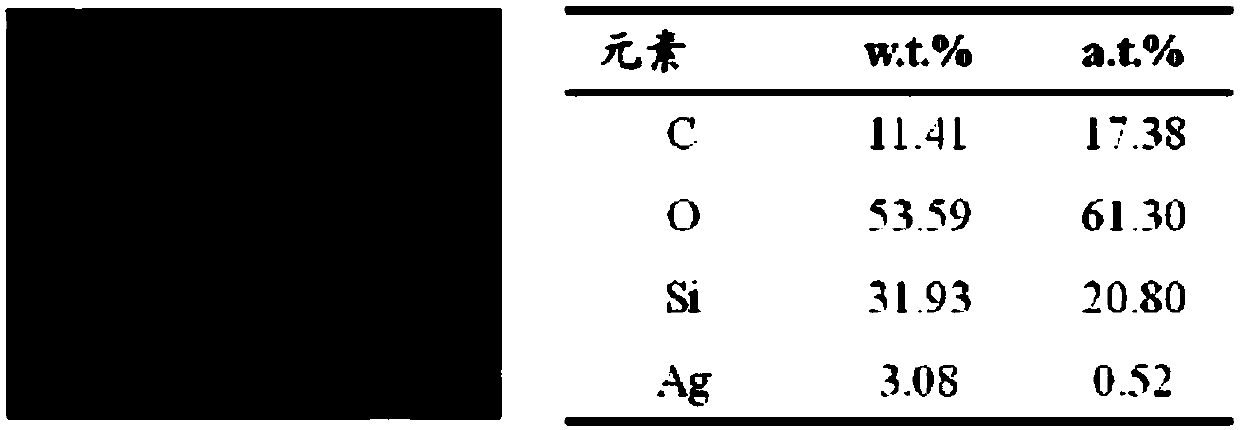
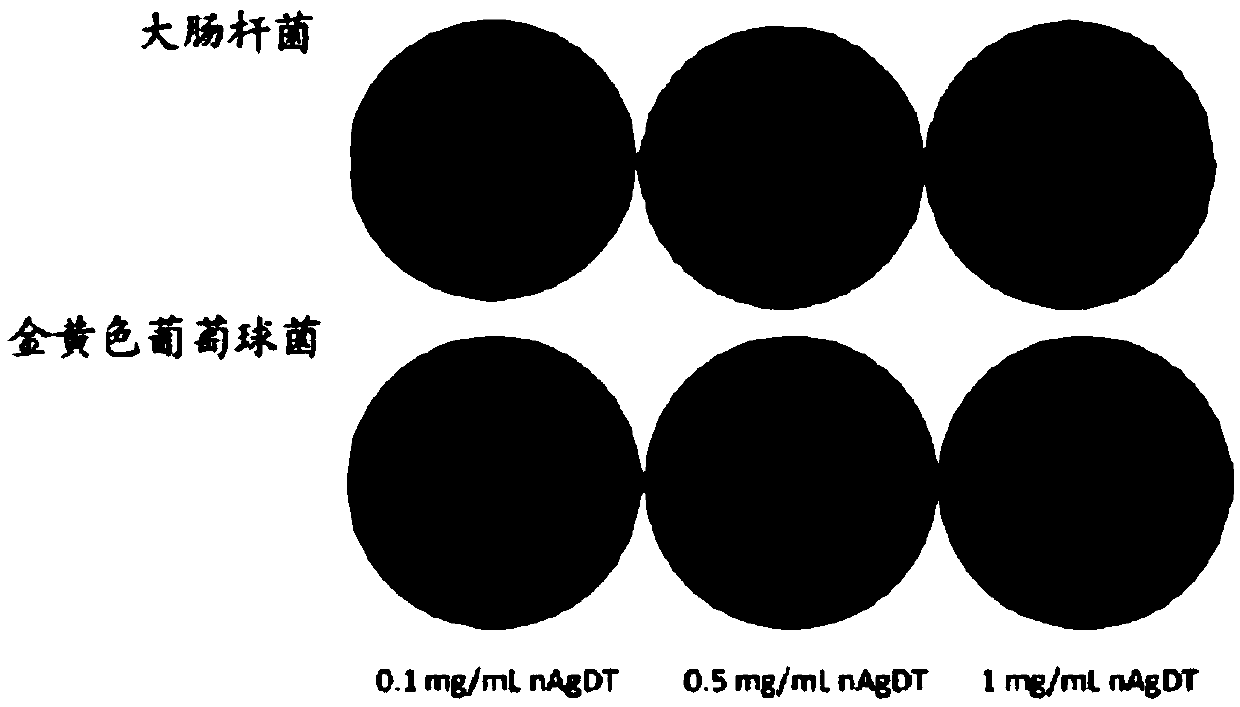
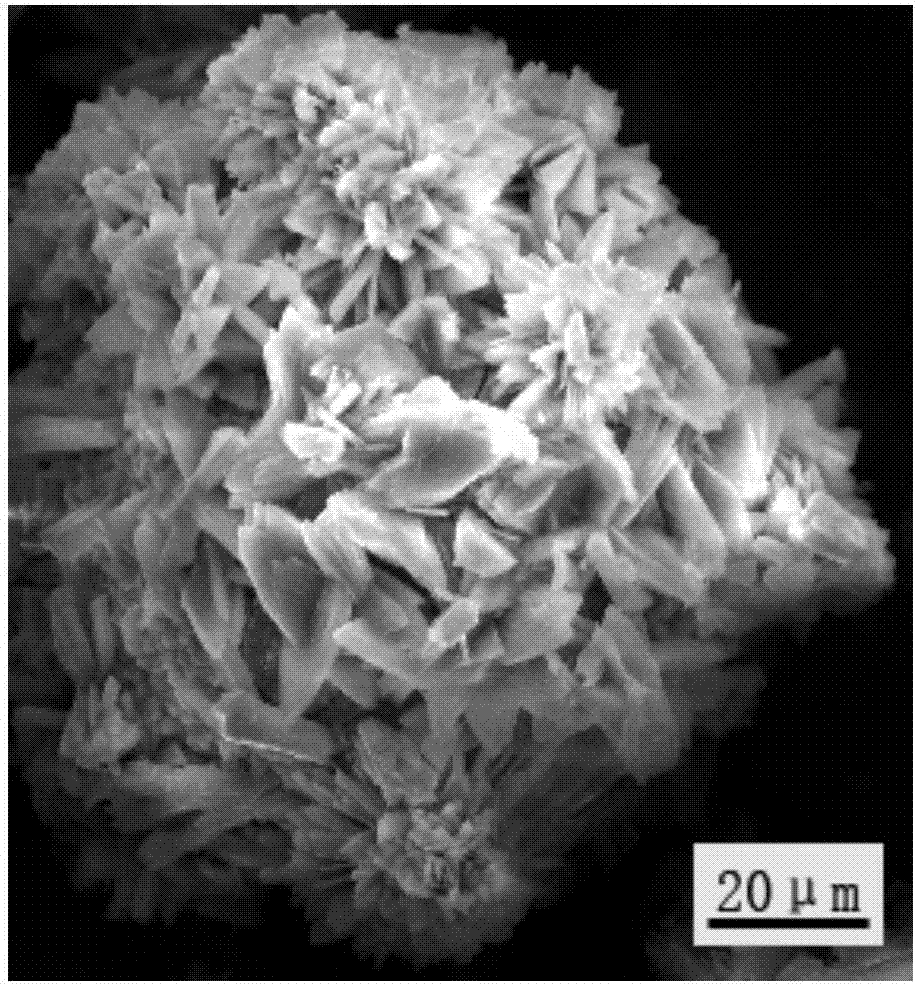
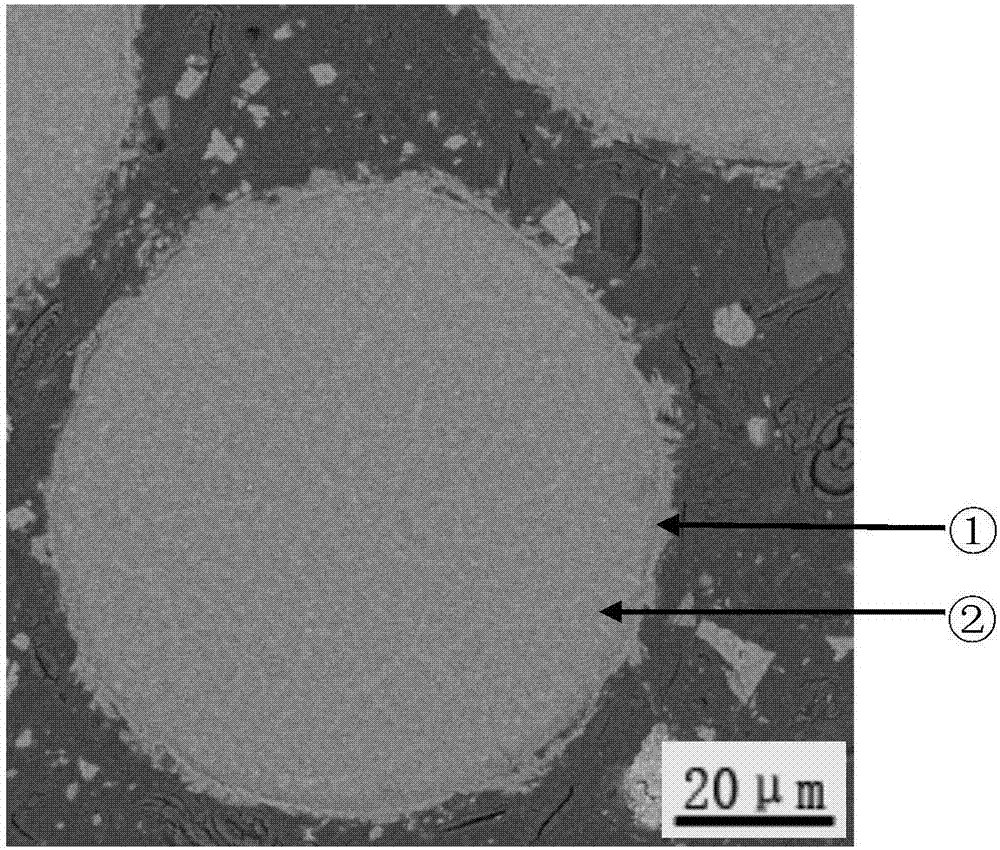
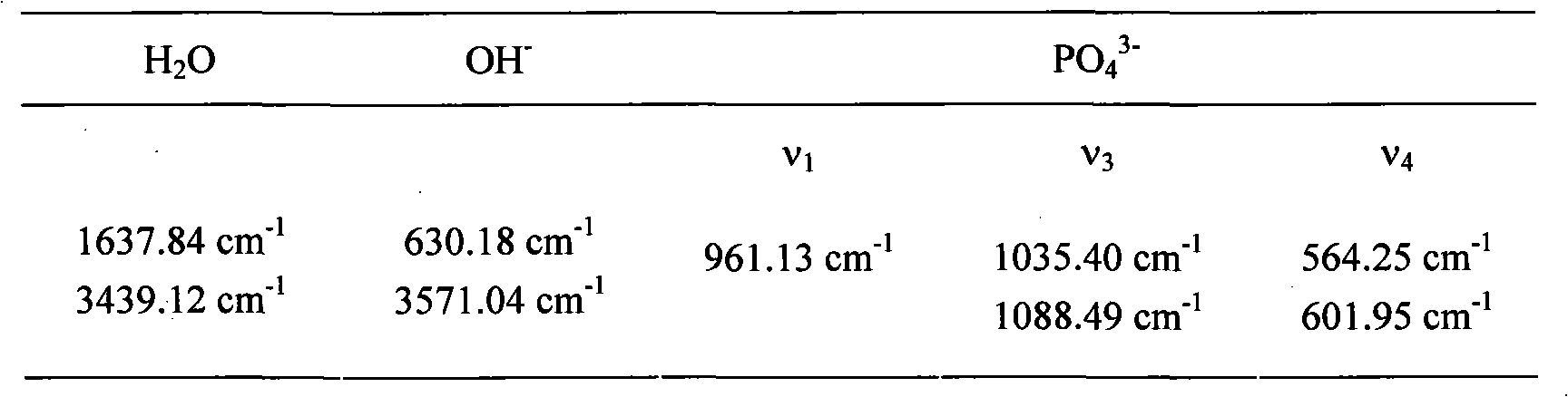
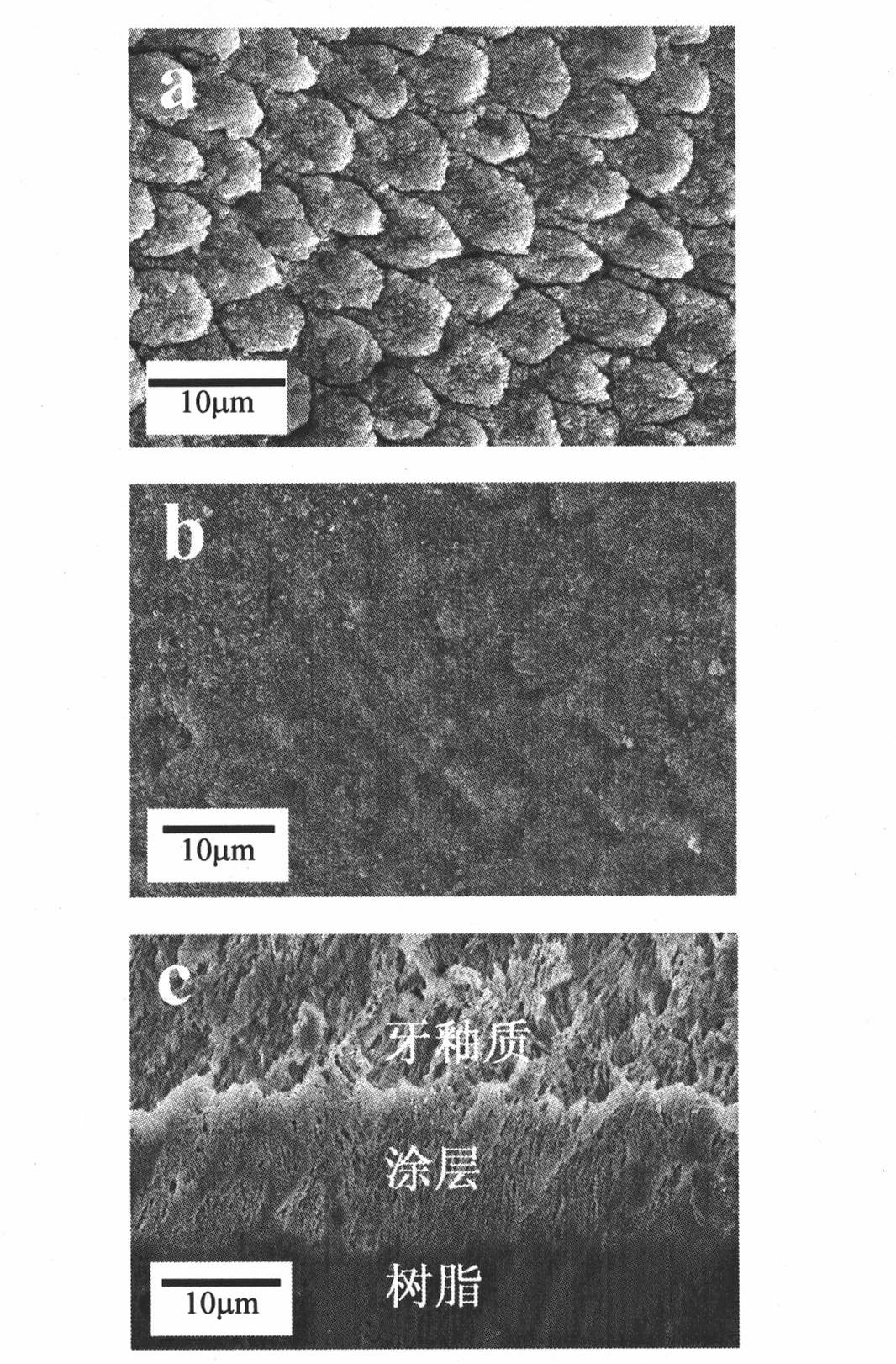
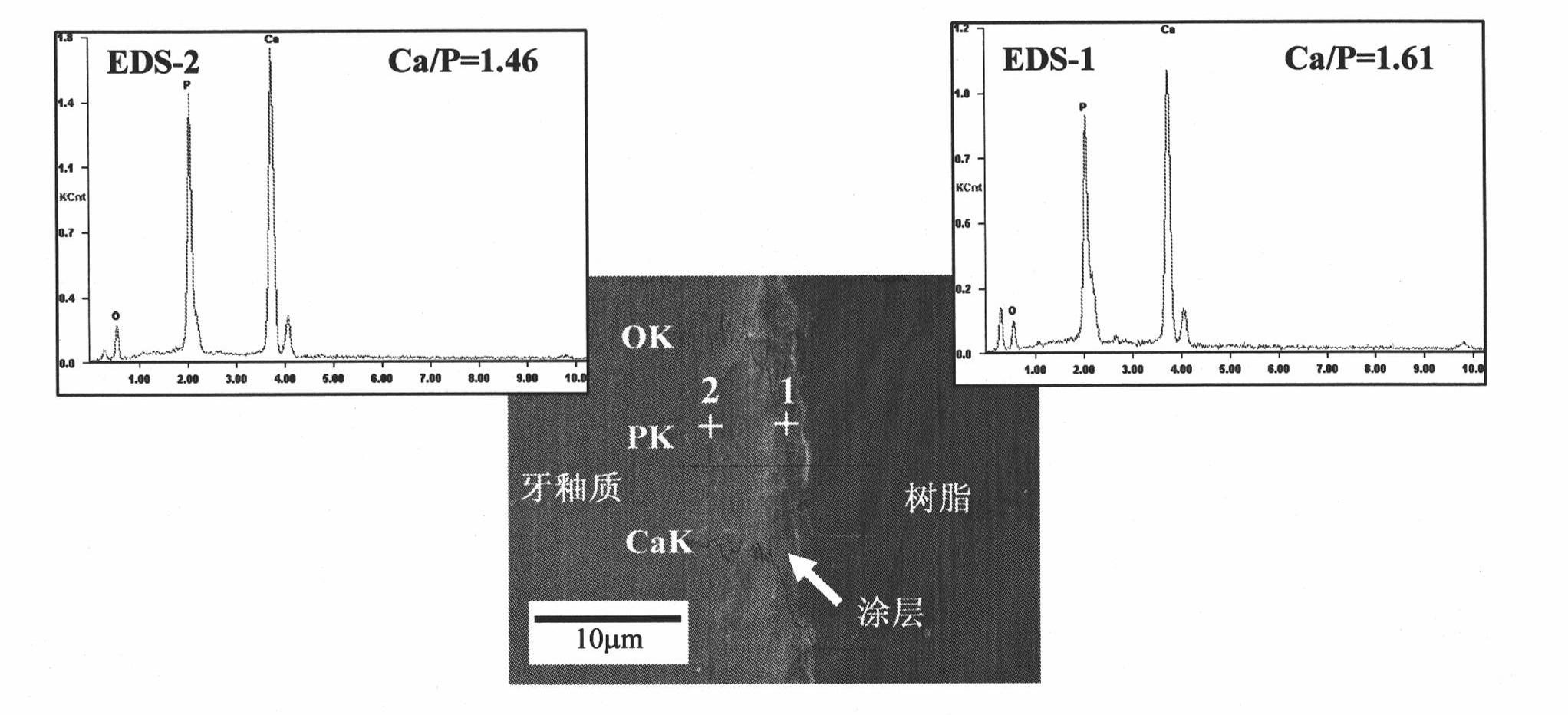
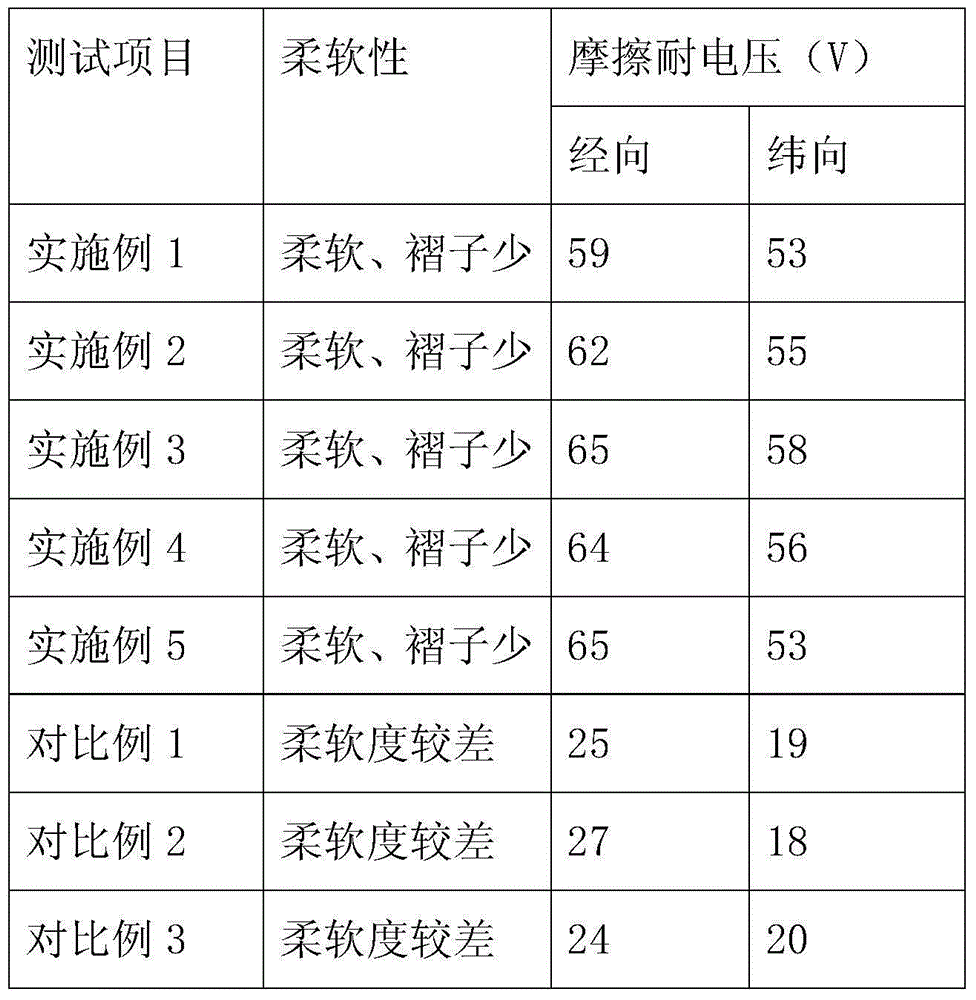
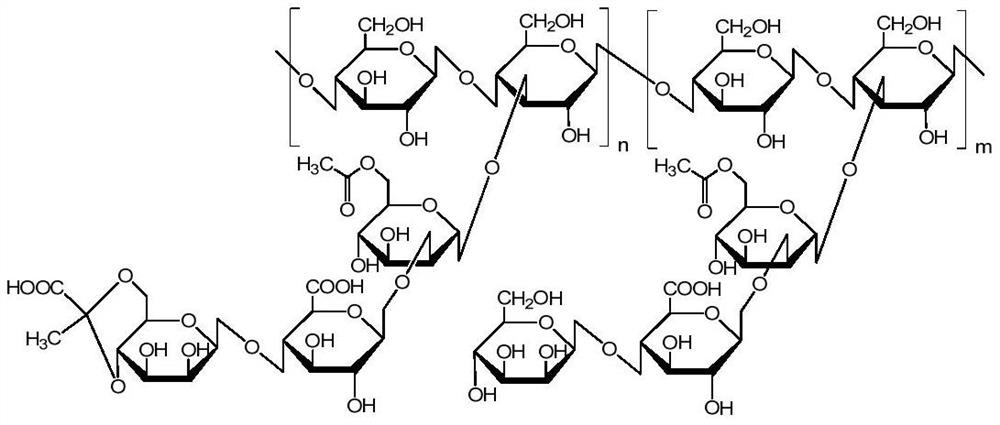
Patents
Literature
31 results about "Dicalcium phosphate dihydrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
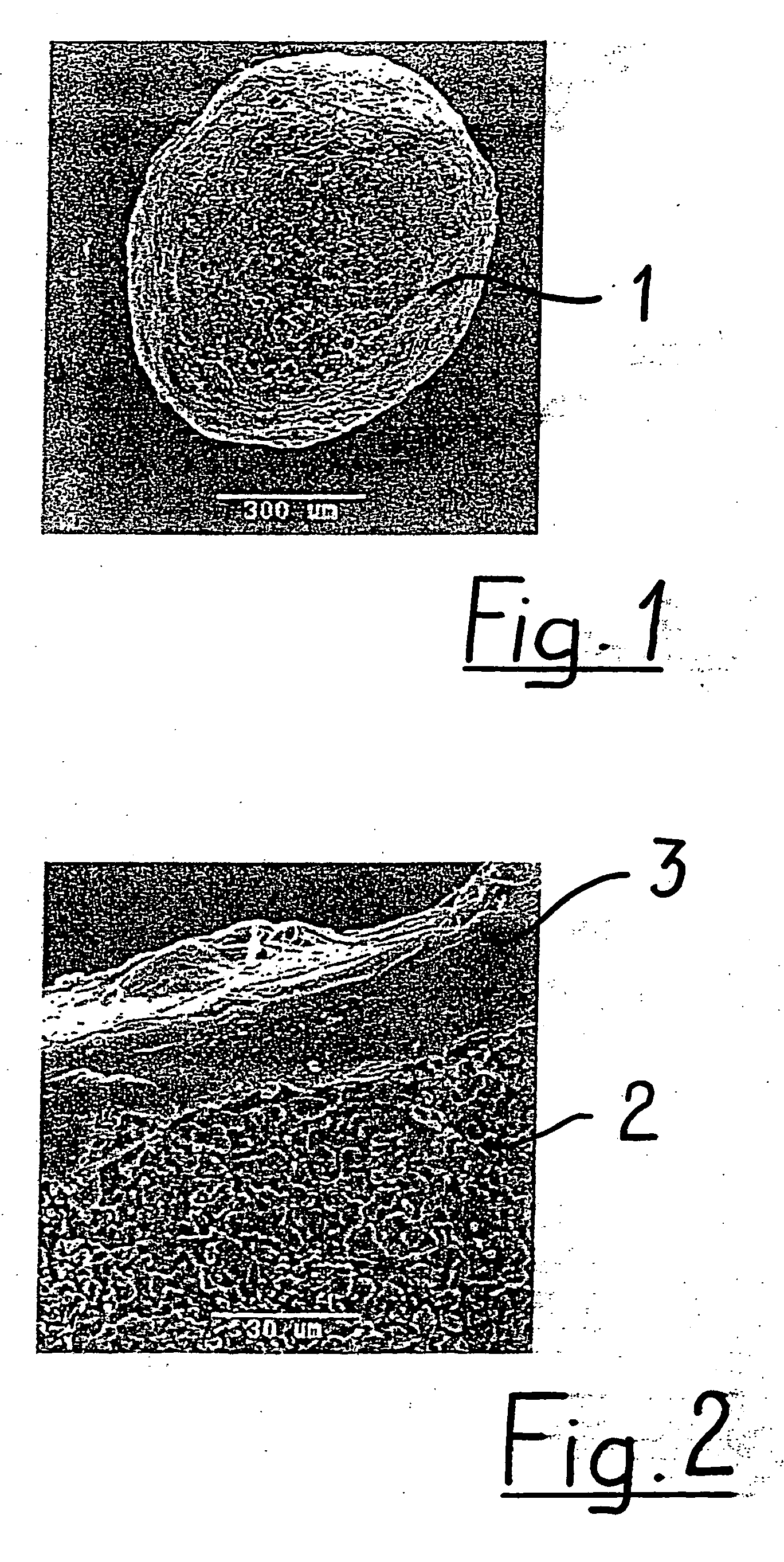
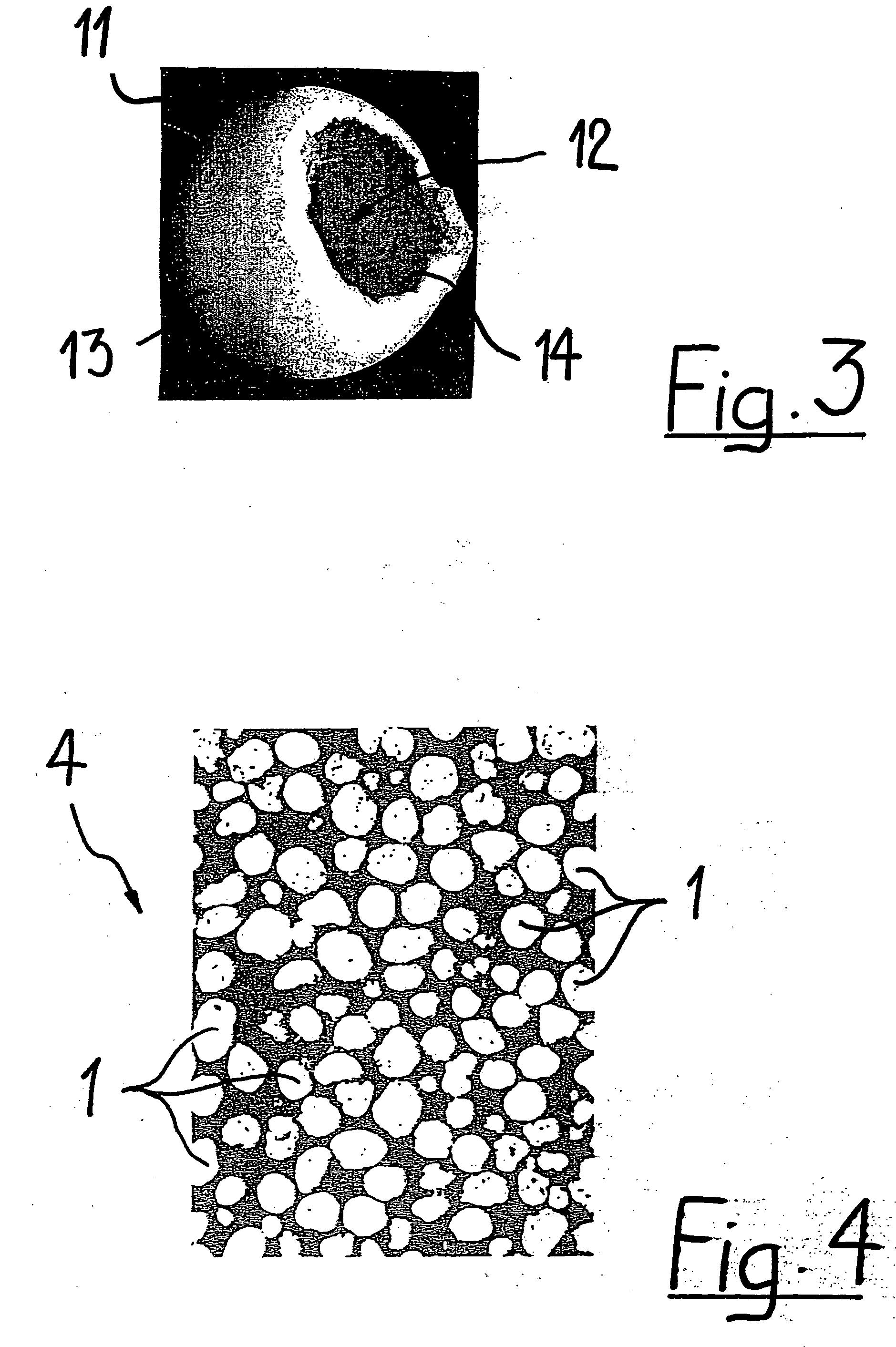
Porous biocompatible implant material and method for its fabrication
a biocompatible and biodegradable implant for a cavity in a bone of a living organism is made of a biocompatible and biodegradable granules which are selected from the group including biopolymers, bioglasses, bioceramics preferably calcium sulfate, calcium phosphate such as monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, tetracalcium phosphate, calcium orthophosphate phosphate, α-tricalcium phosphate, β-tricalcium phosphate, apatite such as hydroxyapatite, or a mixture thereof. The biocompatible and biodegradable granules are provided with a coating, which comprises at least one layer of a biocompatible and biodegradable polymer which is selected from the group including poly((α-hydroxyesters), poly(orthoesters), polyanhydrides, poly(phosphazenes), poly(propylene fumarate), poly(ester amides), poly(ethylene fumarate), polylactide, polyglycolide, polycaprolactone, poly(glycolide-co-trimethylene carbonate), polydioxanone, co-polymers thereof and blends of those polymers. The biocompatible and biodegradable implants are obtained by fusing together the polymer-coated granules through polymer-linkage of the polymer coatings of neighboring granules.
Owner:COLLAGEN MATRIX
Dentinal tubule sealant and method for producing the same
InactiveUS20130189337A1Impart caries resistanceSubstance may accumulateCosmetic preparationsImpression capsCalcium biphosphateDentinal Tubule
A dentinal tubule sealant comprises poorly-soluble calcium phosphate particles (A), a phosphorus-free calcium compound (B), and water (C), wherein the particles (A) are at least one member selected from the group consisting of dicalcium phosphate anhydrous [CaHPO4] particles, α-tricalcium phosphate [α-Ca3(PO4)2] particles, β-tricalcium phosphate [β-Ca3(PO4)2] particles, amorphous calcium phosphate [Ca3(PO4)2.nH2O] particles, calcium pyrophosphate [Ca2P2O7] particles, calcium pyrophosphate dihydrate [Ca2P2O7.2H2O] particles, octacalcium phosphate pentahydrate [Ca8H2(PO4)6.5H2O] particles, and dicalcium phosphate dihydrate [CaHPO4.2H2O] particles, and the dentinal tubule sealant contains 30 to 76% by weight of the particles (A), 0.001 to 4% by weight of the compound (B), and 23 to 69% by weight of the water (C). Thus, there is provided a dentinal tubule sealant capable of sealing dentinal tubules of an exposed dentin and also remineralizing the surrounding dentin after the sealing.
Owner:KURARAY NORITAKE DENTAL
Calcium phosphate cement
The present invention relates to a calcium phosphate composition comprising at least one calcium phosphate mineral, at least one reaction retarder, at least one binder and at least one sodium phosphate compound, and to a calcium phosphate cement , comprising a first powder component of stabilized dicalcium phosphate dihydrate containing 10-60 ppm magnesium, a second powder component comprising at least one other calcium phosphate mineral in addition to said stabilized dicalcium phosphate dihydrate, and a third powder component comprising water.
Owner:HOWMEDICA OSTEONICS CORP
Method for stabilization of heavy metals and odor control with dicalcium phosphate dihydrate powder
InactiveUS20070225541A1Reduce wasteSolid waste disposalTransportation and packagingOdor controlPhosphoric acid
This invention provides a method for stabilization and treatment of heavy metal bearing materials and wastes subject to acid leaching tests or leach conditions and odor limits by addition of acid semi-soluble DiCalcium Phosphate DiHydrate such that the leaching potential is inhibited to desired levels and odors are reduced to desired levels and the material or waste is free flowing, more permeable, less weight and permits immediate handling and disposal or reuse. The resultant material or waste after stabilization is deemed suitable for on-site reuse, off-site reuse or disposal as RCRA non-hazardous waste.
Owner:FORRESTER KEITH E
Method for stabilization of heavy metals and odor control with dicalcium phosphate dihydrate powder
InactiveUS20090209800A1Reduce wasteSolid waste disposalTransportation and packagingOdor controlPhosphoric acid
This invention provides a method for stabilization and treatment of heavy metal bearing materials and wastes subject to acid leaching tests or leach conditions and odor limits by addition of acid semi-soluble pulverized or fine particle DiCalcium Phosphate DiHydrate such that the leaching potential is inhibited to desired levels and odors are reduced to desired levels and the material or waste is free flowing, more permeable, less weight and permits immediate handling and disposal or reuse. The resultant material or waste after stabilization is deemed suitable for on-site reuse, off-site reuse or disposal as RCRA non-hazardous waste.
Owner:FORRESTER KEITH E
Method for stabilization of heavy metals and odor control with dicalcium phosphate dihydrate powder
This invention provides a method for stabilization and treatment of heavy metal bearing materials and wastes subject to acid leaching tests or leach conditions and odor limits by addition of acid semi-soluble pulverized or fine particle DiCalcium Phosphate DiHydrate such that the leaching potential is inhibited to desired levels and odors are reduced to desired levels and the material or waste is free flowing, more permeable, less weight and permits immediate handling and disposal or reuse. The resultant material or waste after stabilization is deemed suitable for on-site reuse, off-site reuse or disposal as RCRA non-hazardous waste.
Owner:FORRESTER KEITH E
Method for stabilization of heavy metals in incinerator bottom ash and odor control with dicalcium phosphate dihydrate powder
This invention provides a method for stabilization and treatment of heavy metal bearing materials and wastes subject to acid leaching tests or leach conditions and odor limits by addition of acid semi-soluble DiCalcium Phosphate DiHydrate such that the leaching potential is inhibited to desired levels and odors are reduced to desired levels and the material or waste is free flowing, more permeable, less weight and permits immediate handling and disposal or reuse. The resultant material or waste after stabilization is deemed suitable for on-site reuse, off-site reuse or disposal as RCRA non-hazardous waste.
Owner:FORRESTER KEITH E
Flat bread dough composition and method for making flat breads
InactiveUS20120263854A1Dough composition to relaxDough treatmentPre-baking dough treatmentSodium aluminum phosphateSodium phosphates
The present invention relates to a flat bread dough composition, comprising: A) flour; B) shortening; C) water; and D) a leavening system having a leavening base and a leavening acid having a combination selected from the group consisting of a) sodium aluminum phosphate and dicalcium phosphate dihydrate; b) sodium aluminum phosphate and sodium acid pyrophosphate; c) sodium acid pyrophosphate and dicalcium phosphate dihydrate; d) sodium acid pyrophosphate, monocalcium phosphate, and calcium acid pyrophosphate; e) dicalcium phosphate dehydrate, monocalcium phosphate, and calcium acid pyrophosphate; f) sodium aluminum phosphate, sodium acid pyrophosphate, and dicalcium phosphate dihydrate; g) sodium aluminum sulfate and dicalcium phosphate dihydrate; and h) sodium aluminum sulfate and sodium acid pyrophosphate, the sodium acid pyrophosphate exhibiting an evolved percentage of carbon dioxide of about 30 or less at room temperature prior to baking, and the slower reacting of the leavening acids in the combination being at least 10 wt % of the total weight of the acid combination.
Owner:INNOPHOS INC
Hydroxyapatite coating with biological activity and hierarchical structure on surface of degradable magnesium-based endosteal implant and preparation method
ActiveCN111973812AHigh bonding strengthAccelerated corrosionPharmaceutical delivery mechanismMetallic material coating processesMg alloysDrug biological activity
The invention provides a hydroxyapatite coating with biological activity and a hierarchical structure on the surface of a degradable magnesium-based endosteal implant and a preparation method. The coating comprises an inner fluorinated film and an outer hydroxyapatite conversion coating. The method comprises the following steps of: soaking magnesium and magnesium alloy in hydrofluoric acid to generate a chemical conversion film MgF2, soaking the chemical conversion film MgF2 in solution containing calcium phosphate, and generating a dicalcium phosphate dihydrate coating by a chemical deposition method; and finally, converting the dicalcium phosphate dihydrate coating into a thick hydroxyapatite coating with a micro-nano hierarchical structure and a composite surface appearance in situ under high temperature and pressure by a hydrothermal conversion method. The hydroxyapatite coating has high bonding strength with magnesium and magnesium alloy matrixes, and the corrosion resistance andbiological activity of the magnesium matrixes are obviously improved. The preparation method is simple, convenient and feasible, has low cost, and can be applied to magnesium-based endosteal implant devices with any complex shapes.
Owner:SHANGHAI JIAO TONG UNIV
Bone cement as well as preparation method and application thereof
ActiveCN107899073APharmaceutical delivery mechanismProsthesisAlpha-tricalcium phosphateGlucocorticoid
The invention relates to bone cement as well as a preparation method and application thereof. The bone cement is prepared from 50 to 92 weight percent of alpha-tricalcium phosphate, 3 to 10 weight percent of dicalcium phosphate dihydrate, 2 to 30 weight percent of starch and 0.5 to 15 weight percent of nano-silver loaded diatomite. The bone cement disclosed by the invention has the advantages of injection, antibacterial property and degradability, and can be used for treating vertebral compression fracture caused by osteoporosis induced by glucocorticoid.
Owner:PEKING UNIV FIRST HOSPITAL
Preparation method of calcium phosphate-coated magnesium powder
ActiveCN107236940AImprove performanceGood biocompatibilityTransportation and packagingMetal-working apparatusMagnesium phosphateMonopotassium phosphate
The invention relates to a preparation method of calcium phosphate-coated magnesium powder. According to the method, a deposition solution prepared from calcium nitrate, monopotassium phosphate and disodium ethylene diamine tetraacetic acid in a certain ratio is used, and gas-atomized pure magnesium powder is used for a deposition reaction under the deposition conditions that the pH value ranges from 3.6 to 4.8, the deposition temperature ranges from 10 DEG C to 20 DEG C and the stirring speed ranges from 200 rpm to 400 rpm. With the preparation method, a complete and uniform coating layer is prepared on the surface of the pure magnesium powder, the main phase of the coating layer is dicalcium phosphate dihydrate and a small amount of magnesium hydrogen phosphate, and the corrosion resistance of the magnesium powder in a chlorine-containing environment can be improved.
Owner:CHONGQING UNIV
Method for stabilization of hazardous wastes with dilute acid semi-soluble dicalcium phosphate dihydrate powder
This invention provides a method for stabilization of heavy metal bearing materials and wastes subject to acid leaching tests or leach conditions by addition of acid semi-soluble DiCalcium Phosphate DiHydrate such that the leaching potential is inhibited to desired levels and the material or waste is free flowing, more permeable, less weight and permits immediate handling and disposal or reuse. The resultant material or waste after stabilization is deemed suitable for on-site reuse, off-site reuse or disposal as RCRA non-hazardous waste.
Owner:FORRESTER KEITH EDWARD
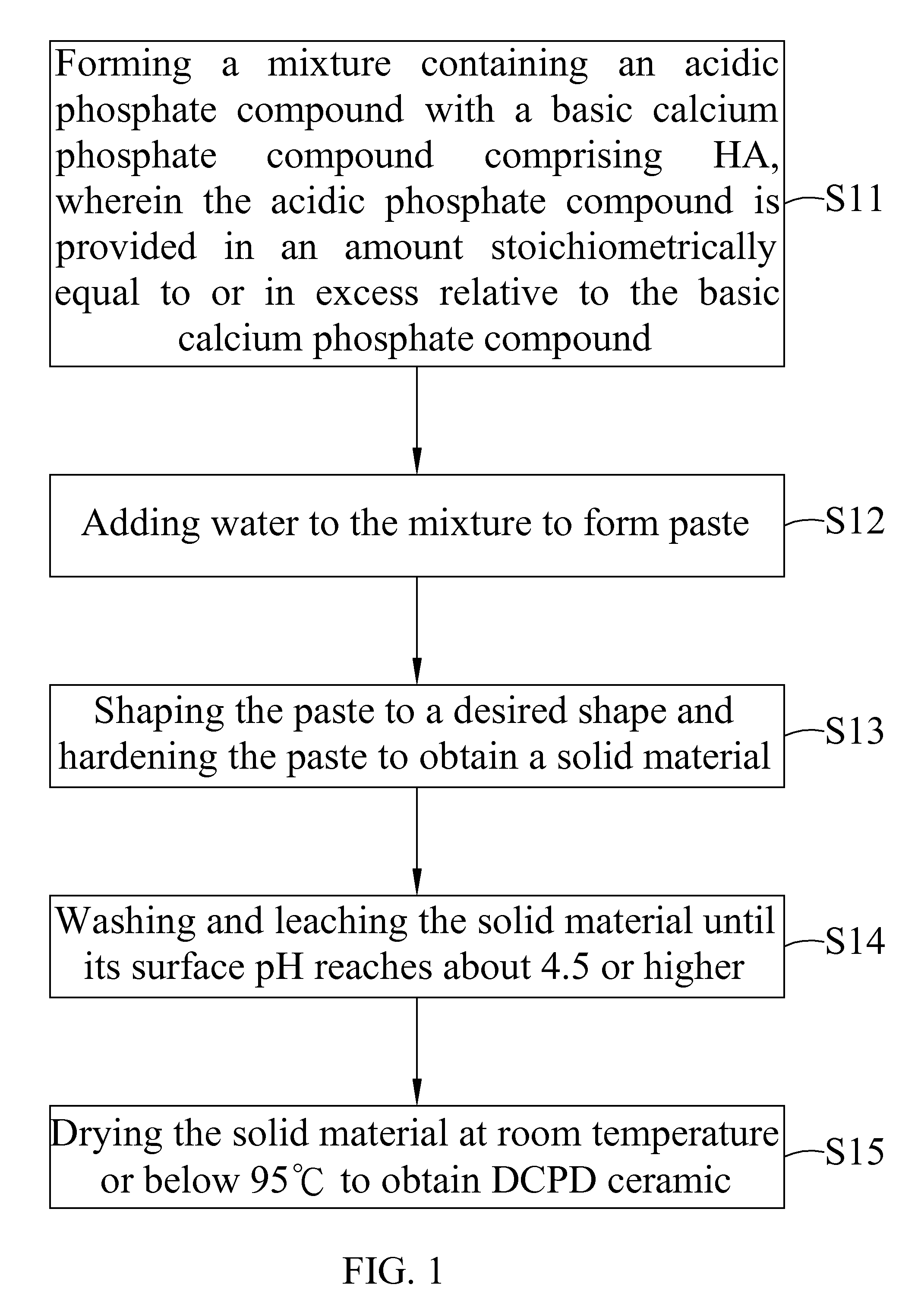
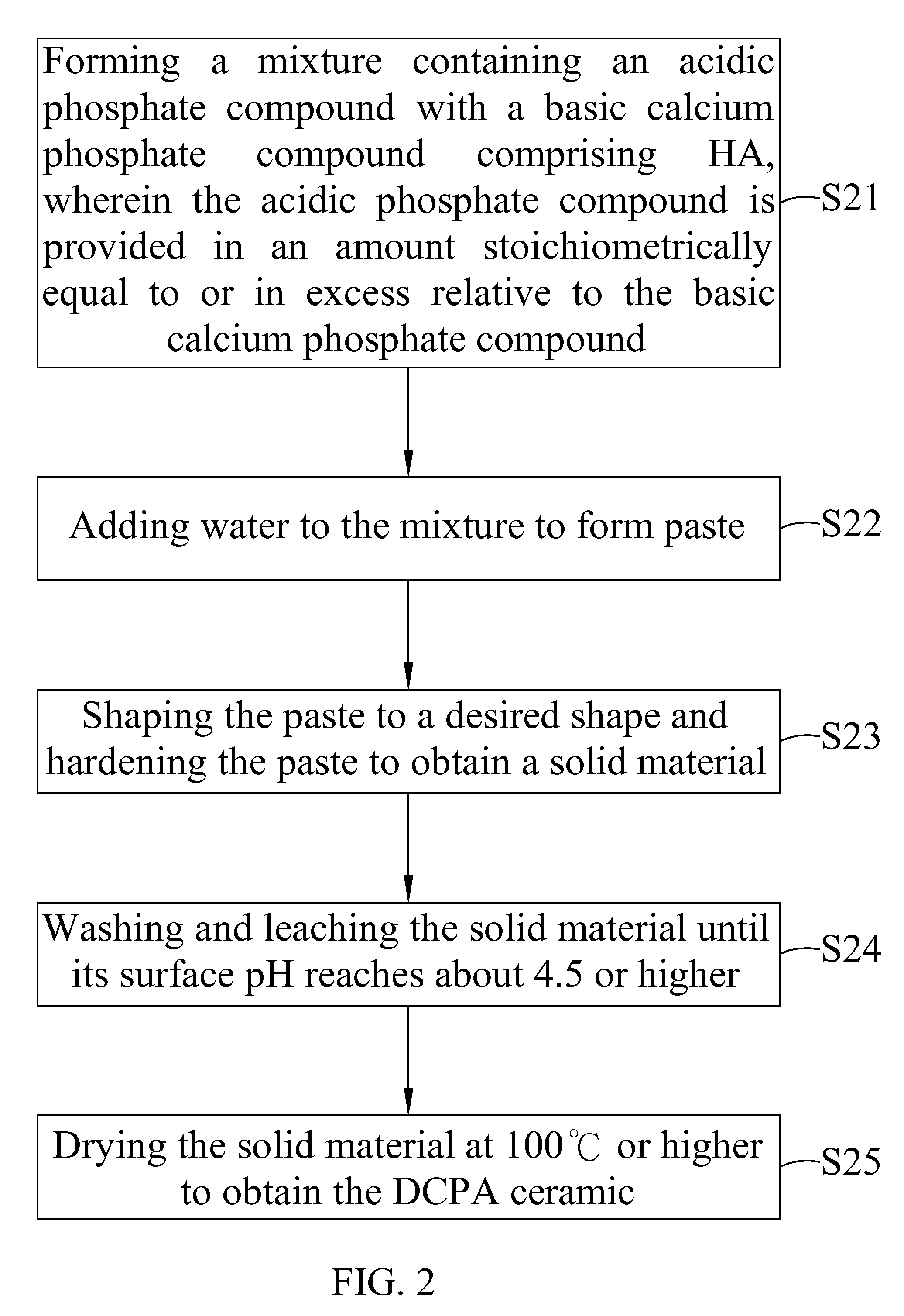
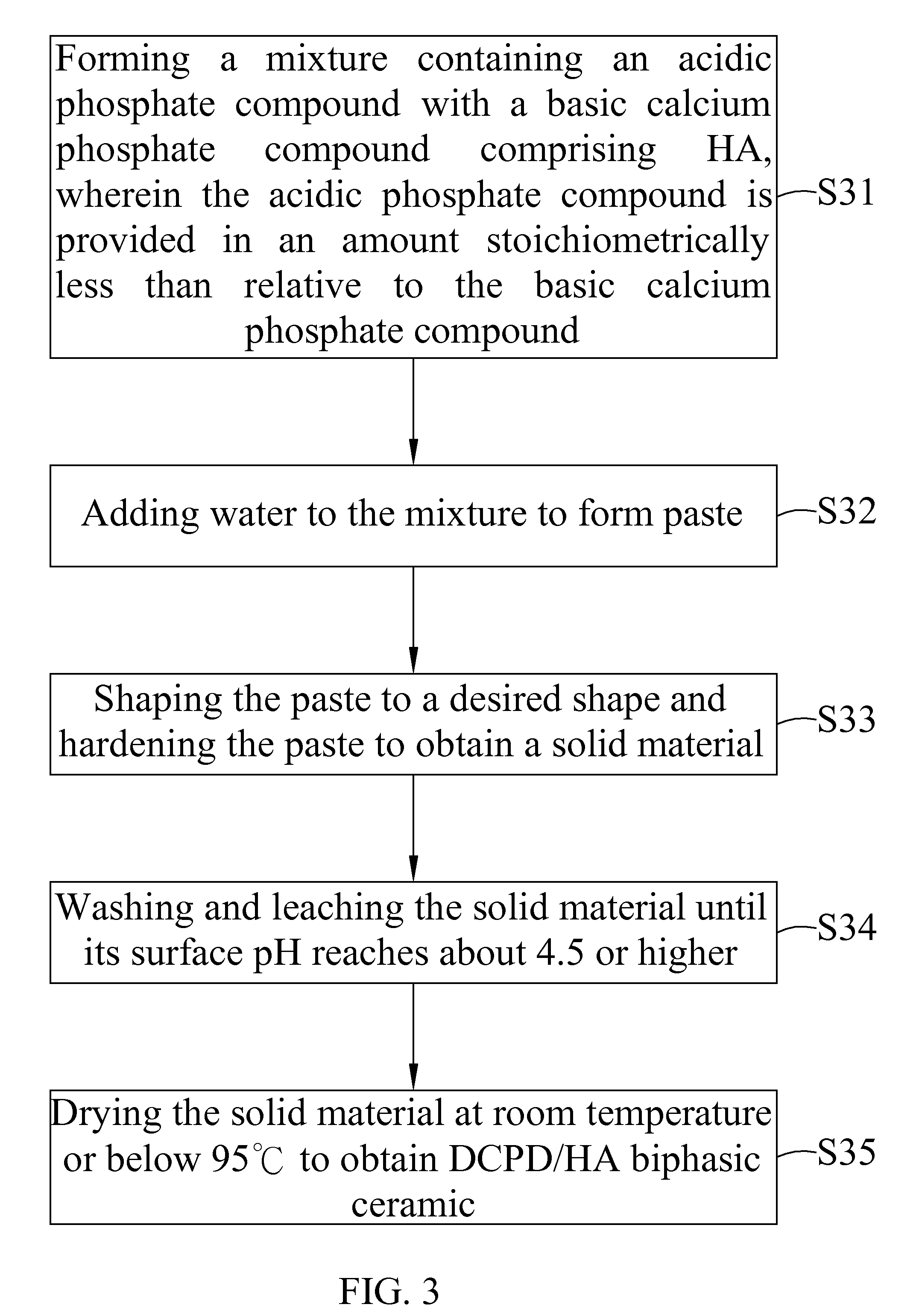
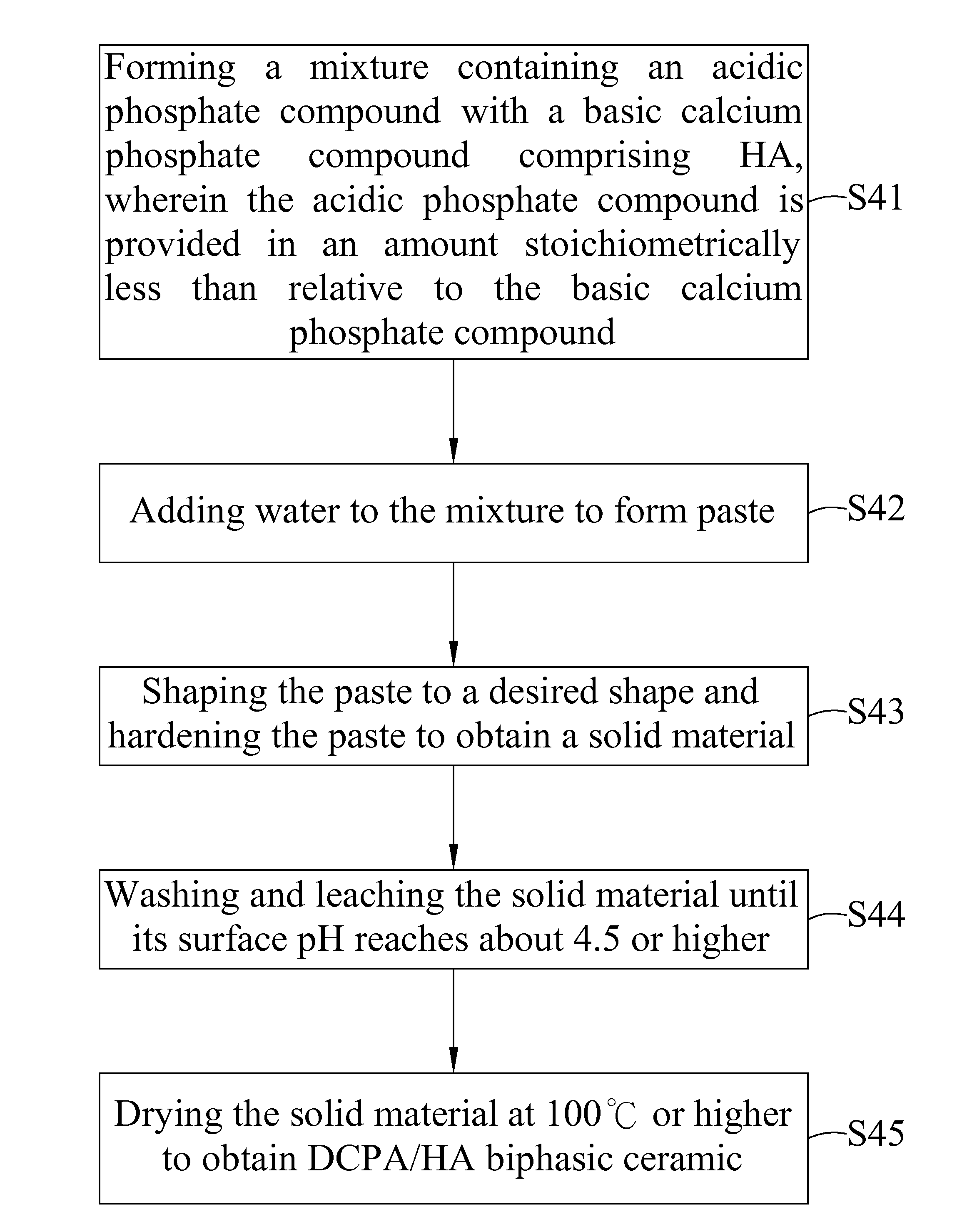
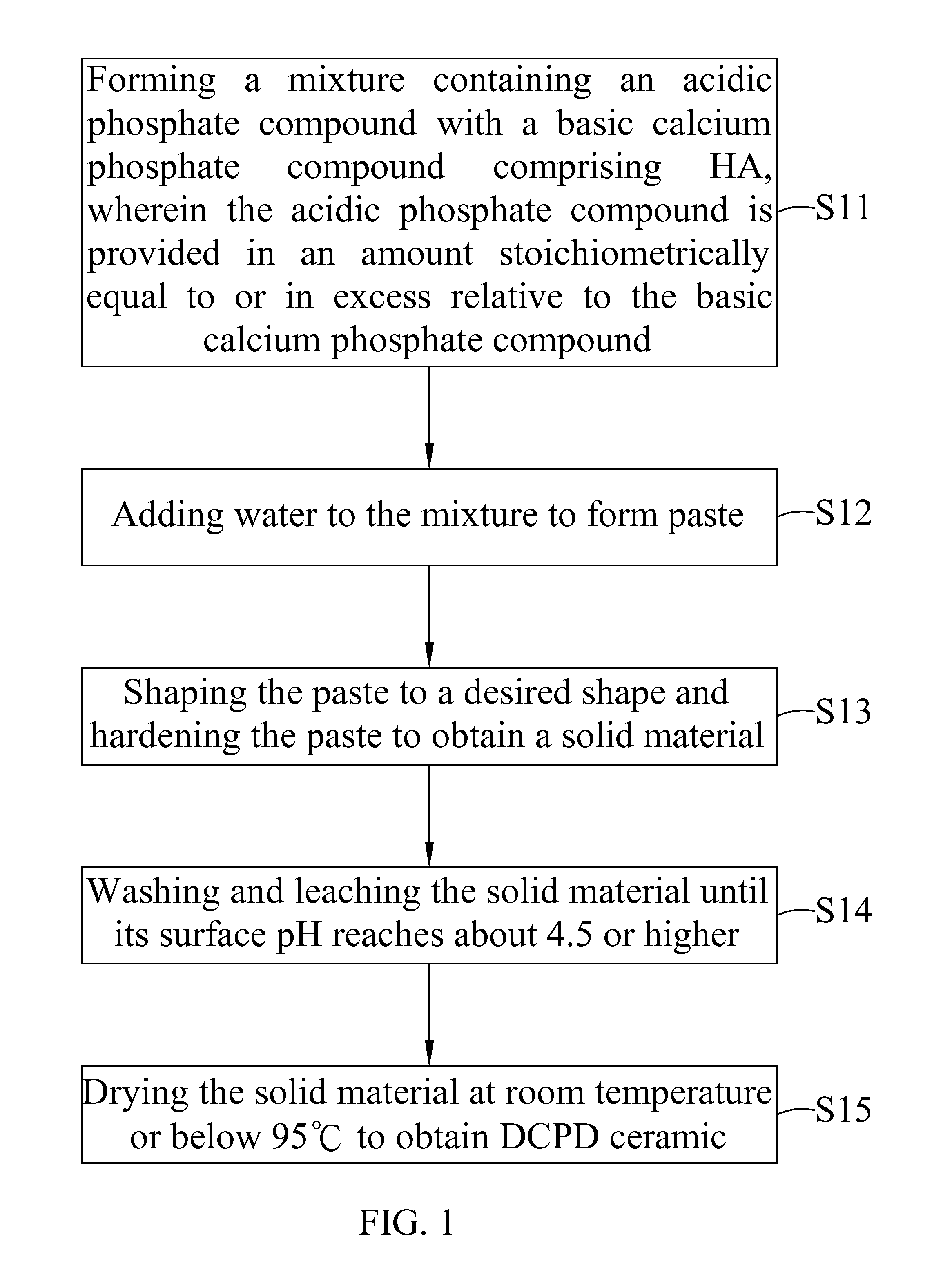
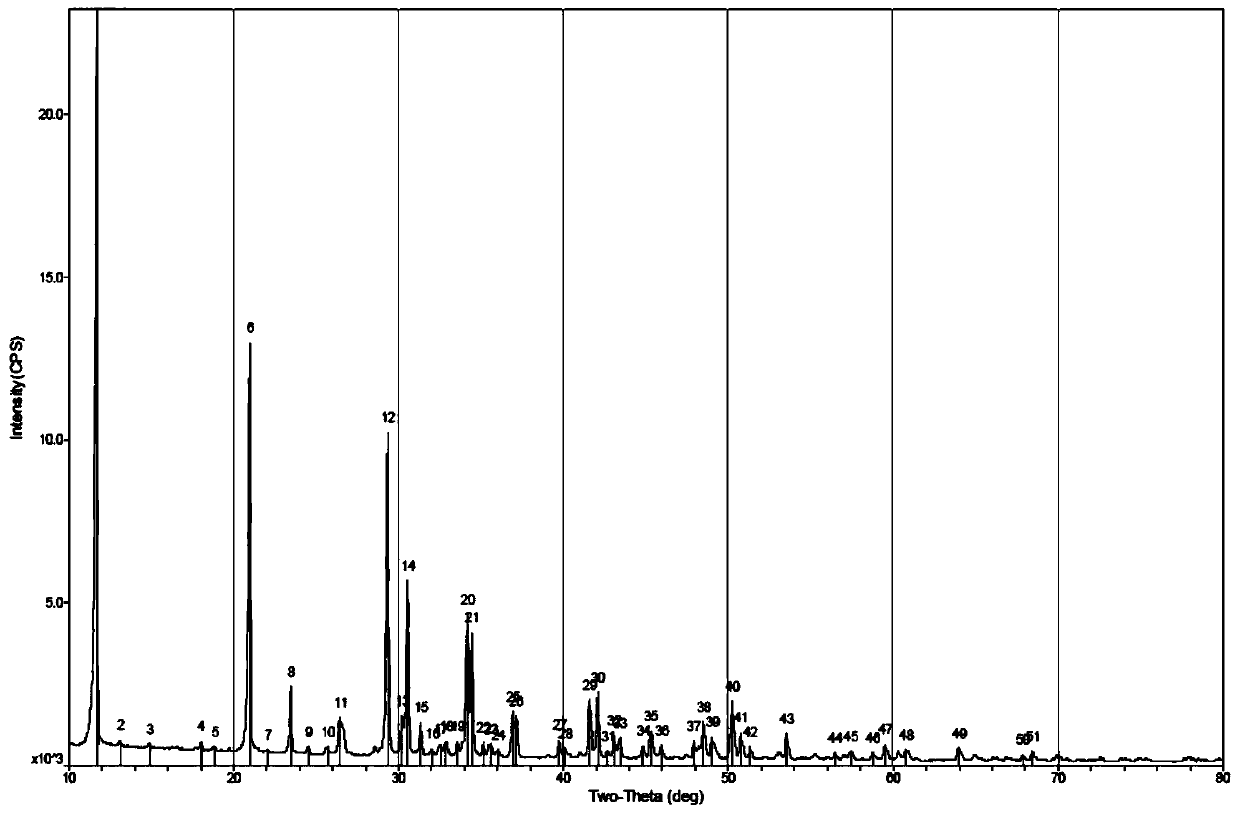
Dicalcium phosphate ceramics, dicalcium phosphate/hydroxyapatite biphasic ceramics and method of manufacturing the same
ActiveUS8962037B2Good biocompatibilityIncrease ratingsCosmetic preparationsBiocideCalcium biphosphatePhosphoric acid
The present invention discloses a method of manufacturing pure dicalcium phosphate ceramics or dicalcium phosphate / hydroxyapaite (HA) biphasic ceramics for medical applications in hard tissue areas to be used as implant materials. These ceramic implant materials are in granular form or in block form, and are prepared by using an acidic phosphate compound, a basic calcium phosphate compound comprising HA, and water. The dicalcium phosphate ceramic comprises either dicalcium phosphate dihydrate (CaHPO4.2H2O, DCPD) or dicalcium anhydrous (CaHPO4, DCPA). Wherein, when the acidic phosphate compound is provided in an amount stoichiometrically equal to or in excess relative to the basic calcium phosphate compound, a reaction product is the DCPD or DCPA ceramic; when the acidic phosphate compound is provided in the amount stoichiometrically less than the basic calcium phosphate compound, the reaction product is the DCPD / HA or DCPA / HA biphasic ceramic.
Owner:MAXIGEN BIOTECH
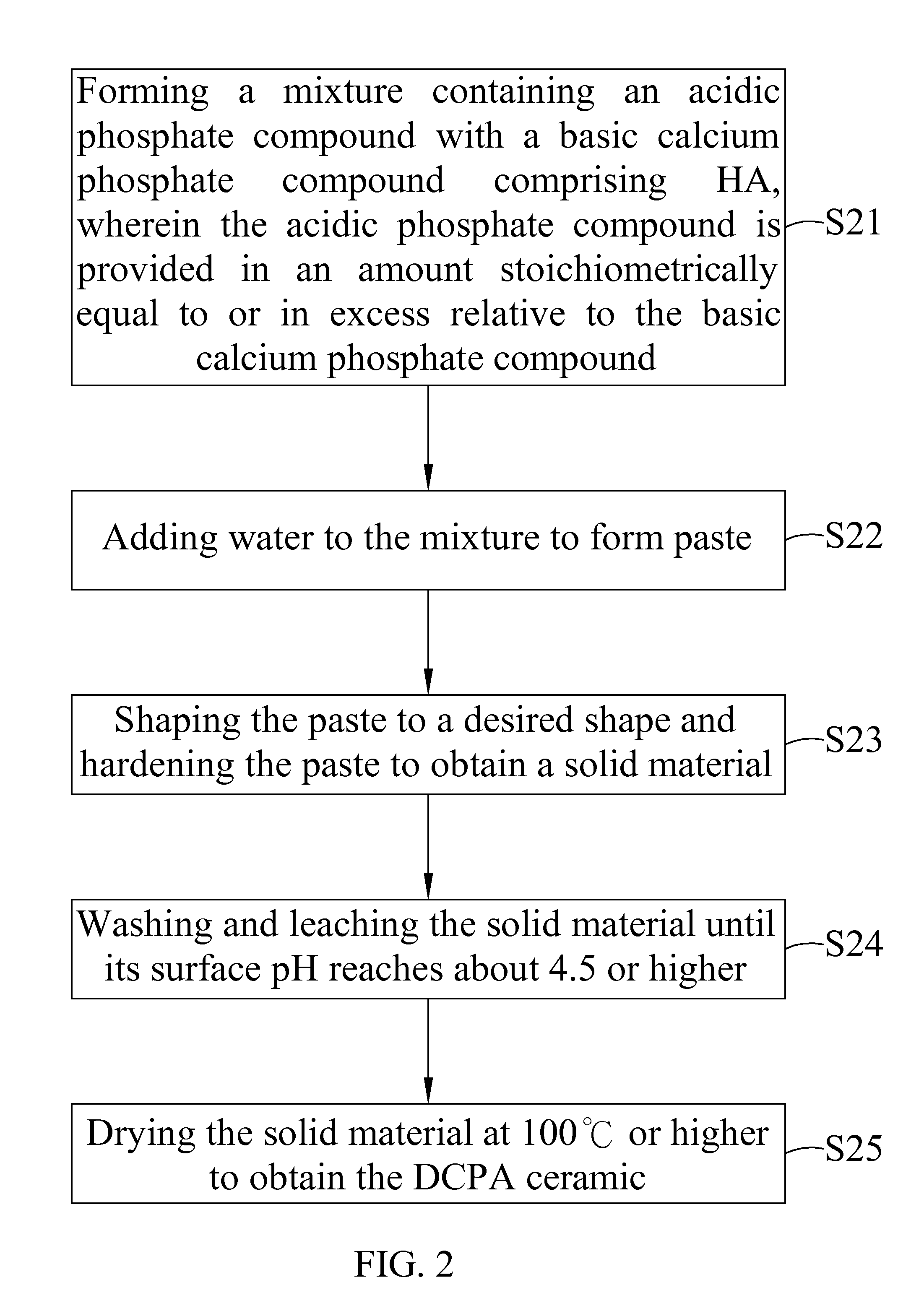
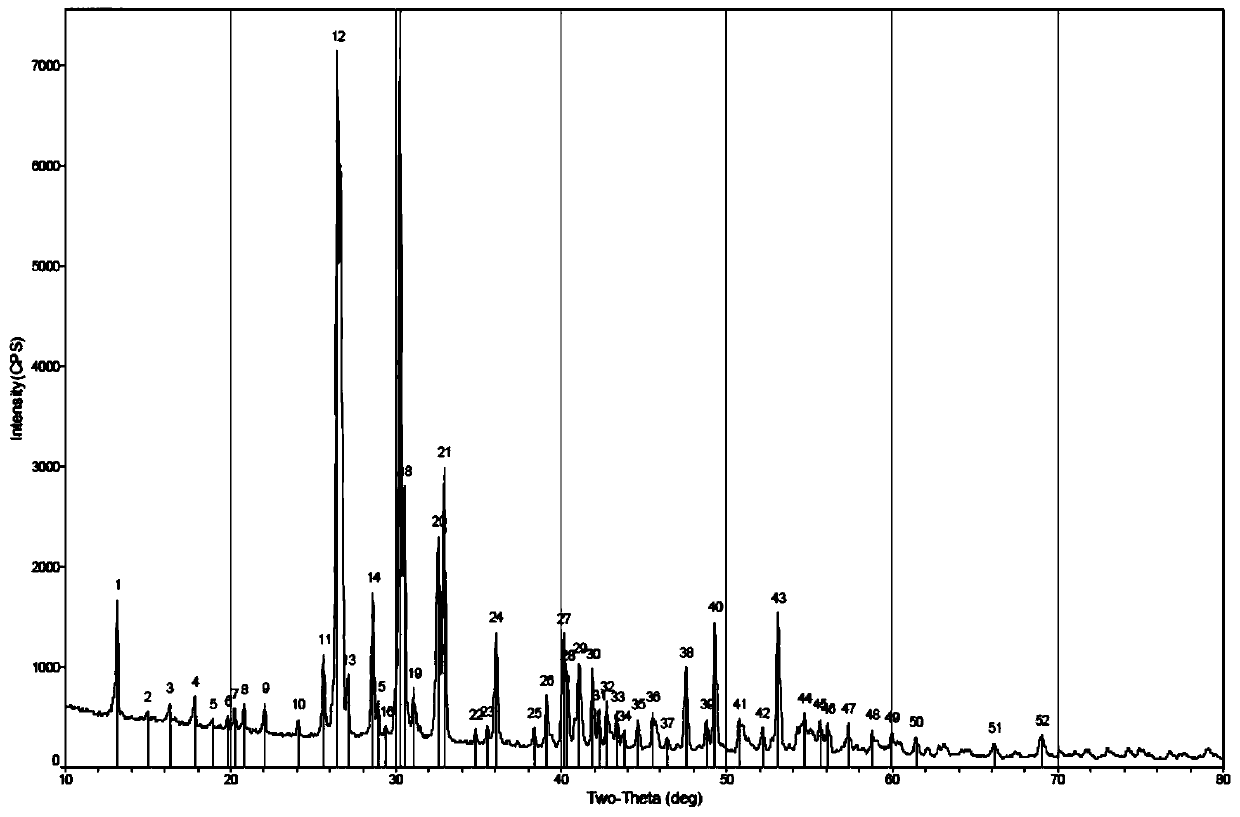
Dicalcium Phosphate Ceramics, Dicalcium Phosphate/Hydroxyapatite Biphasic Ceramics and Method of Manufacturing the Same
ActiveUS20120201870A1Good biocompatibilityExcellent bioresorption rateBiocidePhosphatesCalcium biphosphatePhosphoric acid
The present invention discloses a method of manufacturing pure dicalcium phosphate ceramics or dicalcium phosphate / hydroxyapaite (HA) biphasic ceramics for medical applications in hard tissue areas to be used as implant materials. These ceramic implant materials are in granular form or in block form, and are prepared by using an acidic phosphate compound, a basic calcium phosphate compound comprising HA, and water. The dicalcium phosphate ceramic comprises either dicalcium phosphate dihydrate (CaHPO4.2H2O, DCPD) or dicalcium anhydrous (CaHPO4, DCPA). Wherein, when the acidic phosphate compound is provided in an amount stoichiometrically equal to or in excess relative to the basic calcium phosphate compound, a reaction product is the DCPD or DCPA ceramic; when the acidic phosphate compound is provided in the amount stoichiometrically less than the basic calcium phosphate compound, the reaction product is the DCPD / HA or DCPA / HA biphasic ceramic.
Owner:MAXIGEN BIOTECH
Stable paroxetine hydrochloride tablet and preparation method thereof
ActiveCN110037995AInhibition of reddeningHave an unexpected effectOrganic active ingredientsNervous disorderSucroseFiller Excipient
The invention belongs to the technical field of medicines, and provides a stable paroxetine hydrochloride tablet and a preparation method thereof. The paroxetine hydrochloride tablet comprises the following components in parts by weight: 7.6 parts of paroxetine hydrochloride, 67.4-81.9 parts of a filler, 2-5 parts of an adhesive, 3-10 parts of a stabilizer, 5-7 parts of a disintegrant and 0.5-3 parts of a lubricant, wherein the stabilizer is sucrose, the filler is calcium hydrogen phosphate dihydrate, the disintegrant is sodium carboxymethyl starch, the adhesive is povidone K30, and the lubricant is magnesium stearate. The preparation method of the paroxetine hydrochloride tablet adopts a wet granulation process. The method solves the problems that paroxetine hydrochloride products turn red in the wet granulation process and the hardness of paroxetine hydrochloride tablets is poor at high temperature when calcium phosphate dihydrate is used as a main filler.
Owner:山东启荣科技有限公司 +1
Bone cement, its preparation method and use
The present invention relates to bone cement, its preparation method and use. The bone cement of the present invention contains: 50-92wt% α-tricalcium phosphate; 3-10wt% calcium hydrogen phosphate dihydrate; 2-30wt% starch; and 0.5%-15wt% nanosilver-loaded diatomite. The bone cement of the present invention has the advantages of injectability, antibacterial properties and degradability, and can be used to treat spinal vertebral body compression fractures caused by glucocorticoid-induced osteoporosis.
Owner:PEKING UNIV FIRST HOSPITAL
Whitening compound
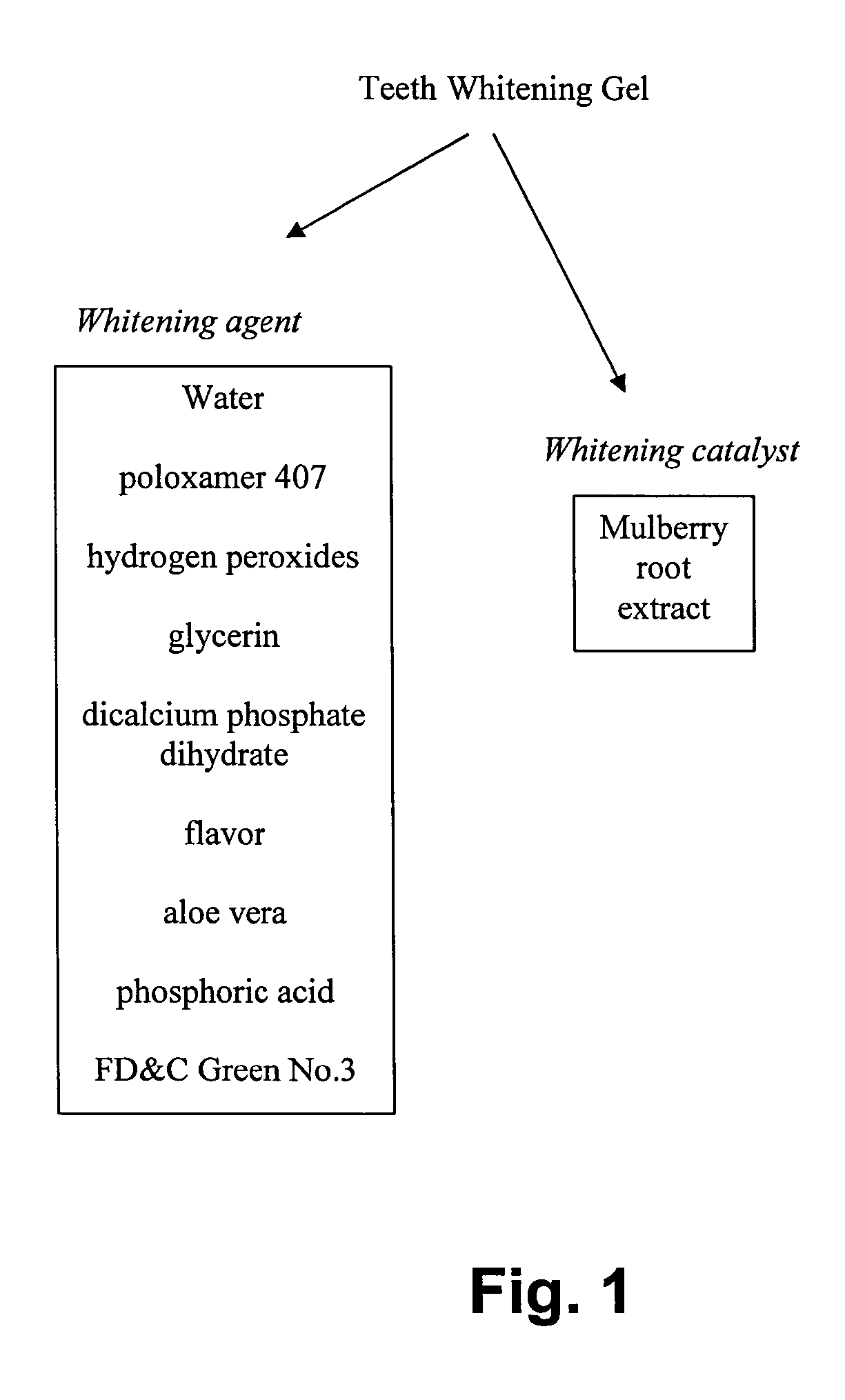
InactiveUS20050042185A1Enhancing effectiveness and efficiency and stabilityStabilizing teeth whitening processCosmetic preparationsToilet preparationsO-Phosphoric AcidWhitening Agents
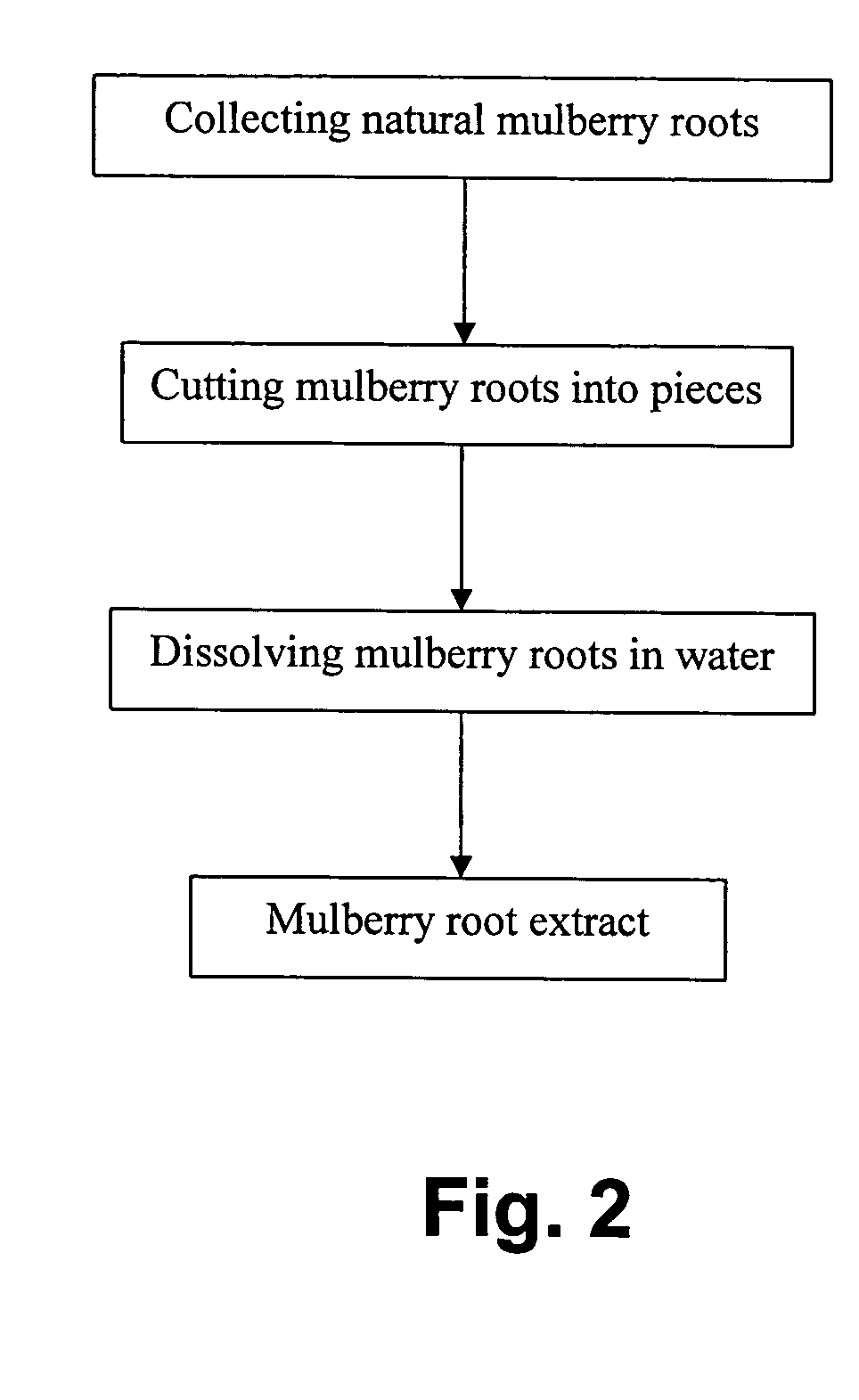
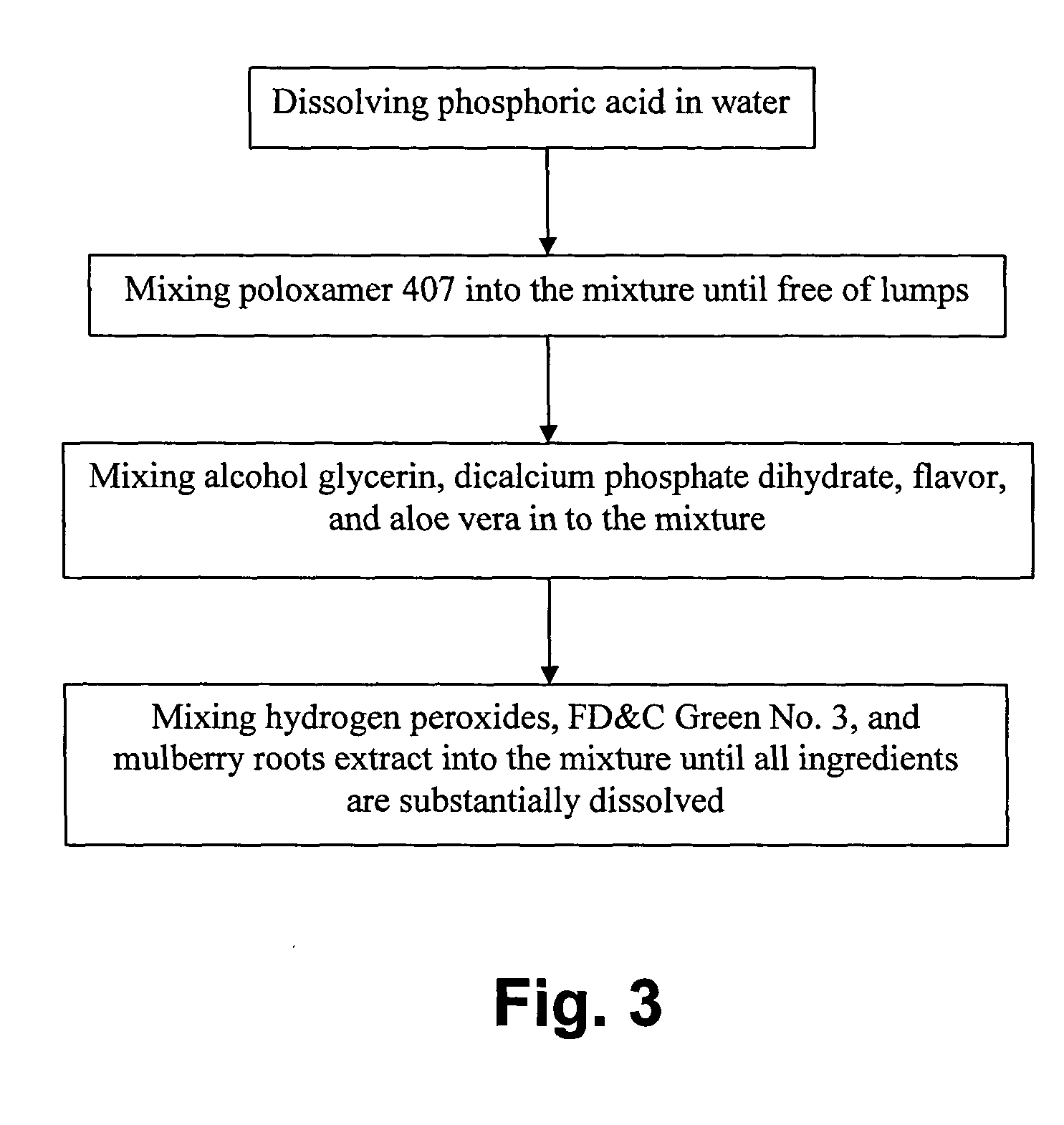
A tooth whitening compound includes a predetermined amount of whitening agent and whitening catalyst, wherein the whitening compound includes, a predetermined composition by weight water of 50% to 70%; poloxamer 407 of 10% to 30%; hydrogen peroxide of 3% to 6%; glycerin of 7% to 10%; dicalcium phosphate dihydrate of 1% to 2%; a predetermined kind of flavor of 1%; aloe vera of 0.1% to 1%; phosphoric acid of 1% to 2%; Food, Drug & Cosmetic Green No. 3 of 0.1% to 1%; and mulberry root extract of 0.1% to 1%. The whitening catalyst is the mulberry root extract as a teeth whitening catalyst for enhancing effectiveness, efficiency and stability of teeth whitening process of the whitening compound.
Owner:ZHAO JINGWEI
Pharmaceutical composition
InactiveCN104706615AImprove the operating environmentSolve the problem of large differences in dissolution between different batchesOrganic active ingredientsNervous disorderStearic acidCroscarmellose sodium
The invention relates to a pharmaceutical composition containing a dexzopiclone tablet, belonging to the technical field of medicine. Commercially available dexzopiclone tablets are of three specifications, i.e., 1 mg, 2 mg and 3 mg. Due to small specifications of the dexzopiclone tablets, content uniformity of prepared tablets can hardly achieve standards; and since the dexzopiclone is hardly soluble in water, reproducibility of the dissolution data of tablets of different batches is poor. The technical scheme of the invention is as follows: the dexzopiclone tablet is characterized by containing, by mass, 1% of dexzopiclone with D90 of below 30 mu m, 0 to 77.5% of dicalcium phosphate dihydrate, 20 to 97.5% of microcrystalline cellulose, 1% of croscarmellose sodium and 0.5% of magnesium stearate. The technical scheme of the invention overcomes the above-mentioned problems in the prior art.
Owner:WEIHAI DISU PHARMA CO LTD +1
A galanthamine hydrobromide tablet
InactiveCN107744508ASolving Content Uniformity ProblemsSimple processNervous disorderInorganic non-active ingredientsMagnesium stearatePharmaceutical formulation
Owner:WEIHAI GUANBIAO INFORMATION TECH
Composition for tooth-surface anticaries repair and preparation method thereof
InactiveCN101810540ATightly boundNo gapsImpression capsDentistry preparationsMass ratioPhosphoric acid
The invention provides a composition for tooth-surface anticaries repair and a preparation method thereof, and relates to a composition. The invention provides the composition taking tetracalciumphosphate and dicalcium phosphate as main raw materials for tooth-surface anticaries repair, and the preparation method thereof. The composition comprises calcification agent powder and solidification liquid, wherein the mass ratio of the calcification agent powder to the solidification liquid is 0.1 to 10; the calcification agent powder comprises tetracalciumphosphate powder and dicalcium phosphate powder; the particle size of the calcification agent powder can be less than or equal to 50 mu m; the dicalcium phosphate can be dicalcium phosphate dihydrate or anhydrous dicalcium phosphate and the like; the solidification liquid may be at least one of water-soluble phosphate solution, citric acid solution, sodium alginate solution and the like; and the pH of the composition for tooth-surface anticaries repair can be 5 to 9. During preparation, the tetracalciumphosphate powder and the dicalcium phosphate powder are mixed and ground to obtain the calcification agent powder; the solidification liquid is prepared; and the calcification agent powder is mixed with the solidification liquid to obtain the composition for tooth-surface anticaries repair.
Owner:XIAMEN UNIV
Novel antistatic and sterilization fiber treatment material and preparation method thereof
InactiveCN105220499AImprove anti-static effectImprove flexibilityFibre treatmentFiberPhosphoric acid
The invention discloses a novel antistatic and sterilization fiber treatment material and a preparation method thereof. The novel antistatic and sterilization fiber treatment material is prepared from the following components in parts by weight: 50-60 parts of polyethylene glycol, 5-8 parts of 2-ethyl hexyl ester of epoxidized soybean oil, 3-5 parts of hydroxymethyl cellulose, 2-4 parts of dihydrocarveol, 1-3 parts of polyethylene wax, 1-3 parts of dicalcium phosphate dihydrate, 1-2 parts of polymethylcyclotetrasiloxane, 0.5-2 parts of quininic acid, 0.5-1 part of polydimethylsiloxane diquaternary ammonium salt, 0.5-1 part of 4-guanidyl-1-butanol and 0.02-0.08 part of bergapten. The invention further provides a preparation method of the antistatic and sterilization fiber treatment material.
Owner:付淑珍
Benzenesulfonate amlodipine dispersible tablet and preparation method thereof
InactiveCN109106691AReduce adverse reactionsDisintegrates quicklyOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchDissolution
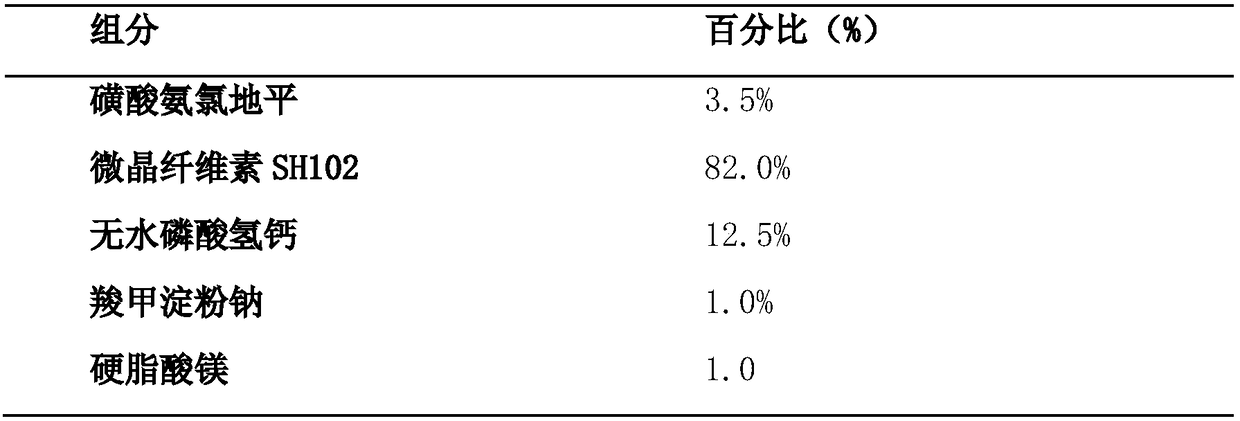
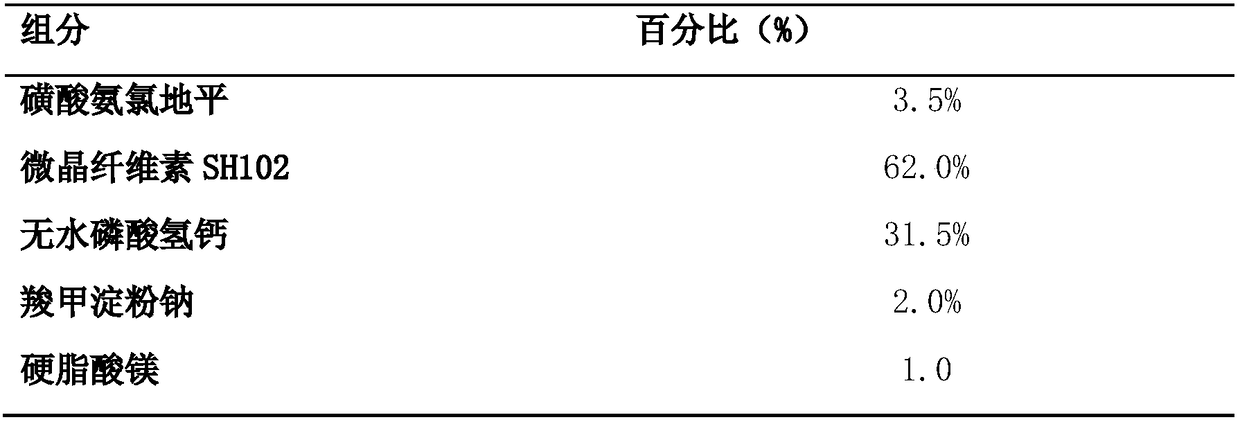
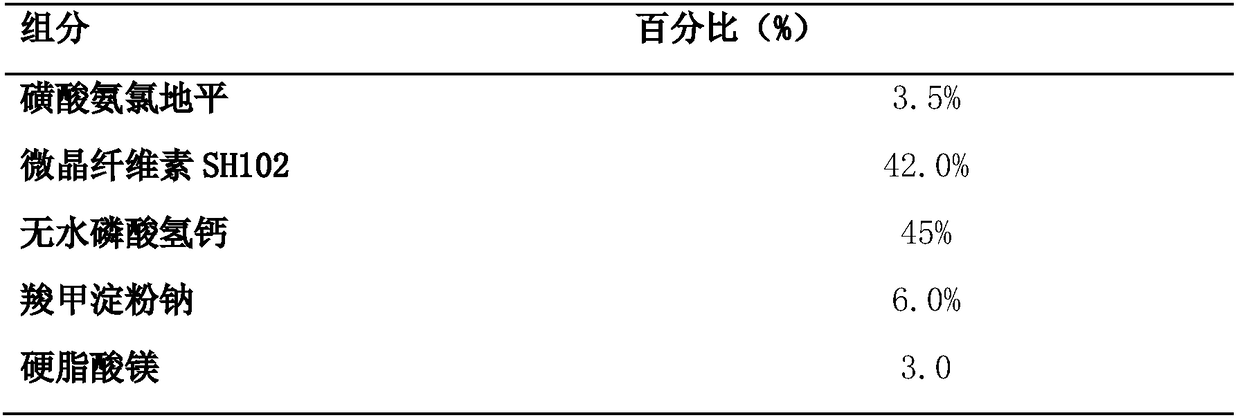
The invention discloses a benzenesulfonate amlodipine dispersible tablet, which is prepared from the following ingredients in percentage by mass: 3.5% of benzenesulfonate amlodipine, 42-82% of microcrystalline cellulose SH102, 12.5-45% of dicalcium phosphate anhydrous, 1.0-6.0% of carboxymethyl starch sodium and 1.0-3.0% of magnesium stearate. The preparation method comprises the following steps:firstly, adding benzenesulfonate amlodipine, carboxymethyl starch sodium and 1 / 3 of microcrystalline cellulose of a prescription dosage to be mixed for 8-15 minutes, sieving by a sieve of 40 meshes, and adding a residual quantity of microcrystalline cellulose to be mixed for 8-15 minutes; then, adding the dicalcium phosphate anhydrous of the prescription dosage to be mixed for 15-25 minutes, finally, mixing with magnesium stearate for 2-5 minutes, and tableting. The benzenesulfonate amlodipine dispersible tablet provided by the invention has the advantages of the tablet and liquid preparation,is convenient in taking, is quick in disintegration, is quick in absorption and higher in blood pressure reduction effect and is high in bioavailability, blood pressure is more effectively controlled, and the adverse reaction of the medicine can be lowered. A 5-min dissolution rate is as high as 97.8%, and therefore, the benzenesulfonate amlodipine dispersible tablet has the advantages of high accumulated dissolution rate, high bioavailability, good stability, simple preparation technology and the like and is suitable for industrialized production and the like.
Owner:南通久和药业有限公司
Tooth filling composite material and preparation method thereof
InactiveCN108478441AHigh compressive strengthShort setting timeImpression capsDentistry preparationsCalcium phosphate dibasicMagnesium sulphate heptahydrate
The invention discloses a tooth filling composite material and a preparation method thereof. The preparation method comprises the steps that nano silicon powder, ethyl acetate, hydroxy cyclohexyl acetophenone and an acrylate monomer are mixed, after a dispersion medium is steamed out, illumination solidification is conducted, after crushing, a composite filler is obtained, and dicalcium phosphatemonohydrate, anhydrous dicalcium phosphate, dicalcium phosphate dihydrate, calcium phosphate dibasic anhydrous, anhydrous magnesium sulfate, sodium chloride and sodium bicarbonate are mixed and then ground to obtain mixed powder; the mixed powder, solvent, a binding agent, fibrefill, a biological filler and the composite filler are mixed to obtain the tooth filling composite material. The problemsare solved that setting time of traditional filling materials is longer, and the compressive strength of the traditional filling materials is relatively inadequate.
Owner:ANHUI AOZI INFORMATION TECH CO LTD
Oral bacteriostatic composition
InactiveCN109528608AEfficient killingGrowth inhibitionCosmetic preparationsToilet preparationsOral mucous membraneOral ulcers
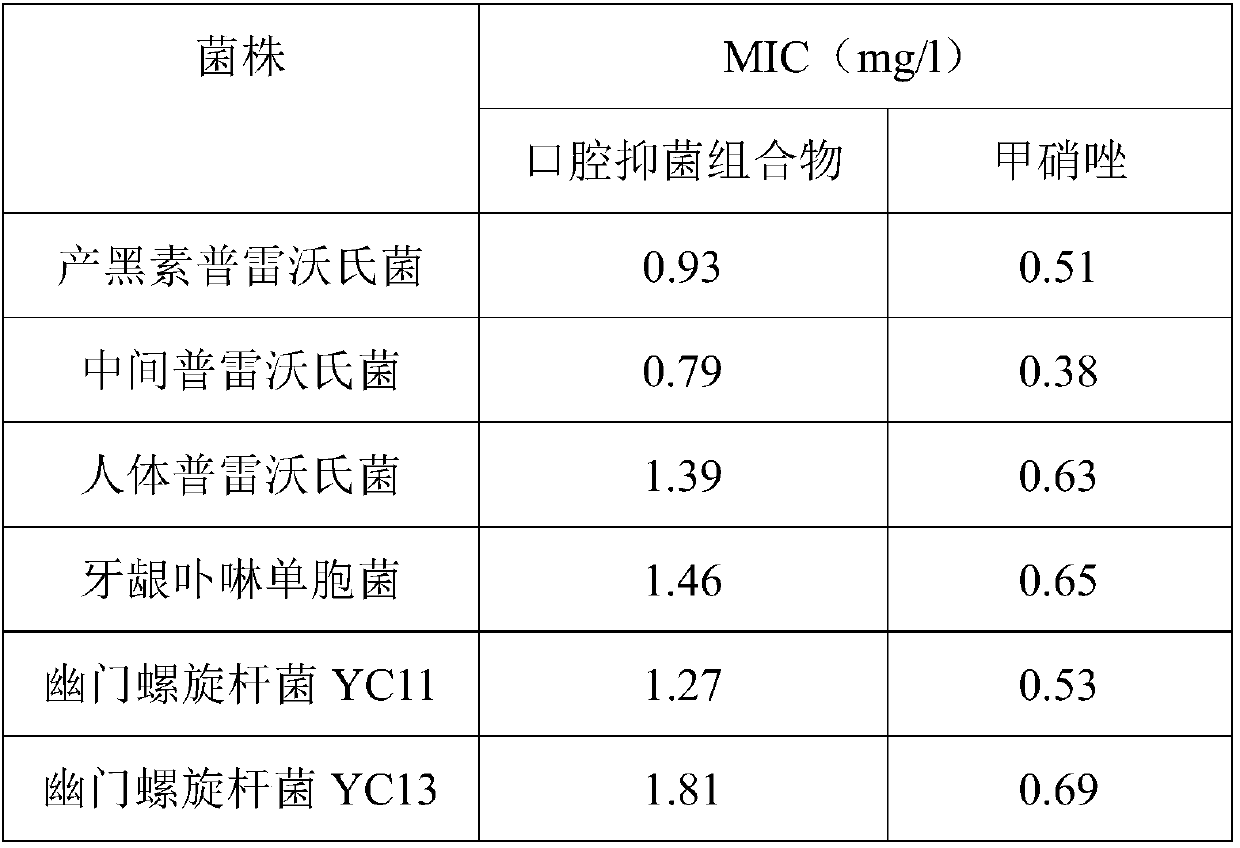
The invention provides an oral bacteriostatic composition. The composition comprises, in parts by weight as follows: 10-30 parts of traditional Chinese medicine extract, 20-30 parts of longan seed powder, 0.1-0.5 parts of sodium saccharin, 15-30 parts of sodium polyoxyethylene lauryl alchohol ether sulfate, 15-30 parts of sodium phytate, 5-10 parts of glucose oxidase, 5-18 parts of dicalcium phosphate dihydrate, 1-8 parts of silica and 2-8 parts of xylitol. The invention provides the oral bacteriostatic composition, which is prepared by a conventional method and capable of effectively inhibiting the growth of harmful bacteria, improving oral microecology, promoting the repair and defense of oral mucous membrane, killing HP and preventing it, the function of treating dental caries, particularly for HP in oral cavity, has a good killing or inhibiting effect. In addition, the composition can also effectively remove internal heat, treat oral ulcer, sore throat, dental calculus and halitosis, and has broad application prospects.
Owner:北京方诣生物医药有限公司
Dentinal tubule sealant and method for producing the same
InactiveUS20150209251A1Impart caries resistanceSubstance may accumulateCosmetic preparationsImpression capsPhosphoric acidSealant
A dentinal tubule sealant comprises poorly-soluble calcium phosphate particles (A), a phosphorus-free calcium compound (B), and water (C), wherein the particles (A) are at least one member selected from the group consisting of dicalcium phosphate anhydrous [CaHPO4] particles, α-tricalcium phosphate [α-Ca3(PO4)2] particles, β-tricalcium phosphate [β-Ca3(PO4)2] particles, amorphous calcium phosphate [Ca3(PO4)2.nH2O] particles, calcium pyrophosphate [Ca2P2O7] particles, calcium pyrophosphate dihydrate [Ca2P2O7.2H2O] particles, octacalcium phosphate pentahydrate [Ca8H2(PO4)6.5H2O] particles, and dicalcium phosphate dihydrate [CaHPO4.2H2O] particles, and the dentinal tubule sealant contains 30 to 76% by weight of the particles (A), 0.001 to 4% by weight of the compound (B), and 23 to 69% by weight of the water (C). Thus, there is provided a dentinal tubule sealant capable of sealing dentinal tubules of an exposed dentin and also remineralizing the surrounding dentin after the sealing.
Owner:KURARAY NORITAKE DENTAL
Brushite water-hardening gelling material stabilized by magnesium salt
A brushite cement for surgical purposes includes a first component including a basic calcium phosphate, a second component including an acidic phosphate, a third component including water, and a fourth component including a source of magnesium used to stabilize an end-product of the setting reaction between the components. The solubility of the source of magnesium is smaller than 100 g / L. The components are chosen in such an amount that (i) the pH of the cement past during setting is lower than 6.0; and (ii) the end-product of the setting reaction comprises dicalcium phosphate dihydrate.
Owner:H C 罗伯特·马泰斯·斯蒂夫腾 +1
Composition of materials for tooth remineralisation
InactiveUS20190365615A1Efficacious occlusion of dentinal tubulesRaise the ratioCosmetic preparationsToilet preparationsSilicic acidDivalent metal ions
Present invention refers to tooth re-mineralization materials incorporating calcium phosphates and calcium-free silicon compounds. Materials are efficacious in re-mineralization of teeth and occlusion of exposed dentinal tubules either when used alone or in dentifrice preparations for oral healthcare. Preferred calcium phosphates include anhydrous dicalcium phosphate, dicalcium phosphate dihydrate, hydroxyapatite and amorphous calcium phosphate that can incorporate partial substitutions of calcium by divalent metallic ions such as Mg++, Zn++, Ba++, Fe++, Sn++ and Sr++. Preferred calcium-free silicon compounds include silicon oxide, silica gel, methasilicic acid, orthosilicic acid, silicic acid, and combinations thereof.
Owner:HELICON MEDICAL SL
Nefopam hydrochloride tablet and preparation method thereof
InactiveCN110638780AGuaranteed stabilityEnsure safetyOrganic active ingredientsAntipyreticNefopam HydrochlorideMagnesium stearate
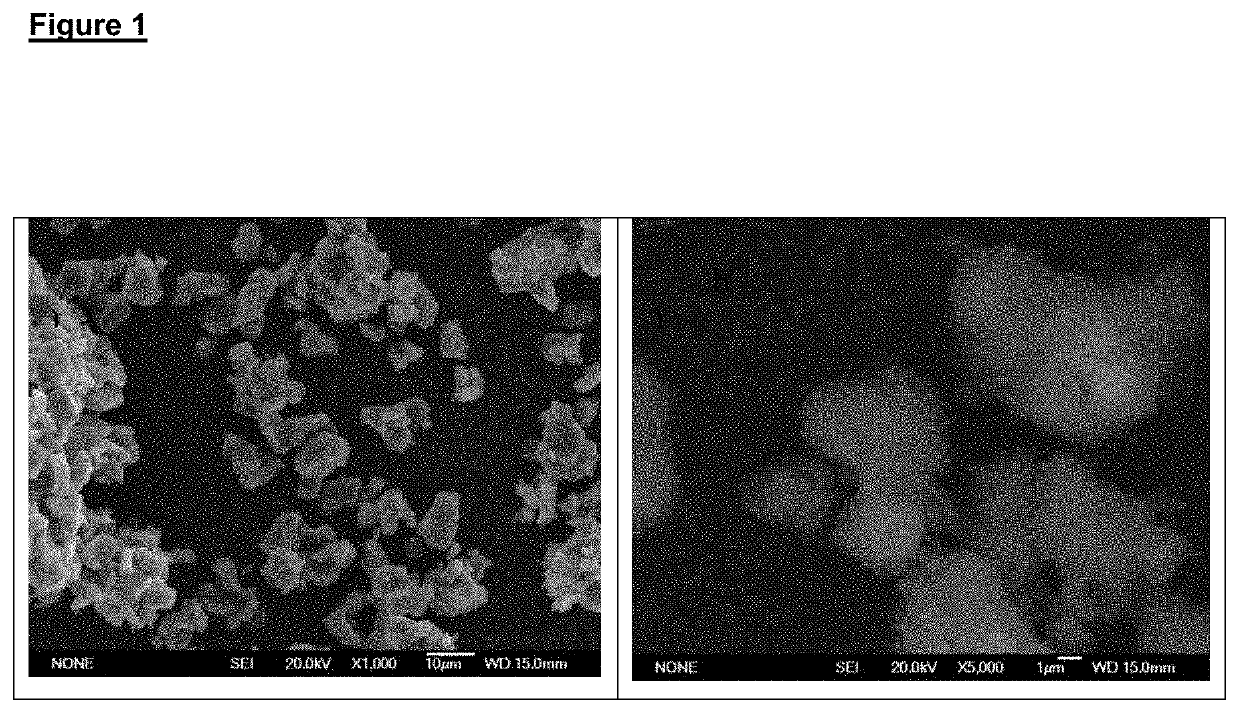
The invention discloses a nefopam hydrochloride tablet and relates to the technical field of pharmaceutical preparation. The nefopam hydrochloride tablet is prepared from the following raw materials in parts by weight: 30-50 parts of dicalcium phosphate dihydrate, 25-50 parts of microcrystalline cellulose, 1-5 parts of hydroxypropyl methylcellulose, 1-8 parts of anhydrous silica gel, 1-6 parts ofmagnesium stearate, 10-38 parts of nefopam hydrochloride and 2-20 parts of white Opadry 20A58806. According to the nefopam hydrochloride tablet, the problems that an existing nefopam hydrochloride tablet is not coated, the quality of the processing process is not well controlled, the produced nefopam hydrochloride tablet is prone to being polluted, and consequently, the drug efficacy is affected are solved.
Owner:HUAYI PHARMA ANHUI CO LTD
Preparation of brushite and octacalcium phosphate granules
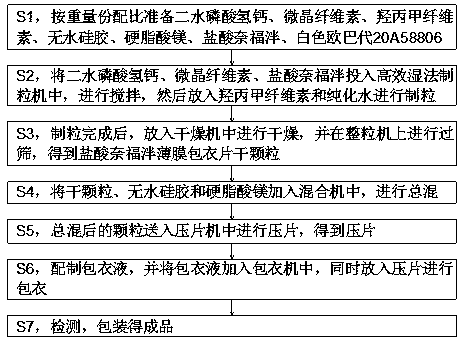
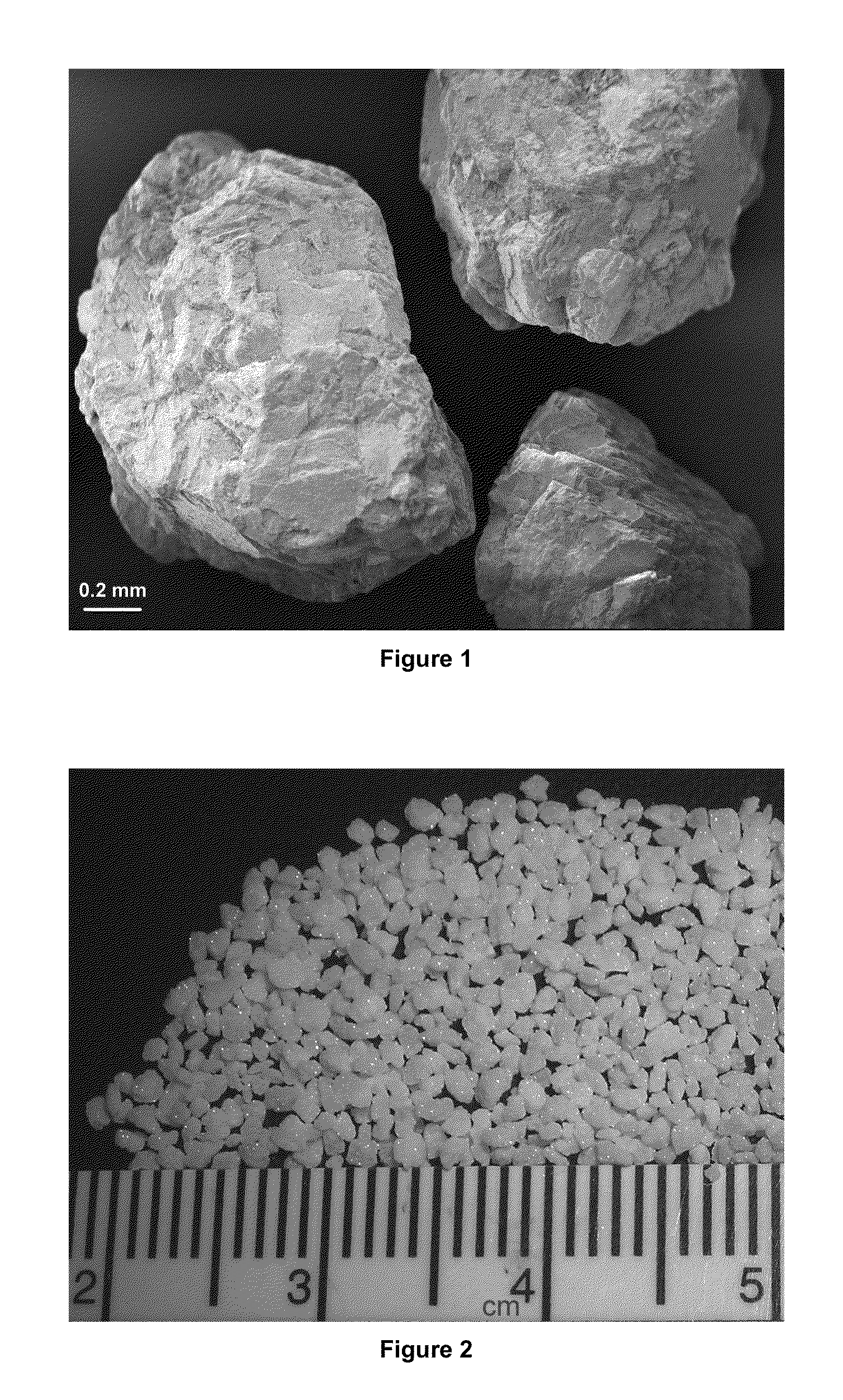
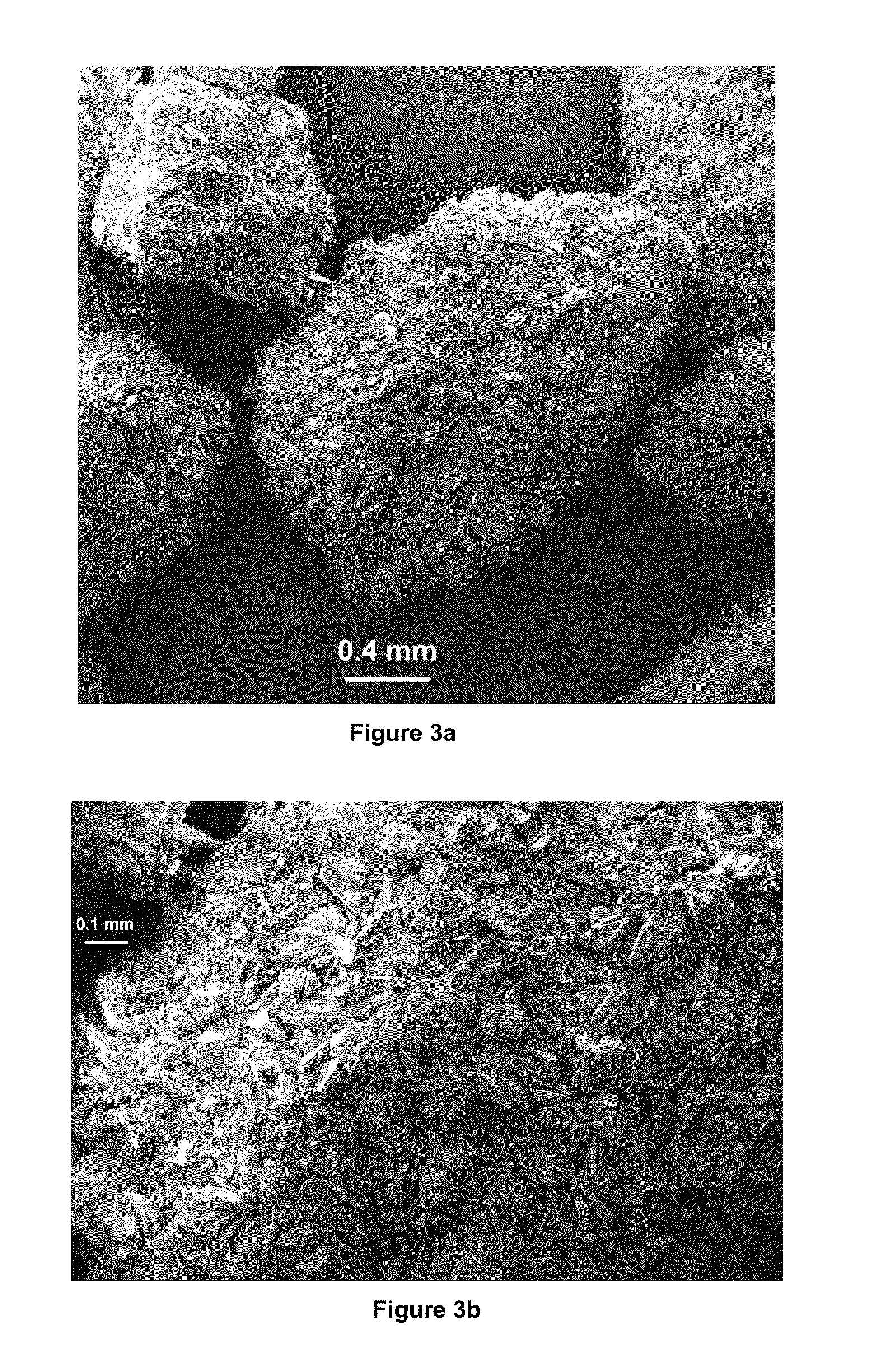
Brushite (DCPD, dicalcium phosphate dihydrate, CaHPO4-2HH2O) and octacaicium phosphate (OCP, Ca8(HPO4)2(PO4)4-5H2O) granules in the millimetre size range were prepared by using calcium carbonate granules of marble-origin as the starting material. The method of the invention comprised of soaking the marble granules in aqueous solutions containing phosphate and / or calcium ions at temperatures between 20° and 75° C. The load-bearing DCPD and OCP granules of this invention are useful in maxillofacial and orthopedic void / bone defect filiing and grafting applications.
Owner:TAS AHMET CUNEYT
oral care composition
Disclosed is an oral care composition comprising 5-80% by weight of calcium silicate, 0.4-2% by weight of xanthan gum and / or its derivatives, a benefit agent and a physiologically acceptable carrier, wherein the The benefit agent is selected from the group consisting of coloring agents, biomineralizers, antibacterial agents or mixtures thereof, and wherein said biomineralizer is amorphous calcium phosphate, alpha-tricalcium phosphate, beta-tricalcium phosphate; calcium carbonate, calcium deficient Type hydroxyapatite (Ca 9 (HPO 4 )(PO 4 ) 5 OH), dicalcium phosphate (CaHPO 4 ), dicalcium phosphate dihydrate (CaHPO 4 2H 2 O), hydroxyapatite (Ca 10 (PO 4 ) 6 (OH) 2 ), monocalcium phosphate monohydrate (Ca(H 2 PO 4 ) 2 ·H 2 O), octacalcium phosphate (Ca 8 h 2 (PO 4 ) 6 ·5H 2 O) and tetracalcium phosphate (Ca 4 (PO 4 ) 2 O) or mixtures thereof.
Owner:UNILEVER IP HLDG BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com