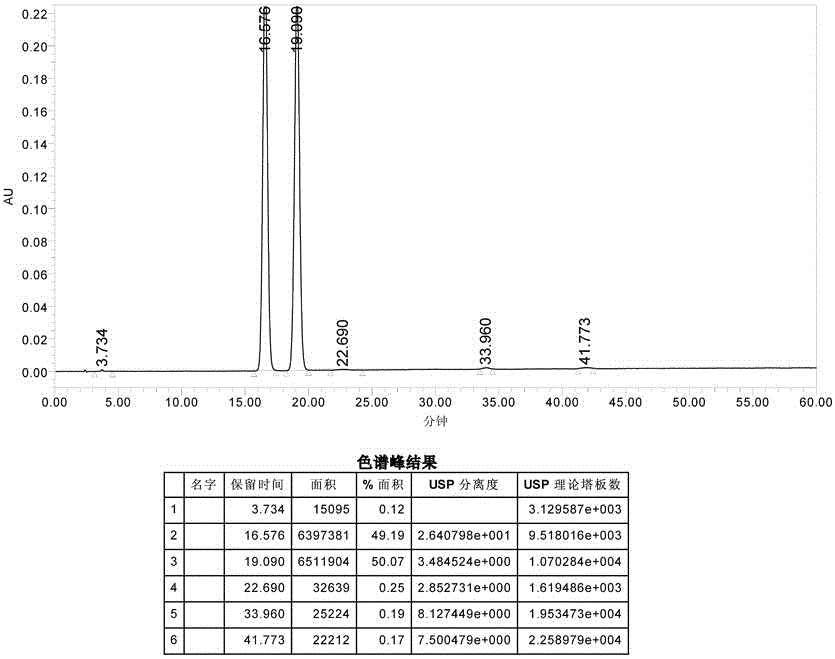
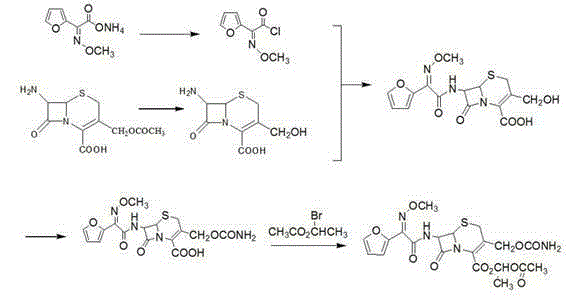
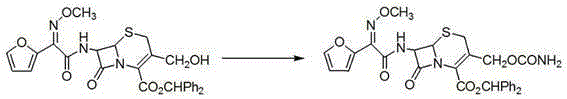Preparation method of cefuroxime axetil
A technology of cefuroxime axetil and cefuroxime axetil, which is applied in the field of preparation of cefuroxime axetil, and can solve problems such as difficult control of related substances, low product color grade, unstable quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Implementation example 1 decarbamoyl cephalosporanic acid diphenyl ester is synthesized
[0023] 1L three-neck flask, add 38.1g (0.1mol) decarbamyl cefuroxime acid, 381ml ethyl acetate, 38.1mL DMF, stir and cool to 0-5°C, slowly add 97.5g (0.5mol) diphenyldiazomethane 290mL of ethyl acetate solution, raised to 20-25°C and stirred overnight after addition. Add 380mL of water, stir and separate layers. After separation, the aqueous layer was extracted twice with 75 mL of ethyl acetate, the combined organic phases were washed twice with 380 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 49.7 g (91%) of the product.
Embodiment 2
[0024] Implementation example 2 cefuroxime diphenylmethyl ester is synthesized
[0025] 1L three-neck flask, add 27.4g (0.05mol) decarbamyl cephalosporanic acid diphenyl ester, 274mL acetone, stir to dissolve, cool to -10°C, slowly add 7.5mL trichloroacetyl isocyanate and 22.5mL The mixed solution of acetone was raised to room temperature and stirred for 2h after the dropwise completion, and the solid was collected and washed with acetone. Transfer the solid into a bottle, add a mixed solution of 5.4g sodium carbonate, 108g water, and 432mL THF, stir at room temperature for 3h, distill off THF under reduced pressure, extract the remaining aqueous solution twice with 275mL ethyl acetate, combine the oil phases, and wash with saturated brine. Dry over sodium sulfate and distill under reduced pressure. The remainder was recrystallized with dichloromethane / n-hexane to obtain 17.1 g of product (58%).
Embodiment 3
[0026] Implementation example 3 cefuroxime acid is synthesized
[0027] Add 20g of cefuroxime diphenylmethyl, 40mL of dichloromethane, 30mL of TFA, 8mL of tertiary methyl ether into a 1L three-necked flask, stir at room temperature for 30min, concentrate under reduced pressure after stirring to remove dichloromethane and TFA, add 80mL of tertiary methyl ether after concentration, After stirring, a solid was precipitated, filtered, washed three times with 40 mL of tertiary methyl ether, and dried to obtain 13.7 g (96%) of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com