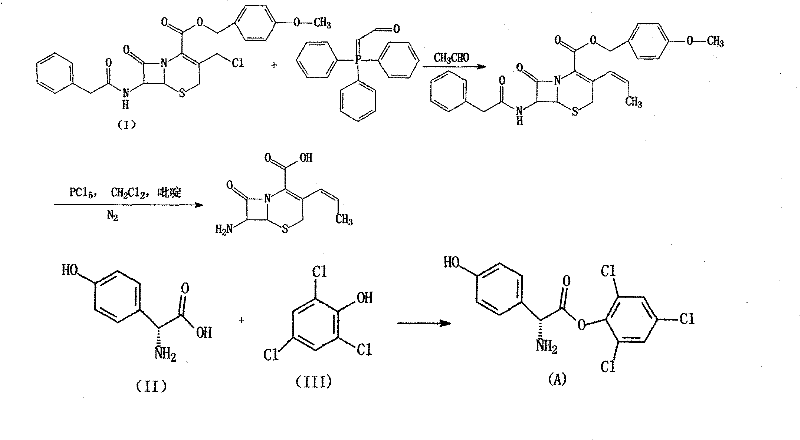
Synthesis method for 7-neophedan-3-chloromethyl cephalosporanic p-methoxy benzyl ester
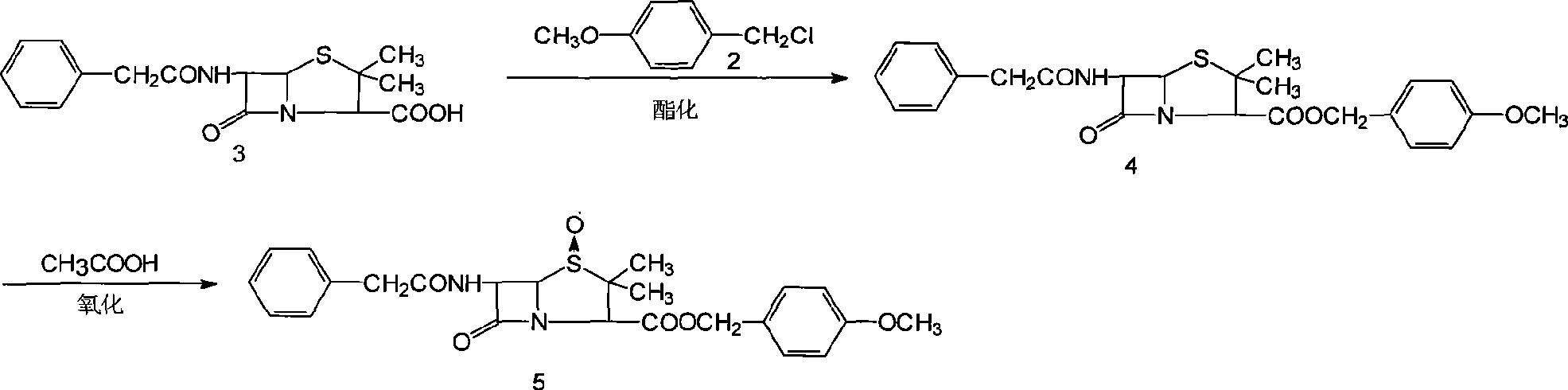
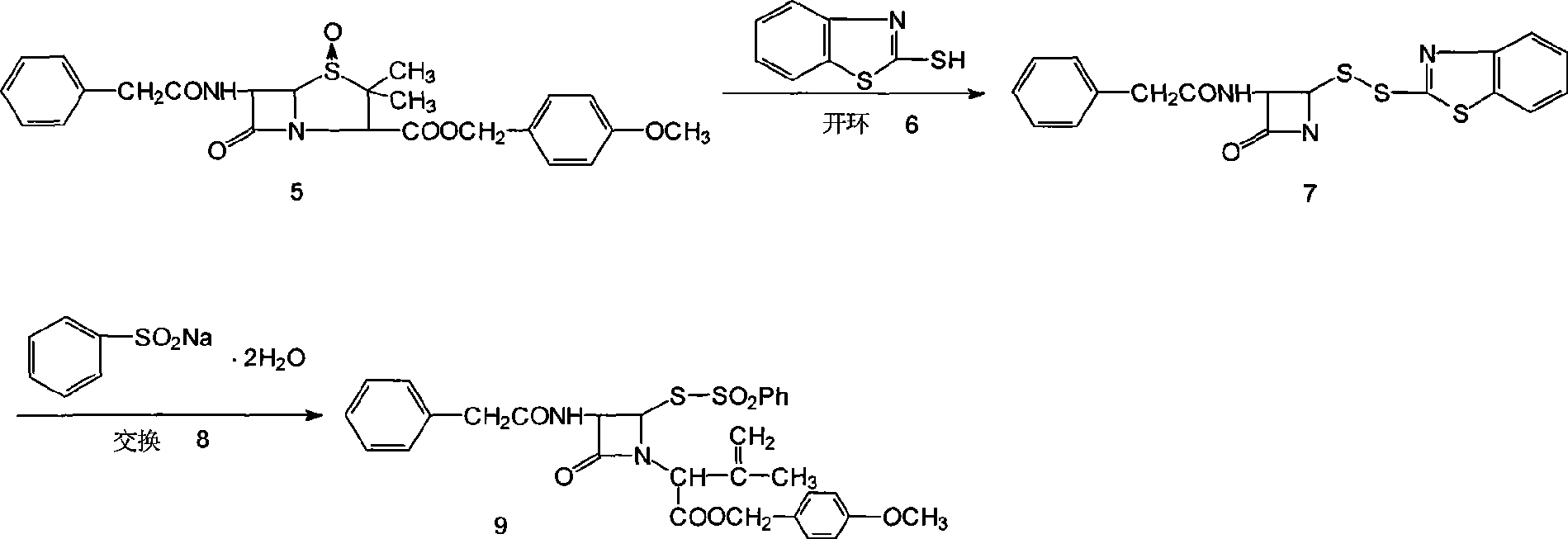
The invention provides a method for synthesizing 7-phenylacetamide-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (GCLE). The method produces the GCLE by taking methoxybenzyl alcohol as a raw material through steps such as esterification and oxidation, ring opening and exchange, chlorization and ring closure and so on. The method has the advantages that the method has a simplified solvent and easy recovery, obviously improves the quality, ensures that the content is more than or equal to 95 percent, reduces the cost, has convenient operation, and is easy for industrialized production.
Owner:上海五洲药业股份有限公司 +1
New method for preparing cefuroxime sodium compound
ActiveCN101671349AImprove responseSuitable for large-scale industrial productionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCephalosporanic AcidsTriphenylphosphine oxide
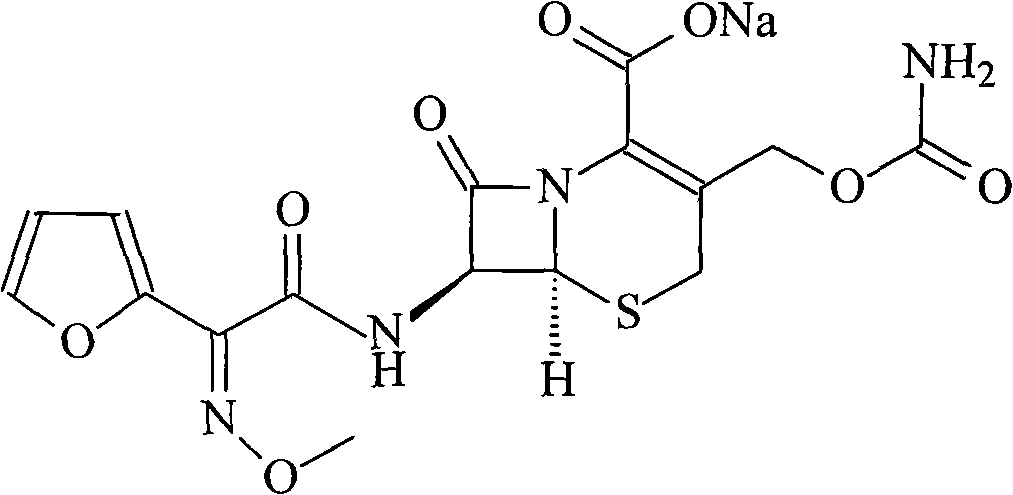
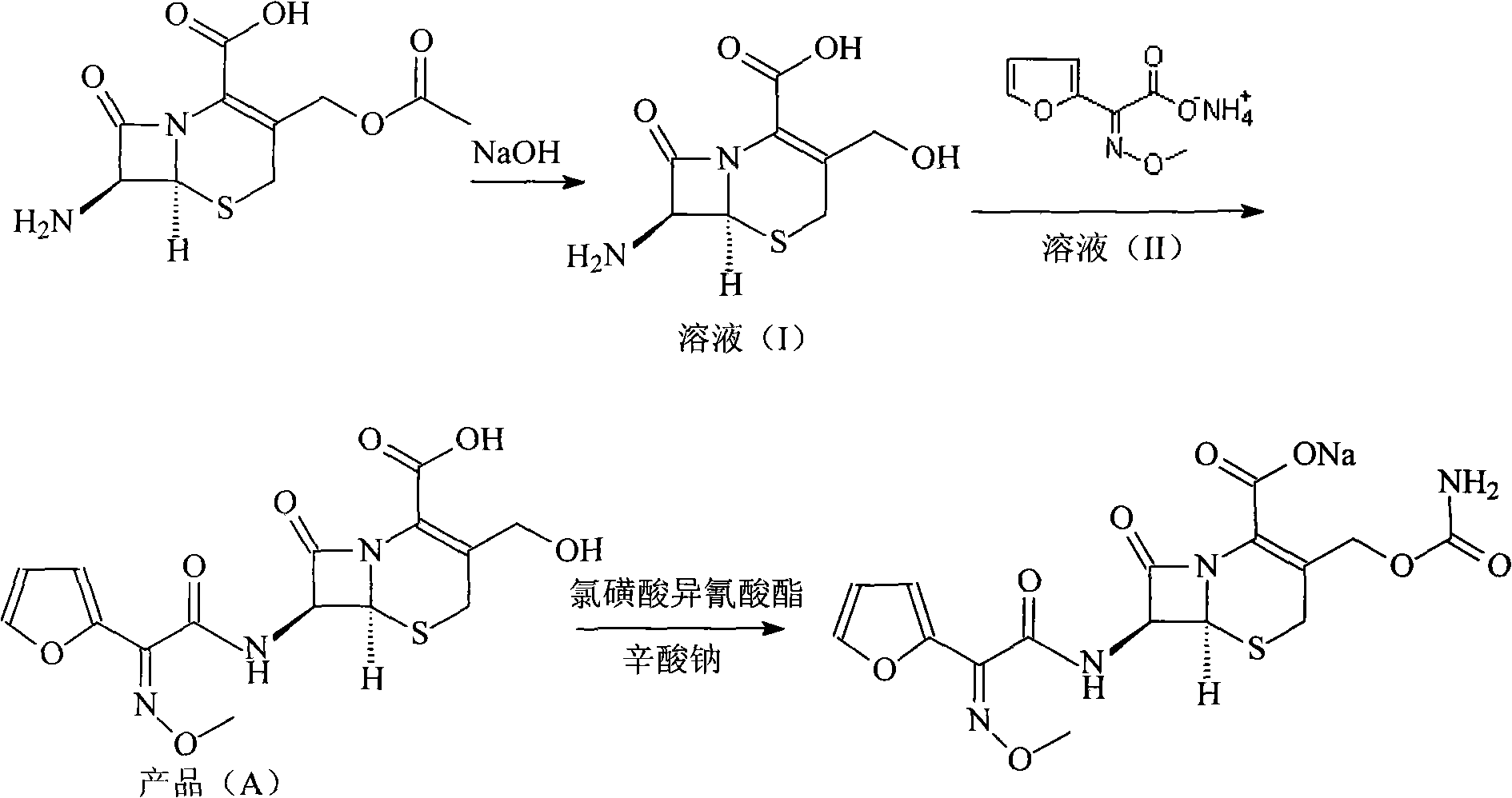
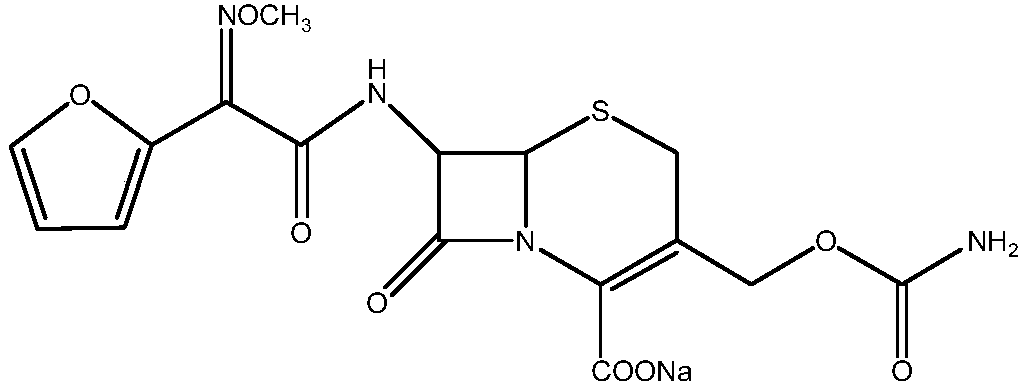
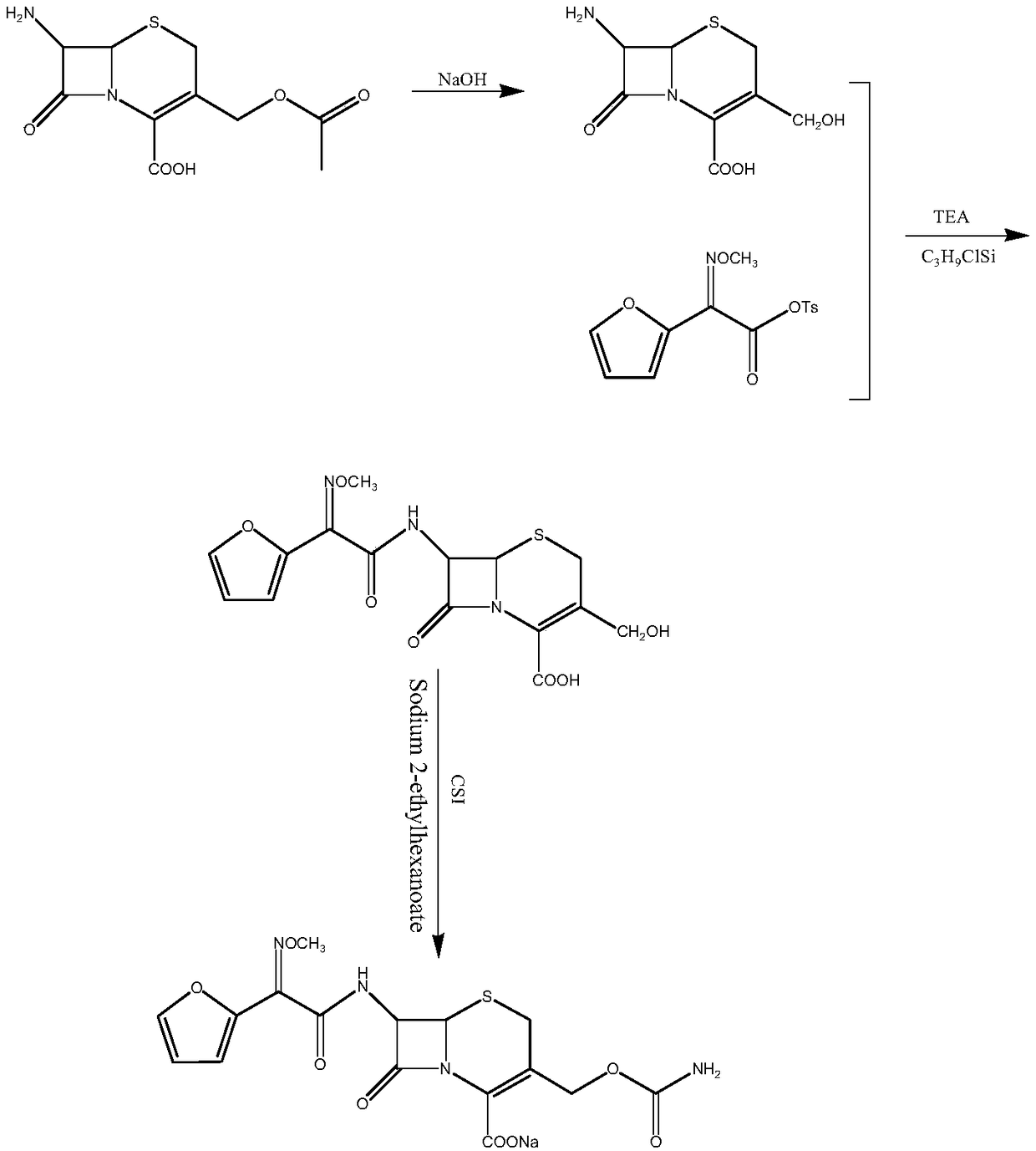
The invention relates to a new method for preparing a cefuroxime sodium compound. A target product is prepared by using triphosgene and triphenylphosphine oxide as catalysts, reacting 7-amino-cephalosporanic acid with (Z)-methoxyl imido furylacetic acid ammonium salt and sequentially adding chlorosulfonyl isocyanate and sodium iso-octoate for reaction. The cefuroxime sodium compound prepared by the method is greatly enhanced in purity and yield coefficient and has advantages of inexpensive using materials, simple synthesizing technology and equipment and easy production separation and purification.
Owner:灵康药业集团股份有限公司
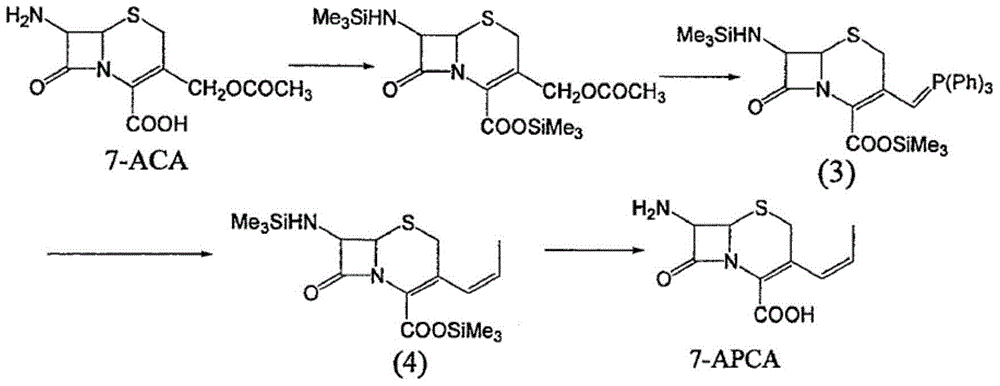
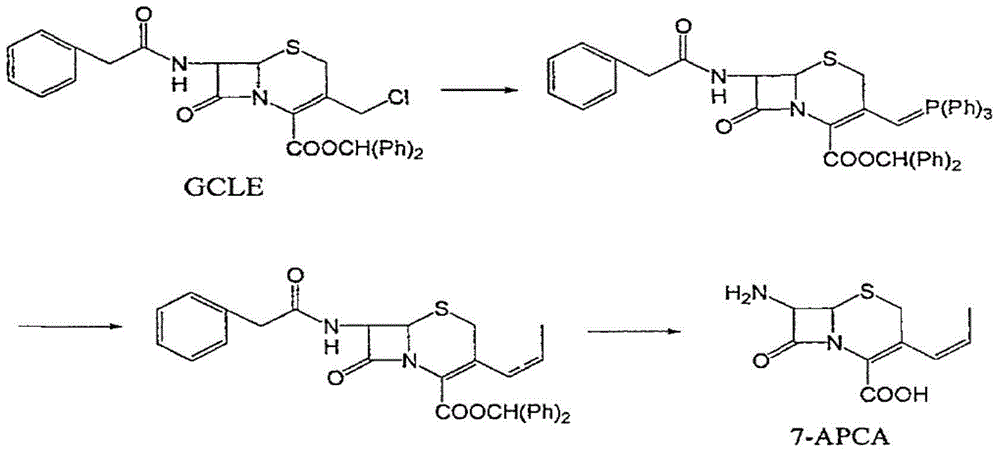
Method for preparing 7-amido-3-vinyl cethalosporanic acid
InactiveCN101182326ALow costSimple production methodOrganic chemistryWittig reactionTriethylphosphite
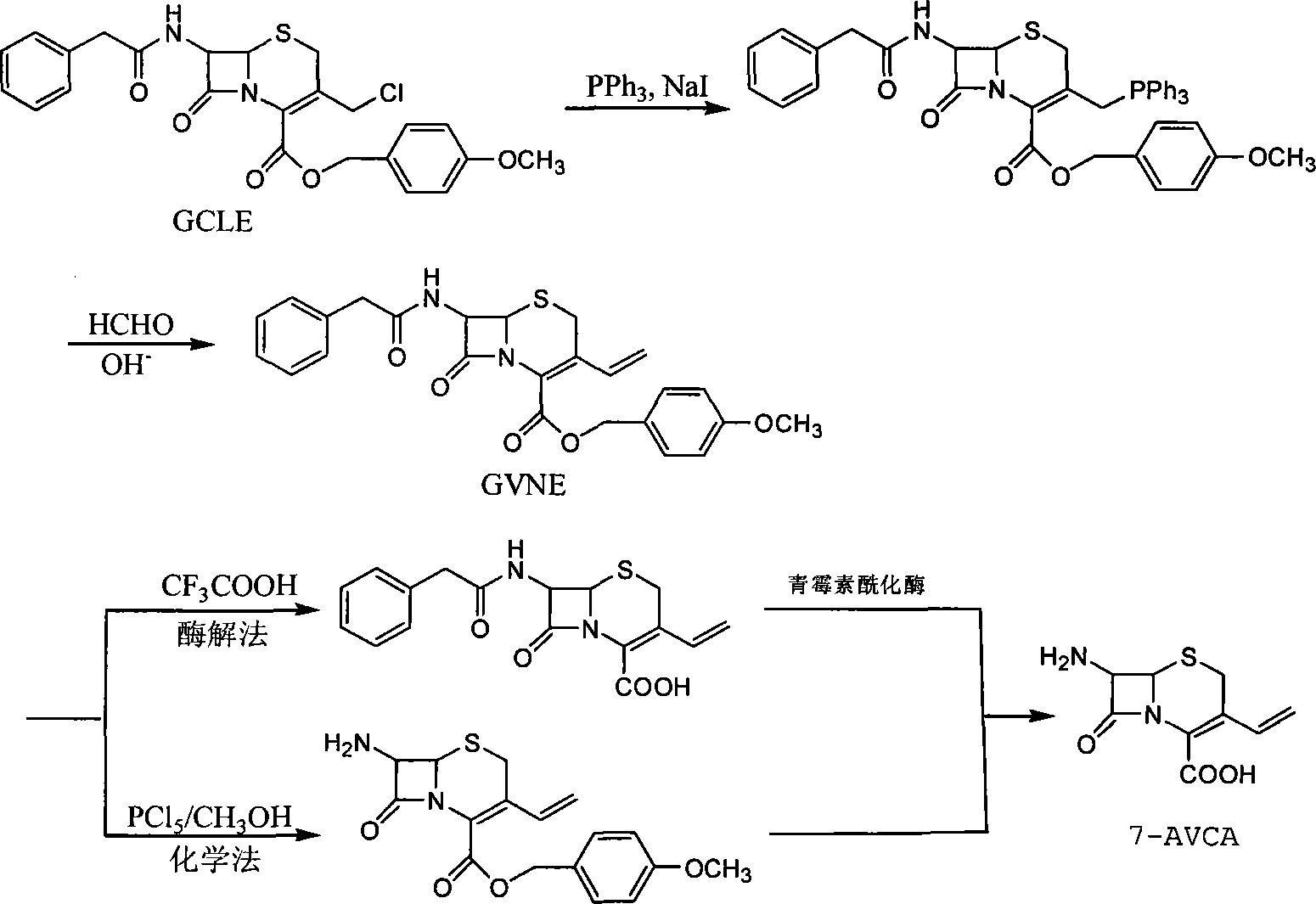
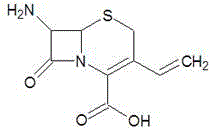
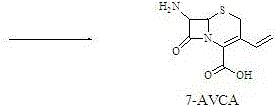
A method of preparing for 7-amido-3-ethylene cephalosporanic acid considers 7-phenylacetyl amino-3-chloromethyl cephalosporanic acid p-methoxybenzyl ester (GCLE) as a starting material and adopts the Wittig reaction. Ethylene is introduced on position 3 to obtain 7-phenylacetyl amino-3- ethylene-4- cephalosporanic acid p-methoxybenzyl ester, and a product GVNE is obtained. The GVNE is used to prepare for 7-amido-3-7 alkenyl cephalosporanic acid (7-AVCA). The present invention has the main technical characteristic that the triethyl phosphite with quite low price is used to replace triphenyl-phosphonium in the preparation of the GVNE to realize the purpose of introducing the ethylene on the position 3 of GCLE to prepare for the GVNE. The production method is simple; the cost of the main materials is low; the yield is high; the comprehensive benefit efficiency is high; the present invention is applicable to the large scale industrialized preparation of 7-AVCA.
Owner:SHANDONG JINCHENG PHARMA & CHEM
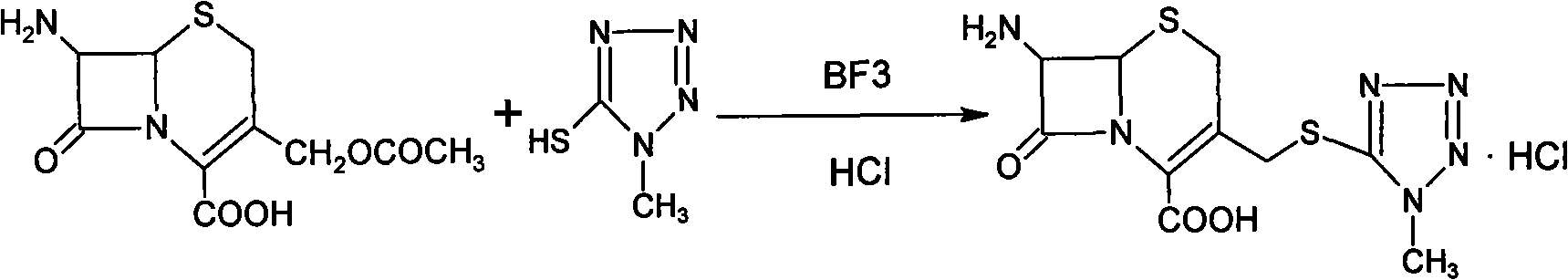
Preparation process of cefamandole nafate
The invention discloses a preparation process of cefamandole nafate. The preparation process comprises steps of: heating and stirring 7-amino cephalosporanic acid, 5-mercapto-1-methyltetrazole and a catalyst boron trifluoride acetonitrile complex for a reaction; and carrying out a cooling post-treatment to obtain cefditoren nuclear parent; conducting a heating reflux reaction on the cefditoren nuclear parent and a silanizing agent until the solution turns to a clarified state; adding N, N-dimethyl aniline under the protection of inert gas at a low temperature, dropwise adding D-(-)-O-formyl mandeloyl chloride for reaction, and carrying out post-treatment to obtain formyl cefamandole acid; and reacting the formyl cefamandole acid with an organic acid salt, and recrystallizing to obtain the cefamandole nafate. By the above way, the preparation process of cefamandole nafate provided by the invention employs a simple and easily implemented process to obtain high-yield cefditoren nuclear parent with low impurity content; dichloromethane is used as a solvent to obtain the cefamandole acid with greatly enhanced color grade and yield; and dosage of activated carbon in the post-treatment is obviously reduced, so as to reduce the production cost.
Owner:苏州盛达药业有限公司
Method for synthesizing cephalosporin intermediate
The invention discloses a method for synthesizing a cephalosporin intermediate, which comprises the following steps: 1, adding 1-methyl-5-mercaptotetrazole and 7-amino-cephalosporanic acid into acetonitrile with stirring, heating the solution, and adding a boron trifluoride complex compound into the solution; 2, adding a proper amount of active carbon into the solution, stirring and filtering thesolution, washing the carbon by using aqueous solution of acetone containing hydrochloric acid, and merging filtrate; 3, adding the merged filtrate into the aqueous solution of acetone, stirring the solution, slowly dripping alkali solution till the pH value of the solution is 2.0 to 3.5, and growing crystals for 1 to 2 hours; and 4, filtering the solution, transferring the filtrate to another container, reclaiming the acetonitrile and the acetone, washing the obtained crystals twice to trice by using the acetone, filtering the solution, and then drying the crystals under vacuum. Aiming at the problem that the conventional method for synthesizing the cephalosporin intermediate is not suitable for industrialized production, the invention provides the method for synthesizing the cephalosporin intermediate; the method is simple and convenient to operate, is suitable for industrialized production and has high yield; and the prepared 7-ATCA.HCl has high purity.
Owner:SHANGHAI NEW ASIA PHARMA
Method for synthesizing 7-phenylacetamide-3-chloromethyl-4-cephalosporanic acid p-methoxybenzyl ester
InactiveCN101260116AHigh yieldEasy to operateOrganic chemistryPenicillin G sulfoxideSynthesis methods
The invention discloses a synthesis method of 7- phenylacetamide-3-chloromethyl-4- cephalsporanic acid p- methoxybenzyl ester(GCLE). The invention takes the penicillin G kali salts mass made in China as starting materials; firstly, the penicillin G kali salts reacts with the p- methoxy benzyl chloride to obtain p- methoxybenzyl ester; secondly, the p- methoxybenzyl ester is oxidized by peroxy acetic acid to obtain penicillin sulfoxide ester; the penicillin sulfoxide ester firstly reacts with the 2- mercaptobenzothiazole, and then reacts with benzene sulfinic acid to obtain ring opening products; the ring opening products reacts with chlorine gases to obtain chlorination products; the chlorination products reacts with ammonia gases to obtain 7- phenylacetamide-3-chloromethyl-4- cephalsporanic acid p- methoxybenzyl ester(GCLE).
Owner:HUNAN NANXIN PHARMA CO LTD
Preparation process of 3-deacetylated-7-amino-cephalosporanic acid
The invention discloses a preparation process of 3-deacetylated-7-amino-cephalosporanic acid and belongs to the technical field of medicine. A method of the preparation process comprises the steps that: cephalosporin acylase and cephalosporin esterase are mixed, then, cephalosporin C extracting solution is added, next, acid is added for crystallization, washing and drying, and the solid 3-deacetylated-7-amino-cephalosporanic acid is obtained. The method only needs the one-step normal-temperature cracking reaction and one-step crystallization process, the process route is short, the pollution is little, the product mol yield is higher than 85 percent, and the preparation process is suitable for industrial production.
Owner:石药集团内蒙古中诺药业有限公司
Mutation penicillin ring enlargement enzyme and process preparing 7-ADCA using same
The present invention is mutant expandase with even higher activity to benzyl penicillin used for preparing phenylacetyl-7-aminodeacetoxy cephalosporanic acid (7-ADCA). The mutant expandase has one or several amino acid substitutes selected from M73T, S79E, V275I, L277K, C281Y, G300V, N304K, I305L and I305M.
Owner:骏翰生化股份有限公司
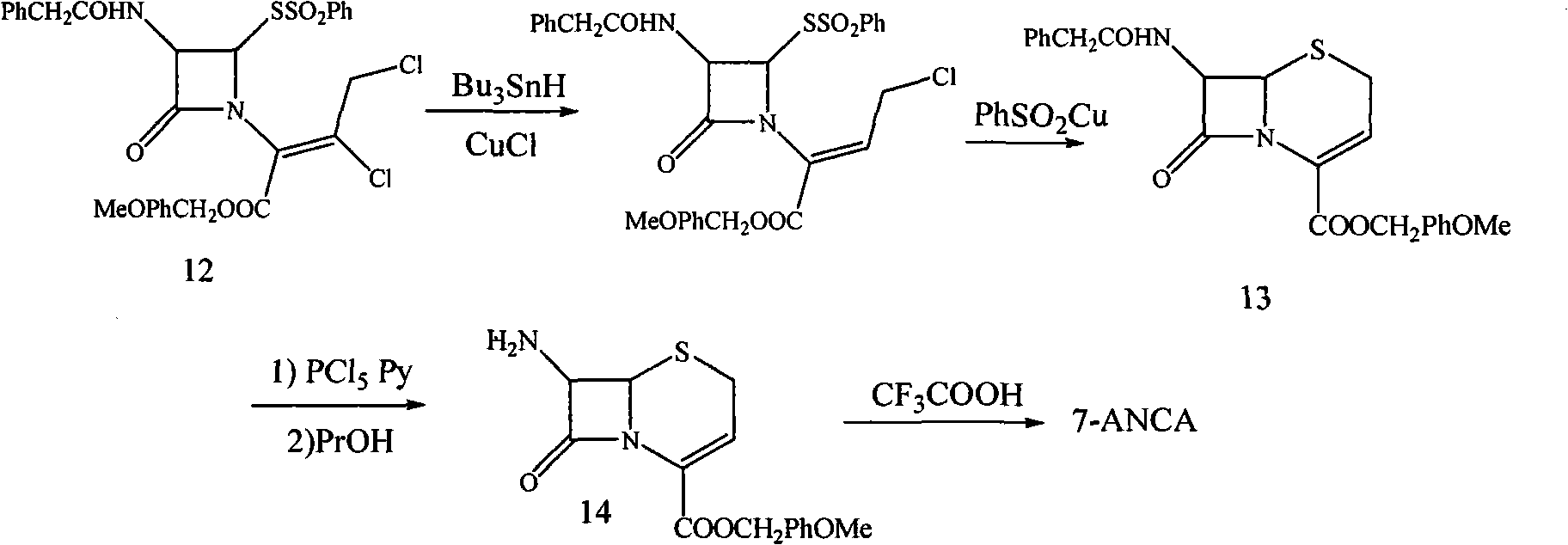
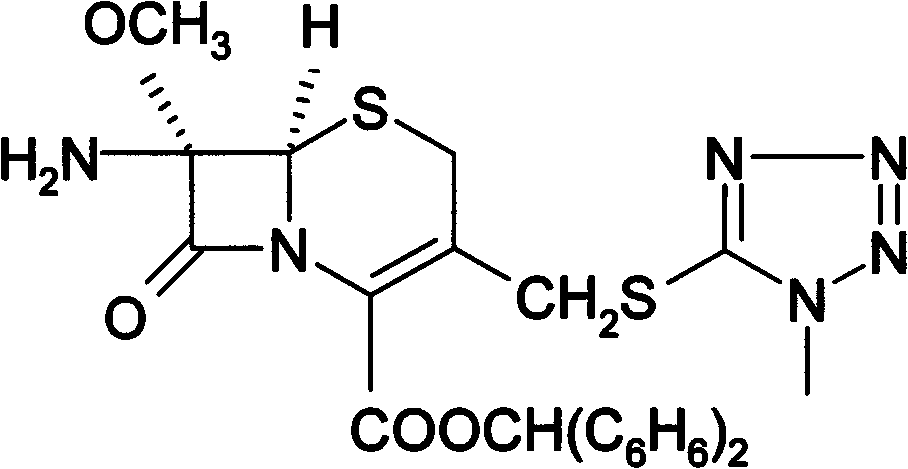
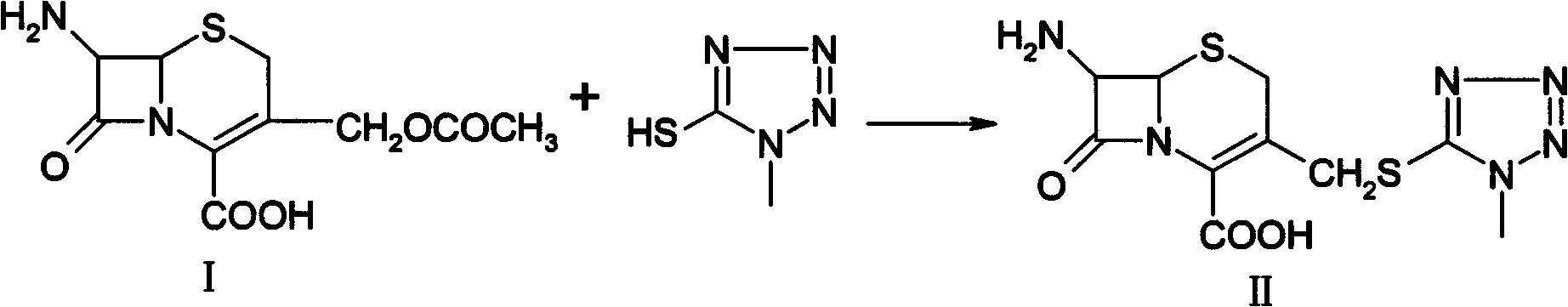
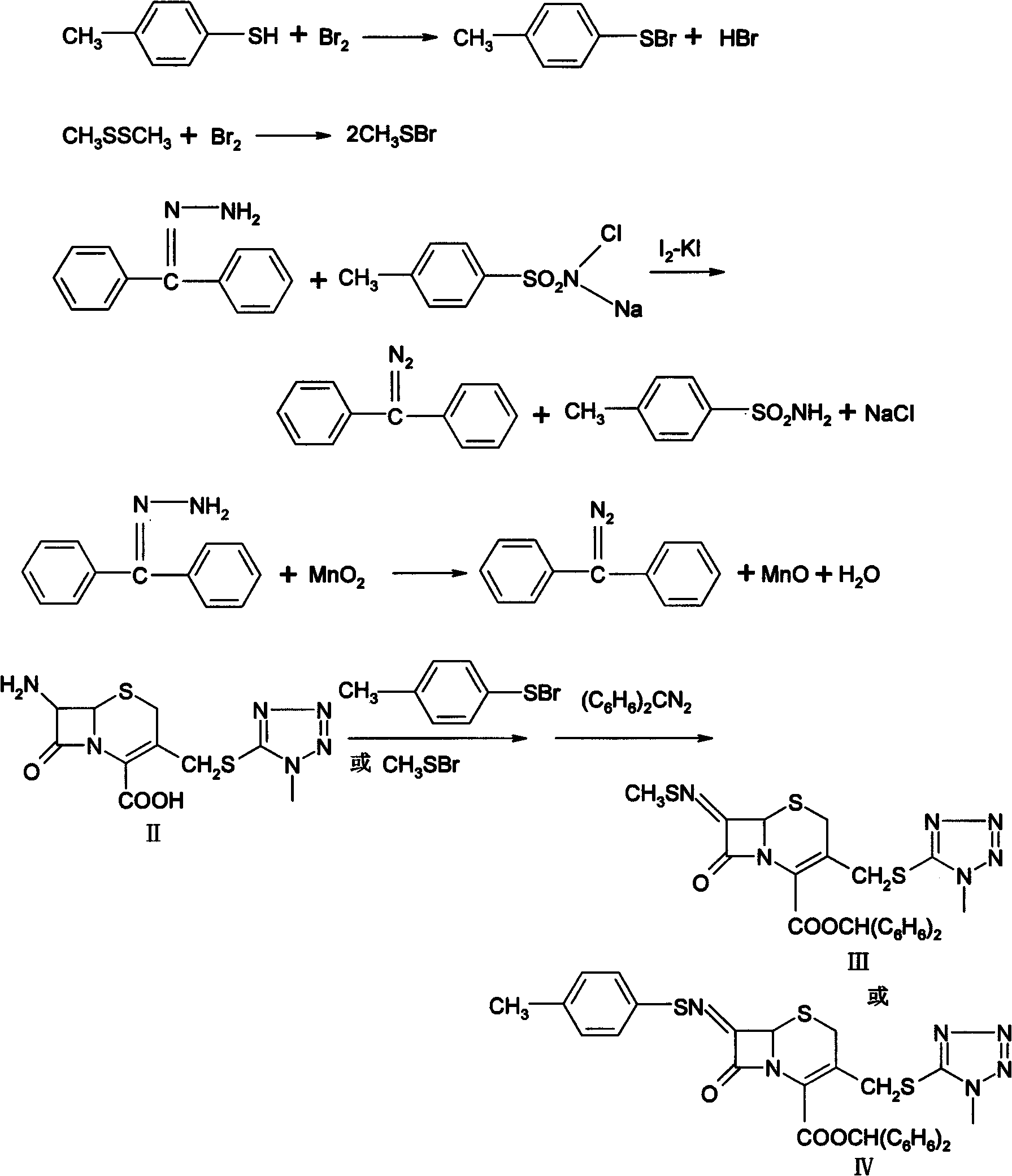
Preparation method for 7-amino-3-non-3-cephem-4-carboxylic acid(7-ANCA)
ActiveCN102180888AMild reaction conditionsHigh yieldOrganic chemistryBulk chemical productionChemical industryPhenylacetic acid
The invention belongs to the fields of chemical industry and pharmacy and relates to a preparation method for 7-amino-3-non-3-cephem-4-carboxylic acid (7-ANCA, I). The 7-ANCA is prepared from 7-phenylacetamide-3-hydroxy cephalosporanic acid diphenyl methyl ester (II-1) or 7-phenylacetamide-3-hydroxy cephalosporanic acid paramethoxy benzyl ester (II-2) serving as a raw material by the following steps of: (1) performing 3-hydroxyl sulfonylation, (2) eliminating, (3) removing 4-position protective group and (4) removing 7-position phenylacetyl amino; and phenylacetic acid is recovered. The raw materials II-1 and II-2 used in the process can be prepared from corresponding 3-hydroxy cephalosporanic acid through reduction reaction by the known method. The whole process is mild in reaction condition, high in yield, low in equipment investment, light in environmental pollution and suitable for industrialized production.
Owner:河北爱弗特精细化工有限责任公司
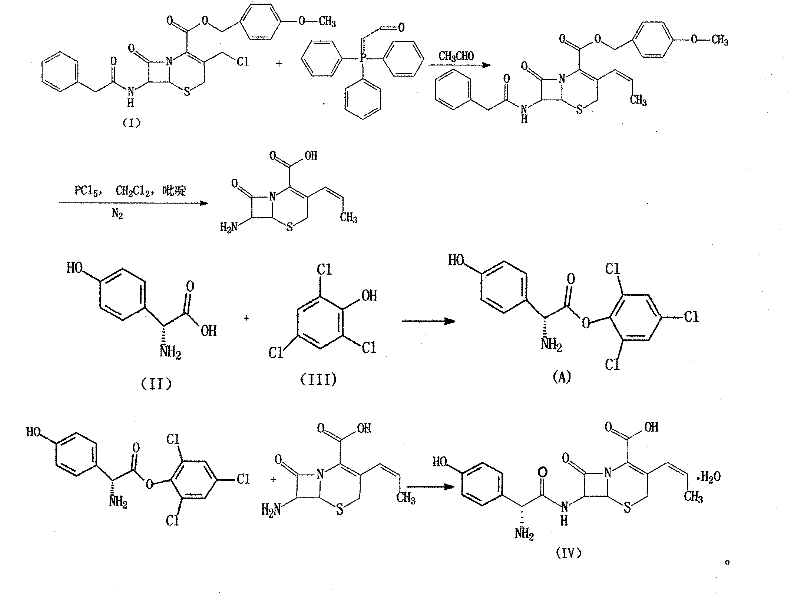
Method for synthetizing 7-amino-3-vinyl-cephalosporin ring-4-carboxylic acid
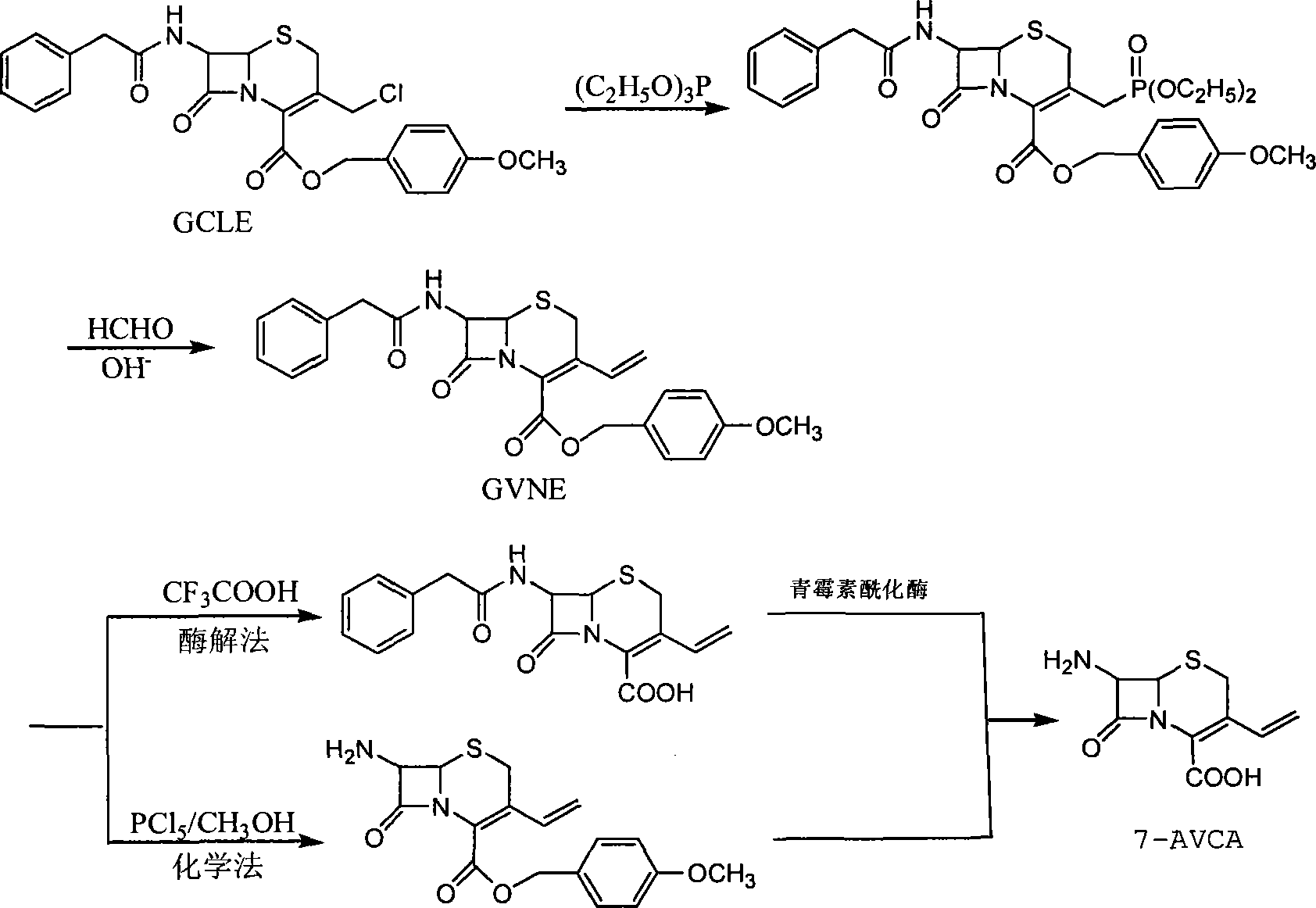
InactiveCN104073543AImprove conversion rateHigh yieldOrganic chemistryFermentationReaction temperatureCarboxylic acid
The invention discloses a method for synthetizing 7-amino-3-vinyl-cephalosporin ring-4-carboxylic acid. According to the technical scheme, the method comprises the following steps: reacting 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxy benzyl ester (GCLE) serving as a raw material with triphenylphosphine under the action of strong alkali to generate 7-phenylacetamido-3-vinyl-4-cephalosporanic acid p-methoxy benzyl ester (GVNE), carrying out hydrolysis reaction on the GVNE in a phenol solution to remove the carboxyl protecting group, adjusting the PH to alkaline without separating, and adding immobilized penicillin acylase to carry out enzymolysis reaction to remove the carboxyl protecting group to obtain the 7-amino-3-vinyl-4-cephalosporanic acid. The method has the advantages that the product conversion ratio is high; the extracting solvent is low in toxicity and easy to recycle; the reaction temperature is moderate and easy to control; moreover, the immobilized penicillin acylase can be recycled; the production cost is low; the environmental pollution is little; and the harm to the health of operators is hardly caused.
Owner:GUANGDONG LIGUO PHARMACY
Process method for preparing methoxycephems intermediate 7-MAC (7-methoxycephalosporin)
InactiveCN102250122ARaw materials are easy to getStable operation processOrganic chemistrySodium bicarbonateSynthesis methods
The invention discloses a process method for preparing a methoxycephems intermediate 7-MAC (7-methoxycephalosporin), belonging to the technical field of chemical engineering. The process method provided by the invention comprises the following steps of: condensing 7-ACA (7-amino Cephalosporanic Acid) and 1-methyl-5-sulfydryl tetrazole under the catalysis of boron trifluoride-methanesulfonic acid to prepare 7-TMCA (7-amino-3-[(1-methyltetrazol-5-yl)thiomethyl]cephem-4-carboxylic Acid); then protecting carboxyl of the 7-TMCA with BSA (Bovine Serum Albumin) or HMDS (Hexamethyl Disilazane), and subjecting the protected 7-TMCA and methyl sulfur bromide to imidization; then subjecting a product and diphenyldiazomethane to an esterification; then reacting the obtained intermediate with methanol to obtain the methoxycephems intermediate 7-MAC under the catalysis of aluminum trichloride-sodium bicarbonate-triphenyl phosphorus. The catalysts of all steps are optimally screened. Side reactions and the generation of impurities are tracked and analyzed so that a method for avoiding the side reactions is found. The process method provided by the invention is an improved industrial synthesis method of the 7-MAC.
Owner:江苏力达宁化工有限公司
Preparation method of 7-phenylacetamido-3-vinyl-4-p-methoxy benzyl ester cefotaximate
ActiveCN103923104AReduce pollutionHigh purityOrganic chemistryBulk chemical productionBenzeneSodium iodide
The invention discloses a preparation method of 7-phenylacetamido-3-vinyl-4-p-methoxy benzyl ester cefotaximate and belongs to the Chemical medicine field. The preparation method comprises the following steps: carrying out quaternary phosphorization and vinyl reaction at the same time on 7-phenylacetamido-3-chloromethyl-4-p-methoxy benzyl ester cefotaximate (GCLE), sodium iodide, triphenylphosphine and formaldehyde solution in a mixed system of dichloromethane, acetone and water to obtain 7-phenylacetamido-3-vinyl-4-p-methoxy benzyl ester cefotaximate; and removing 7-amino and 4-carboxyl protecting group according to known methods to prepare cefixime and cefdinir nuclear parent 7-AVCA (7-amino-3-vinyl-4-cephalosporanic acid). The aqueous phase comprising sodium iodide and excessive formaldehyde can be repeatedly applied, so that the utilization ratio of sodium iodide is remarkably improved, the production cost is lowered, and the environmental pollution caused by formaldehyde is reduced. The preparation method disclosed by the invention is low in production cost, simple in operating method and suitable for industrialized production and the raw materials are easily available in the preparation process.
Owner:湖北凌晟药业股份有限公司
Mutated cephalosporin hydroxylase and its application in deacetylcephalosporanic acid synthesis
The present invention relates to a mutant hydroxylase with increased activity and greater substrate specificity towards phenylacetyl-7-ADCA derivatives for the production of phenylacetyl-7-HACA derivatives, which carries one or more amino acid modification at residue positions when compared with the wild type hydroxylase from the following group of residues, Proline at position 7, Alanine at position 40, Threonine 51, Methionine at position 53, Glutamic acid at position 82, Arginine at position 91, Threonine at position 96, Glycine at position 108, Isoleucine at position 149, Valine at position 171, Alanine at position 177, Arginine at position 182, Methioinine at position 184, Isoleucine at position 193, Phenylalanine at position 195, Glutamine at position 209, Alanine at position 210, Valine at position 226, Methionine at position 233, Leucine at position 236, Alanine at position 237, Alanine at position 241, Valine at position 249, Arginine at position 250, Serine at position 251, Glycine at position 255, Glutamic Acid at position 258, Serine at position 260, Phenylalanine at position 267, Alanine at position 280, Valine at position 307 and Asparagine at position 313.. The invention further provides a process for the preparation of deacetyl cephalosporanic acid from the corresponding deacetoxy cephalosporanic acid using an enzyme of the present invention. The invention also provides the method for producing and processing of such enzymes.
Owner:ORCHID CHEM & PHARM LTD
Cefuroxime sodium synthesizing method
The invention relates to the technical field of pharmaceutical and chemical industries and particularly discloses a cefuroxime sodium synthesizing method. The synthesizing method comprises the steps:dropwise adding alkali solution into 7-aminocephalosporanic acid aqueous solution for hydrolysis reaction, and then subjecting the mixture and (Z)-2-furyl-2-methoxyimino acetic acid p-toluene sulfonicanhydride to amidation to obtain 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid; dissolving the 7-[(Z)-2-furyl-2-methoxyiminoacetamido]-3-hydroxymethyl-4-cephalosporanic acid into organic solvents to sequentially perform nucleophilic addition and hydrolysis reaction with chlorosulfonyl isocyanate, then adding sodium iso-octoate solution, and performing devitrification, filtering, washing and drying to obtain cefuroxime sodium. According to the synthesizing method, raw materials are wide and easy to obtain, the cost is low, the steps are simple, operation is simple, and side reaction is less.
Owner:湖北凌晟药业股份有限公司 +1
Preparation method of cephalosporin C sodium salt and 7-amino-cephalosporanic acid
ActiveCN110423241AQuality improvementImprove qualityOrganic chemistryFermentationFiltrationFermentation
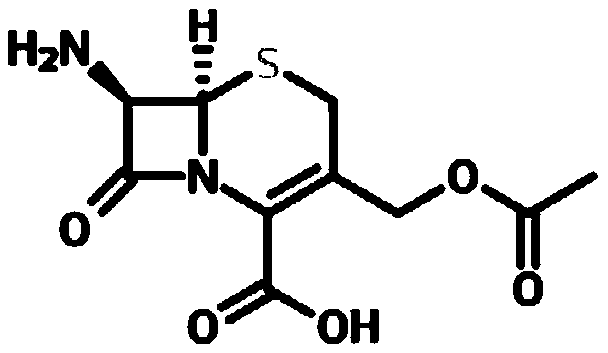
The invention relates to a preparation method of cephalosporin C sodium salt and 7-aminocephalosporanic acid. The method comprises the following steps: separating a cephalosporin C filtrate from a fermentation liquid by a membrane filtration manner; then extracting cephalosporin C from the filtrate by using an organic solvent to obtain a cephalosporin C extraction solution; mixing the cephalosporin C extraction solution and sodium 2-ethylhexanoate, carrying out a salt formation reaction, performing standing stratification, and collecting a heavy phase; adding an anti-solvent into the heavy phase for crystallization to obtain cephalosporin C sodium salt; and then dissolving cephalosporin C sodium salt by using purified water to obtain an aqueous solution of cephalosporin C sodium salt, adding an immobilized cephalosporin C acylase, and performing enzymatic hydrolysis, crystallization, filtration, washing and drying on cephalosporin C sodium salt to obtain 7-amino-cephalosporanic acid. Cephalosporin C sodium salt and 7-aminocephalosporanic acid prepared by the method are significantly superior to a conventional process, the product is used for preparation of cephalosporin downstreamproducts, the obtained products have higher quality, and medication is safer.
Owner:SHANXI WEIQIDA PHARMA IND
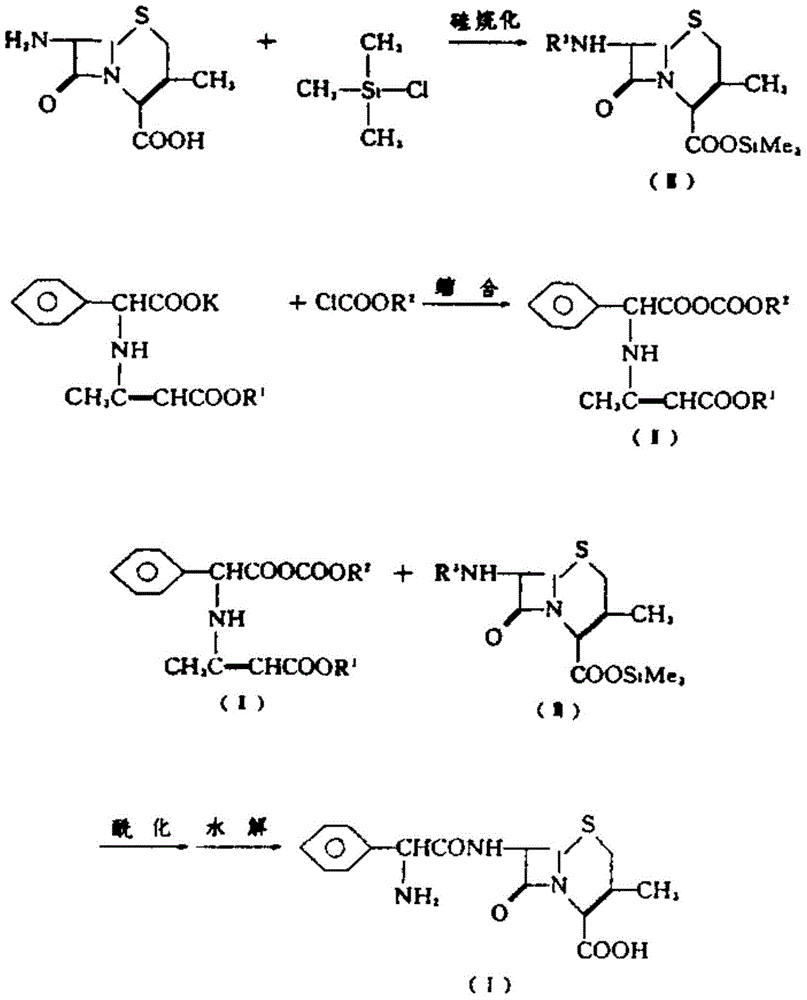
Improvement method of cefalexin synthesis process
The invention belongs to the field of pharmaceutical synthesis, and particularly relates to an improvement method of a beta-lactam antibiotic cefalexin synthesis process. According to the method, a 7-ADCA (amino desacetoxy cephalosporanic acid) is taken as a raw material, the method comprises the steps that the 7-ADCA is subjected to silane protection of carboxyl, and then the 7-ADCA has a condensation reaction with alpha-amino benzeneacetyl chloride or hydrochlorides thereof under the catalysis of 4-dimethylaminopyridine; the obtained object is subjected to hydrolyzed aftertreatment so as to obtain cefalexin; and after the cefalexin is treated by using an organic solvent and subjected to alkali adjustment treatment, an appropriate pH value of the cefalexin is controlled by using a hydrochloric acid, so that cefalexin crystals with an extremely high purity are obtained. The method has the advantages that the method is simple in operation, mild in reaction condition and has small possibility of causing side effects, and can effectively remove impurities and prepare high-purity cefalexin.
Owner:孙丽华
Method for preparing cefprozil compound
InactiveCN101798312BSimple processHigh purityOrganic compound preparationAmino-carboxyl compound preparationChlorobenzeneAklanonic acid
Owner:HAINAN MEIDA PHARMA
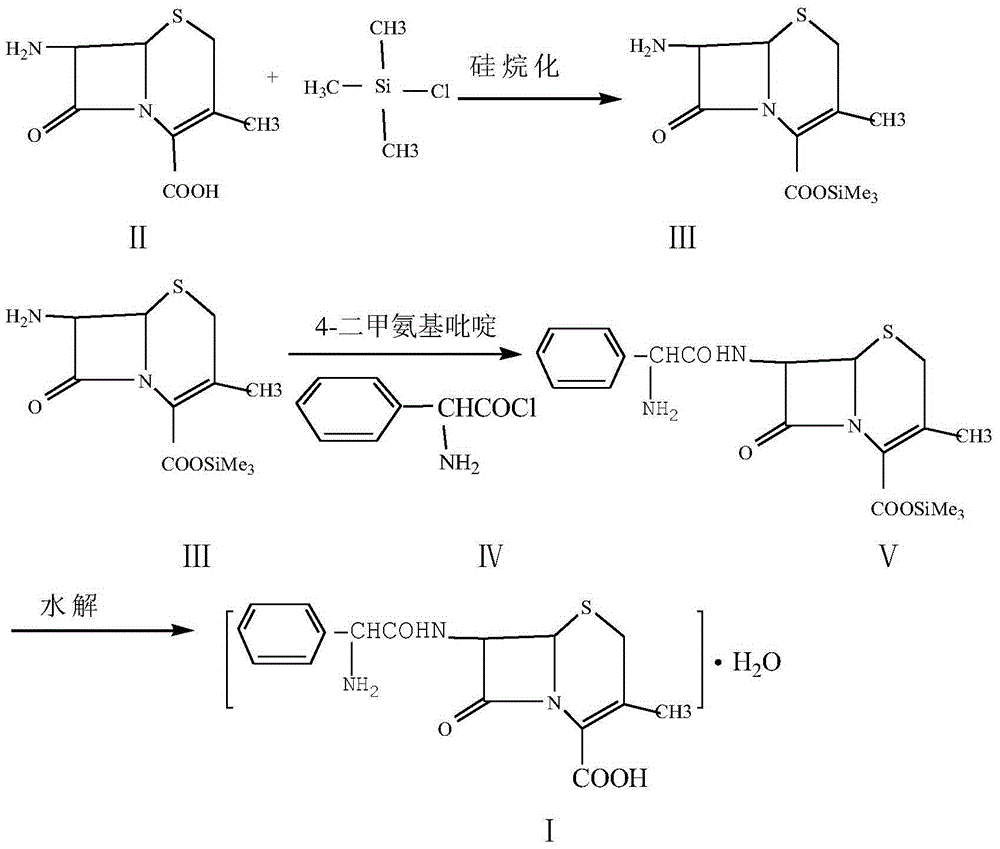
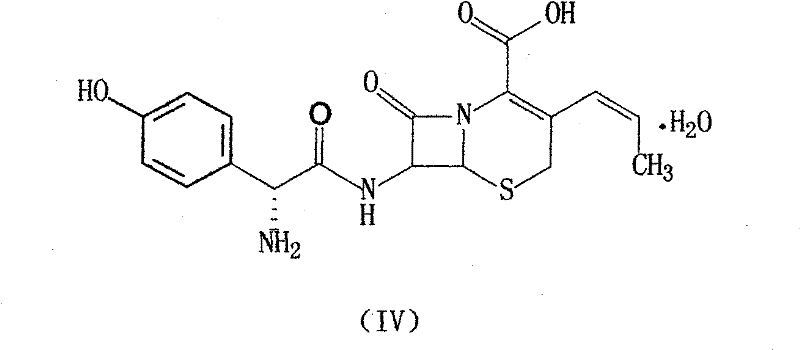
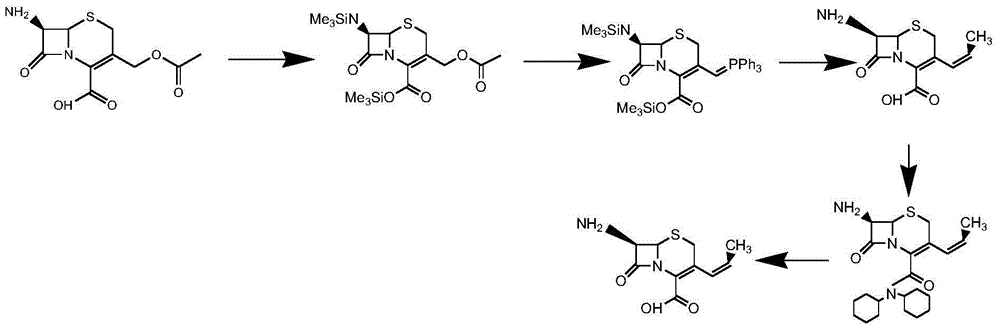
Method for preparing cefprozil mother nucleus 7-amino-3-acryl cephalosporanic acid
ActiveCN105001238AEasy to separateNot easy to patinaOrganic chemistryWittig reactionCephalosporanic Acids
The invention relates to a method for preparing cefprozil mother nucleus 7-amino-3-acryl cephalosporanic acid. According to the method, 7-amino cephalosporanic acid is adopted as a starting raw material, silylation protection, phosphorusylide formation, wittig reaction and silylation protection group removing are sequentially performed to obtain a cefprozil mother nucleus crude product, and a cefprozil mother nucleus crude product refining post-treatment process is added, wherein the cefprozil mother nucleus crude product refining post-treatment process comprises: a, amine salt forming, b, decolorization treatment, and c, cefprozil mother nucleus refined product preparing. According to the present invention, the prepared cefprozil mother nucleus has characteristics of high purity, good crystalline form, good color and high yield, the ratio of the E isomer content to the Z type isomer content is optimal so as to ensure the improved quality of the cefprozil prepared at the latter stage, the cefprozil mother nucleus purity can achieve 99.7%, the 7-ADCA is less than or equal to 0.15%, the crystalline form is hexagonal columnar, separation is easy, the patina is not easily generated, the color is less than or equal to Y-4 and is basically bright white, the yield is high, the quality is good, and the mass yield is more than 65%.
Owner:HEBEI JIUTIAN BIOLOGICAL PROD CO LTD
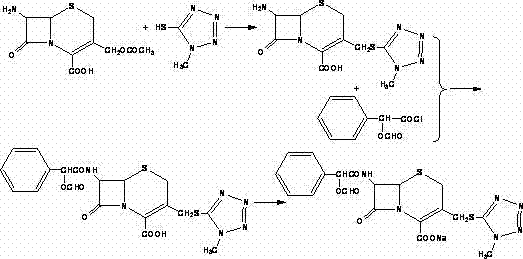
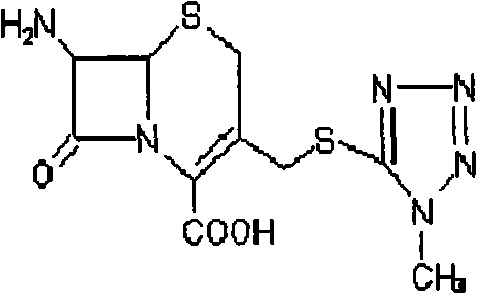
Synthesis method of cefoperazone acid
InactiveCN102532168AHigh yieldReduce generationOrganic chemistrySynthesis methodsPolyethylene glycol
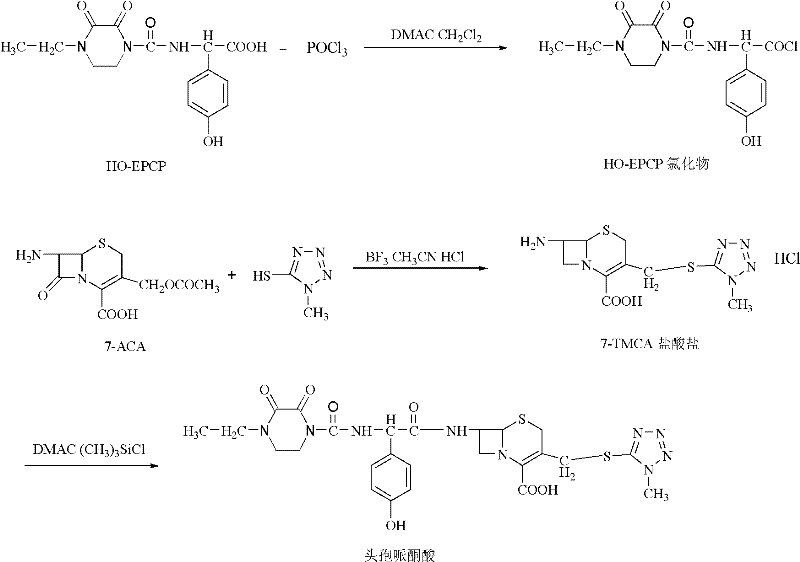
The invention relates to a synthesis method of cefoperazone acid, and belongs to the technical field of medicines. The method comprises the following steps: (1) adopting 7-ACA (7-aminocephalosporanic acid) and 1-methyl-5-mercapto-1,2,3,4-tetrazole as raw materials, allowing reaction between the two raw materials in the catalysis of a boron trifluoride-acetonitrile solution to obtain 7-TMCA (7-amino-3-methyl tetrazolyl cephalosporanic acid) hydrochloride, and performing the protection of carboxyl group and amino group on 7-TMCA hydrochloride with trimethylchlorosilane; (2) allowing reaction of HO-EPCP (2-[(4-ethyl-2,3-dioxopiperazinyl)carbonylamino]-2-(4-hydroxyphenyl)acetic acid) and phosphorus oxychloride in a DMAC (dimethylacetamide) and dichloromethane solution in the protection of nitrogen gas to obtain HO-EPCP chloride; and (3) allowing N-acylation reaction of the 7-TMCA hydrochloride subjected to the protection of carboxyl group and amino group obtained by the step (1) and HO-EPCP chloride obtained by the step (2) with polyethylene glycol 800 as a phase transfer catalyst in a dichloromethane-water mixed solution, regulating pH with a hydrochloric acid solution, and crystallizing to obtain cefoperazone acid. In the invention, the yield of 7-TMCA hydrochloride under the catalysis of boron trifluoride is improved by 3%. The addition of the phase transfer catalyst reduces occurrence of side reaction, improves the reaction yield by 5%, makes the final product yield reach above 69.0%, and improves purity to above 99%.
Owner:YIYUAN XINQUAN CHEM
Preparation method of cefuroxime axetil
InactiveCN105131016ALow impurity contentGood colorOrganic chemistryBulk chemical productionCephalosporanic AcidsTrichloroacetyl isocyanate
The invention relates to a preparation method of cefuroxime axetil. The preparation method comprises the following steps: (1) reacting de-ammoniated formyl cephalosporanic acid with diphenyl diazomethane to generate acetyl-diphenyl methyl cephalosporanate; (2) reacting diphenyl methyl de-ammoniated formyl cephalosporanate with trichloroacetic isocyanate to generate diphenyl methyl cefuroxime ester; (3) hydrolyzing diphenyl methyl cefuroxime ester to obtain cefuroxime acid; (4) reacting cefuroxime acid with 1-acetoxyl-bromoethane to generate cefuroxime axetil. During the preparation process, the carboxyl group is protected by diphenyl diazomethane, the generation of impurities is reduced, and the product quality is improved.
Owner:JIANGSU QINGJIANG PHARMA
Penicillin fermentation broth treating technology
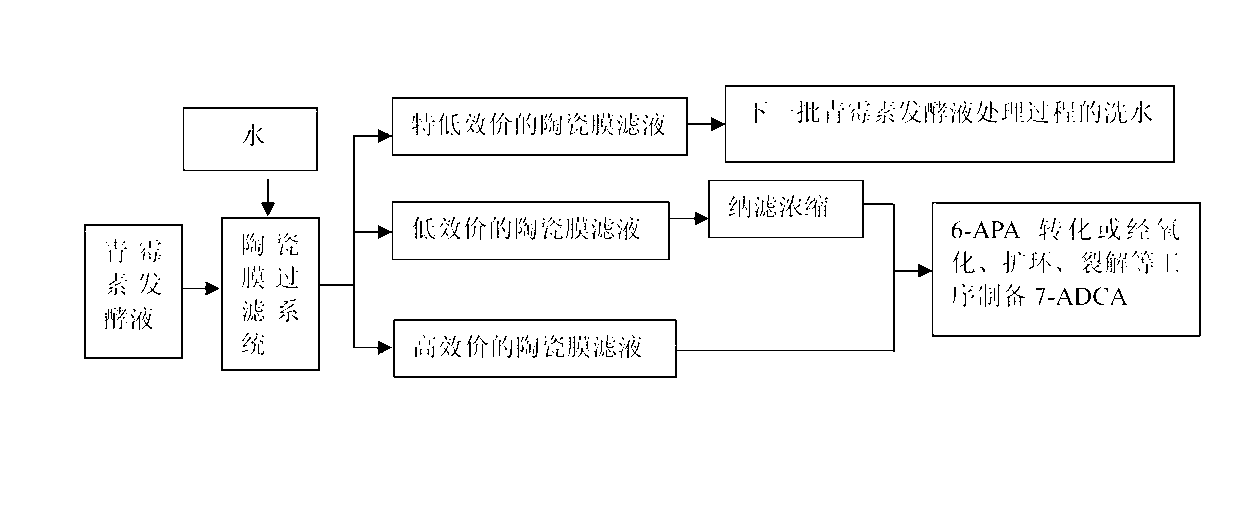
ActiveCN103214498ADetermining the concentrationDetermine the quantitySemi-permeable membranesOrganic chemistryCross-flow filtrationCephalosporanic Acids
The invention discloses a penicillin fermentation broth treating technology, which comprises the following steps of: cooling an original penicillin fermentation broth, filtering the cooled penicillin fermentation broth by a closed ceramic-membrane cross-flow filtration system, and collecting high-titer ceramic-membrane filtrate; during filtration, when the wet solid content in the penicillin fermentation broth is enhanced to 1.8-2 times that of the original fermentation broth, adding water with weight accounting for 2 times that of the original fermentation broth for dialyzing to obtain and collect low-titer ceramic-membrane filtrate, and then nano-filtering, concentrating and dewatering the low-titer ceramic-membrane filtrate for later use; continuing to add water with weight accounting for 2 times that of the original fermentation broth for dialyzing to obtain and collect ultra-low-titer ceramic-membrane filtrate; stopping filtration until the titer of penicillin in the penicillin fermentation broth is low to 500-800U; collecting bacterium dregs intercepted by the ceramic-membrane filtration system; putting the high-titer ceramic-membrane filtrate in 6APA (6-aminopenicillanic acid) for conversion or oxidization, ring enlargement and cracking, thus preparing 7-ADCA (7-aminodeacetoxy cephalosporanic acid); and collecting the obtained bacterium dregs and adding engineering bacteria for decomposing the bacterium dregs.
Owner:河北美邦工程科技股份有限公司 +1
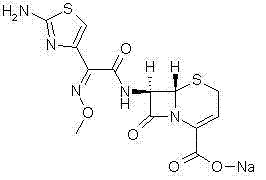
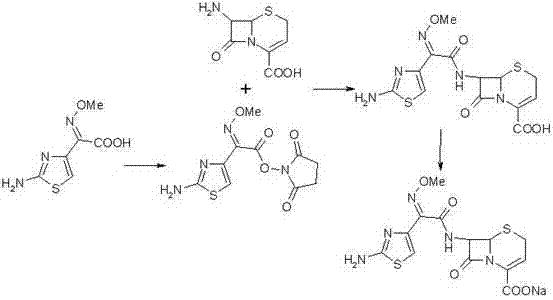
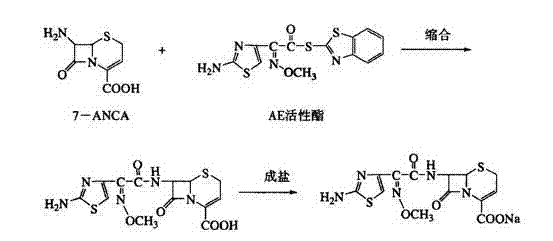
Method for preparing ceftizoxime
The invention provides a method for preparing ceftizoxime. The method comprises the following steps of: (1) dissolving 2-(2-amino-4-thiazolyl)-2-methoxy-imine acetic acid in an organic solvent, and reacting with N-succinimide under the catalysis of dicyclohexyl carbodiimide / dimethylamino pyridine (DCC / DMAP) to obtain active ester; (2) dissolving the active ester in dichloromethane, and adding 7-amino-3-demethylation-3-cephalosporanic acid (7-ANCA) and alkali for reacting to obtain ceftizoxime acid; and (3) reacting the ceftizoxime acid with a salifying agent to obtain the ceftizoxime, wherein the salifying agent is sodium acetate, sodium ethoxide, sodium 2-ethylhexanoate, sodium isocaproate, sodium bicarbonate or sodium hydroxide.
Owner:SUZHOU ERYE PHARMA CO LTD
Monohydrater cefotiam hydrochloride compound and pharmaceutical composition thereof
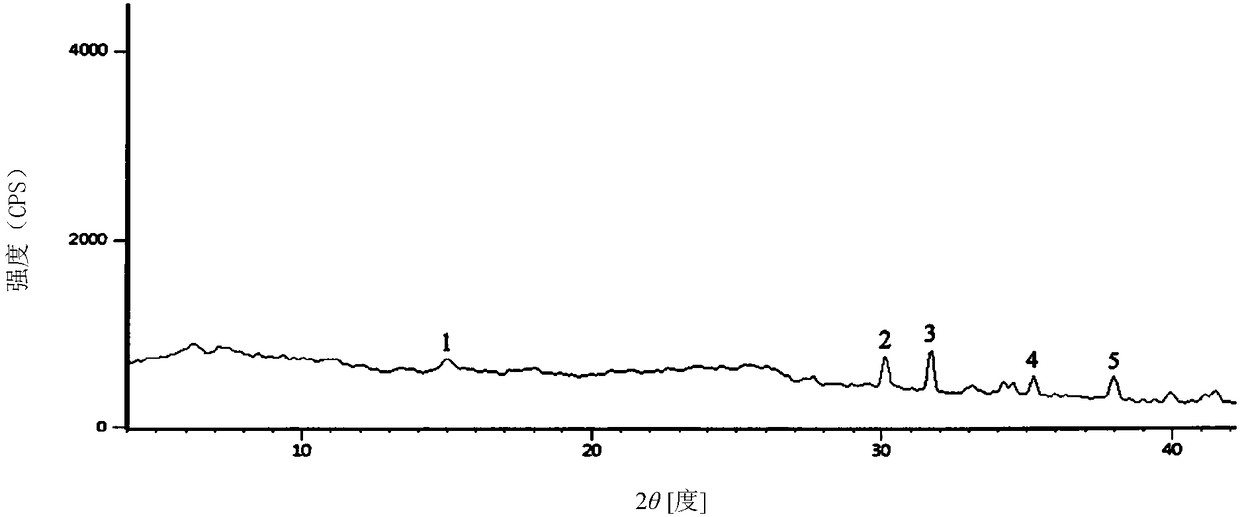
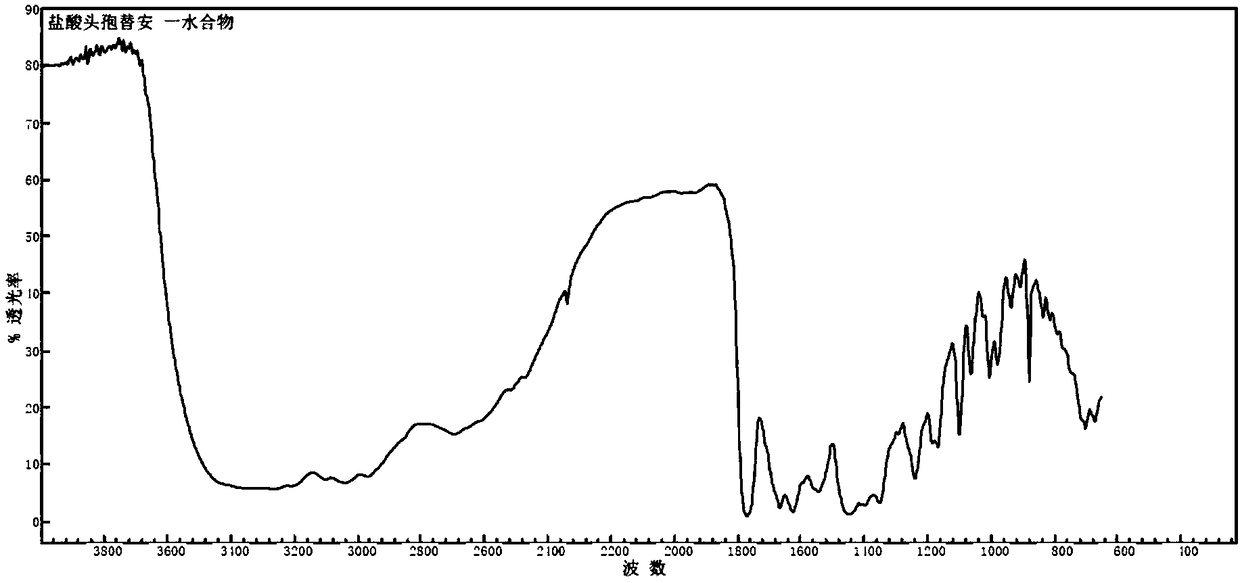
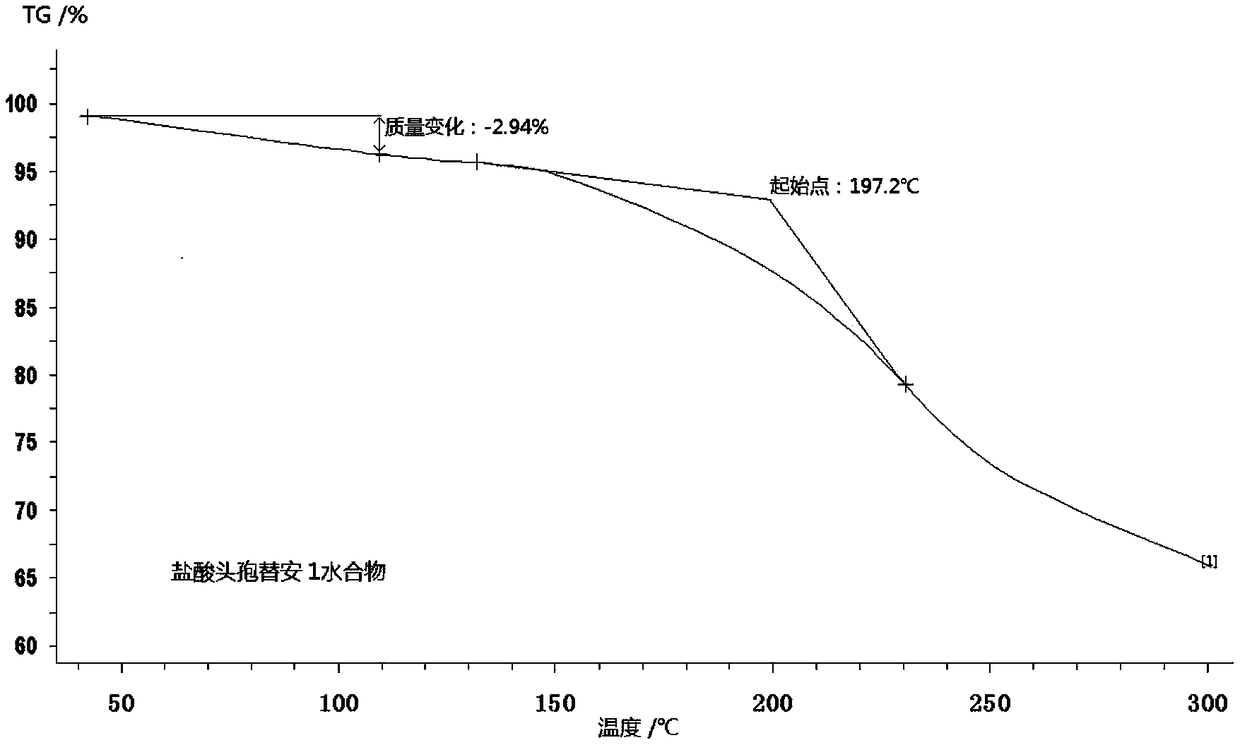
The invention discloses a monohydrate cefotiam hydrochloride compound and a preparing method thereof. One mole of cefotiam hydrochloride contains one mole of water, and an x-ray diffraction spectrogram of the compound has characteristic peaks at the positions with the diffraction angles 2theta of 14.82-15.22 degrees, 29.92-30.32 degrees, 31.48-31.88 degrees, 35.01-35.41 degrees and 37.79-38.19 degrees. 7-amino-cephalosporanic acid (7ACA) serving as a starting material is condensed with 1-(2-dimethylaminoethyl)-5-thiotetrazole under the catalytic effect of organic solvents dimethyl carbonate boron trifluoride and the like to prepare an intermediate at a C-3 site; then 2-(2-aminothiazol-4-yl) acetic acid hydrochloride reacts with methylene chloride and concentrated hydrochloric acid gas to prepare acyl chloride at a 7 site, and the one-water cefotiam hydrochloride compound is synthesized. The operation is simple and environmentally friendly, the reactants are easily obtained, the reaction conditions are mild, and the yield is high. The one-water cefotiam hydrochloride compound has low hygroscopicity and impurity content, good fluidity and thermodynamic stability and wider applicationprospects.
Owner:宁应
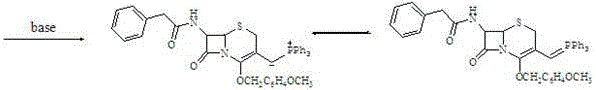
7-amino-3-vinyl cephalosporanic acid preparation method
The invention belongs to the technical field of medicine synthesis, and especially discloses a method for preparing 7-amino-3-vinyl cephalosporanic acid. The preparation method takes GCLE as an initial raw material, the GCLE and sodium iodide and triphenylphosphine are reacted in a mixing system of an organic solvent and water to generate phosphine salt, a certain amount of alkali lye is added in an organic phase for reacting to generate the corresponding phosphorus ylide, excess free alkali in an organic phase is removed through washing, formaldehyde and ylide are added for a wittig reaction, the organic phase is concentrated to a certain weight, phenol is added for removing carboxyl protection, amino protection is removed, crystallization is carried out to obtain 7-AVCA. The synthesis method does not require a mixing organic solvent, can realize recovery of sodium iodide and formaldehyde, has the advantages of high product yield, good quality, no three wastes generation, and environmental protection, and is suitable for large scale industrial production.
Owner:QILU ANTIBIOTICS PHARMA
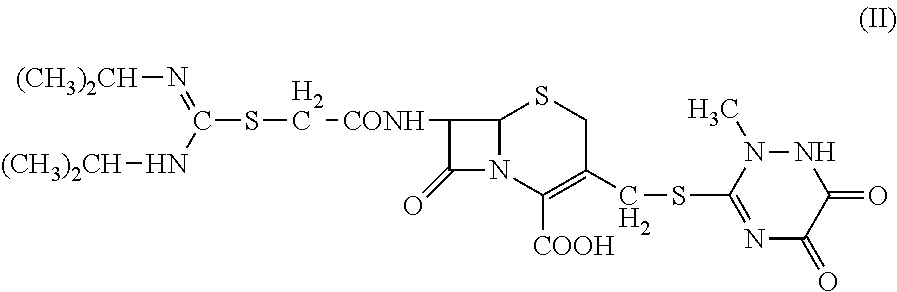
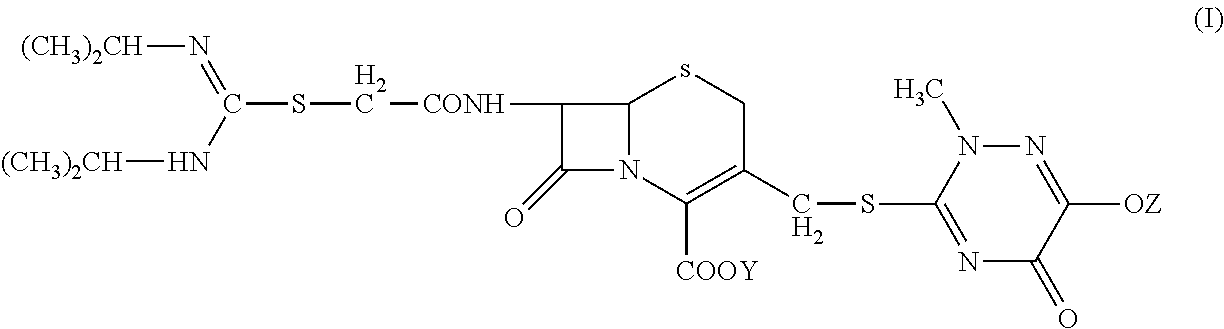
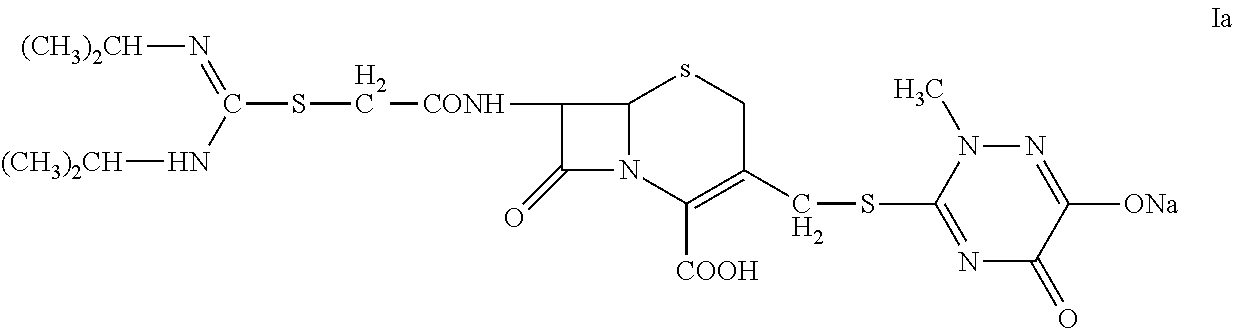
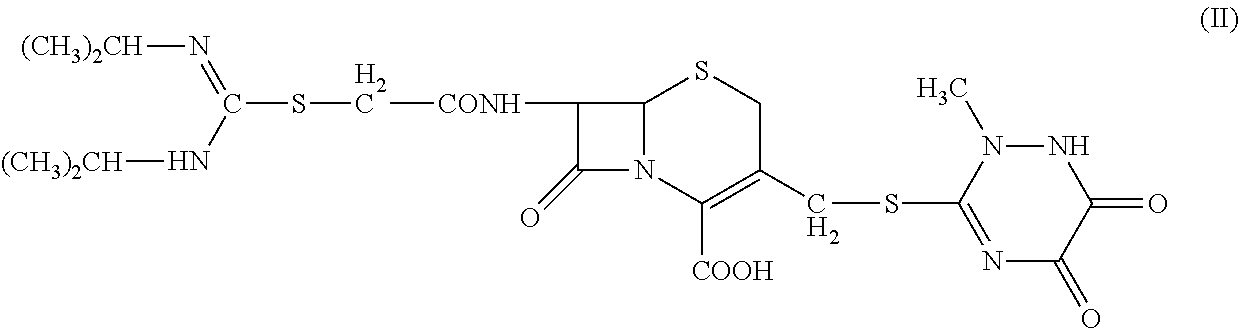
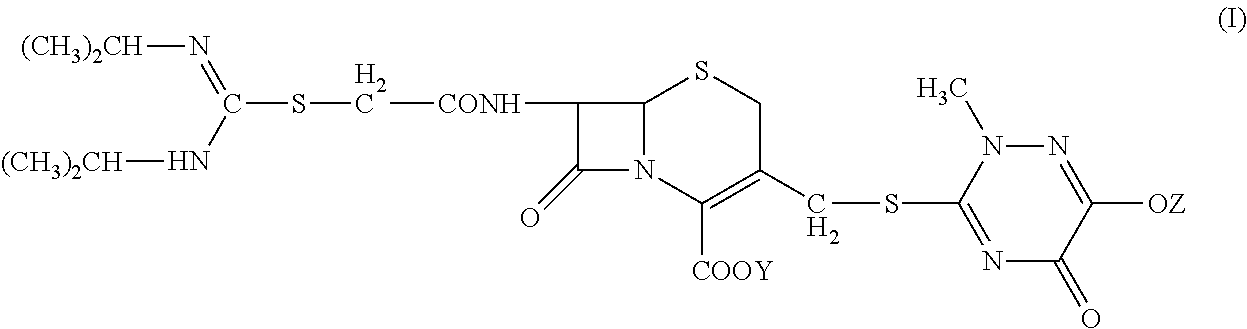
N-heterocyclic substituent-containing antibiotic, preparation and use thereof
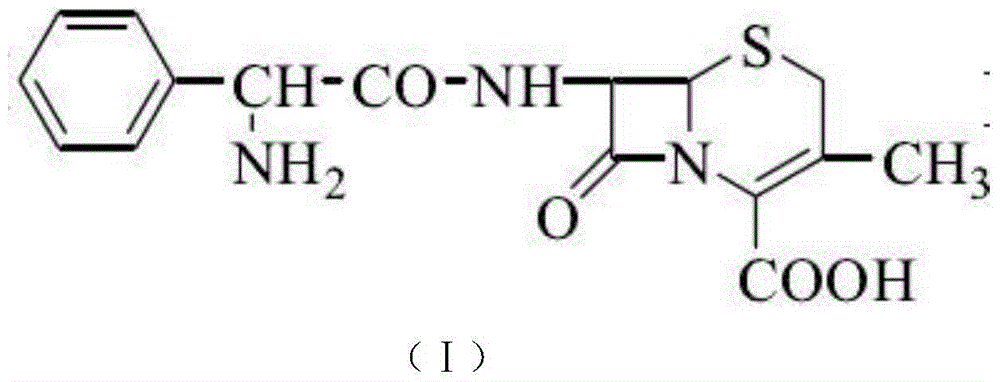
The invention relates to N-heterocyclic substituent-containing antibiotics, their preparation, and their use. Disclosed are sodium and potassium salts of 7-(α-((N,N′-diisopropylamidino)thio)acetylamino)-3-(((1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-1,2,4-triazin-3-yl)thio)methyl) cephalosporanic acid as presented by the general structure (I), their preparation, and their use. The antibiotics of the invention can be used to treat diseases caused by Gram-positive or Gram-negative bacteria such as septicaemia, gastrointestinal tract infection, and urinary tract infection. They have increased half-life in blood and lowered toxicity. They can reduce the frequency of drug use and lower medical treatment costs. They have improved stability and can be stored at ambient temperatures. The method of the invention is simple, and it produces high purity products which can meet the requirements of clinical use.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY +1
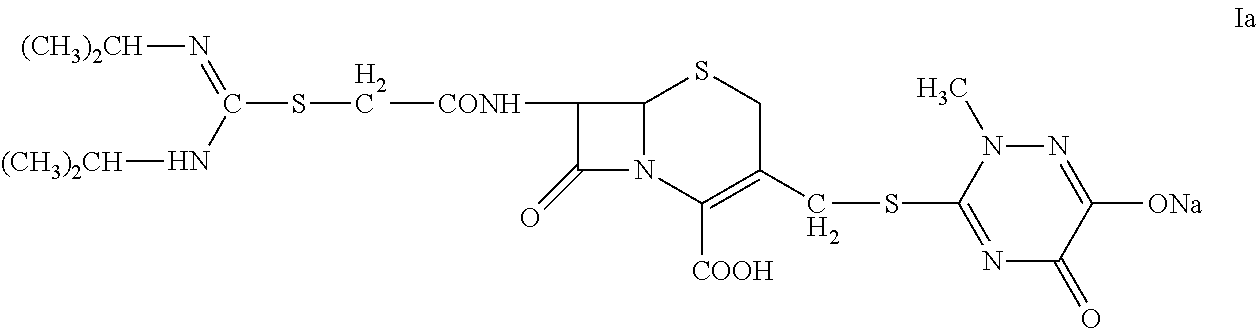
N-Heterocyclic Substituent-Containing Antibiotic, Preparation and Use Thereof
InactiveUS20140128359A1High purityLow purityOrganic active ingredientsOrganic chemistryDiseaseUpper urinary tract infection
The invention relates to N-heterocyclic substituent-containing antibiotics, their preparation, and their use. Disclosed are sodium and potassium salts of 7-(α-((N,N′-diisopropylamidino)thio)acetylamino)-3-(((1,2,5,6-tetrahydro-2-methyl-5,6-diox o-1,2,4-triazin-3-yl)thio)methyl) cephalosporanic acid as presented by the general structure (I), their preparation, and their use. The antibiotics of the invention can be used to treat diseases caused by Gram-positive or Gram-negative bacteria such as septicaemia, gastrointestinal tract infection, and urinary tract infection. They have increased half-life in blood and lowered toxicity. They can reduce the frequency of drug use and lower medical treatment costs. They have improved stability and can be stored at ambient temperatures. The method of the invention is simple, and it produces high purity products which can meet the requirements of clinical use.
Owner:GUANGZHOU PHARMA INDAL RES INSTI +1
A kind of method for preparing cephalexin
The invention discloses a method for preparing cefalexin. The method comprises the steps of charging a D-2-phenylglycine ester derivative and 7-ADCA (amino desacetoxy cephalosporanic acid) in a molar ratio of (1.15-1.6):1, and adding a catalyst penicillin acylase with amount of 1-2 times of amount of the 7-ADCA for acylation reaction, wherein the coded gene sequence of the penicillin acylase is shown as SEQIDNO:3. The method effectively overcomes reverse reaction during enzyme condensation reaction, so as to greatly reduce the using amount of side chains, avoid the phenomenon that more impurities are generated when high side chains are consumed, and solve the problem that the objective product is difficult to purify; and besides, the 7-ADCA conversion rate can be greatly improved to be up to more than 98%, the product quality is further improved and the production cost is reduced.
Owner:NORTH CHINA PHARMA COMPANY
Enzymatic synthesis method of cefaclor
ActiveCN111394415AReduce solubilityAvoid hydrolysisOrganic chemistryFermentationGlycineCephalosporanic Acids
The invention provides an enzymatic synthesis method of cefaclor. The enzymatic synthesis method of the cefaclor comprises the following steps: adding 7-amino-3-chloro cephalosporanic acid into a mixed solvent, adjusting a pH value to be 8.1-8.3, adding an immobilized cefaclor synthetase, adding D-p-phenylglycine methyl ester hydrochloride and methyl (R)-aminophenylacetate, carrying out an enzymatic synthesis reaction, separating out reaction liquid and the immobilized cefaclor synthetase after the reaction is completed to obtain a crude product of the cefaclor, and conducting recrystallization to obtain a cefaclor product, wherein the mixed solvent comprises methanol, glutaraldehyde and a soluble phosphate. According to the provided production method, a pH value of the reaction process does not need to be controlled, the number of times of recycling of the recoverable synthetase in the reaction is significantly increased, the purity of the produced cefaclor product can reach 99% or above, the bulk density can reach 6.2 g / mL or above, the number of times of recycling of the recoverable synthetase can reach 200, the production technology is simplified, the production cost is reduced, and the enzymatic synthesis method of the cefaclor is suitable for large-scale industrial production.
Owner:TIANJIN UNIV +1
Method for fermentation preparation of 7-amino-3-deacetoxy cephalosporanic acid
The disclosed fermentation method for 7-amido-3-deacetoxylcephalosporanic acid comprises: adding penicillin, water, defoamer, fermenting enzyme and nutrient solution into the kettle; after fermentation, extracting the water phase with CHCl3 then reverse extracting the organic phase with water, dewatering, cracking, crystallizing, and suction filtering to obtain the product. This invention increases yield / product content up to 42.5% / 98.5%, and reduces cost and pollution.
Owner:周新基
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com