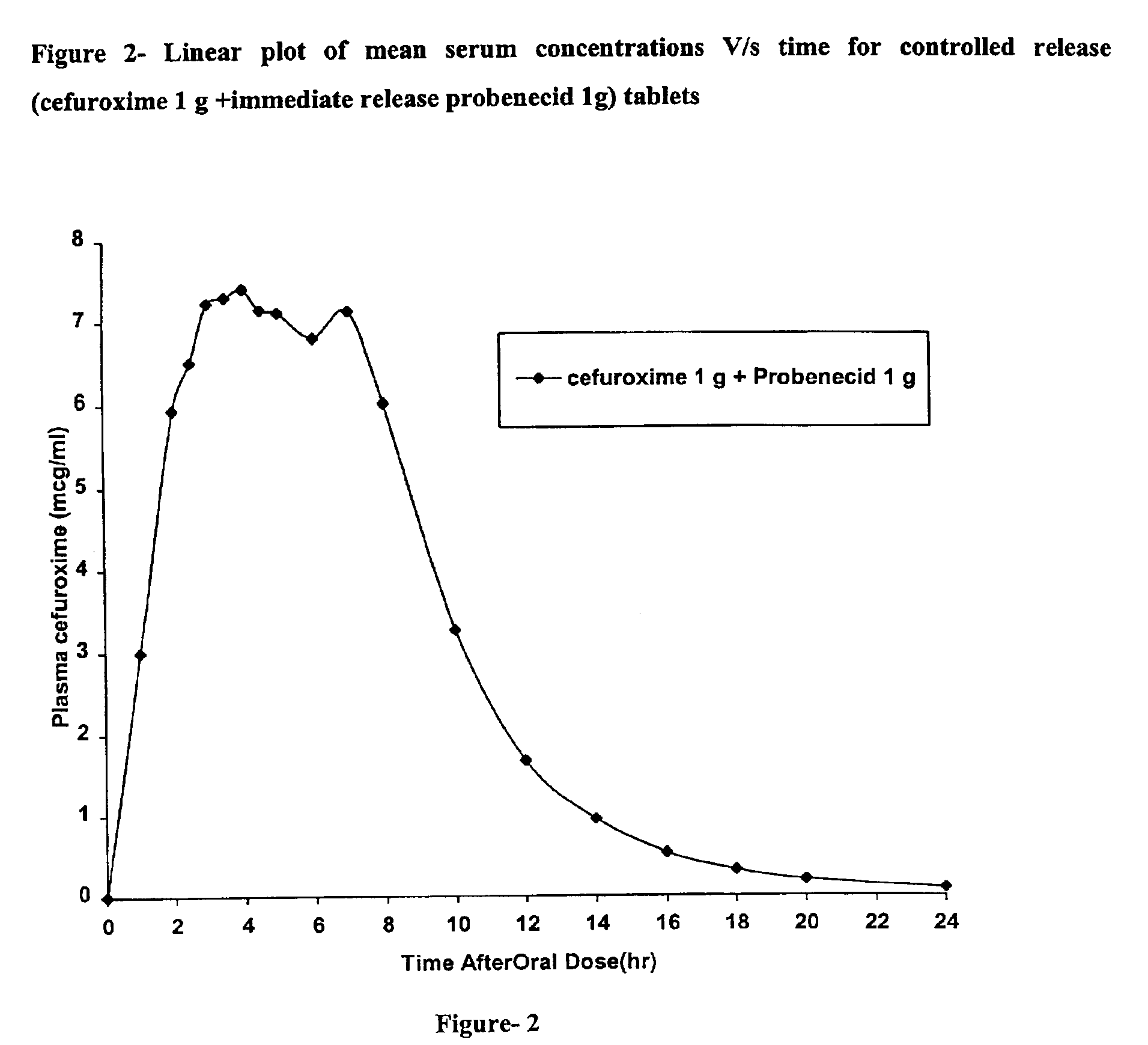
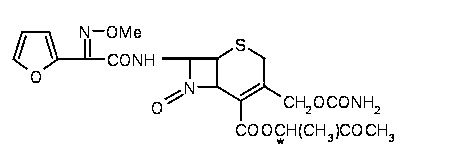
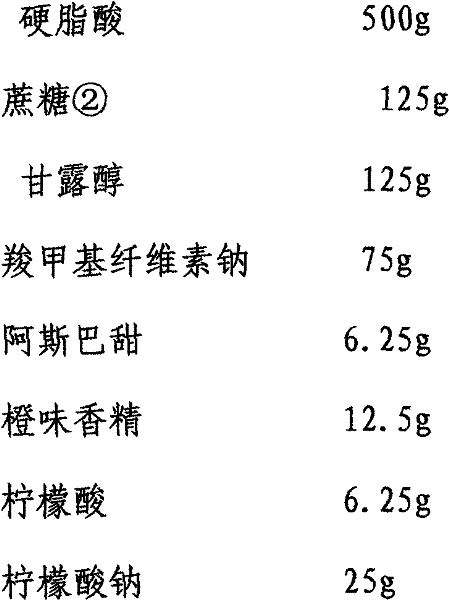
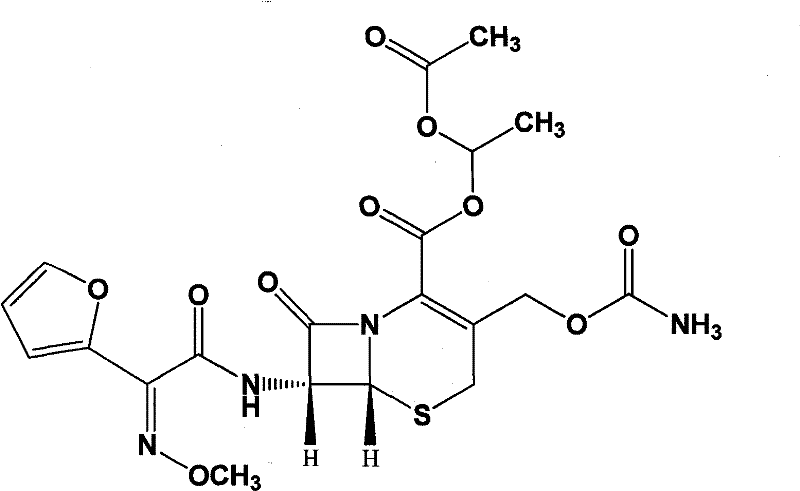
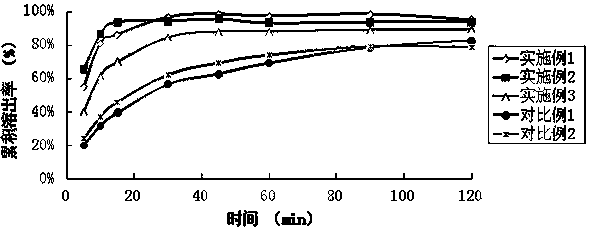
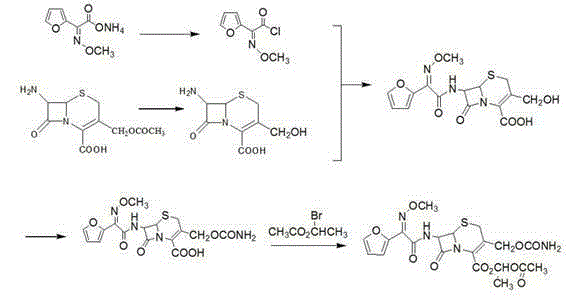
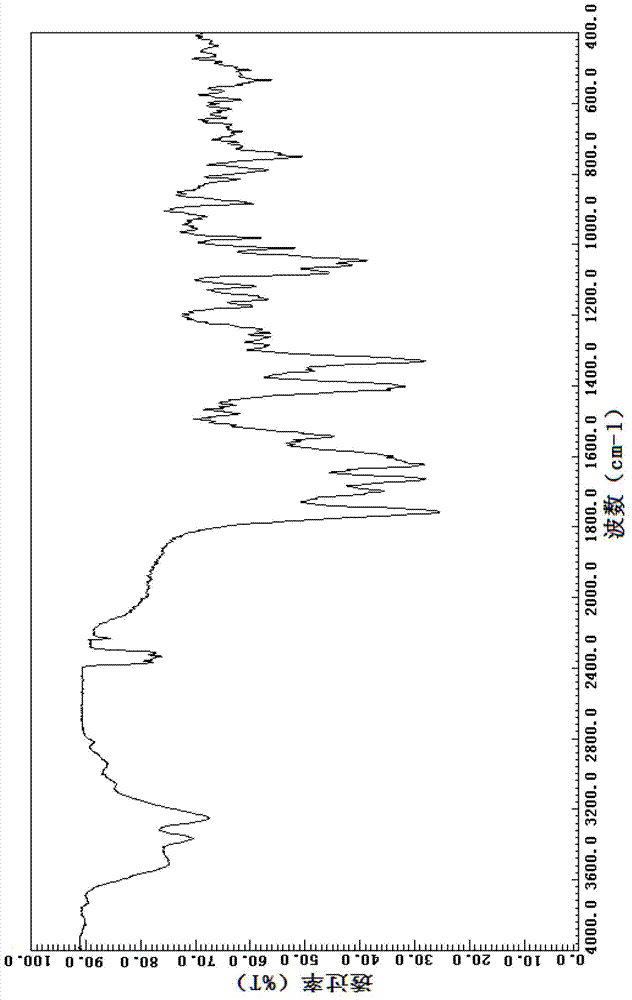
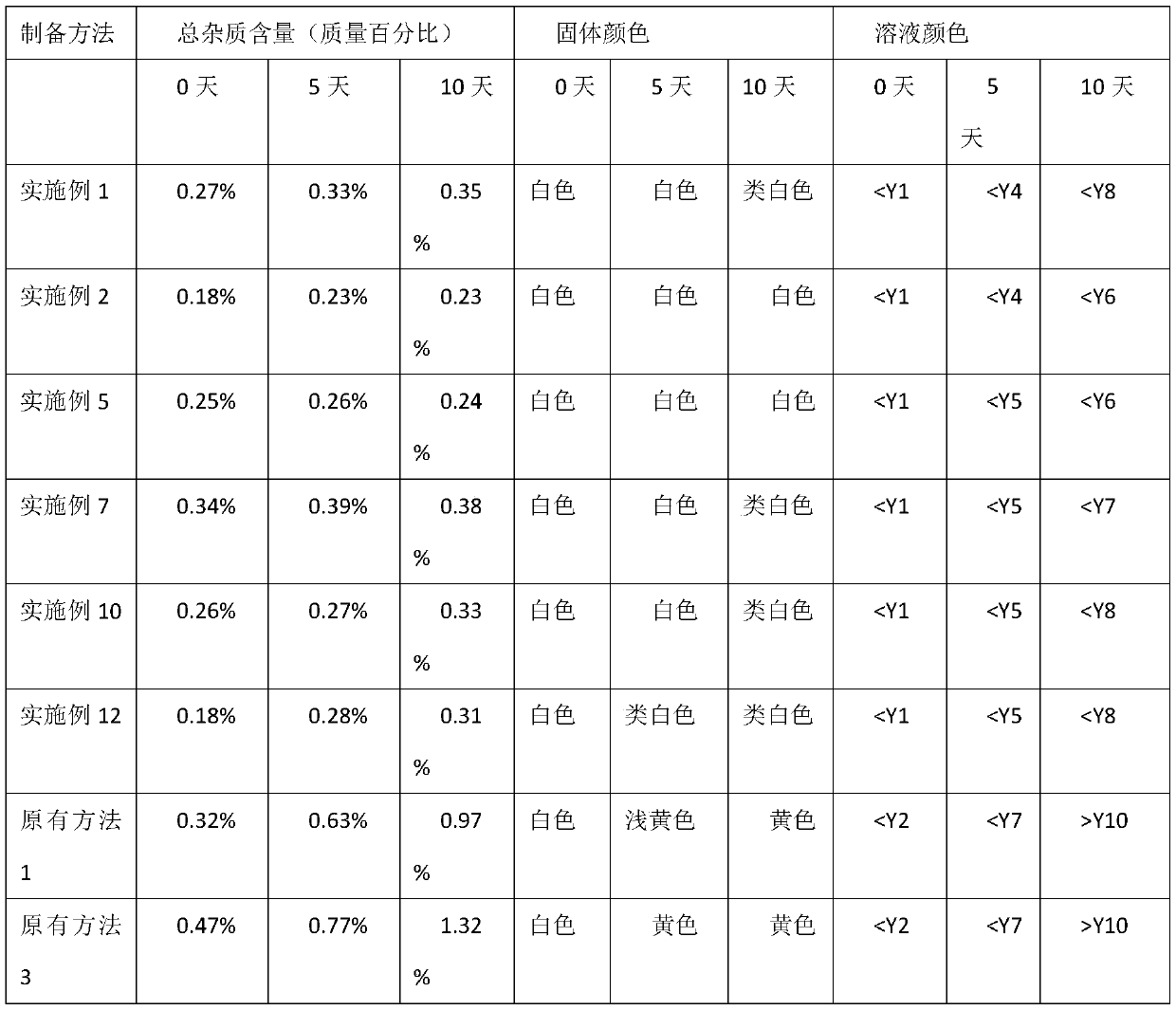
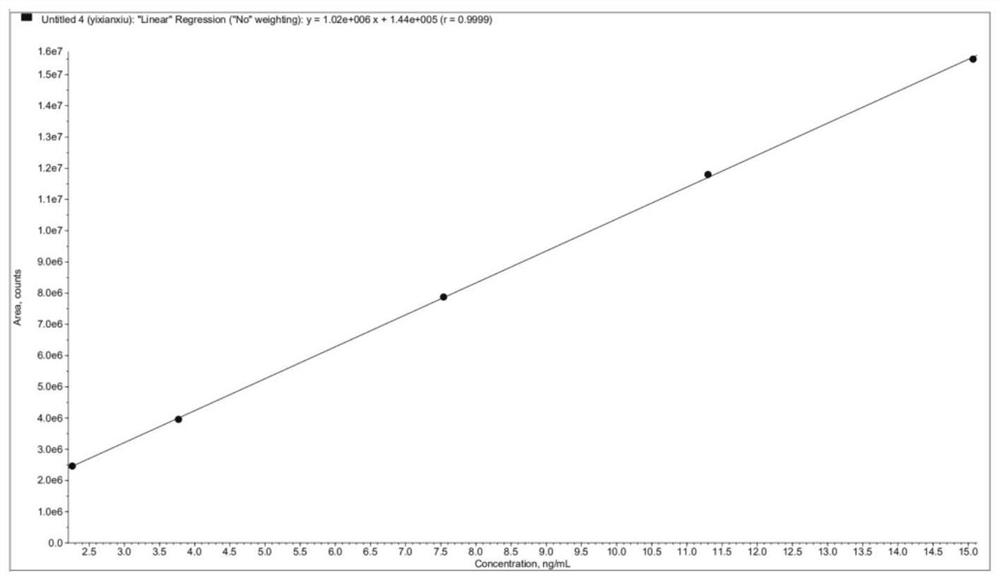
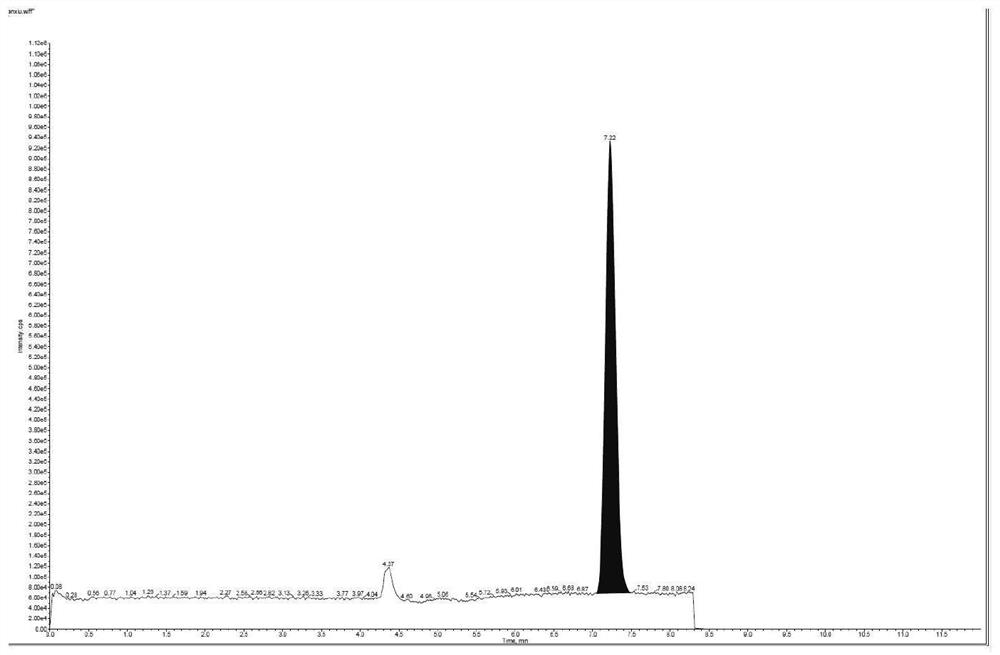
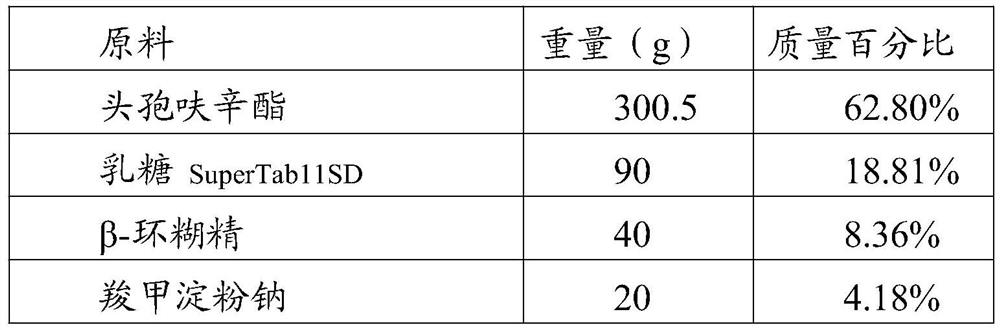
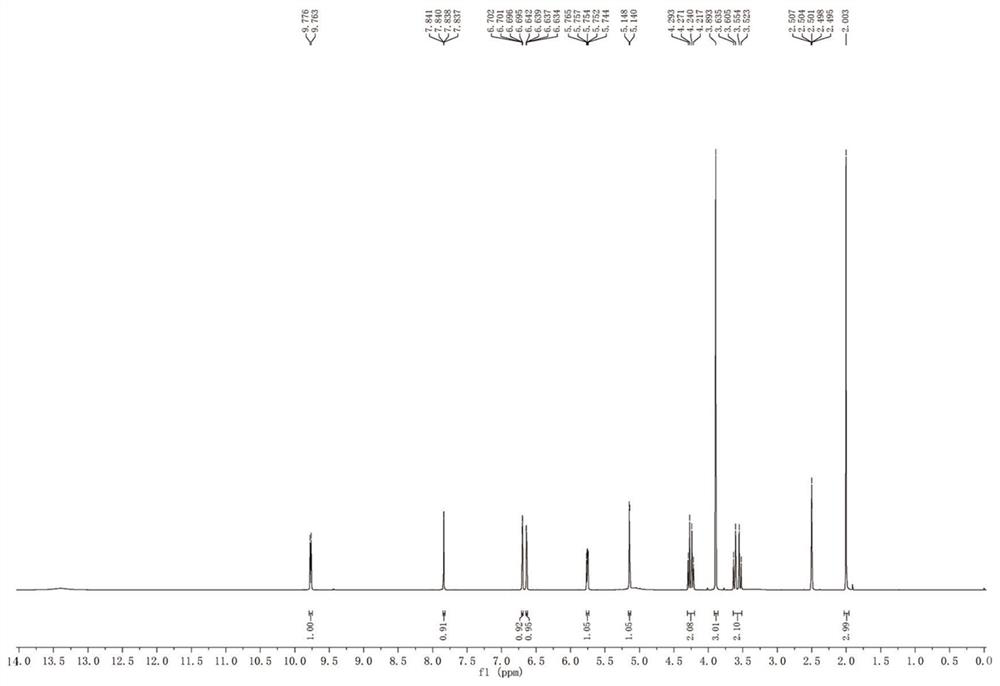
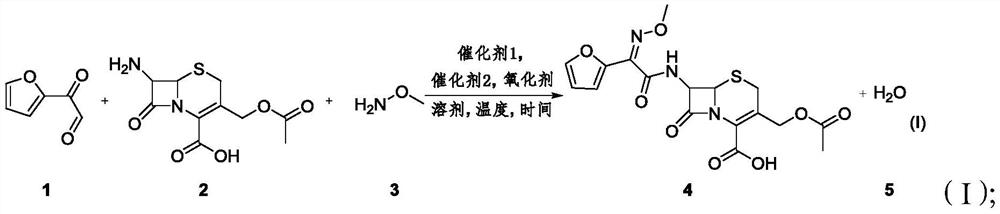
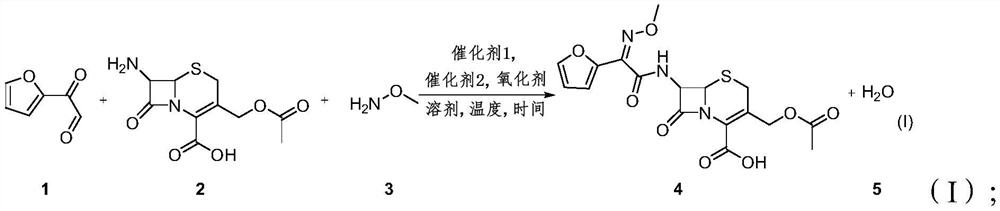
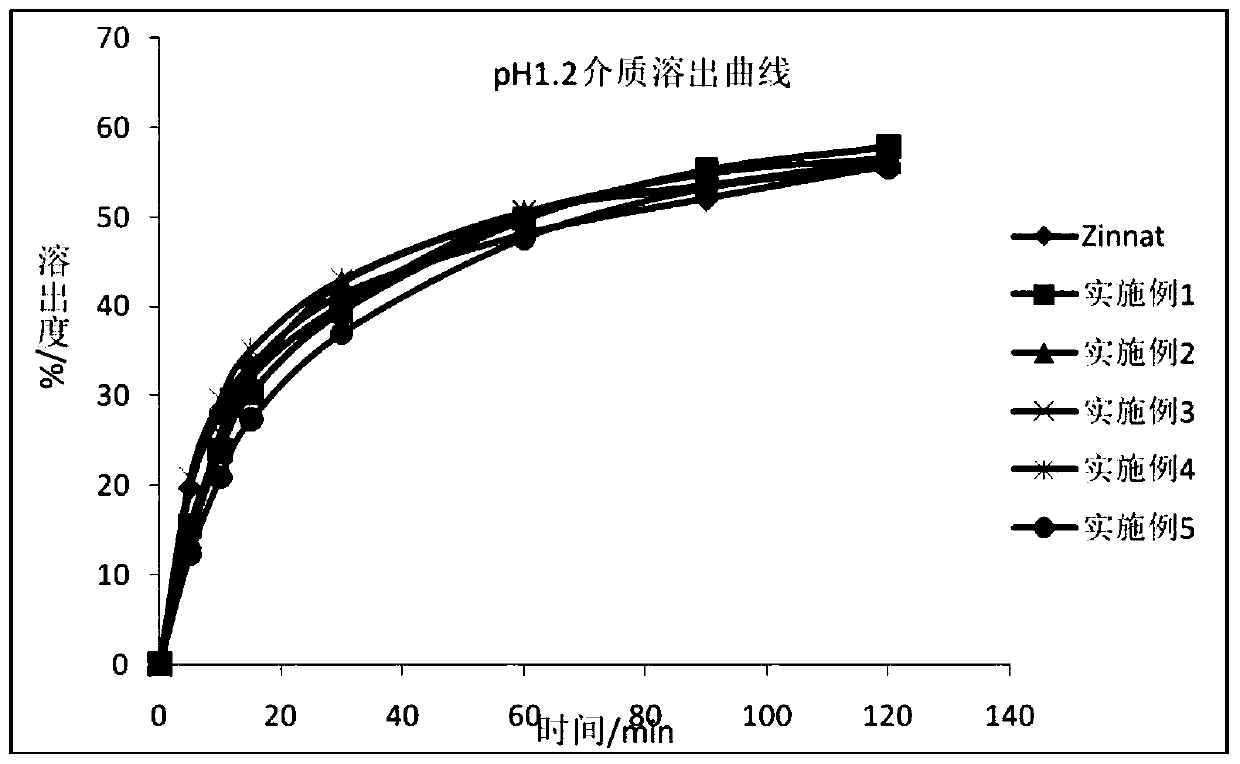
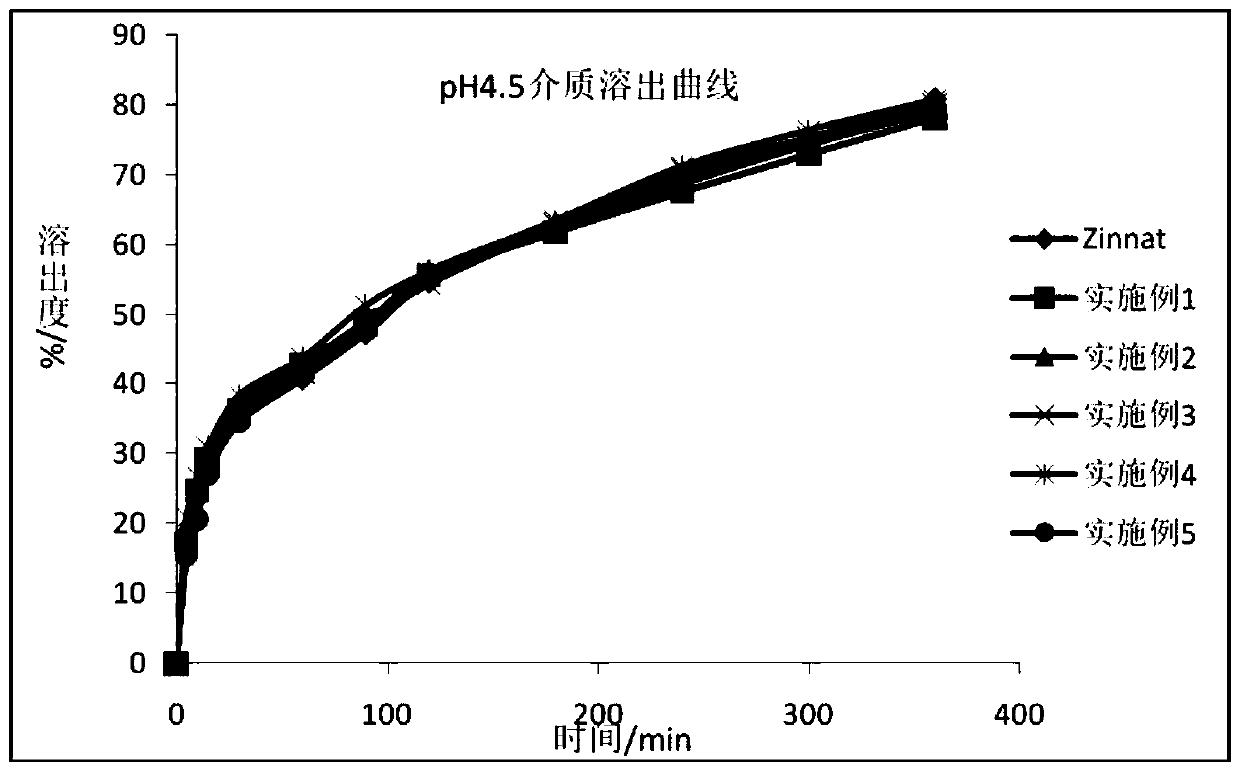
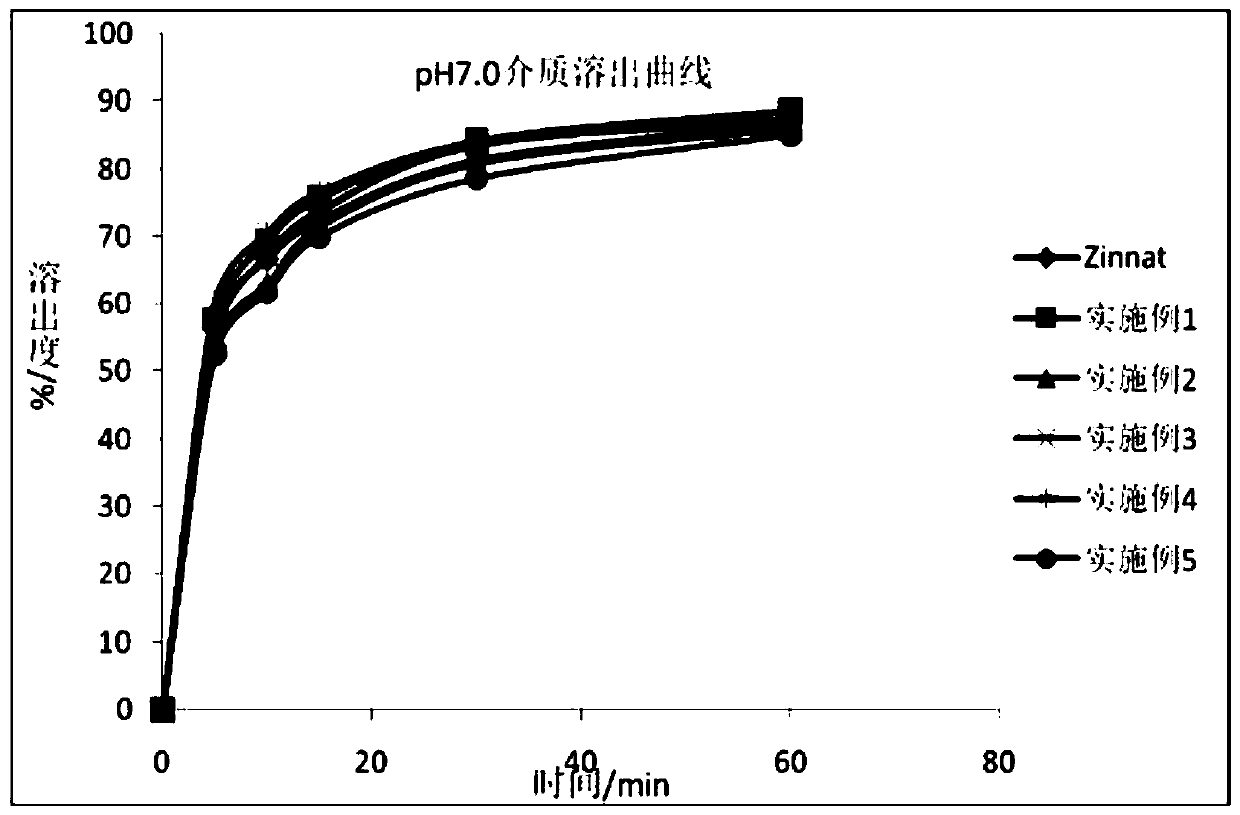
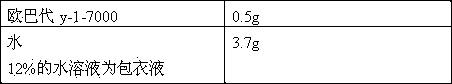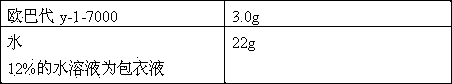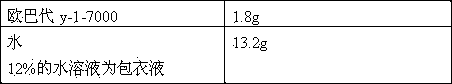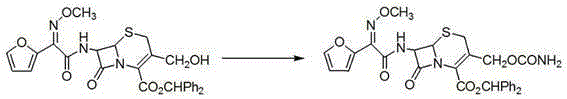Patents
Literature
43 results about "Cefuroximum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Wet granulation technology for cefuroxime axetil tablets
InactiveCN101120927AEasy to separateGood disintegrationAntibacterial agentsOrganic active ingredientsHydroxypropylmethyl celluloseMagnesium
The present invention discloses a wet particle process for a cefuroxime axetil tablet. Firstly, 163g cefuroxime axetil, 25g carboxyl methyl starch sodium, 75g starch and 10g lactoseare are selected, followed by crossing the screen with 60 holes and mixing . Secondly, 20g silica gel particle is added and well mixed. Thirdly, 100ml hydroxypropyl methylcellulose of 2 percent is produced into the flexible material and particles are produced by a nylon screen with 16 holes. The product is dried at 60 DEG C for 5 to 6 hours and the particles are selected by a nylon screen with 14 holes. Followed by homogeneous mixing of 25g carboxyl methyl starch sodium, 5g magnesium separate and tabletting the core by 125mg cefuroxime axetil per tablet. Finally, the packaging liquid can spray slowly and the tablet core is dried at 40 DEG CC to 50 DEG C at any time. The packaging liquid for 1000 tablet cores is 100ml pharmaceutical film coating premixed agent of 20 percent. In the wet particle process of the present invention, the carboxymethyl sodium particle is added by internal and external addition. Therefore the tablet core is quickly divided into particles and further divided into thin powder, and then the biological activity is released quickly.
Owner:JIANGSU QINGJIANG PHARMA
Cefuroxime axetil composition and preparation method thereof
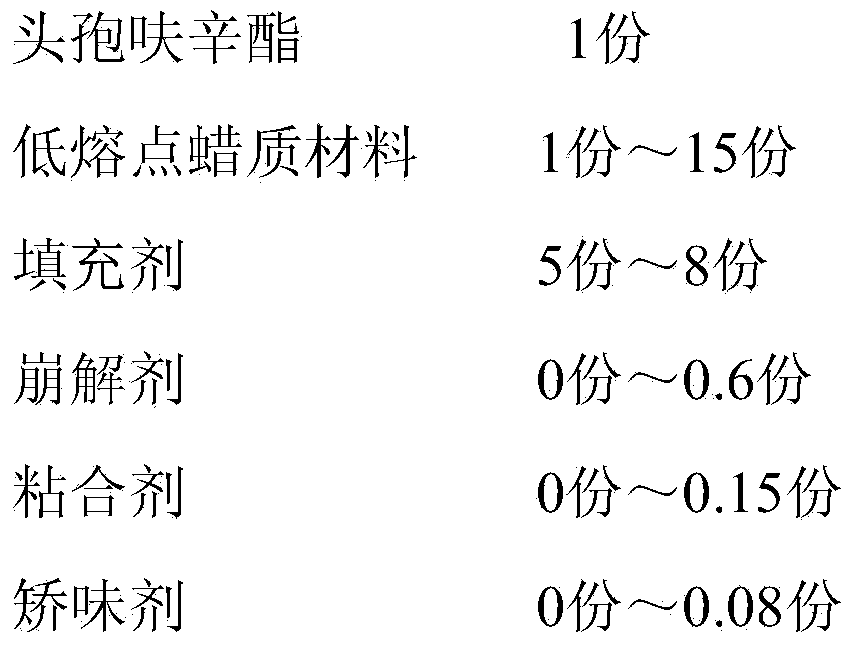
The invention discloses a cefuroxime axetil composition which comprises the following components in parts by weight: 1 part of cefuroxime axetil, 1-15 parts of a low-melting-point wax material, 5-8 parts of filler, 0-0.6 part of a disintegrating agent, 0-0.15 part of a binder and 0-0.08 part of a corrigent. The invention further discloses a preparation method of the cefuroxime axetil composition. The cefuroxime axetil composition obtained can better cover bitter taste of the medicine and is good in dissolution rate, so that the medication compliance of patients is further improved.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Rapidly disintegrating sustained release cefuroxime axetil composition
InactiveUS6932981B2Increased blood levelsGood curative effectPharmaceutical non-active ingredientsMicrocapsulesMethacrylateControlled release
A fast disintegrating controlled release oral composition comprising a core material containing cefuroxime axetil present as controlled release form, the cefuroxime axetil being provided with an outer coating of a copolymer selected from aqueous dispersions of enteric methacrylic acid and methacrylic acid esters anionic copolymers having carboxyl group as the functional group or mixtures thereof and an inner coating of a sustained-release copolymer selected from aqueous dispersions of acrylate and methacrylate pH independent copolymers having quaternary ammonium group as a functional group or mixtures thereof, and optionally probenecid. Additionally, the coating composition may contain plasticizers. The composition is suitable for once daily administration.
Owner:LUPIN LABORATORIES LTD
Preparation method of cefuroxime axetil
The invention discloses a preparation method of cefuroxime axetil. The method comprises the following steps: completely dissolving cefuroxime acid in dimethylformamide, and carrying out esterification reaction with 1-bromethylacetate under the catalytic action of cupric chloride; and hydrolyzing with ethyl acetate and sodium chloride solution, extracting, carrying out vacuum distillation, crystallizing with cyclohexane, carrying out vacuum filtration, and drying to obtain high-purity cefuroxime axetil. The cupric chloride, which has the advantages of no toxicity, no harm and high catalytic efficiency, is preferably used as the catalyst; the cyclohexane for crystallization is easy to recover and reutilize, thereby lowering the production cost; and meanwhile, the method has the advantages of mild and controllable reaction conditions, short production cycle and lower energy consumption, and is suitable for industrial production.
Owner:GUANGDONG LIGUO PHARMACY
Pharmaceutical composition of cefuroxime axetil for suspension and preparation method thereof
InactiveCN102440960AImprove bitternessEasy to acceptAntibacterial agentsOrganic active ingredientsUse medicationSucrose
The invention relates to a pharmaceutical composition of cefuroxime axetil for suspension for pharmaceutical purposes and a preparation method thereof. The pharmaceutical composition of the cefuroxime axetil for suspension disclosed by the invention comprises 100 parts by weight of cefuroxime axetil (calculating according to the weight of cefuroxime), 50-250 parts by weight of cane sugar and 400-650 parts by weight of stearic acid. The invention further provides the method for preparing the composition. According to the pharmaceutical composition for suspension disclosed by the invention, the bitter taste of the cefuroxime axetil is effectively covered up; and the pharmaceutical composition is applied to adults, more importantly, the problem for difficultly taking medicines of children, old people and patients suffering from dysphagia is solved, and the compliance of medicine taking patients is greatly increased.
Owner:SHANDONG LUKANG PHARMA +1
Cefuroxime axetil dispersible tablet
InactiveCN103845298ASimple manufacturing processProcess stabilityAntibacterial agentsOrganic active ingredientsBitter tasteDrug product
The invention provides a cefuroxime axetil dispersible tablet. The cefuroxime axetil dispersible tablet prepared by the invention can be released within a short time; the formula is reasonable; bitter taste of the cefuroxime axetil dispersible tablet is masked; the mouth feel of the cefuroxime axetil dispersible tablet is improved; the medicinal compliance is improved; furthermore, the preparation process is simple and feasible, strong in operability and steady in process; the bioavailability and the curative effect of the cefuroxime axetil dispersible tablet disclosed by the invention are increased.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Cefuroxime axetil composition and preparation method thereof
ActiveCN103816123AMask bitternessImprove complianceAntibacterial agentsPowder deliveryPharmaceutical drugBitter tastes
The invention discloses a cefuroxime axetil composition and a preparation method thereof. The cefuroxime axetil composition can effectively cover bitter taste of a cefuroxime axetil medicine so as to improve the delivery compliance of the patient. Meanwhile, the medicine containing the cefuroxime axetil composition has higher dissolution rate and high bioavailability. The composition disclosed by the invention can be further used for preparing dry suspensions or granules which are more beneficial for infants, children and patients without the ability of swallowing to take. The composition disclosed by the invention is simple in preparation method, free from any solvents, uniform in medicine content, short in heating time, small in energy consumption and easy to realize continuous large-scale production.
Owner:GUANGDONG PHARMA UNIV
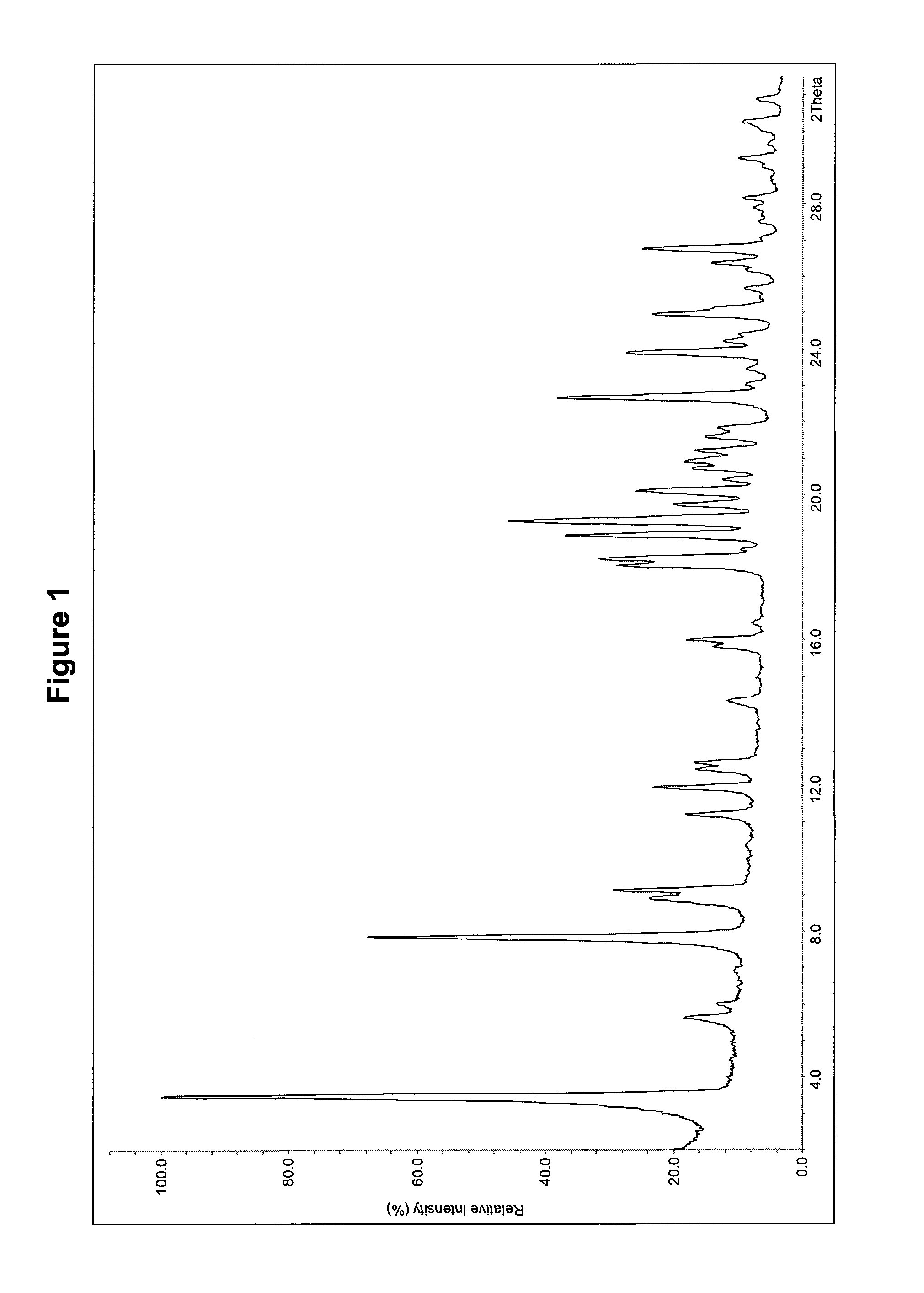
Novel crystal form of cefuroxime acid and preparation method thereof
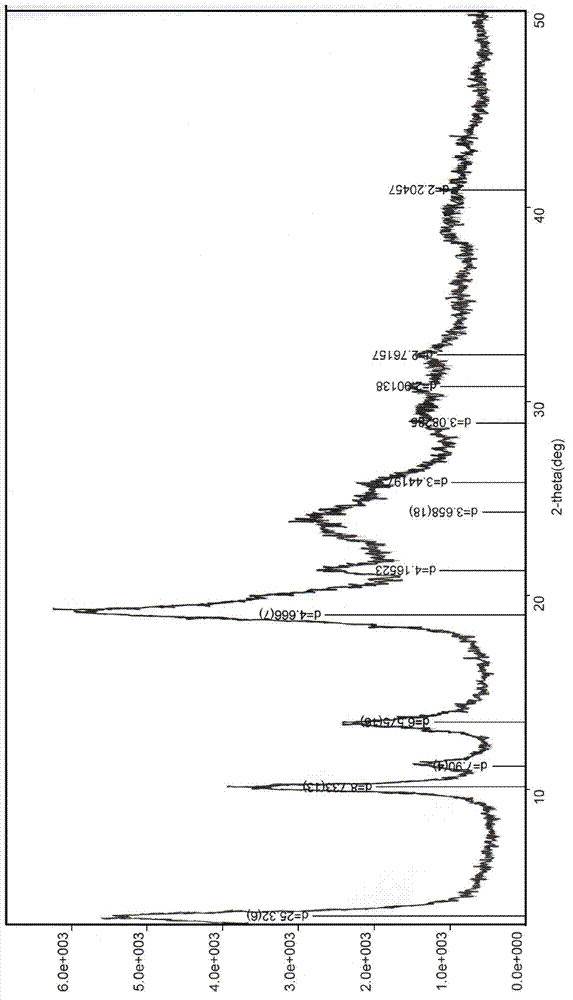
The invention relates to a novel crystal form of cefuroxime acid and a preparation method thereof. In X ray powder diffraction, the novel crystal form of cefuroxime acid has characteristic peaks at diffraction angles 2 theta of 8.06, 9.96, 10.44, 11.84, 12.82, 16.34, 19.02, 20.02, 21.86, 23.22, 25.46, 25.98, 33.12, 34.24 and 36.40 degrees. The preparation method comprises the following steps of adding cefuroxime raw materials into an acetonitrile mixed solvent to obtain a solution having a concentration of 0.1 to 0.2g / ml, stirring at a temperature of 25 to 35 DEG C for complete dissolution, continuously stirring for 30 to 60 minutes, carrying out filtration and decoloration, transferring filtrate to a crystallizer, adding a hydrochloric acid solution into the crystallizer to adjust a pH value of the mixed solution to 3-4, cooling to a temperature of 10 to 15 DEG C, adding cefuroxime acid seed crystals into the crystallizer, carrying out crystallization for 1 to 2 hours, adding water as a solventing-out agent into the crystallizer, sequentially cooling to a temperature of 0 to 5 DEG C, carrying out crystallization for 1 to 3 hours, filtering, washing filter cake by a washing solvent, and drying to obtain products. The novel crystal form of cefuroxime acid has HPLC content above 99.3%, avoids crystal accumulation, has large particle sizes, wherein a main particle size is more than 100 microns, and also has uniform particle size distribution and a single-cycle mole yield above 88% in crystallization.
Owner:TIANJIN UNIV
Preparation method of cefuroxime axetil
InactiveCN105131016ALow impurity contentGood colorOrganic chemistryBulk chemical productionCephalosporanic AcidsTrichloroacetyl isocyanate
The invention relates to a preparation method of cefuroxime axetil. The preparation method comprises the following steps: (1) reacting de-ammoniated formyl cephalosporanic acid with diphenyl diazomethane to generate acetyl-diphenyl methyl cephalosporanate; (2) reacting diphenyl methyl de-ammoniated formyl cephalosporanate with trichloroacetic isocyanate to generate diphenyl methyl cefuroxime ester; (3) hydrolyzing diphenyl methyl cefuroxime ester to obtain cefuroxime acid; (4) reacting cefuroxime acid with 1-acetoxyl-bromoethane to generate cefuroxime axetil. During the preparation process, the carboxyl group is protected by diphenyl diazomethane, the generation of impurities is reduced, and the product quality is improved.
Owner:JIANGSU QINGJIANG PHARMA
Cefuroxime sodium crystal compound and composition powder injection thereof
The invention provides a cefuroxime sodium crystal compound and a composition powder injection thereof, and relates to the fields of medicament and preparation method technology of medicament, and cefuroxime sodium crystal compound composition belongs to a new crystal type-amorphous. The crystal form of cefuroxime sodium is amorphous and the preparation method thereof is as follows: reacting cefuroxime acid with sodium bicarbonate to obtain a solution and adding active carbon to discolor it, and then quick-freezing for 2 hours at -40-50 DEG C after sterile filtration using a filter membrane with aperture of 0.45 mu m and 0.22 mu m, followed by vacuum drying (drying temperature below 15 DEG C, vacuum degree 10-20 Pa, time 30-35 hours). The components of the cefuroxime sodium composition powder injection are: 95-100 parts of cefuroxime sodium crystal compound and 5-10 parts of mannitol. The component is packaged into bottles of 0.25 g, 0.75 g, 1.5 g, 2.25 g or 2.5 g as power injection preparation for clinic injection. The cefuroxime sodium crystal compound has the advantages of a simple preparation method, good stability, high solubility and dissolving quickly in water, and is convenient for clinical use.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
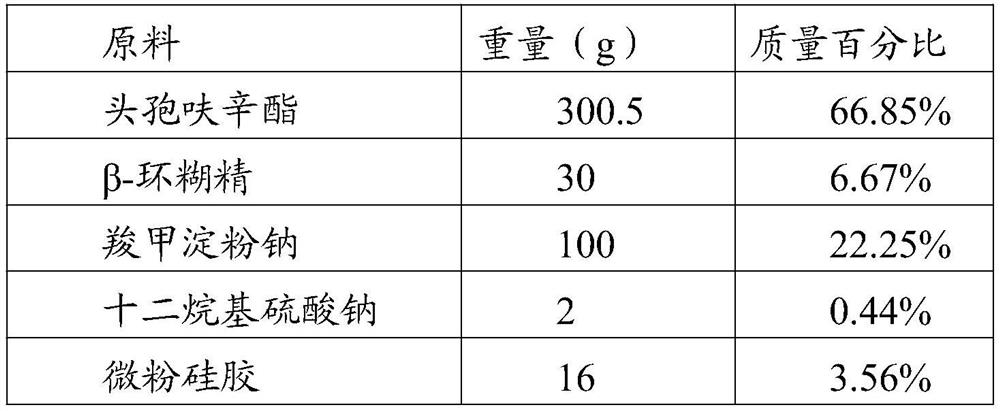
Cefuroxime axetil pharmaceutical composition
The invention discloses a cefuroxime axetil pharmaceutical composition which is a capsule. Every 1000 capsules are prepared by the following components in percentage by weight: 80 g to 180 g of cefuroxime axetil, 10 g to 80 g of superfine silica powder, 20 g to 120 g of carboxymethyl starch sodium and 0.5 g to 9 g of lauryl sodium sulfate; in addition, the invention further discloses a preparation method of the cefuroxime axetil pharmaceutical composition. The cefuroxime axetil pharmaceutical composition has the characteristics of good stability, high dissolution rate, simple formula and high relative bioavailability, and is better suitable for industrial production.
Owner:ZHEJIANG RUIXIN PHARMA
Preparation method and application of cefuroxime sodium
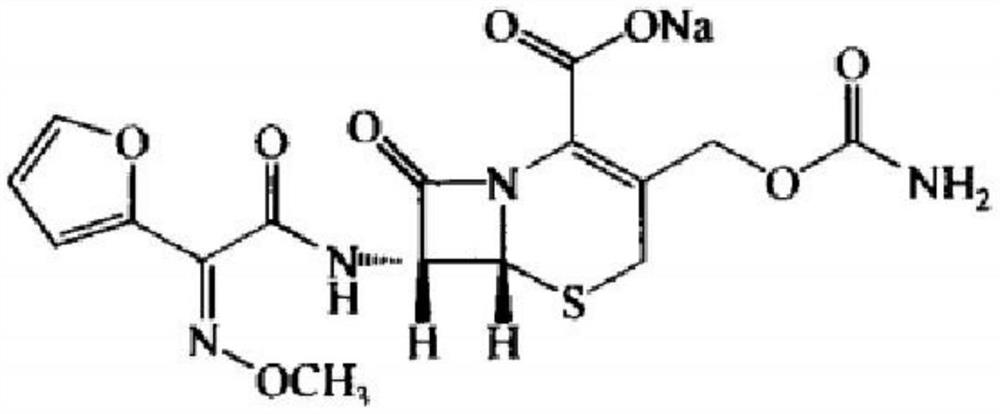
InactiveCN110437258AImproved color stabilityHigh yieldAntibacterial agentsOrganic active ingredientsCefuroxime SodiumAqueous solution
The invention belongs to the technical field of medicines, and relates to a preparation method and application of cefuroxime sodium. The preparation method of the cefuroxime sodium comprises the following steps: dissolving cefuroxime acid in a solvent, adding a stabilizer aqueous solution, and adding a salt former to obtain the cefuroxime sodium. The cefuroxime sodium prepared by the method disclosed by the invention has good color stability. In addition, seldom residual solvent exists in the preparation method of the cefuroxime sodium; the yield is high; and water and total solvent consumption are reduced, production is facilitated, and the production cost is greatly reduced.
Owner:SHANGHAI LONGXIANG BIO MEDICINE DEV CO LTD
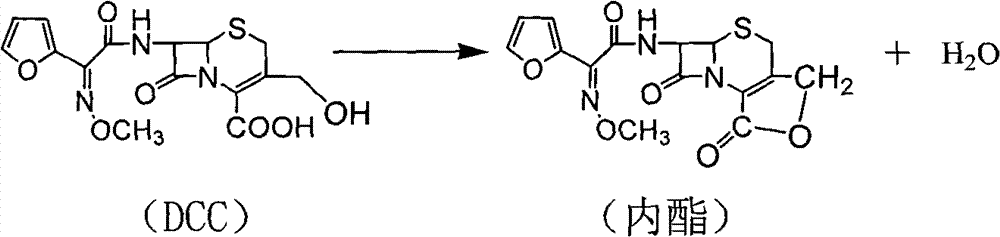
Optimized impurity removal method for cefuroxime intermediate (3-decarbamoyl-cefuroxime acid)
The invention relates to an optimized impurity removal method for cefuroxime intermediate (3-decarbamoyl-cefuroxime acid). The method comprises the following steps: fully stirring and mixing a mixed solvent containing many impurities (mainly being trans-3-decarbamoyl-cefuroxime acid and 3-decarbamoyl-cefuroxime lactone), water, ester and alcohol, removing the solvent, mixing and stirring the obtained material and a mixed solvent of water, ester and dichloromethane, removing the solvent, mixing the obtained material with dichloromethane, and drying the obtained mixture to obtain highly-pure 3-decarbamoyl-cefuroxime acid. The method improves the purity of the cefuroxime intermediate (3-decarbamoyl-cefuroxime acid), so that high-impurity disqualified intermediates are recycled, and the utilization rate of the disqualified intermediates is increased.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Cefuroxime axetil tablet and preparation method thereof
InactiveCN103505434ALow requirements for excipientsImprove pass rateAntibacterial agentsOrganic active ingredientsCelluloseEngineering
The invention discloses a cefuroxime axetil tablet and a preparation method thereof. The cefuroxime axetil tablet is characterized by comprising the following components: cefuroxime axetil, starch, lactose, micropowder silica gel, a 2% hydroxypropyl methyl cellulose aqueous solution, sodium carboxymethyl starch and magnesium stearate, and the cefuroxime axetil tablet is prepared by reasonably allocating a proportion of the components according to a wet method pelleting tabletting technology. The method solves the problems that cefuroxime axetil meets water or alcohol to generate very high viscosity, and dry method pelleting tabletting or direct powder tabletting can only by adopted generally; special equipment and special auxiliary materials are not required; a qualification rate is high in a production process; and the production cost is lowered.
Owner:JIANGSU QINGJIANG PHARMA
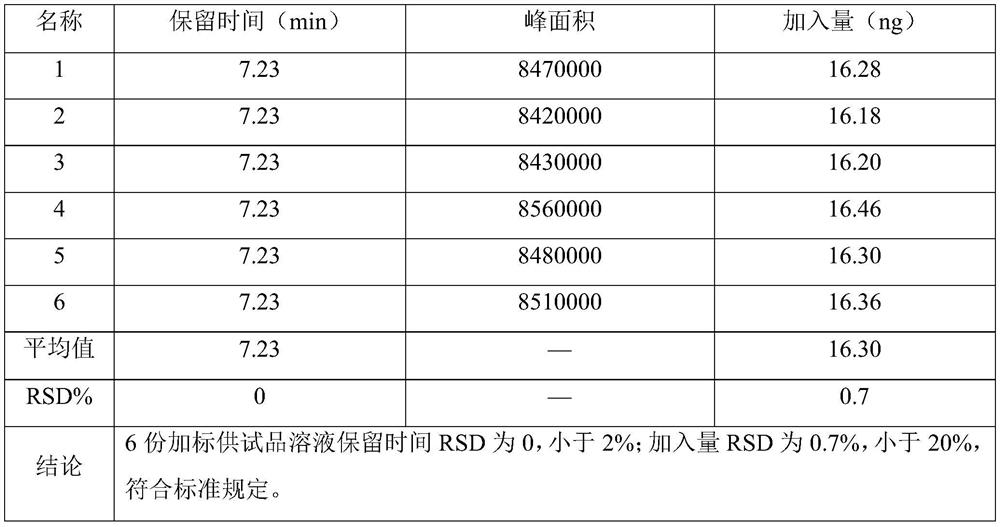
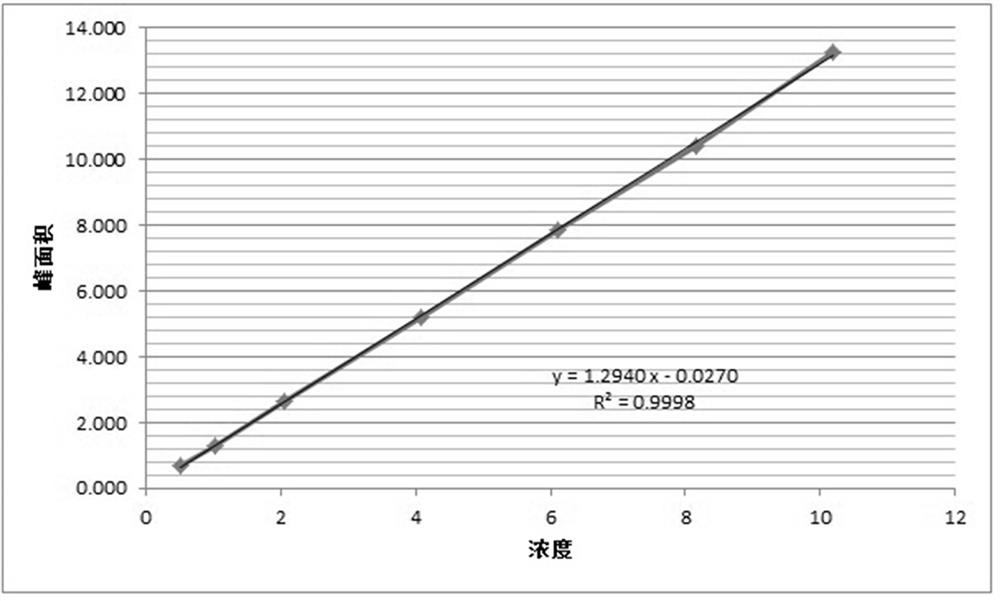
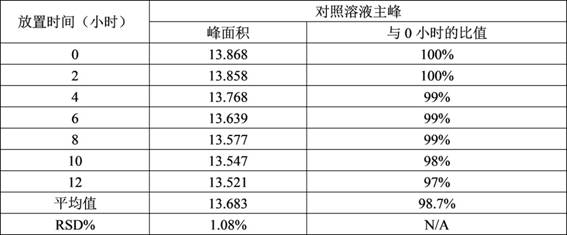
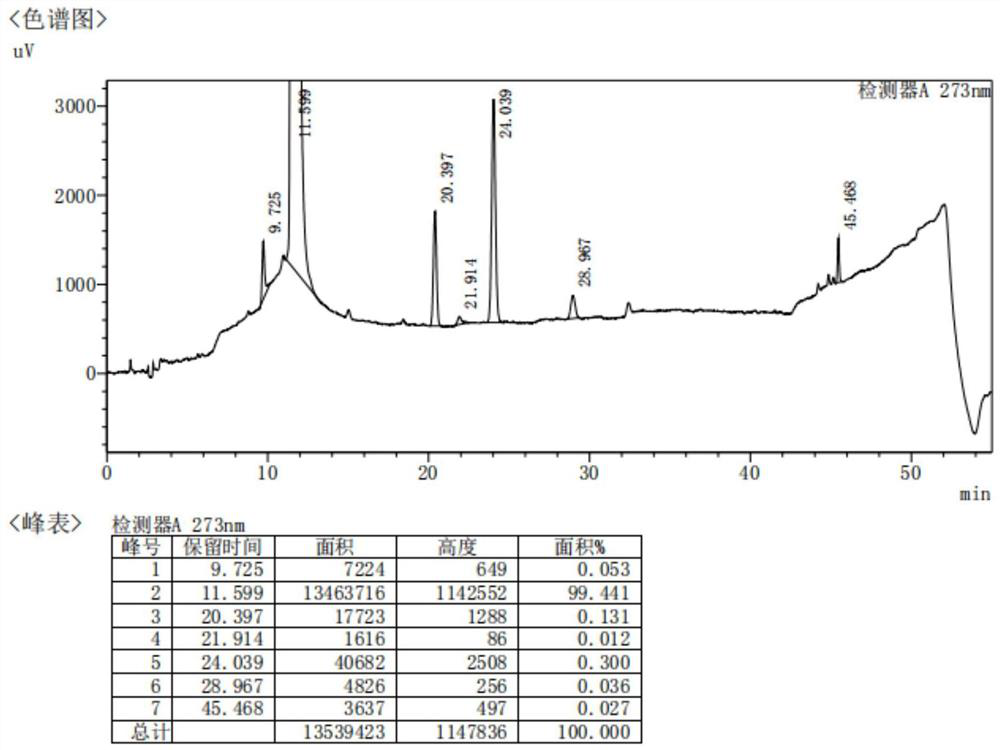
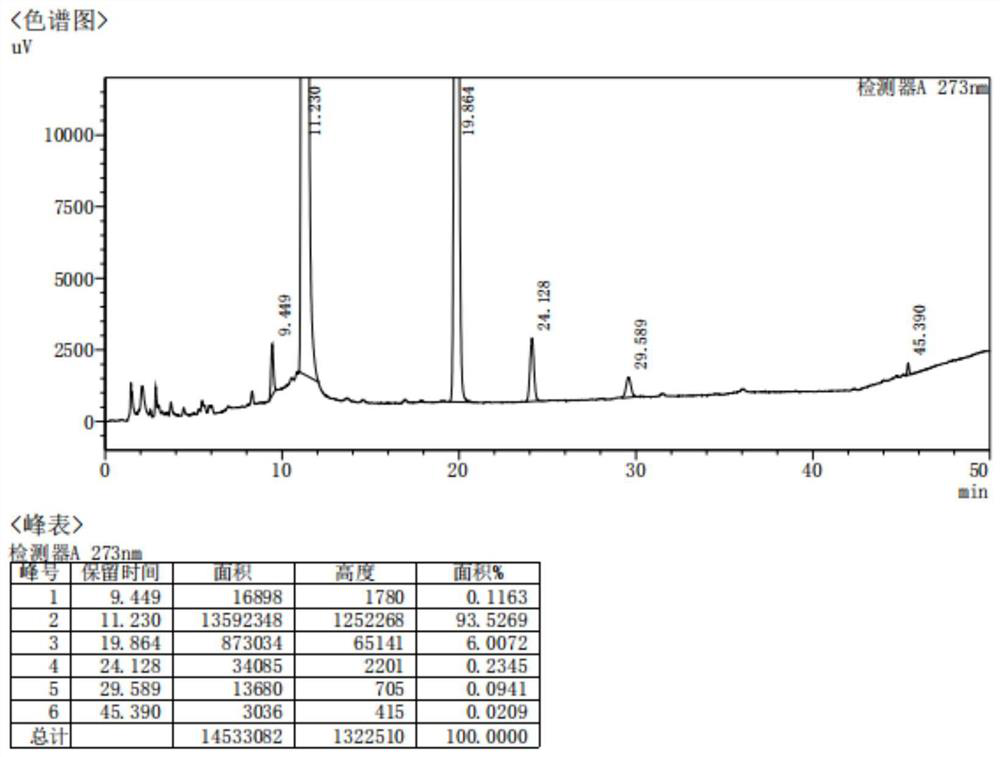
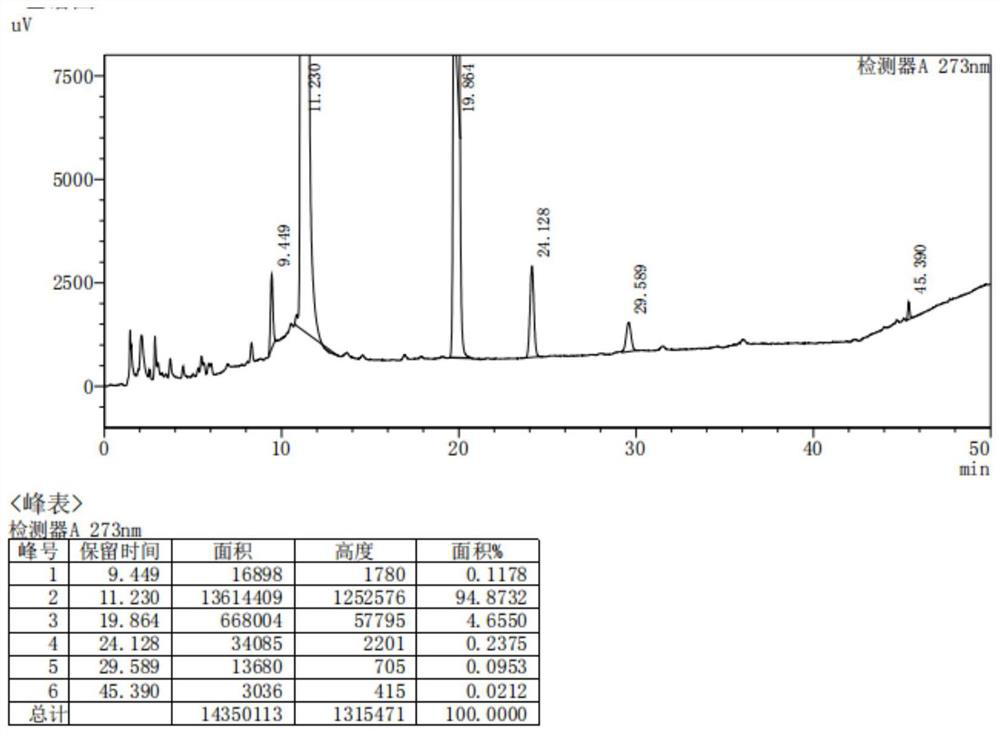
Method for analyzing content of acetyl bromide in cefuroxime axetil
ActiveCN114354800ASolve the problem that the nature is lively and cannot be directly determined by liquid massShort reaction timeComponent separationHydrolysisCefuroximum
The invention relates to the technical field of analysis and detection, in particular to a method for analyzing the content of acetyl bromide in cefuroxime axetil. According to the analysis method, the content of acetyl bromide in cefuroxime axetil is measured by using a triple four-stage liquid chromatograph-mass spectrometer, acetyl bromide is used as a reference substance, 2, 4-dinitrophenylhydrazine is used as a derivatization reagent, and the content of acetyl bromide in cefuroxime axetil is analyzed by using a standard curve method. According to the analysis method disclosed by the invention, the problems that acetyl bromide is extremely active, easy to hydrolyze and difficult to measure are solved through a pre-column derivatization method, and the detection method is efficient and accurate.
Owner:SHANDONG UNIV +1
Preparation method of high-purity cefuroxime acid
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylchoride at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
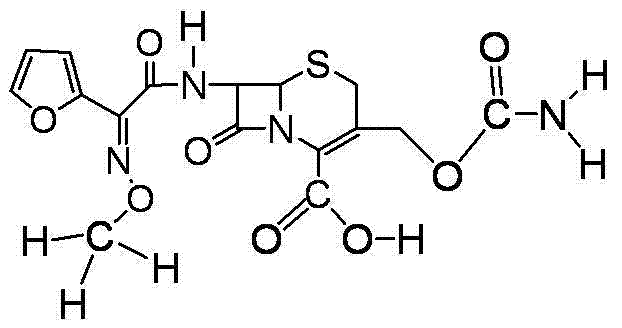
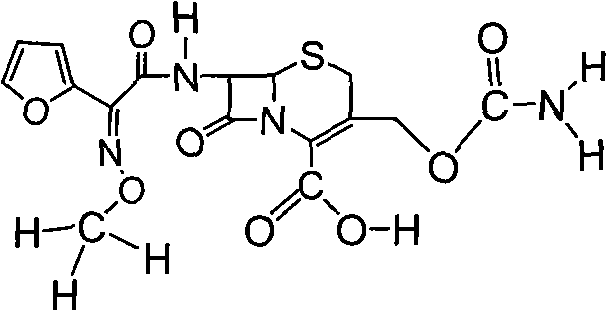
Cefuroxime lysine and preparation thereof
ActiveCN103145734AImprove stabilityLow impurity contentAntibacterial agentsOrganic active ingredientsCefuroximeImpurity
The invention relates to a cefuroxime lysine compound. The cefuroxime lysine contains 98-99.99% by weight of cefuroxime lysine and 0.01-2% of trans-cefuroxime acid, and the molecular formula of the trans-cefuroxime acid is as shown in formula I. The obtained cefuroxime lysine disclosed by the invention has very strong stability which is higher than that of the prior art; and the impurity content and the polymer content are lower than those of the cefuroxime lysine in the prior art, thus the cefuroxime lysine is very suitable for clinical applications.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for determining high-molecular polymer in cefuroxime axetil tablet
The invention discloses a method for determining a high-molecular polymer in a cefuroxime axetil tablet, which comprises the following steps: chromatographic conditions comprise a high-performance gelchromatographic column, a detector is UV, and a mobile phase is an N, Ndimethylformamide solution of 0.03 mol / L lithium halide containing 25-35% of monohydric alcohols with the number of carbon atomsbeing 38. The method provided by the invention has good solution stability, can effectively prevent degradation of cefuroxime axetil to-be-detected components, and ensures the stability of a sample,so that high-molecular polymers in the cefuroxime axetil tablets can be more accurately controlled, the product quality is ensured, anaphylactic reactions caused by the polymers are prevented, and safe medication of patients is better ensured.
Owner:JIANGSU QINGJIANG PHARMA
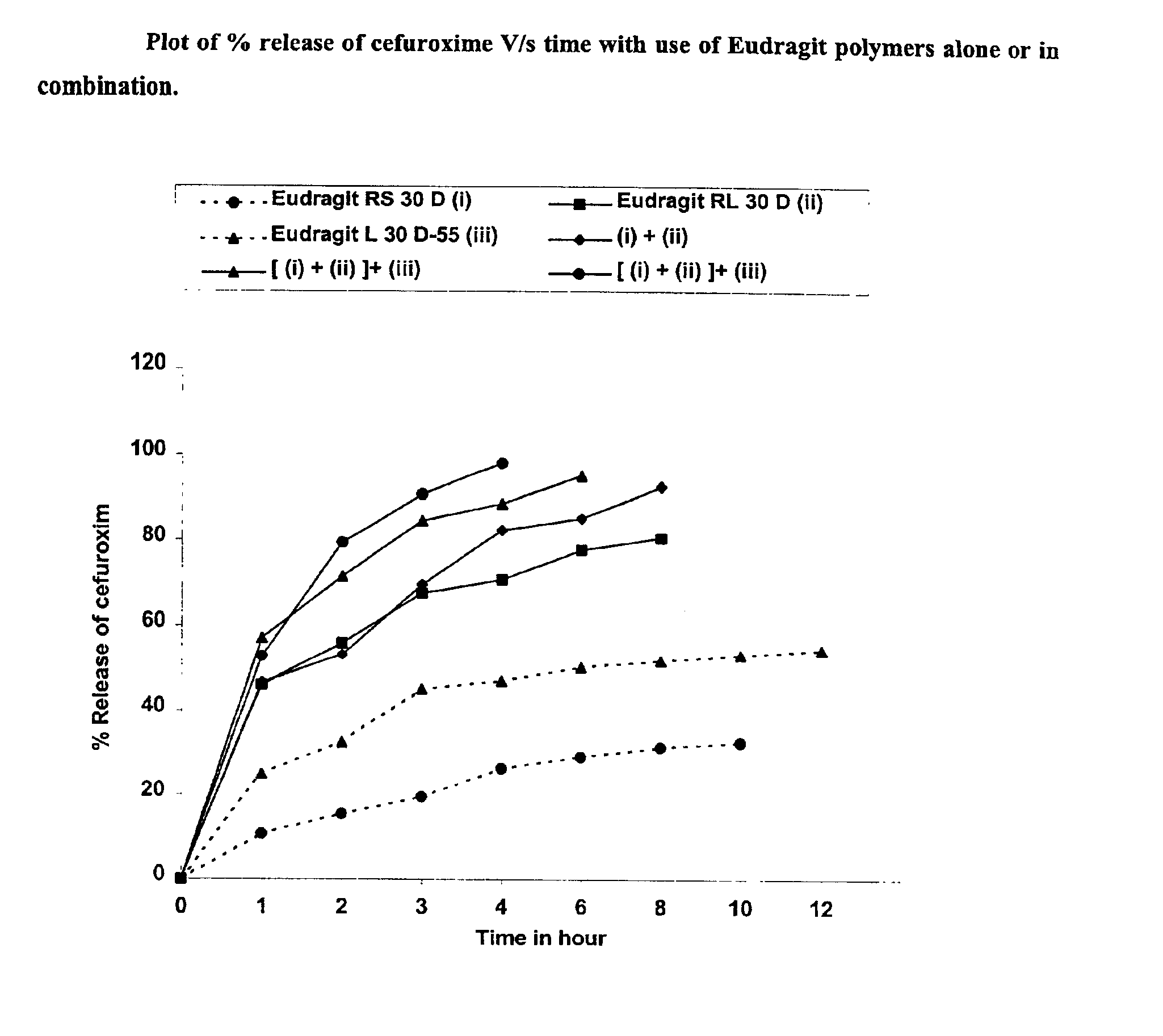
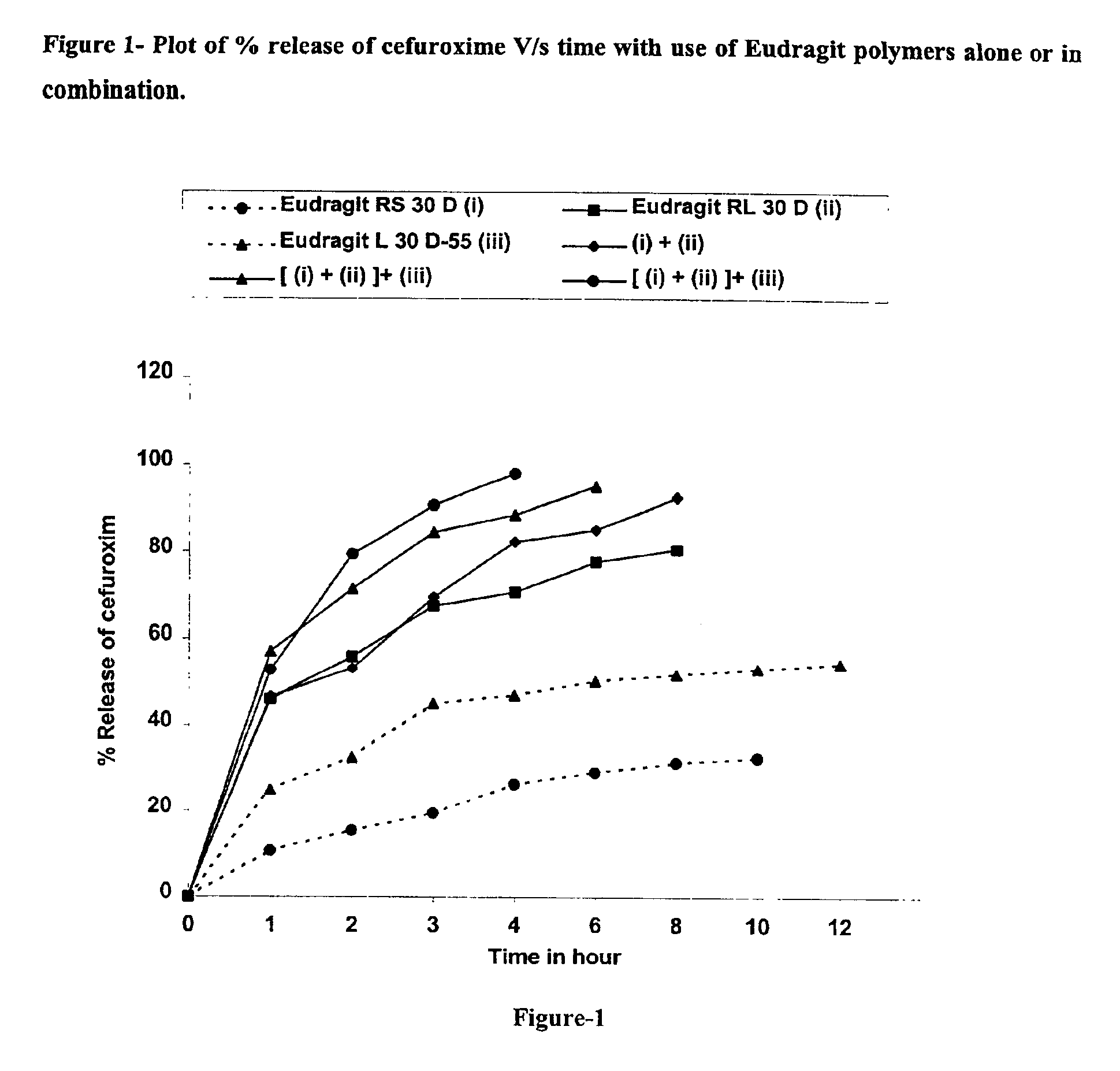
Dosage form with improved release of cefuroximaxetil
InactiveUS8747900B2Improve bioavailabilityEasy to processBiocideOrganic chemistryΛ carrageenanDosage form
The invention relates to a pharmaceutical composition comprising cefuroximaxetil and at least one carrageenan selected from the group consisting of κ-carrageenan, λ-carrageenan and -carrageenan. The invention furthermore relates to pellets, to a multiparticulate, pharmaceutical dosage form and to a novel crystalline modification of cefuroximaxetil.
Owner:GRUNENTHAL GMBH
Method for synthesizing 3-decarbamoyl-acetyl-cefuroxime acid compound
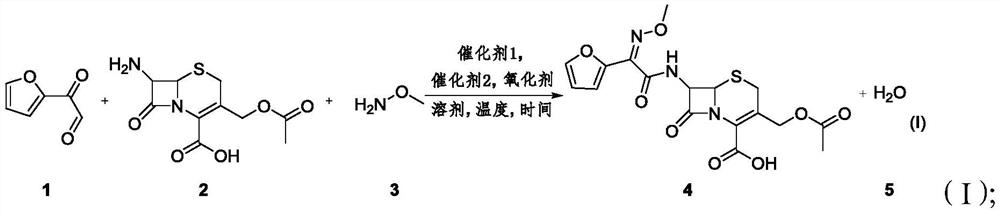
ActiveCN112679527AThe process steps are simpleReduce cost pressureOrganic chemistryFuranPtru catalyst
The invention provides a method for synthesizing a 3-decarbamoyl-acetyl-cefuroxime acid compound. The method comprises the following steps: taking 2-(2-furyl)-2-oxo-acetaldehyde, 7-aminocephalosporanic acid and methoxyamine as raw materials; reacting the raw materials in a reaction solvent under the combined action of a first catalyst, an oxidizing agent and a second catalyst; and carrying out chromatography to obtain the 3-deaminobenzoyl acetyl cefuroxime acid compound. By using the method, the process steps are simple, the byproduct is mainly water, a large amount of other wastes do not exist, and the subsequent treatment cost and the environmental protection pressure are low.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Preparation method of cefuroxime acid
The invention relates to a preparation method of cefuroxime acid, wherein the preparation method comprises the following steps: (1) adding D-7ACA into water, and dropwisely adding an alkali solution to dissolve; adding a methoxyimino furan acetyl chloride dichloromethane solution to carry out acylation reaction, layering to obtain a water phase after the reaction is finished, adding activated carbon to decolorize, filtering, then adding dichloromethane, dropwise adding hydrochloric acid to crystallize, filtering and drying to obtain MDCC; and (2) adding MDCC into an organic solvent, adding chlorosulfonyl isocyanate to carry out aminomethyl acylation reaction, and after the reaction is finished, adding pre-cooling water to hydrolyze to obtain a cefuroxime acid suspension; and dropwise adding an alkaline solution for multiple times, growing crystals after dropwise adding is completed each time, finally adjusting the pH value of the system to 1.0-2.0, filtering, and washing to obtain the cefuroxime acid product. The obtained product is good in stability, good in product flowability and easy to subpackage; the crystallization size phase of the product is greatly improved, and the stability is obviously improved.
Owner:QILU ANTIBIOTICS PHARMA
Preparation method of cefuroxime acid
The invention discloses a preparation method of cefuroxime acid, and belongs to the field of preparation of medical intermediates. The preparation method comprises the following steps: taking a cefuroxime sodium crude product as a raw material, adding into a dissolving agent, stirring, dissolving, adding an adsorbent, stirring, filtering, and crystallizing by using an acidic reagent to obtain the cefuroxime acid. The prepared cefuroxime acid has the advantages of high content, few impurities and good stability, and the preparation method is simple, energy-saving, environment-friendly and suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for recovering cefuroxime acid from cefuroxime acid waste residue liquid
ActiveCN112574232AQuality improvementNot easy to hydrolyzeOrganic chemistryPtru catalystDistillation
The invention relates to the technical field of medicine synthesis, in particular to a method for recovering cefuroxime acid from cefuroxime acid waste residue liquid. The method comprises the steps of: dissolving the cefuroxime acid waste residue liquid in a solvent, filtering, adding a polymer supported catalyst into the filtrate, reacting, and carrying out reduced pressure distillation to obtain the cefuroxime acid. According to the invention, trans-isomer byproducts are directly converted into cis-products, so that the environmental protection problem caused by waste residues is solved, the product yield is increased, the cis-products in the reaction process are not damaged and can be completely recycled by a reaction system, the reaction process is simple, other reaction raw materialsare not consumed in the process, the process is simple, and the production cost is low.
Owner:山东金城昆仑药业有限公司
Cefuroxime axetil capsule and preparation method thereof
PendingCN113952313AAvoid Dissolution Decrease DisadvantagesHigh densityAntibacterial agentsOrganic active ingredientsEngineeringBioavailability
The invention discloses a cefuroxime axetil capsule and a preparation method thereof. The preparation method comprises the following steps: (1) raw material pretreatment: sieving a solubilizer, a flow aid and a lubricant with a 60-100-mesh sieve; sieving the cefuroxime axetil and the filling agent with a 60-100-mesh sieve for later use; (2) premixing: mixing the solubilizer, the flow aid and the lubricant, and sieving with a 60-100-mesh sieve to obtain a mixture A; (3) mixing: uniformly mixing the cefuroxime axetil and the mixture A, then adding the filling agent and the disintegrating agent, and uniformly mixing to obtain intermediate powder; and (4) capsule filling: filling a capsule shell with the intermediate powder according to a filling amount requirement, and then packaging to obtain the cefuroxime axetil capsule. According to the invention, after cefuroxime axetil and auxiliary materials are uniformly mixed, the capsule is directly filled by adopting a full-powder process without a granulation process. The stability of the cefuroxime axetil is improved, and the bioavailability is improved; the production period is shortened and the cost is reduced.
Owner:NINGXIA MEDICAL UNIV
A method for synthesizing 3-decarbamoyl-acetyl-cefuroxin compound
ActiveCN112679527BThe process steps are simpleReduce cost pressureOrganic chemistryFuranPtru catalyst
The invention provides a method for synthesizing 3-decarbamoyl-acetyl-cefuroxin acid compound, comprising: using 2-(2-furyl)-2-oxo-acetaldehyde, 7-aminocephalosporanic acid , and methoxyamine as a raw material; under the joint action of the first catalyst, the oxidizing agent and the second catalyst, the raw material is reacted in a reaction solvent; Cefuroxime Acid Compound. Using the method of the invention, the process steps are simple, the by-product is mainly water, there will not be a large amount of remaining waste, and the cost of subsequent treatment and environmental protection pressure are low.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
A kind of cefuroxime sodium raw material and injection and preparation method thereof
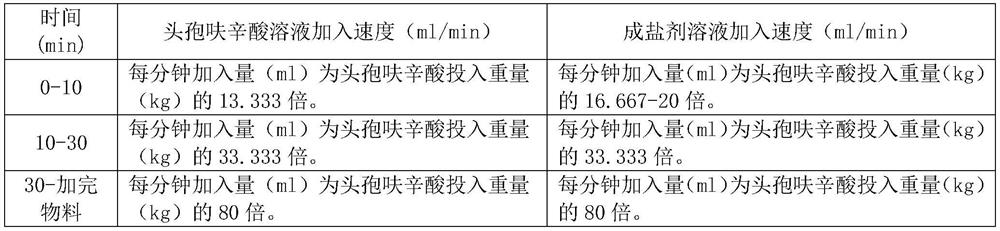
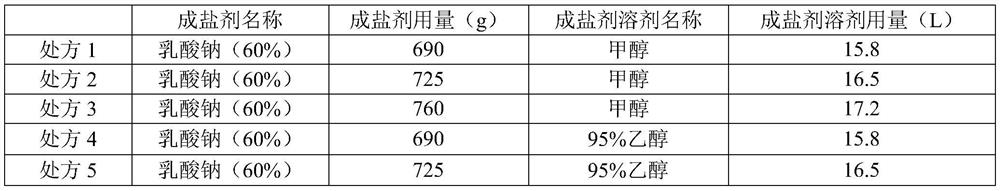
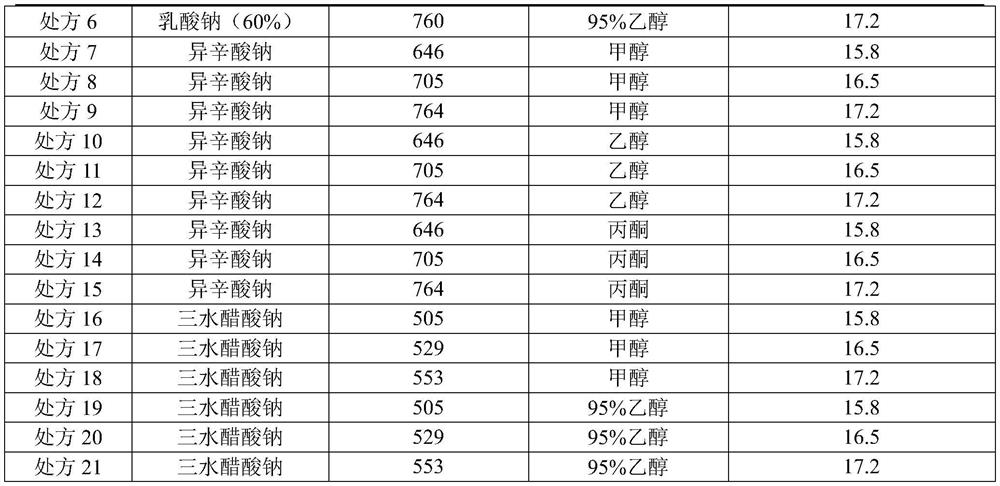
ActiveCN110437260BImprove stabilityAntibacterial agentsPowder deliveryCefuroxime SodiumSodium Acetate Trihydrate
The invention relates to a cefuroxime sodium raw material, an injection and a preparation method thereof, belonging to the technical field of medicine. The cefuroxime sodium raw material of the present invention is passed in a specific solvent environment, with a special dropping rate, dropwise adding cefuroxime acid solution and a 95% ethanol solution of sodium acetate trihydrate prepared according to a specific mixed solvent ratio and a specific concentration at the same time Prepared. The product quality and stability of cefuroxime sodium for injection prepared from this raw material through special process control has been significantly improved, especially the production of related substance 2 has been reduced, and the product quality has been strictly controlled, thereby ensuring the safety and effectiveness of the drug.
Owner:石药集团中诺药业(石家庄)有限公司
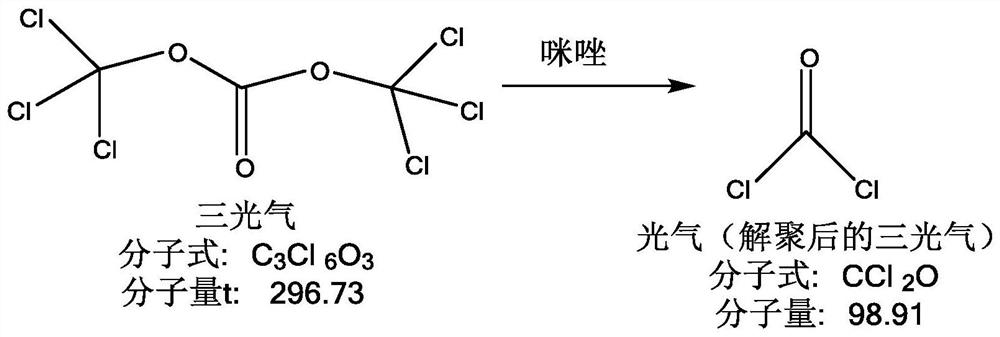
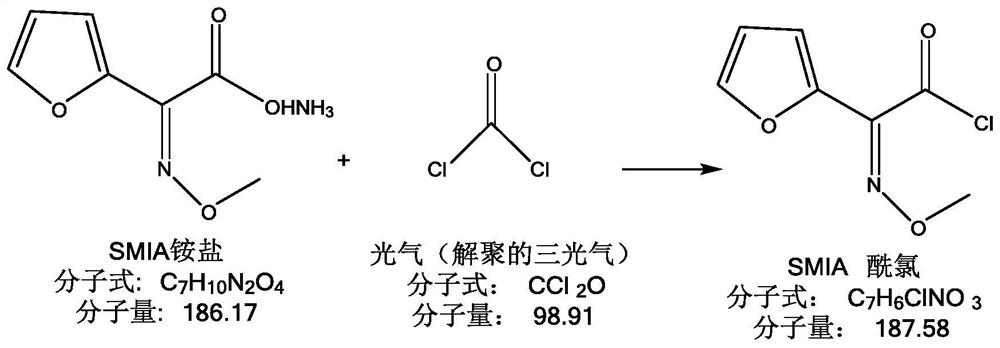
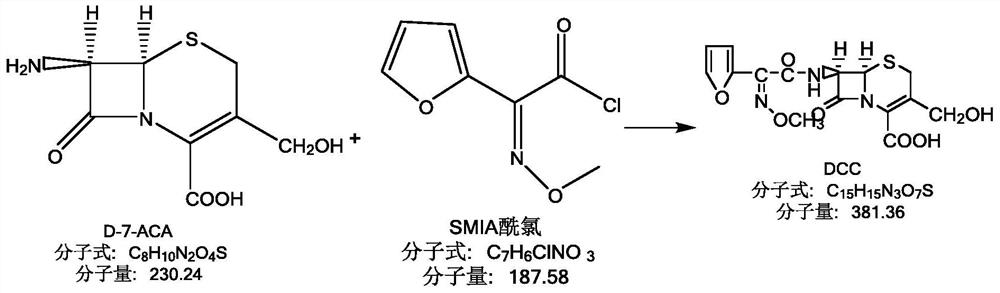
A kind of environment-friendly preparation method of the intermediate of cefuroxime acid
The invention relates to an environmentally-friendly preparation method of an intermediate of cefuroxime acid. The method comprises the following steps: preparing an SMIA acyl chloride solution by utilizing triphosgene, preparing a D-7-ACA solution, and performing a condensation reaction on the SMIA acyl chloride solution and D-7-ACA solution to obtain DCC. The environmentally-friendly preparationmethod of the intermediate of the cefuroxime acid provided by the invention greatly reduces the amount of a chloride salt and a phosphate salt, and does not use high-boiling-point class-2 solvent dimethylacetamide (DMAC) difficult to recover and soluble in water, thereby greatly reducing environmental pressure, greatly reducing production costs, and steadily improving the product quality; the reaction for preparing the acyl chloride from the triphosgene is thorough, and the prepared acyl chloride has high purity, so that the prepared intermediate DCC also has higher purity; and the high-boiling-point class-2 solvent DMAC is not used; therefore the produced cefuroxime acid has a lower residual solvent, a residual solvent of bulk drug cefuroxime sodium and cefuroxime axetil produced by theintermediate of the cefuroxime acid is greatly reduced, the quality of the final product is better improved, and the yield is increased.
Owner:GUANGDONG LIGUO PHARMACY
A kind of cefuroxime lysine and preparation thereof
The invention relates to a cefuroxime lysine compound. The cefuroxime lysine contains 98-99.99% by weight of cefuroxime lysine and 0.01-2% of trans-cefuroxime acid, and the molecular formula of the trans-cefuroxime acid is as shown in formula I. The obtained cefuroxime lysine disclosed by the invention has very strong stability which is higher than that of the prior art; and the impurity content and the polymer content are lower than those of the cefuroxime lysine in the prior art, thus the cefuroxime lysine is very suitable for clinical applications.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
A kind of method for measuring polymer in cefuroxime axetil tablet
The invention discloses a method for determining high-molecular polymers in cefuroxime axetil tablets, which comprises the following steps: the chromatographic conditions include a high-efficiency gel chromatographic column, the detector is UV, and the mobile phase is 0.03 mol / L lithium halide at 25- 35% N,N-dimethylformamide solution containing monoalcohols with 3 to 8 carbon atoms. The method of the present invention has better solution stability, can effectively prevent the degradation of the cefuroxime axetil component to be tested, and ensure the stability of the sample, so that the high molecular polymer in the cefuroxime axetil tablet can be more accurately controlled, and the product can be guaranteed. Quality, prevention of allergic reactions caused by polymers, and better protection of drug safety for patients.
Owner:JIANGSU QINGJIANG PHARMA
Cefuroxime axetil granule and preparation method thereof
PendingCN110664779AGuaranteed releaseMask bitternessAntibacterial agentsOrganic active ingredientsMethacrylic acid-ethyl acrylate copolymerEngineering
The invention provides a cefuroxime axetil granule and a preparation method thereof, and belongs to the field of medicine preparations. A fluidized bed granulation and coating technology is adopted tocarry out fluidized bed granulation and coating on cefuroxime axetil. Since the cefuroxime axetil is unstable when the cefuroxime axetil is under a hot and humid condition, a fluidized bed granulation and coating way adhesive solution volatilizes while the solution is sprayed, direct contact between the cefuroxime axetil and water is effectively avoided, the prepared granules are even, and product quality is stable. In addition, a pore-foaming agent and a methacrylic acid-acrylic acid ethyl ester copolymer at a specific ratio are added into taste-masking coating ingredients to guarantee the releasing of the cefuroxime axetil while granules are accelerated to carry out taste masking. The cefuroxime axetil granule provided by the invention can effectively cover the bitter taste of the cefuroxime axetil, meanwhile, the preparation can be guaranteed to favorably disperse in water, and therefore, the cefuroxime axetil granule is especially suitable for children and people who suffer from dysphagia.
Owner:SHANDONG LUKANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com