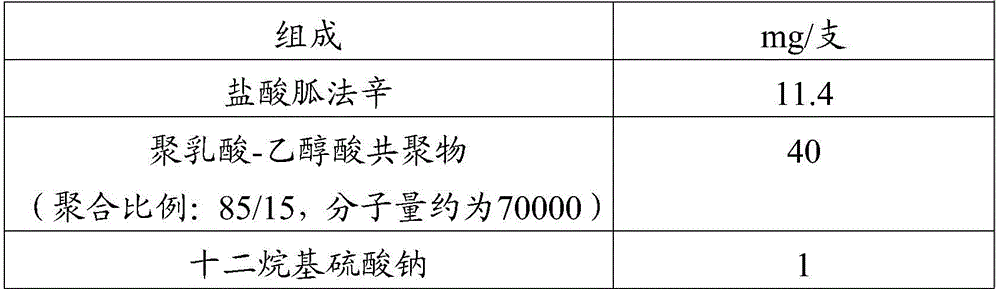
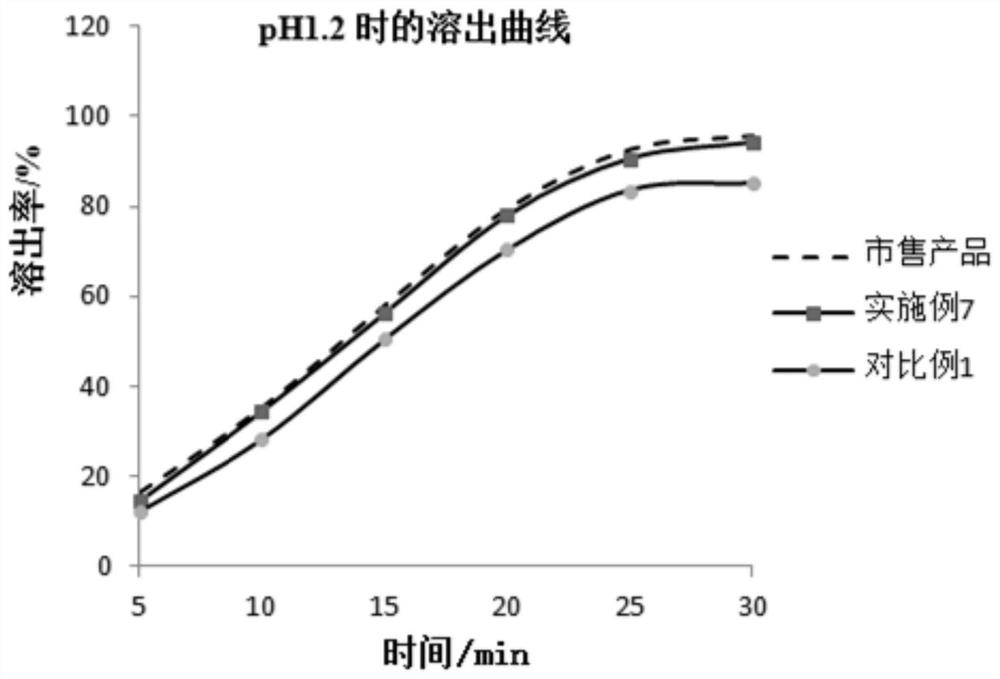
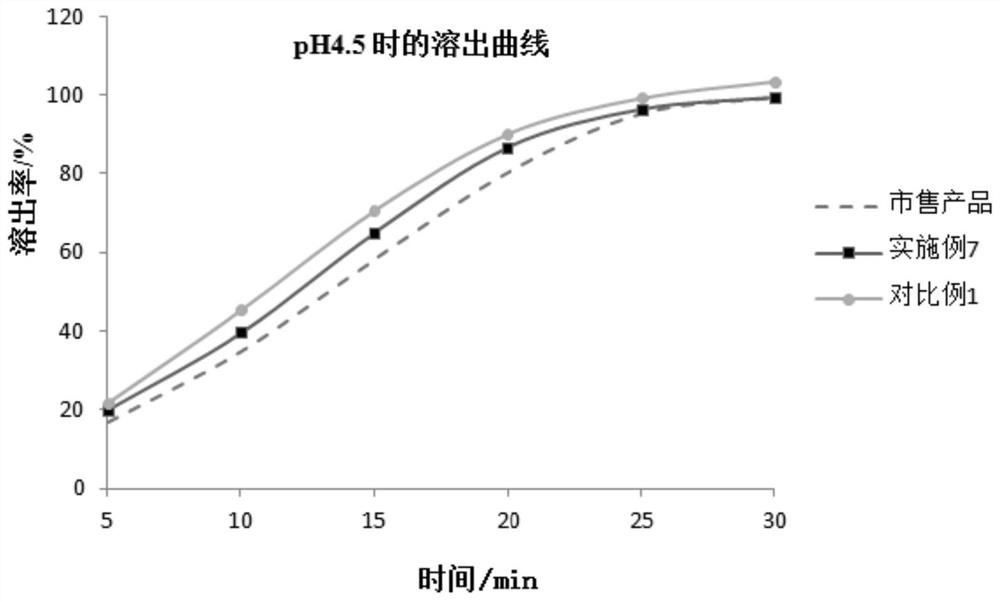
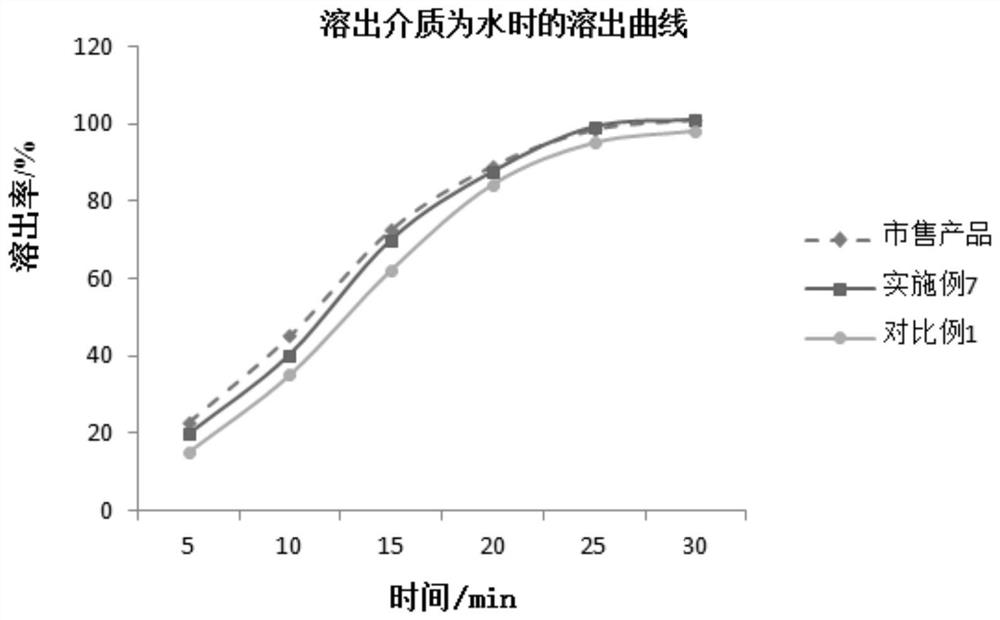
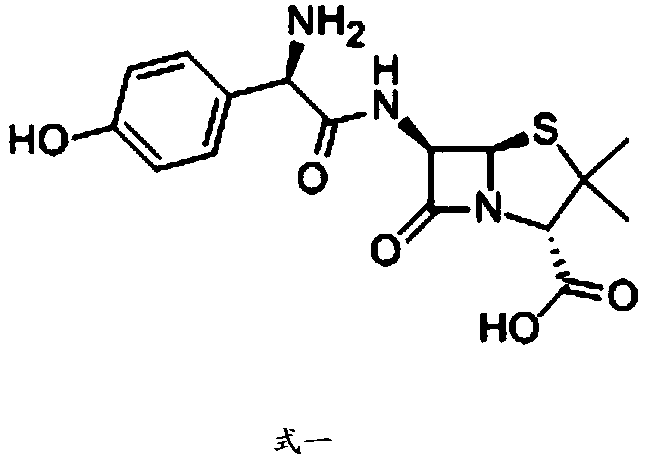
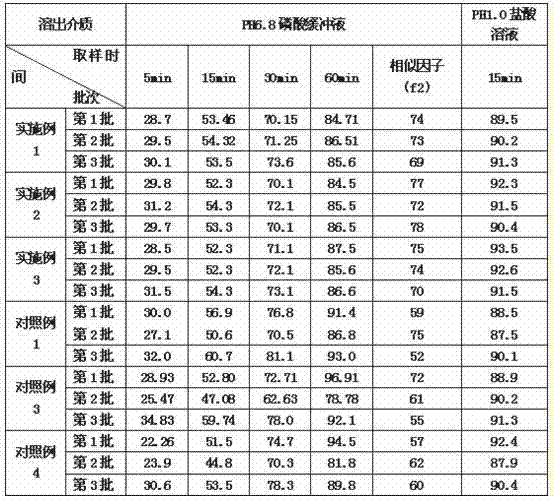
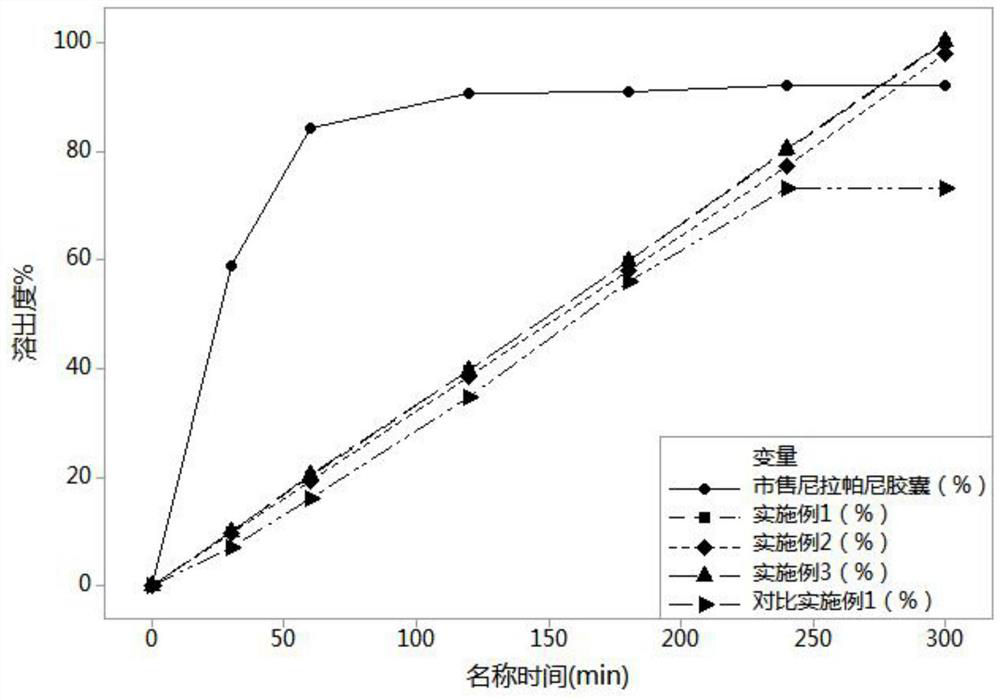
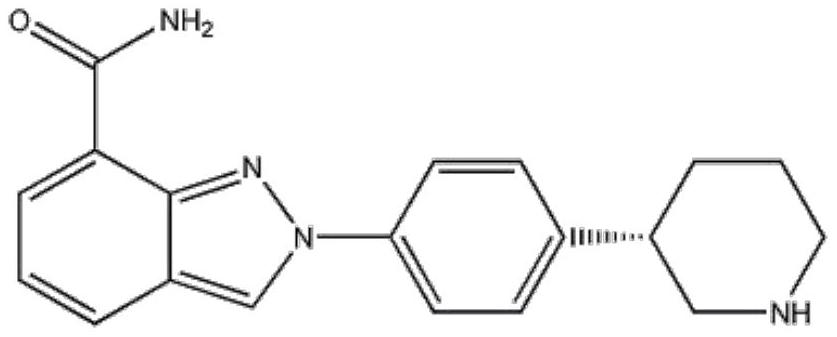
Patents
Literature
82results about How to "Dissolution stability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
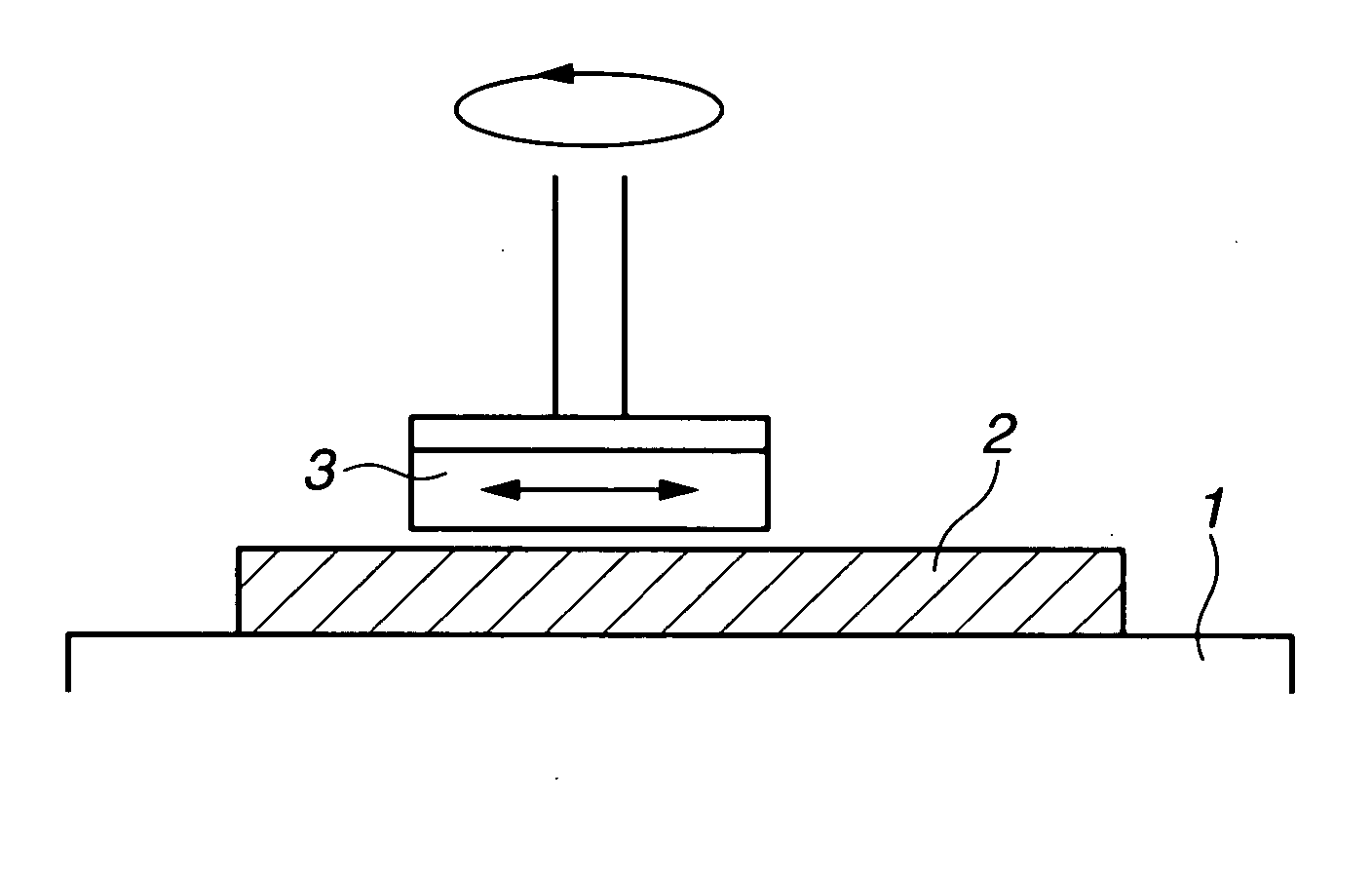
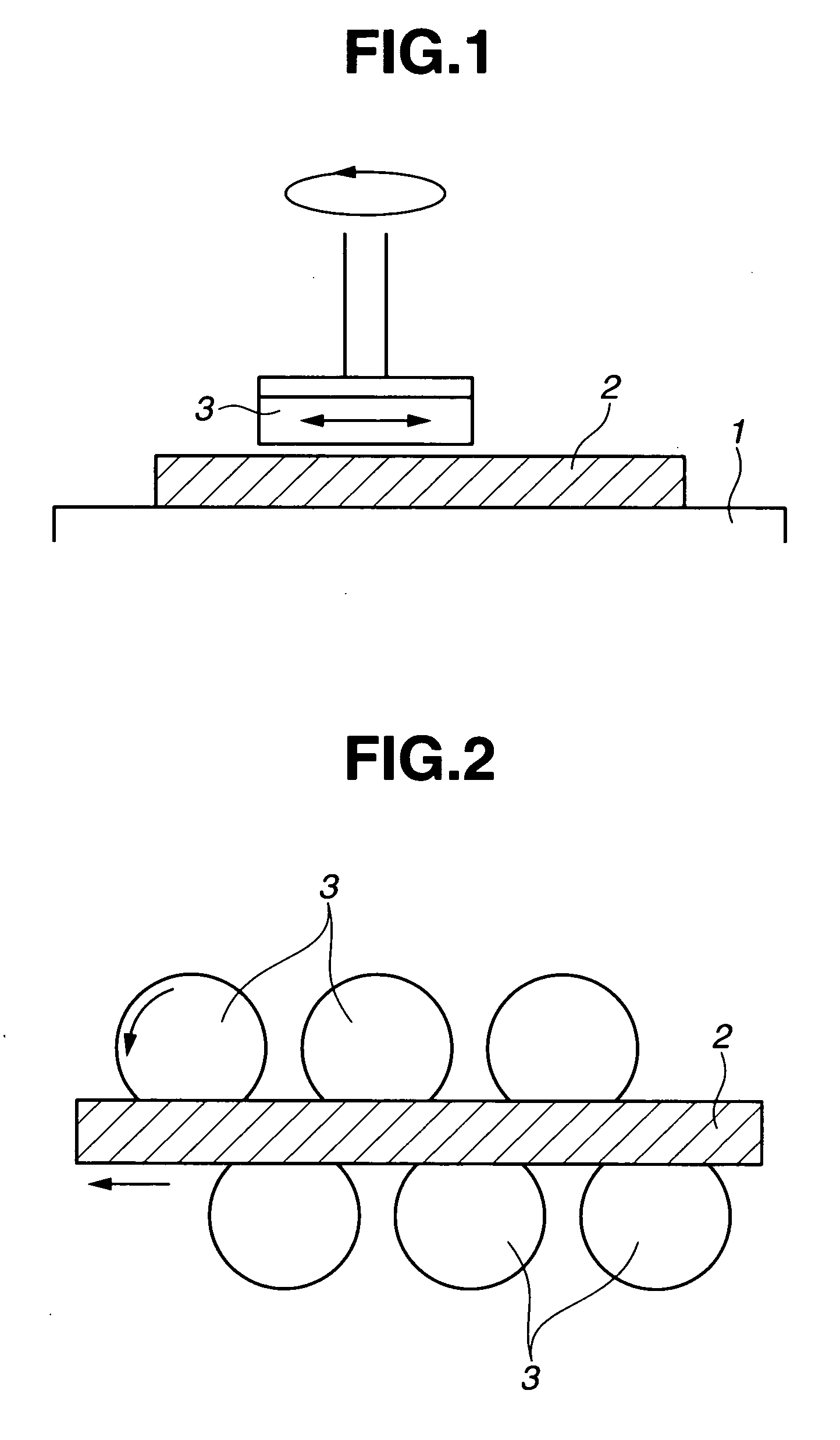
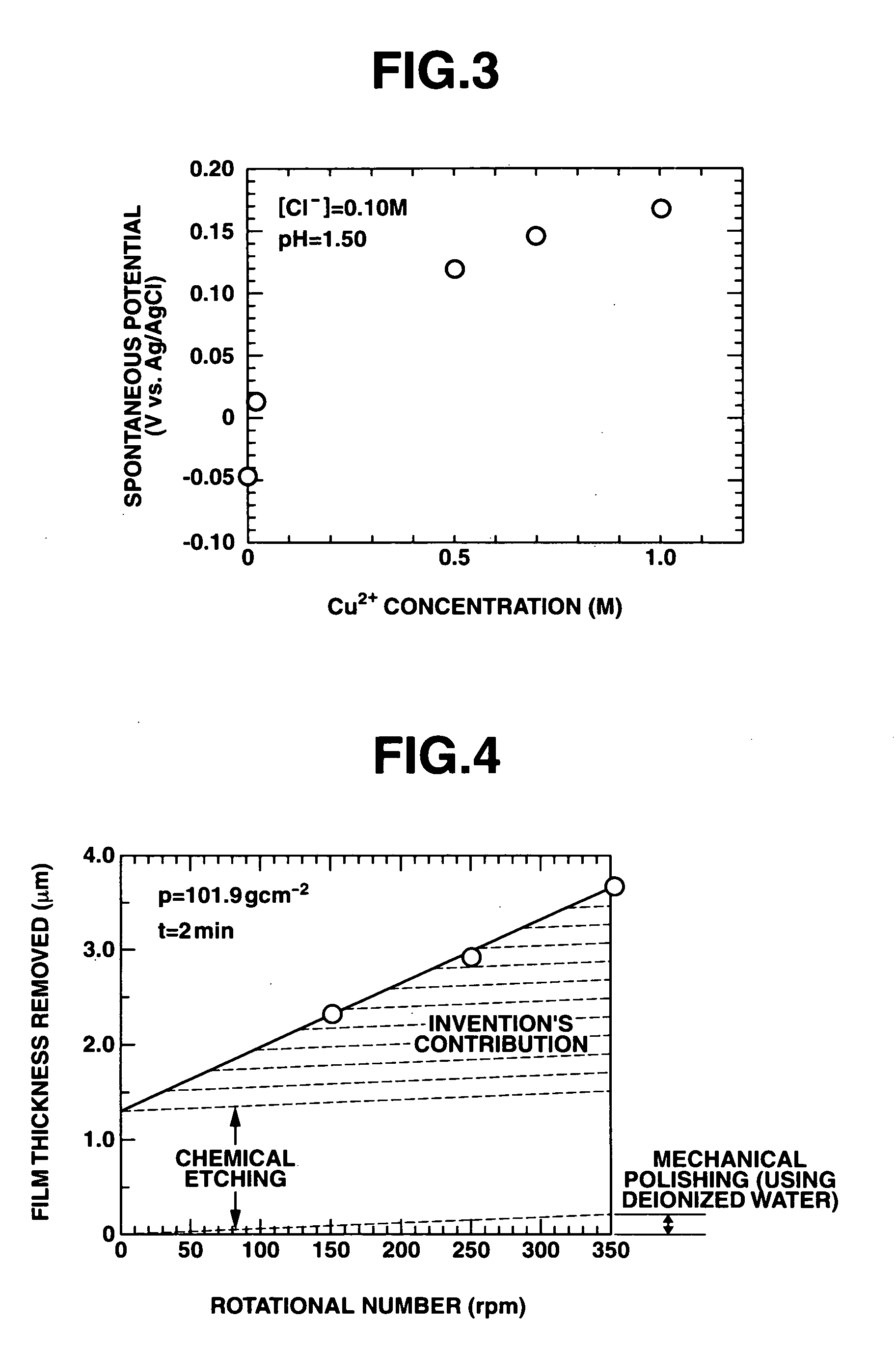
Polishing solution and method of polishing nonferrous metal materials
InactiveUS20050136805A1Low removal rateHigh removal rateOther chemical processesPolishing compositions with abrasivesIonMetallic materials
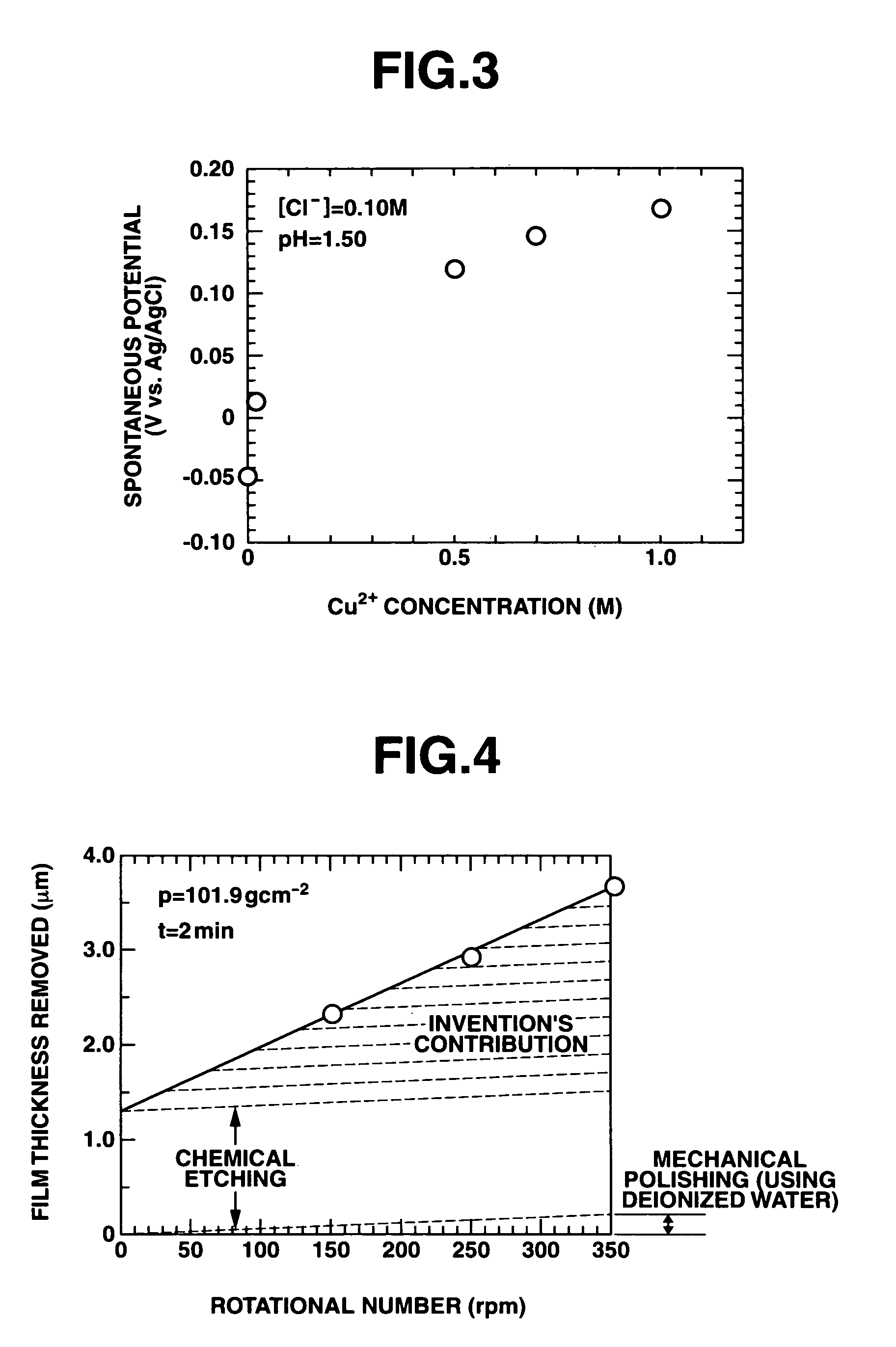
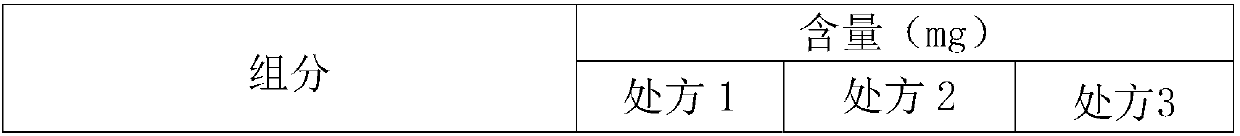
A polishing solution, comprising copper ions and chloride ions in Cu / Cl molar ratio of 10−1 to 103 and at pH 0.5 to 10, is suited for polishing a surface composed of a nonferrous metal material such as copper or copper alloy. Thicker metal film can be polished at high removal rate, so that distribution in film thickness becomes uniform.
Owner:C UYEMURA & CO LTD
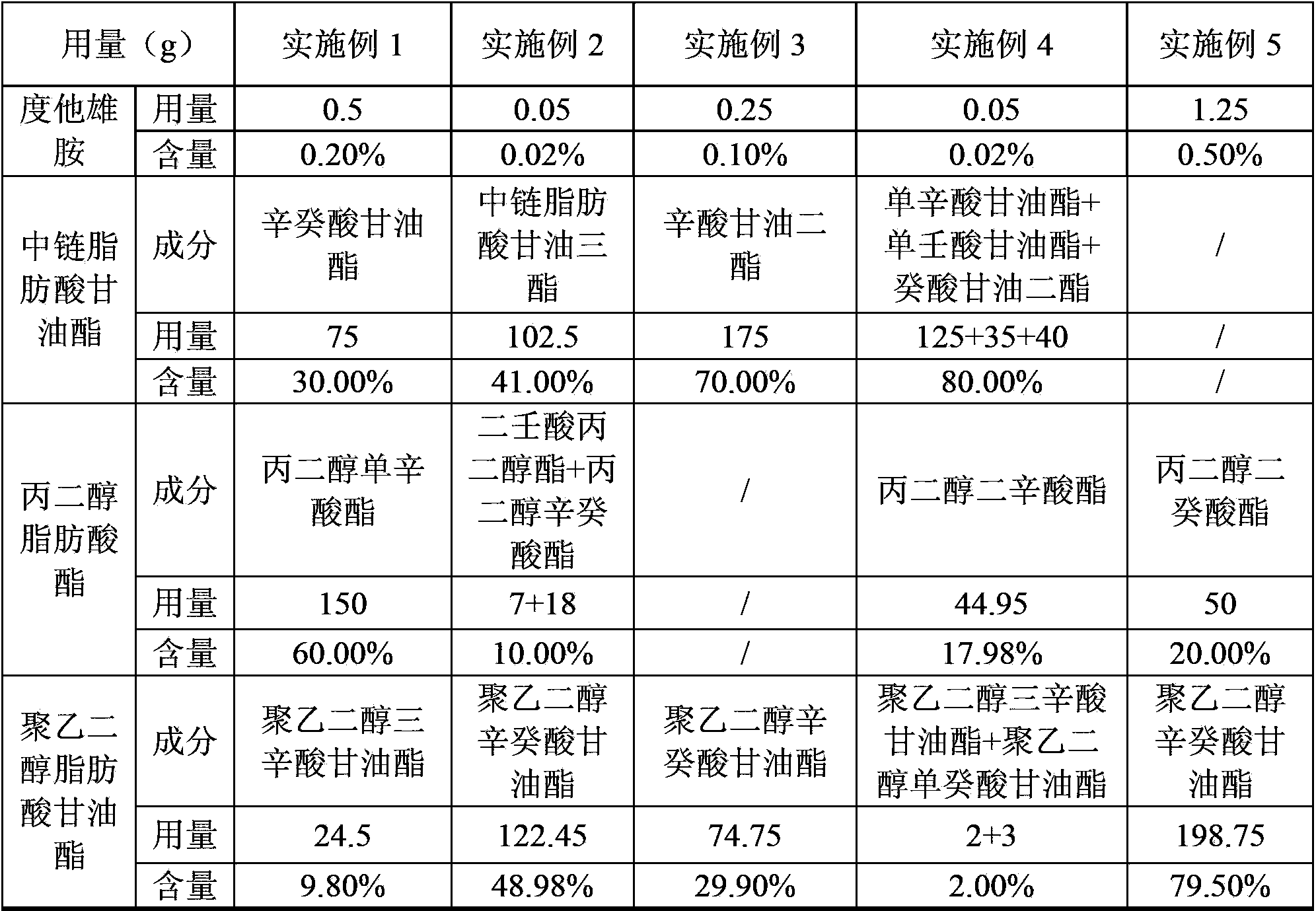
Dutasteride soft capsule with stable quality
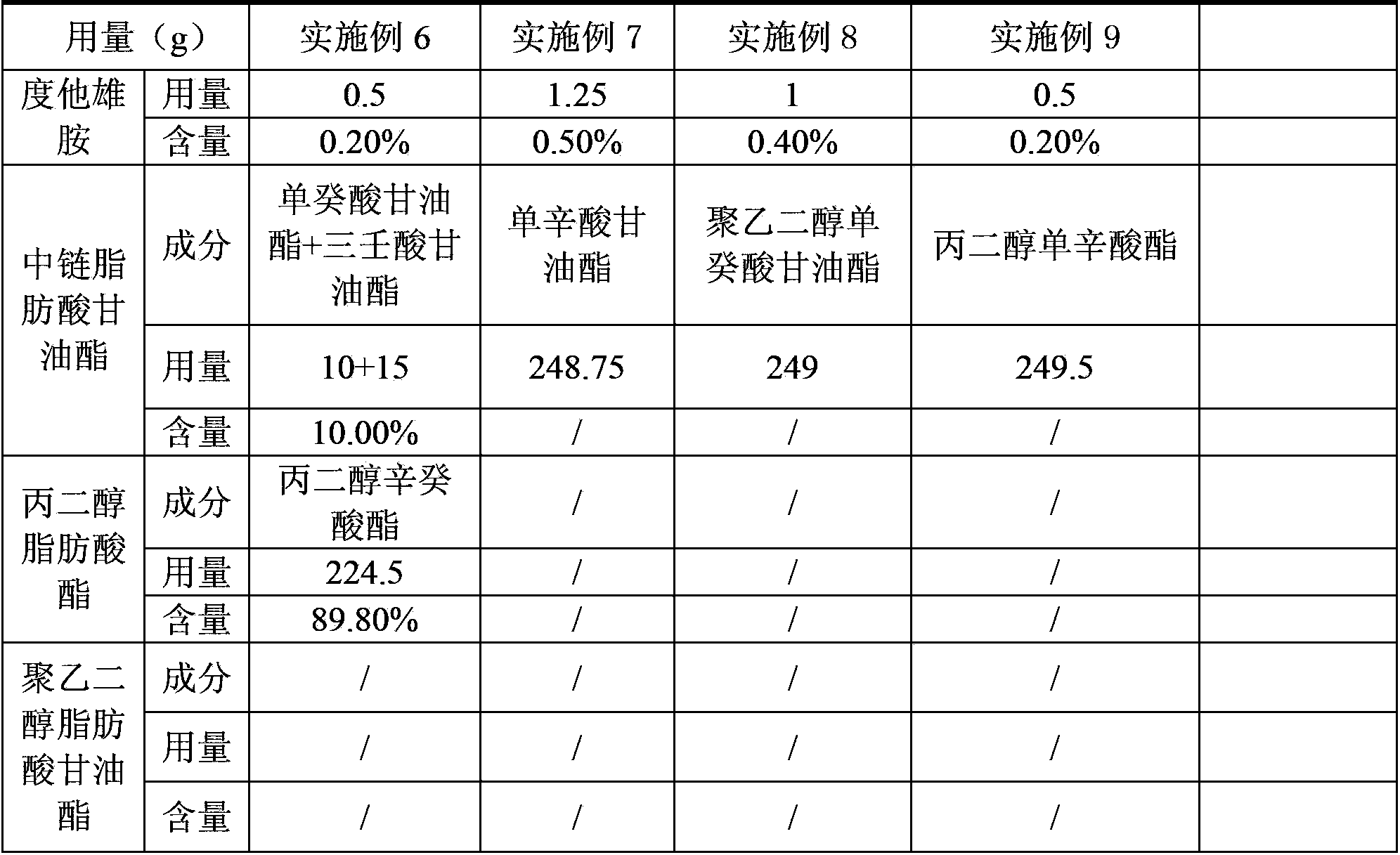
ActiveCN104069084AImprove playbackGood effectOrganic active ingredientsUrinary disorderPolyethylene glycolDissolution
The invention provides a dutasteride soft capsule. The dutasteride soft capsule contains dutasteride and an oleaginous base and is characterized in that the oleaginous base contains polyethylene glycol fatty acid glyceride and / or propylene glycol fatty acid ester, or employs medium-chain fatty acid glyceride. According to the invention, polyethylene glycol fatty acid glyceride and / or propylene glycol fatty acid ester is used as the oleaginous base for dutasteride for the first time; compared with preparations in the prior art, the soft capsule prepared in the invention has a good dissolution rate and high stability.
Owner:CHONGQING HUAPONT PHARMA
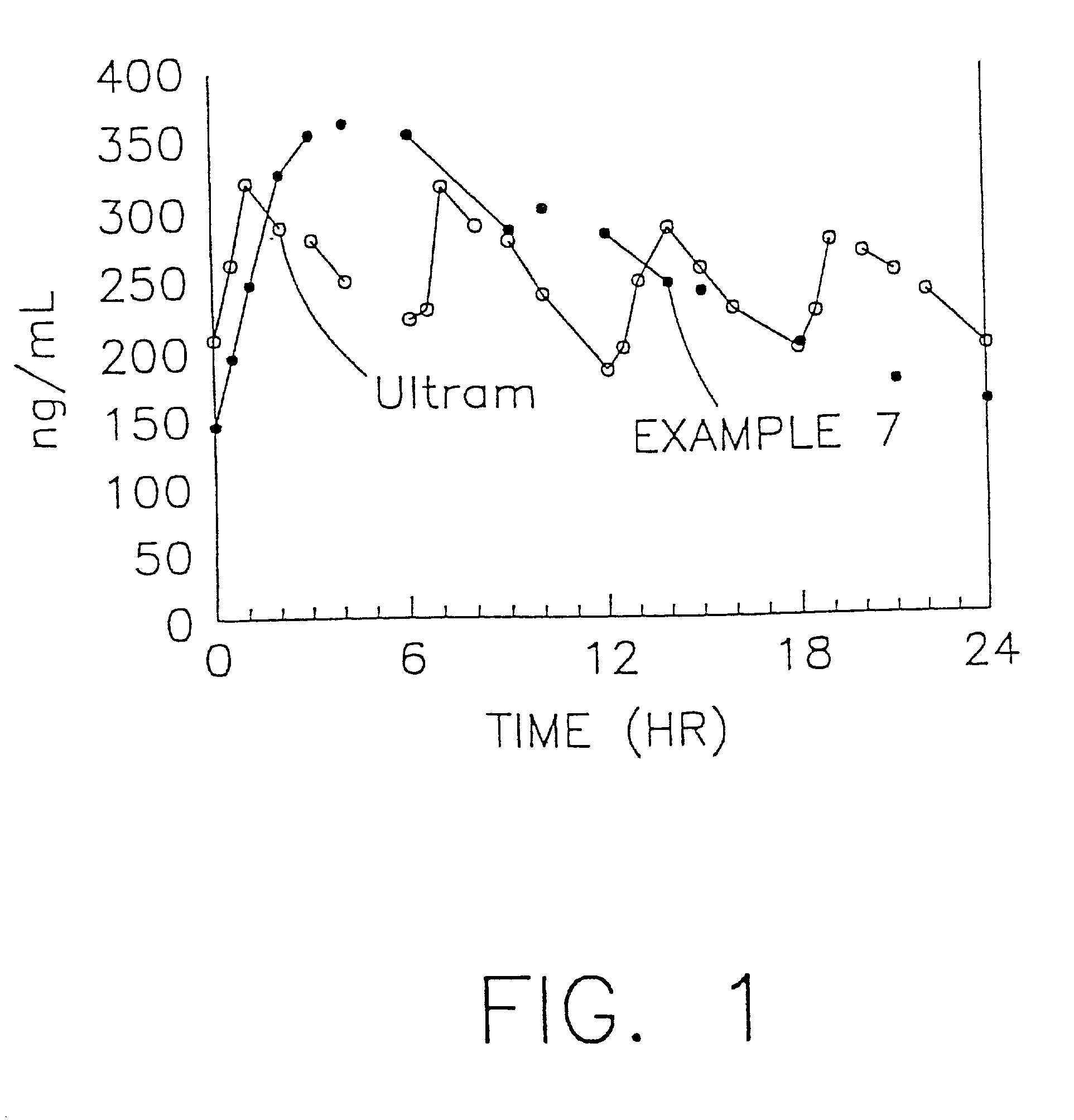
Stabilized sustained release tramadol formulations
InactiveUS20020102302A1Insufficient stabilityPlasticization effectBiocideOrganic active ingredientsWaxDissolution
A stabilized sustained release oral solid dosage form which includes an effective amount of tramadol or a pharmaceutically acceptable salt thereof dispersed in a matrix of a hydrophobic material comprising a wax-like substance which was melted or softened during the preparation of the matrix, is cured at a temperature from about 35° C. to about 65° C. for a time period from about 4 to about 72 hours, such that the formulation, when subjected to in-vitro dissolution after exposure to accelerated storage conditions of at least one month at 40° C. / 75% RH, releases an amount of tramadol which does not vary at any given dissolution time point by more than about 20% of the total amount of tramadol released when compared to in-vitro dissolution conducted prior to subjecting the dosage form to the accelerated storage conditions.
Owner:PURDUE PHARMA LP
Moxifloxacin capsule and its preparation method
ActiveCN1762358AHigh dissolution rateDissolution stabilityAntibacterial agentsOrganic active ingredientsHydroxypropylmethyl celluloseEthyl cellulose
The invention provides a Moxifloxacin capsule, which comprises Moxifloxacin or its salts and / or hydrate, at least a disintegrating agent and at least a lubricating agent, the shell of the capsule contains hydroxy propyl ethyl cellulose. The invention also discloses the process for preparing the capsule.
Owner:JIANGSU TIANYISHI PHARMA
Inkjet ink composition
InactiveUS20050209366A1Good dispersionDissolution stabilityInksDyeing processOrganic chemistryCopolymer
Owner:EI DU PONT DE NEMOURS & CO
Varenicline tablet composition
InactiveCN107753449AUniform contentDissolution stabilityNervous disorderInorganic non-active ingredientsVarenicline TartrateMagnesium stearate
The invention relates to a varenicline tablet composition, which belongs to the technical field of medicine. The technical scheme of the present invention is: the composition of unit dose contains: D50 is 0.5-1mg of varenicline tartrate of 50-76 microns, lactose 50-70mg, microcrystalline cellulose 12-26mg, calcium hydrogen phosphate 6-12mg, Sodium lauryl sulfate 0.8-1.6mg, magnesium stearate 0.8-1.5mg. The technical solution of the present invention obtains a varenicline tartrate tablet with uniform content and stable dissolution.
Owner:WEIHAI GUANBIAO INFORMATION TECH
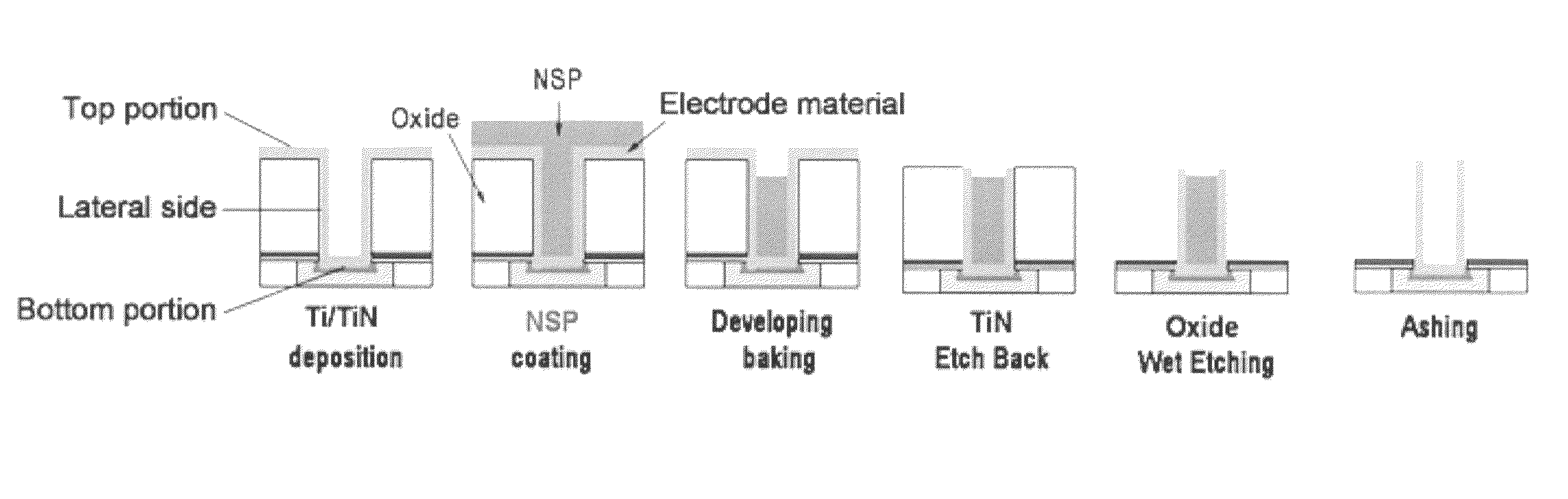
Gap-filling composition with excellent shelf life by end-capping
ActiveUS8642437B2Constant rateDissolution stabilitySemiconductor/solid-state device manufacturingDissolutionPolymer chemistry
A composition with improved shelf life for filling small gaps in a semiconductor device is provided. The composition comprises an end-capped silicone polymer. The molecular weight of the end-capped silicone polymer is not varied during storage. In addition, the dissolution rate (DR) of the composition in an alkaline developing solution is maintained at a desired level during storage. That is, the composition is highly stable during storage. Therefore, the composition is suitable for use in a node separation process for the fabrication of a semiconductor capacitor.
Owner:CHEIL IND INC
Amoxicillin and clavulanate potassium preparation and preparation method thereof
ActiveCN109248150AGood dispersionGood hygroscopicityAntibacterial agentsPharmaceutical non-active ingredientsMedicineCLAVULANATE POTASSIUM
The invention belongs to the technical field medicines, and provides an amoxicillin and clavulanate potassium preparation and a preparation method thereof. According to the dispersed tablet (the preparation) prepared by the technology, the amoxicillin and clavulanate potassium is high in stability, relatively good in dissolution effect and relatively low in related materials. The method provided by the invention can effectively shorten a technological process, reduce a production cycle and explosion time of medicines in time, guarantee stability of medicines and further improve quality of thefinished product (the amoxicillin and clavulanate potassium preparation).
Owner:LUNAN PHARMA GROUP CORPORATION
Polishing solution and method of polishing nonferrous metal materials
InactiveUS7090564B2Uniform thickness distributionHigh removal rateOther chemical processesPolishing compositions with abrasivesNonferrous metalMetallic materials
A polishing solution, comprising copper ions and chloride ions in Cu / Cl molar ratio of 10−1 to 103 and at pH 0.5 to 10, is suited for polishing a surface composed of a nonferrous metal material such as copper or copper alloy. Thicker metal film can be polished at high removal rate, so that distribution in film thickness becomes uniform.
Owner:C UYEMURA & CO LTD
Trimetazine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN109908096AAvoid degradationGuaranteed stabilityOrganic active ingredientsSenses disorderTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazine dihydrochloride sustained-release tablet which comprises trimetazidine dihydrochloride, a sustained-release matrix material, a filler, a binding agent and a lubricating agent. The sustained-release matrix material is a mixture of hydroxypropyl methyl cellulose with different viscosity. By the arrangement, the initial burst release of trimetazidine dihydrochloride in the early stage is stable, shortcomings of poor stability and easy degradation of trimetazidine dihydrochloride tablet are overcome, and the stability of the trimetazine dihydrochloride tabletis improved.
Owner:WUHAN WUYAO SCI & TECH
Effervescent tables for treating thrombus, and its prepn. method
InactiveCN1723971AEasy to takeShort disintegration timeOrganic active ingredientsPill deliverySodium bicarbonatePolyethylene glycol
A medicine Xuesaitong in the form of effervescent tablet for treating thrombus is proportionally prepared from arasaponin, lactose, rebaudiose, tartaric acid, sodium dicarbonate, phlyethanediol, and magnesium stearate. Its preparing process is also disclosed.
Owner:福寿堂制药有限公司 +1
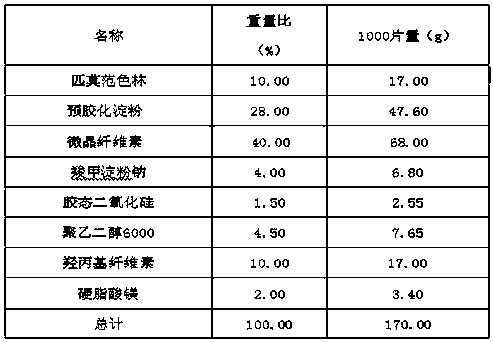
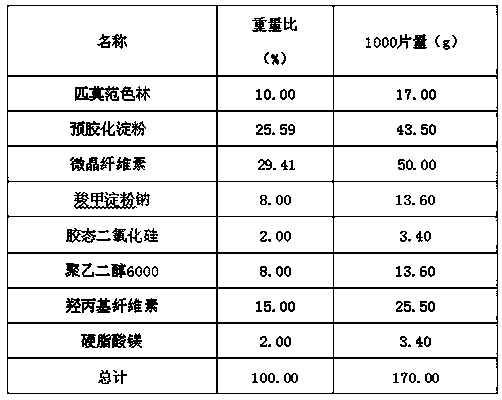
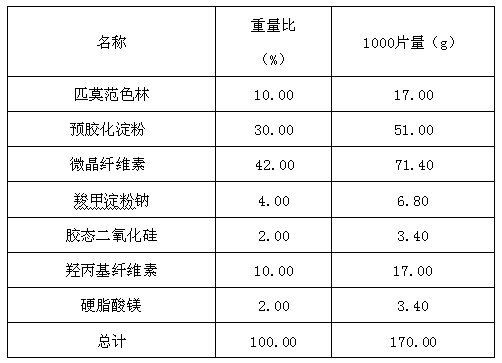
Pimavanserin tablet and preparation method thereof
InactiveCN109568278ADissolution stabilityReduce static electricityOrganic active ingredientsNervous disorderLow-substituted hydroxypropylcellulosePolyethylene glycol
The invention belongs to the field of pharmaceutic preparation, and particularly relates to a pimavanserin tablet and a preparation method thereof. The pimavanserin tablet is composed of active components of pimavanserin, colloidal silicon dioxide, polyethylene glycol, a filling agent selected from dextrin, corn starch, pregelatinized starch, cellulose microciystalline, mannitol and lactose, a disintegrating agent selected from carboxymethyl starch sodium, croscarmellose sodium, low-substituted hydroxypropyl cellulose and crospovidone, and a lubricating agent selected from magnesium stearate,talcum powder, and sodium stearyl fumarate. The pimavanserin tablet has good stability, and can significantly improve drug dissolution and bioavailability.
Owner:BEIJING VENTUREPHARM BIOTECH
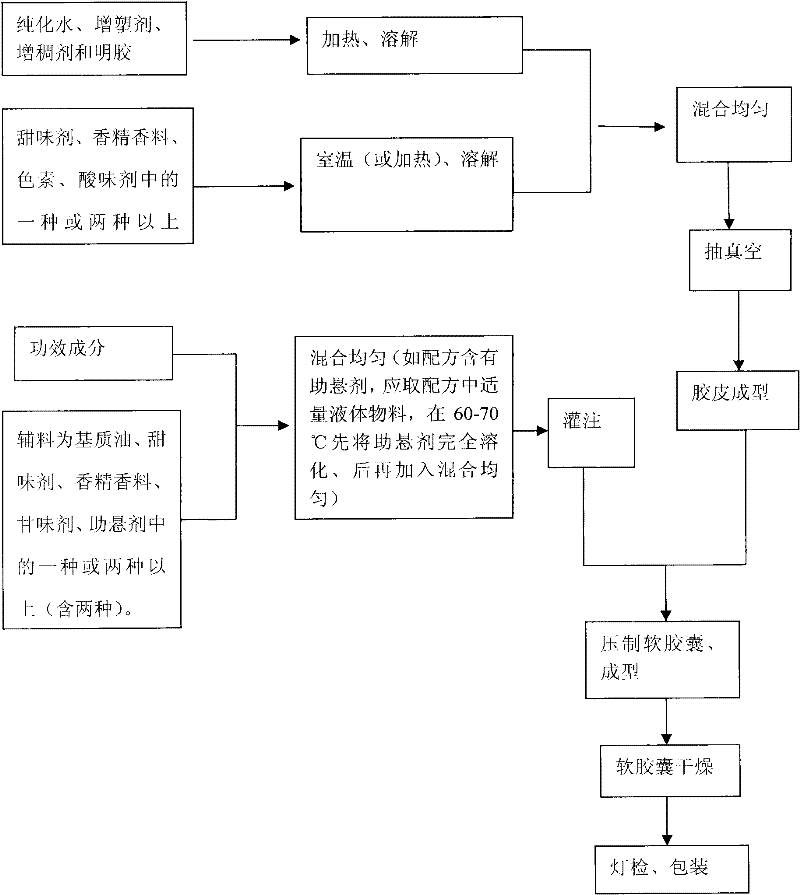
Chewable soft capsules and method for preparing same
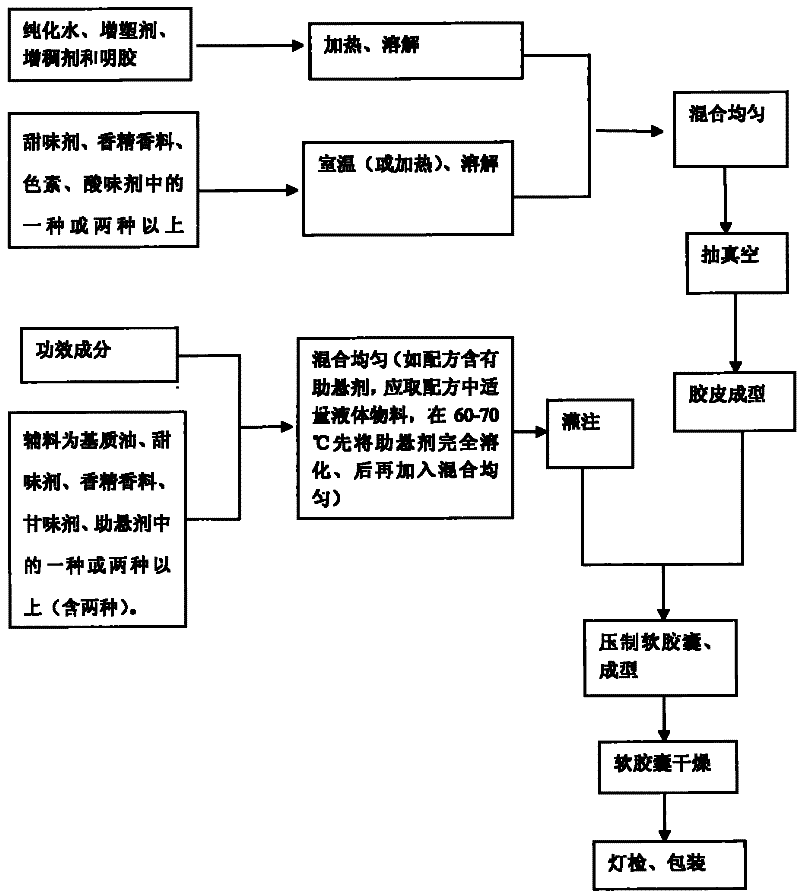
ActiveCN101810336BDissolution stabilityRapid dissolutionCapsule deliveryFood shapingPlasticizerDissolution
The invention discloses chewable soft capsules, which comprise capsule shells and contents sealed in the capsule shells. The chewable soft capsules are characterized by comprising the following raw materials in part by weight: 25 to 65 parts of glutin, 1 to 25 percent of thickening agent, 18 to 65 parts of plasticizer, 4 to 16 parts of water and a mixture containing one or more of 0.005 to 20 parts of sweetening agent, 0.005 to 2 parts of essence, 0.0001 to 10 parts of pigment and 0.0001 to 5 parts of acid additive. The invention also discloses a method for preparing the novel chewable soft capsules. The chewable soft capsules have the advantages of reasonable blending ratio, proper hardness, good temperature resistance, no adhesion, no deformation, stable storage, quick dissolution of effectively components and high chewiness; and when the chewing soft capsules are chewed, tastes of the capsule shells and the contents easily and uniformly spread in the mouth so as to cover up bad taste of raw materials.
Owner:SIRIO PHARMA CO LTD
Pharmaceutical soft gelatin capsule dosage form
InactiveUS20140271837A1Dissolution stabilityOrganic active ingredientsAntipyreticPlasticizerPolyethylene glycol
A pharmaceutical soft gelatin capsule dosage form that includes (a) a shell that includes gelatin and a plasticizer; and (b) a fill that includes at least one active ingredient, polyethylene glycol, polyacrylic acid, a neutralizing agent, and water. The neutralizing agent is a primary amine or a secondary amine, and is present in an amount necessary to provide a pharmaceutical soft gelatin capsule dosage form having stable dissolution after storage.
Owner:APTALIS PHARMA
Moxifloxacin capsule and its preparation method
ActiveCN100363001CHigh dissolution rateDissolution stabilityAntibacterial agentsOrganic active ingredientsMoxifloxacinBiomedical engineering
The invention provides a Moxifloxacin capsule, which comprises Moxifloxacin or its salts and / or hydrate, at least a disintegrating agent and at least a lubricating agent, the shell of the capsule contains hydroxy propyl ethyl cellulose. The invention also discloses the process for preparing the capsule.
Owner:JIANGSU TIANYISHI PHARMA
A kind of itraconazole pellet and its preparation method and preparation
ActiveCN103622918BPromote dissolutionHigh dissolution rateOrganic active ingredientsAntimycoticsOrganic solventItraconazole
The invention provides an itraconazole pellet. The itraconazole pellet is prepared by coating an itraconazole drug-containing layer and an isolated protective layer on the surface of the pellet core of the pellet in sequence, wherein the itraconazole drug-containing layer is prepared from itraconazole, a solid dispersion, a binder, an anti-sticking agent and an organic solvent; the isolated protective layer is prepared from a lubricant and a dispersion solvent. The invention further provides a preparation method of the itraconazole pellet and a preparation of a tablet or a capsule prepared by the itraconazole pellet. The itraconazole pellet disclosed by the invention can realize rapid dissolution, has dissolution rate of dissolving over 85% within 45 minutes, is remarkably superior to the existing itraconazole pellet, and can realize higher bioavailability, so that ideal medical effect is achieved.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
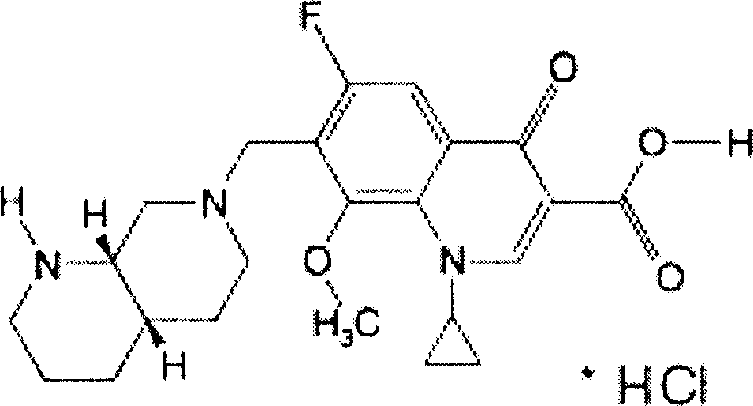
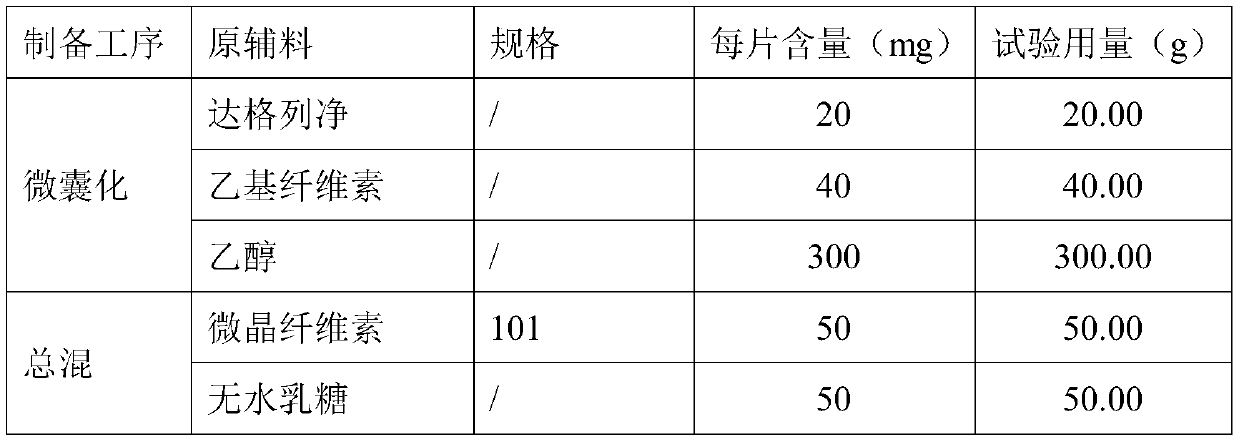
Dapagliflozin microencapsulated sustained-release tablet and preparation method thereof
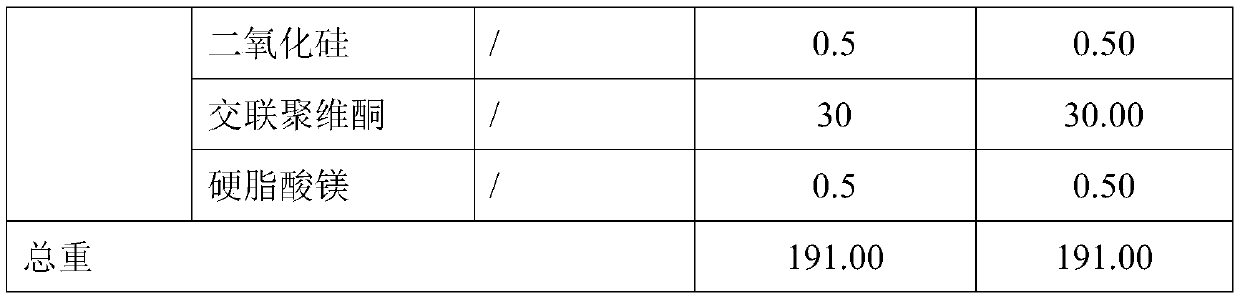
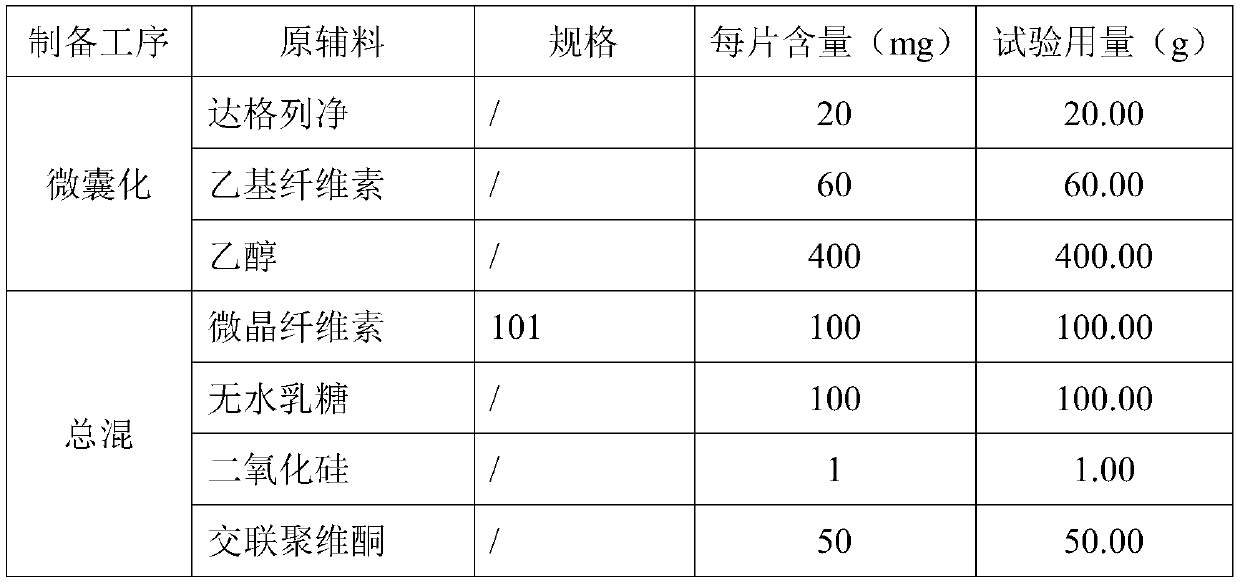
InactiveCN111481522ADissolution stabilityImprove utilizationOrganic active ingredientsMetabolism disorderProlonged-release tabletDrug release
The invention discloses a dapagliflozin microencapsulated sustained-release tablet and a preparation method thereof, and the dapagliflozin microencapsulated sustained-release tablet is prepared by thefollowing steps: preparing active components dapagliflozin and ethyl cellulose into microcapsules, uniformly mixing the microcapsules with other additional auxiliary materials, and tabletting. According to the dapagliflozin microencapsulated sustained-release tablet, a microencapsulation slow-release technology is adopted to control the drug delivery speed so as to prolong the curative effect ofthe drug, and the microencapsulated dapagliflozin raw material is prevented from being contacted with air, so that the interference of air moisture in the tabletting process is solved. Each micro-capsule is an independently controlled drug release unit, and drug release is uniform and stable. The microencapsulated sustained-release tablet can ensure the dissolution of the drug and the stability ofthe drug, and is simple in process and suitable for large-scale production.
Owner:乐普制药科技有限公司
Itraconazole pellet as well as preparation method and preparation thereof
ActiveCN103622918APromote dissolutionHigh dissolution rateOrganic active ingredientsAntimycoticsOrganic solventItraconazole
The invention provides an itraconazole pellet. The itraconazole pellet is prepared by coating an itraconazole drug-containing layer and an isolated protective layer on the surface of the pellet core of the pellet in sequence, wherein the itraconazole drug-containing layer is prepared from itraconazole, a solid dispersion, a binder, an anti-sticking agent and an organic solvent; the isolated protective layer is prepared from a lubricant and a dispersion solvent. The invention further provides a preparation method of the itraconazole pellet and a preparation of a tablet or a capsule prepared by the itraconazole pellet. The itraconazole pellet disclosed by the invention can realize rapid dissolution, has dissolution rate of dissolving over 85% within 45 minutes, is remarkably superior to the existing itraconazole pellet, and can realize higher bioavailability, so that ideal medical effect is achieved.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Probucol orally administered nanometer solid preparation and preparation method for same
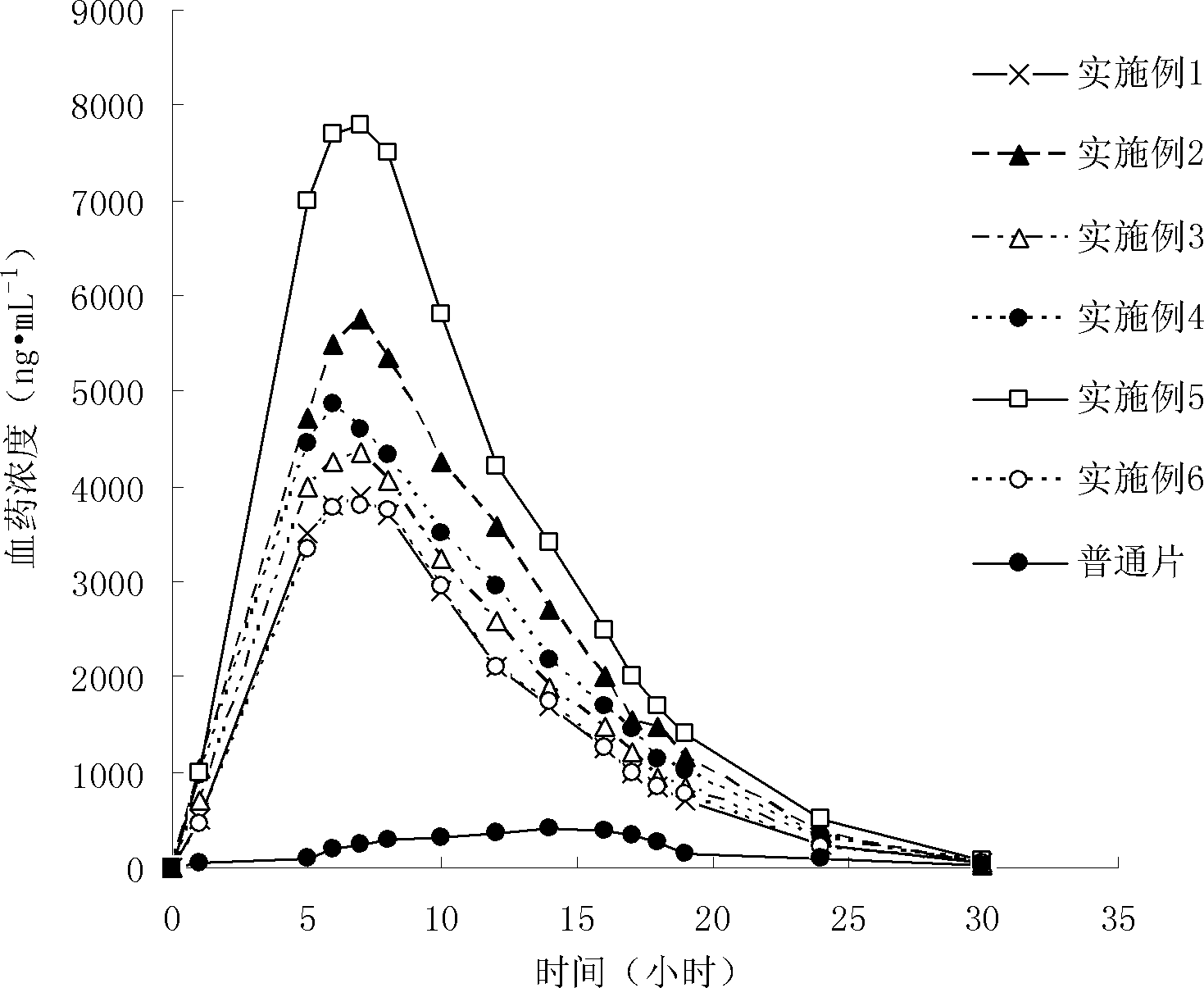
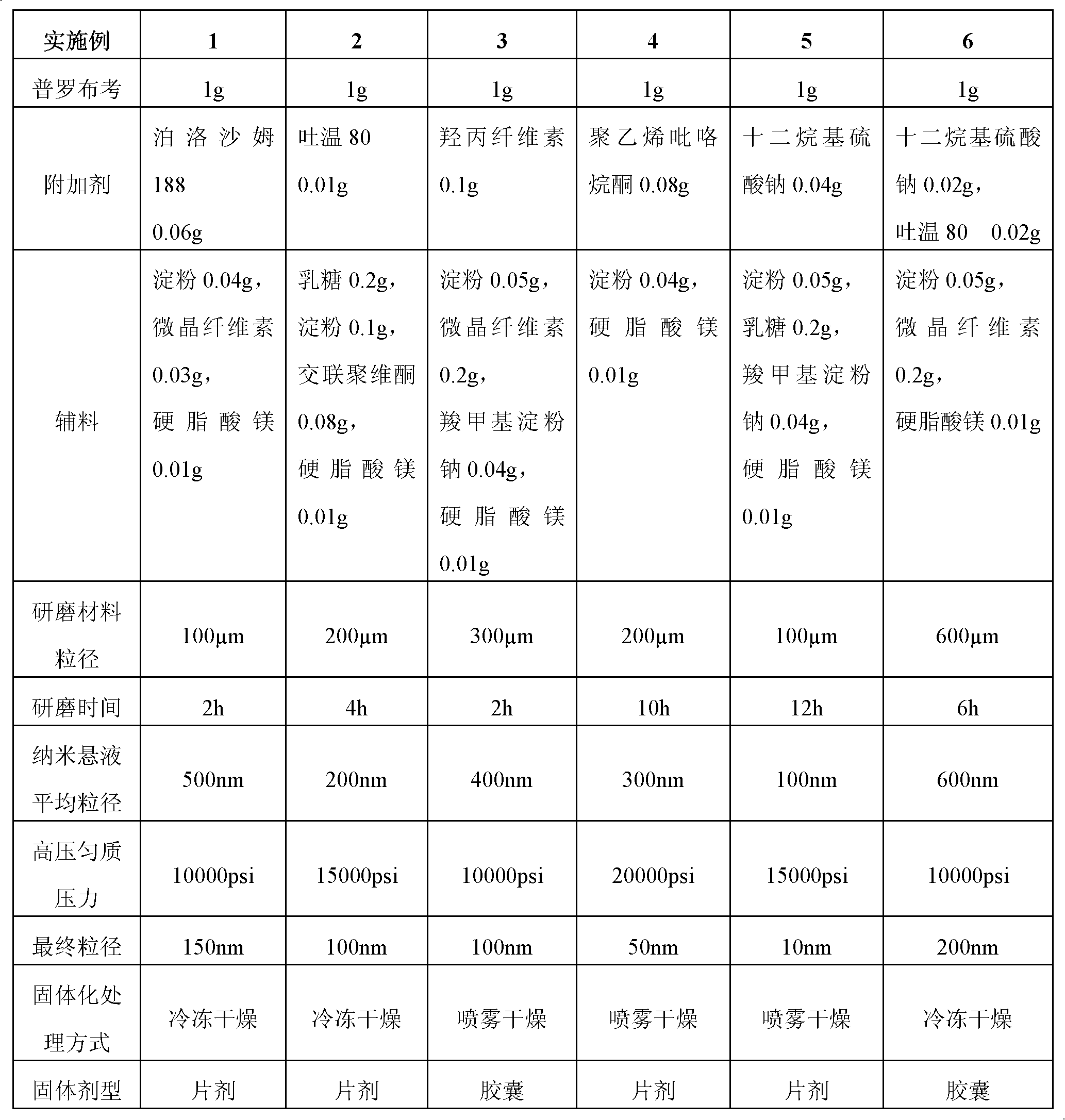
InactiveCN102475688BImprove in vitro dissolutionImprove bioavailabilityPowder deliveryMetabolism disorderHigh dosesDissolution
The invention provides a probucol orally administered nanometer solid preparation, which comprises, by weight, 1 part of probucol, 0.01-0.1 part of additive and 0.05-0.4 part of auxiliary materials. The grain size of the probucol ranges from 10nm to 200nm. The invention further provides a preparation method for the probucol orally administered nanometer solid preparation. The preparation method includes that the probucol and water liquor with 5% of additive are prepared into nanometer suspension with the grain size ranging from 100nm to 600nm by a wet grinding method; the nanometer suspension is homogenized into nanometer grains with the grain sizes ranging from 10nm to 200nm by a high-pressure homogenizing machine; and the nanometer suspension which is homogenized is solidified and is added with the auxiliary materials to be prepared into the orally administered nanometer solid preparation. Compared with a common probucol tablet, the preparation has the advantages that dissolution in vitro of the preparation is increased by 20-30 times, and bioavailability after oral administration is improved by 10-20 times. In addition, the drug loading rate of the preparation is high, and the preparation can meet the requirements of tablet weight clinically needed by high-dose probucol administration. Besides, the preparation is stable, and the dissolution of the preparation can be kept unchanged within two years.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Pharmaceutical composition for edoxaban tablets
InactiveCN108175753ASignificant progressShort disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineBioavailability
The invention provides pharmaceutical composition for edoxaban tablets. The composition is prepared from 10-50 parts of p-toluenesulfonic acid edoxaban monohydrate, 15-35 parts of a disintegrant, 2-10parts of a 5% polyvinylpyrrolidone 95% ethanol solution, 20-45 parts of filler and 0.5-2 parts of a lubricant, and has the advantages of high disintegration speed, high bioavailability, good stability and the like.
Owner:SICHUAN HAISCO PHARMA CO LTD
Cyclosporine-containing soft capsule capsule shell composition and preparation method, and cyclosporine-containing soft capsule prepared therefrom
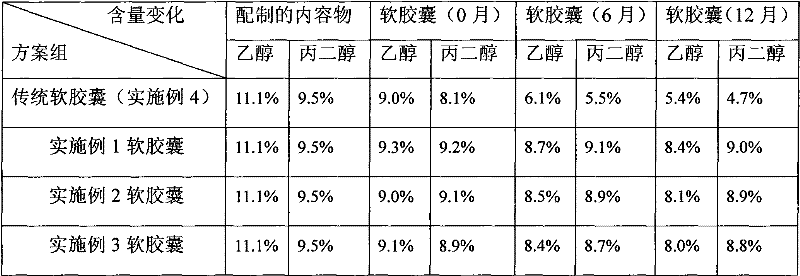
InactiveCN102284062ALower ethanolReduced Propylene Glycol ContentCyclic peptide ingredientsCapsule deliveryCyclosporinsCyclosporin C
The invention relates to a technology of a soft capsule containing ciclosporin, in particular to a capsule shell composition suitably prepared into a soft capsule containing the ciclosporin as well as a preparation method thereof and the soft capsule containing the ciclosporin prepared thereby. Gelatin, glycerin, sorbierite, propanediol, water, hydroxyphenyl ethylester and talcum powder are addedin a glue digester and heated to be completely dissolved to obtain a capsule shell; and the capsule shell and a content solution are wrapped by a soft capsule machine to obtain the soft capsule. According to the capsule shell composition for the soft capsule containing the ciclosporin, which is disclosed by the invention, the contents of ethanol and the propanediol in the content of the soft capsule are ensured not to be greatly reduced in the storage process, the medicament dissolving of the ciclosporin can be ensured and the bioavailability of the ciclosporin is improved. Various raw materials provided for the capsule composition are common varieties and have low price, thus the capsule composition is suitable for large-scale industrial production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Rumen bypass product and production method thereof
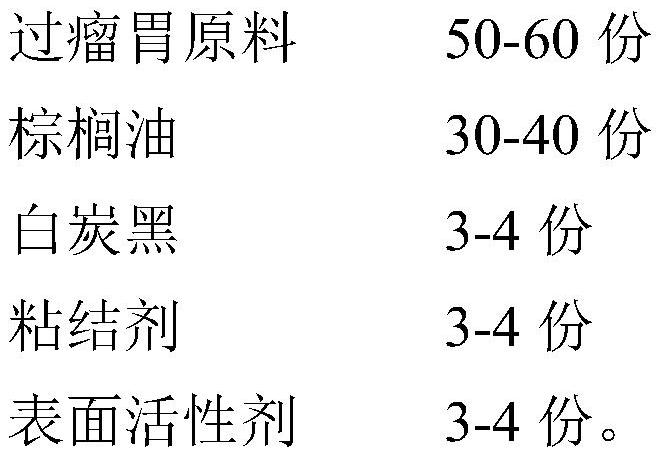
PendingCN112544803AFree from destructionDissolution stabilityAccessory food factorsWorking-up animal fodderBiotechnologyActive agent
The invention relates to a production method for preparing a rumen bypass series product, and belongs to the technical field of feed and feed additives. The rumen bypass series product comprises aminoacid, choline chloride, glucose, urea or ammonium chloride. The production method comprises the following steps of sieving the raw materials, adding auxiliary materials, performing granulating, rounding and drying, heating and melting palm oil, adding a surfactant, spraying the surfactant into the dried materials in a fluidized bed, performing coating, and after coating, performing sieving to obtain the rumen bypass series product. Compared with the prior art, the method has the advantages that the production cost is relatively low, and the prepared rumen bypass series product is regular in shape, relatively narrow in particle size distribution, easy to add and premix and relatively high in product percent of pass.
Owner:WUXI ZHENGDA POULTRY
Clean process for preparing activated aluminum oxide by using pulverized fuel ash
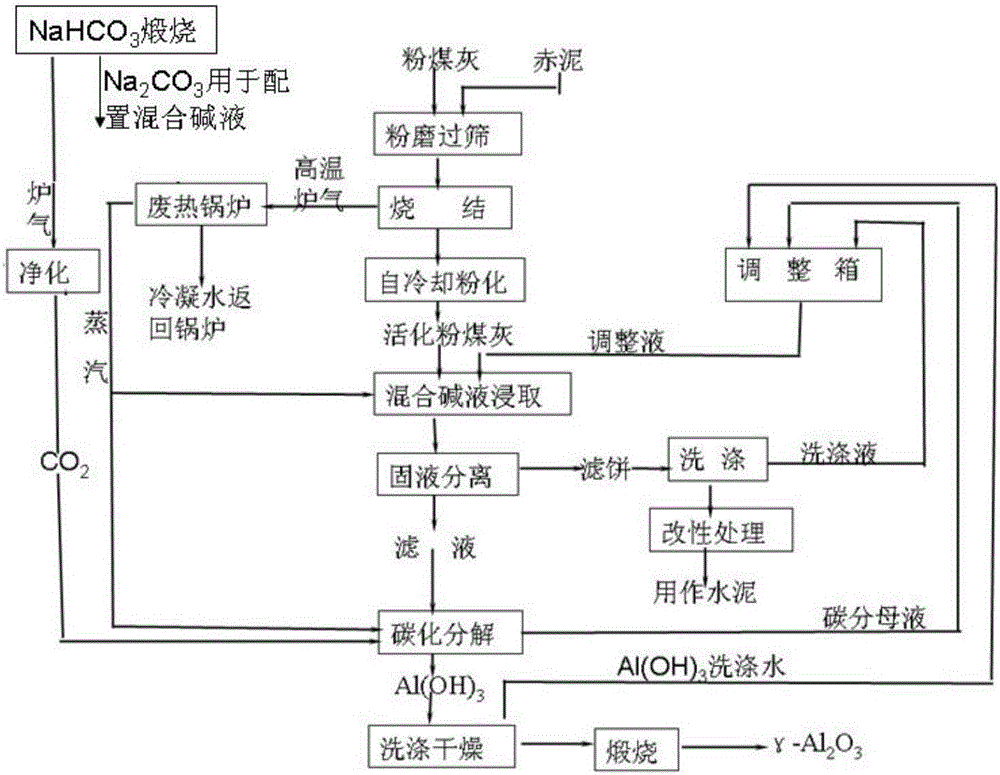
InactiveCN106044813ALow activation temperatureReduce the difficulty of operationAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationThree levelSludge
The invention discloses a clean process for preparing activated aluminum oxide by using pulverized fuel ash. The clean process comprises the following steps that 1, pulverized fuel ash is activated; 2, leaching with a mixed alkaline liquor and solid-liquid separation are performed; 3, carbonating disintergration and wash-drying are performed; 4, calcination is performed. The red sludge from an aluminum oxide enterprise is used for replacing limestone, the pulverized fuel ash is activated by adopting a low-temperature sintering process, and then Al2O3 is dissolved out of the mixed alkaline liquor. Waste residues are modified, waste water is treated, energy is recovered, the resource utilization rate and waste recovery rate are high, and the cleaning degree of the process meets the requirement for three-level clean production in the domestic aluminum oxide industry.
Owner:YIBIN UNIV
Guanfacine implant and preparing method thereof
InactiveCN106236698ADissolution stabilityEffective blood concentrationOrganic active ingredientsNervous disorderMass ratioPolyethylene glycol
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a guanfacine implant and a preparing method thereof. The subcutaneous implant comprises guanfacine or salt and carrier thereof, and the mass ratio of guanfacine to the carrier is 10:(1-100). The carrier is a biodegradable material such as polylactic acid-glycollic acid copolymer, polyethylene glycol-poly lactic acid-glycolic acid segmented copolymer, polylactide, polyglycollide and polycaprolactone, and the average molecular weight of the carrier is 8000-100000. The invention further provides the preparing method of the implant. The blank of the prior art is filled, and the preparation is stable in dissolution, long-acting and safe, and has broad application prospects.
Owner:BEIJING COLLAB PHARMA
Industrial production method of titanium-containing soluble solid solution anode
The invention relates to an industrial production method of a titanium-containing soluble solid solution anode, and belongs to the technical field of titanium smelting. The industrial production method comprises a melting and stirring step, a casting forming step, a cooling step and a shaping step. According to the industrial production method, titanium-containing soluble solid solution are adopted as raw materials, melting into the liquid state under the conditions that the temperature is higher than the melting point of the raw materials and under the protection of the inert atmosphere, andare fully stirring, then pouring the melt into a mold preheated to be 1000 DEG C or higher for casting forming, cooling under the protection of the inert atmosphere at a certain cooling speed, after the cooled anode is subjected to shaping treatment, and the cooled anode can be directly used for molten salt electrolysis to produce the metal titanium. The industrial production method of the titanium-containing soluble solid solution anode is simple in process, high in efficiency and low in cost, and the produced anode has the advantages of being high in density, good in conductivity, free of defects in the interior and the like. By adopting the anode for industrial production to carry out molten salt electrolysis to prepare the metal titanium, the anode can be stably dissolved for a long time, the anode without slag falling during the electrolysis process, and the electrochemical dissolution efficiency of the anode is 90% or higher.
Owner:UNIV OF SCI & TECH BEIJING
Loxoprofen sodium tablet and preparation process thereof
ActiveCN111700868AGood consistencyStable in natureOrganic active ingredientsAntipyreticDrugs preparationsSaccharolipid
The invention discloses a loxoprofen sodium tablet and a preparation process thereof, and belongs to the technical field of pharmaceutical preparations. The loxoprofen sodium tablet is prepared from the following components: loxoprofen sodium, microcrystalline cellulose, sophorolipid, konjac glucomannan, crospovidone and magnesium stearate. The preparation process includes the steps that loxoprofen sodium, microcrystalline cellulose, sophorolipid, konjac glucomannan and crospovidone in the formula amount are evenly mixed to obtain a mixture 1, and a hot melt extruder is used to prepare the mixture 1 into wet granules; after the prepared wet granules are dried, magnesium stearate of the formula amount is added to obtain a mixture 2; the mixture 2 is tableted to obtain plain tablets; and theplain tablets are coated to obtain the loxoprofen sodium tablets. The dissolution rate of the loxoprofen sodium tablet is good in consistency with an original research drug, meanwhile, the property is stable, the dissolution rate remains stable after being placed for a certain period of time, and meanwhile, the hot melt extruder is used for granulation, so that the operation is simple.
Owner:福建东瑞制药有限公司
Amoxicillin tablets and preparation method thereof
InactiveCN108653224AImprove solubilityImprove stabilityAntibacterial agentsPill deliveryDrug productAmoxicillin
The invention provides amoxicillin tablets and a preparation method thereof. According to the method, hydroxypropyl-beta-cyclodextrin and amoxicillin form an inclusion compound, and then, the inclusion compound is granulated, dried and tableted. The prepared amoxicillin tablets have the advantages of good stability, high dissolubility and low related substance content, an auxiliary material formula is optimized, the mixed powder fluidity is good, and sticking is avoided in the tableting process; by means of the preparation method, the technological process is effectively shortened, the production cycle and exposure time of the medicine in the air are shortened, the stability of the medicine is guaranteed, and the product quality is further improved.
Owner:宁波蒙曼生物科技有限公司
Olaparib dissolution enhancing composition
ActiveCN113350349AHigh solubilizing abilityImprove solubilityOrganic active ingredientsPill deliveryEngineeringBULK ACTIVE INGREDIENT
The invention relates to an olaparib dissolution enhancing composition, a preparation method and application thereof, as well as a medicine containing the olaparib dissolution enhancing composition. The olaparib dissolution enhancing composition is prepared from the following components: olaparib, copovidone and a dissolution accelerator, wherein based on 100 parts by weight of olaparib, the copovidone is more than 100 parts by weight and less than 200 parts by weight, and the dissolution accelerator is 20-150 parts by weight. The olaparib dissolution enhancing composition and the medicine prepared from the olaparib dissolution enhancing composition have controllable stability, can be used for improving oral absorption of active ingredients, and can improve the medication convenience of patients since auxiliary dosage is reduced so as to facilitate industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Pharmaceutical composition
InactiveCN104706615AImprove the operating environmentSolve the problem of large differences in dissolution between different batchesOrganic active ingredientsNervous disorderStearic acidCroscarmellose sodium
The invention relates to a pharmaceutical composition containing a dexzopiclone tablet, belonging to the technical field of medicine. Commercially available dexzopiclone tablets are of three specifications, i.e., 1 mg, 2 mg and 3 mg. Due to small specifications of the dexzopiclone tablets, content uniformity of prepared tablets can hardly achieve standards; and since the dexzopiclone is hardly soluble in water, reproducibility of the dissolution data of tablets of different batches is poor. The technical scheme of the invention is as follows: the dexzopiclone tablet is characterized by containing, by mass, 1% of dexzopiclone with D90 of below 30 mu m, 0 to 77.5% of dicalcium phosphate dihydrate, 20 to 97.5% of microcrystalline cellulose, 1% of croscarmellose sodium and 0.5% of magnesium stearate. The technical scheme of the invention overcomes the above-mentioned problems in the prior art.
Owner:WEIHAI DISU PHARMA CO LTD +1
Method for preparing Niraparib microcapsule preparation
InactiveCN111991366AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsPolyvinyl alcoholBoronic acid
The invention belongs to the technical field of pharmaceutical preparations and relates to a method for preparing a Niraparib microcapsule preparation. According to the method, the Niraparib microcapsule preparation is prepared through taking a principal drug Niraparib and an adjuvant boric acid as capsule cores, adding polyvinyl alcohol to prepare Niraparib microcapsules, and then, uniformly mixing the microcapsules with other auxiliaries. The Niraparib microcapsule preparation prepared by the method is high in drug loading capacity and good in entrapment rate, the stability of the Niraparibis improved remarkably, adverse reactions of the Niraparib are reduced, drugs are released stably in vivo, actions of the Niraparib are better exerted, and thus, the Niraparib microcapsule preparationis suitable for being industrially produced on a large scale.
Owner:湖南博隽生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com