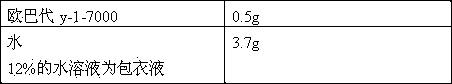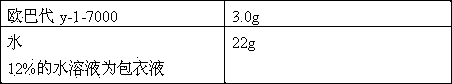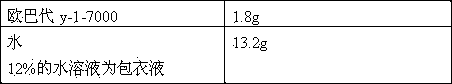Cefuroxime axetil dispersible tablet
A technology of cefuroxime axetil and dispersible tablets, applied in the directions of pill delivery, antibacterial drugs, etc., can solve the problems of high bitterness of cefuroxime axetil, unfavorable for patients to take, low production efficiency, etc., achieve uniform dissolution, improve compliance, Well-formulated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Cefuroxime Axetil Dispersible Tablets
[0024] cefuroxime axetil 150.5g microcrystalline cellulose 77.5g Crospovidone 24g silica 0.15g Sodium dodecyl sulfate 3g stearic acid 18g aspartame 18g Orange Powder Flavor 6g production 1000 pieces
[0025] Opadry y-1-7000 1.5g water 11g 12% aqueous solution is coating liquid
[0026] Preparation:
[0027] Determination of the particle size of cefuroxime axetil raw material requires its particle size d 90 ≤70 μm, pass the cefuroxime axetil raw material through a 100-mesh sieve, and the auxiliary materials through a 80-mesh sieve, and set aside; weigh cefuroxime axetil, microcrystalline cellulose, sodium lauryl sulfate, aspartame, Orange powder flavor, mixed with 50% prescription amount of crospovidone, silicon dioxide, and stearic acid, and mixed for 30 minutes to make it evenly...
Embodiment 2
[0028] Example 2 Preparation of Cefuroxime Axetil Dispersible Tablets
[0029] cefuroxime axetil 3010g microcrystalline cellulose 1550g Crospovidone 480g silica 60g Sodium dodecyl sulfate 60g stearic acid 360g aspartame 360g Orange Powder Flavor 12g production 20000 pieces
[0030] Opadry y-1-7000 182g water 1335g 12% aqueous solution is coating liquid
[0031] The preparation method is the same as in Example 1.
Embodiment 3
[0032] Example 3 Preparation of Cefuroxime Axetil Dispersible Tablets
[0033] cefuroxime axetil 40g microcrystalline cellulose 20g Crospovidone 5.0g silica 0.15g Sodium dodecyl sulfate 0.5g stearic acid 3.0g aspartame 2.0g Orange Powder Flavor 0.5g production 250 tablets
[0034] Coating Solution Prescription:
[0035]
[0036] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com