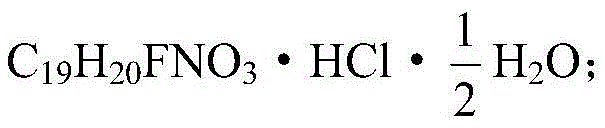Patents
Literature
39 results about "Paroxetine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
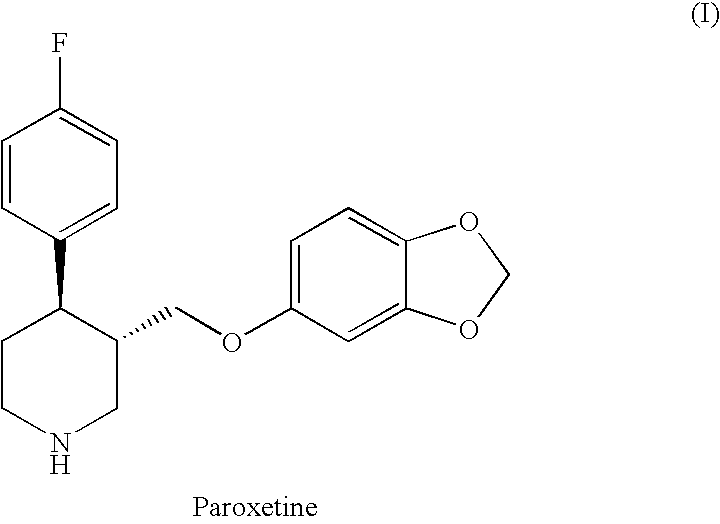
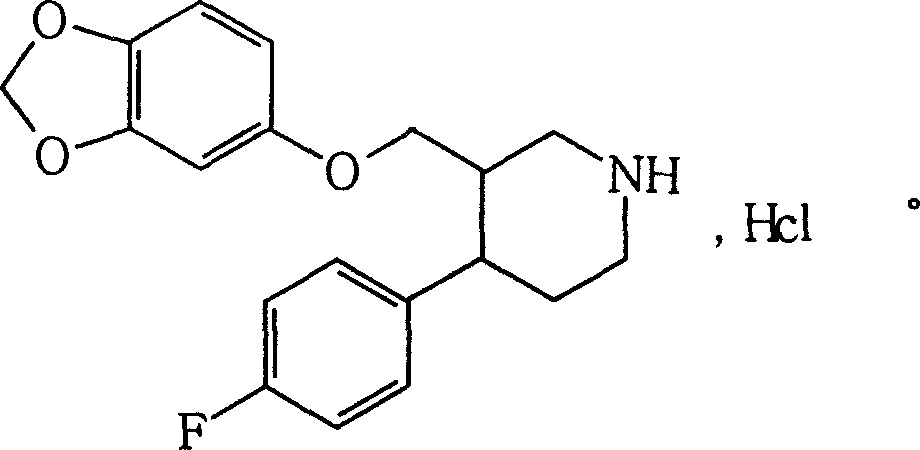
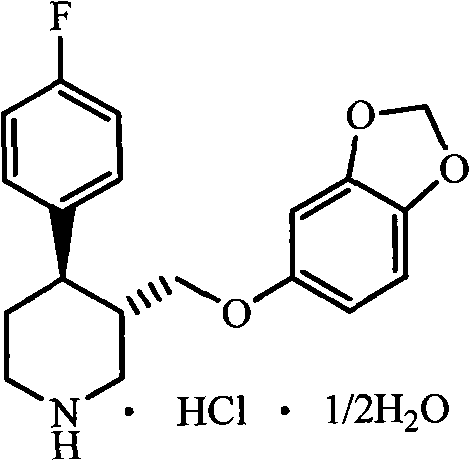
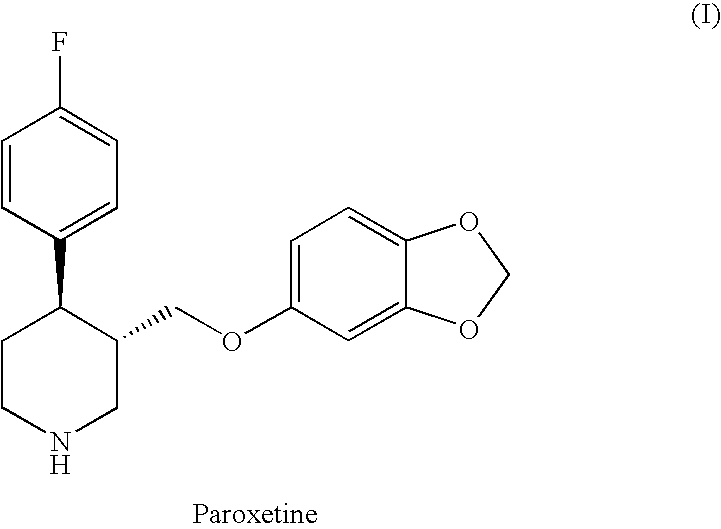
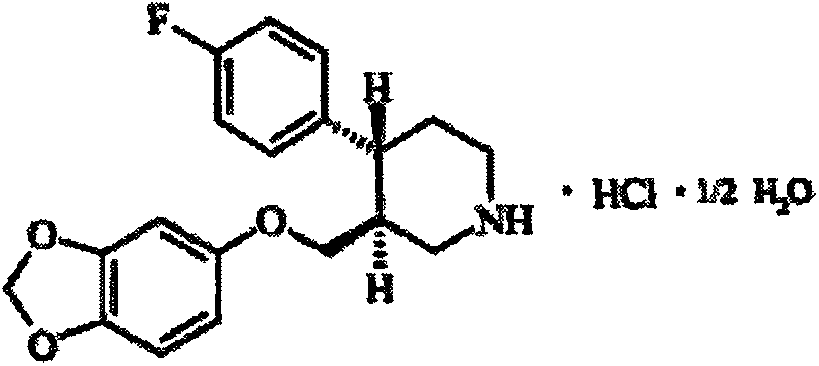
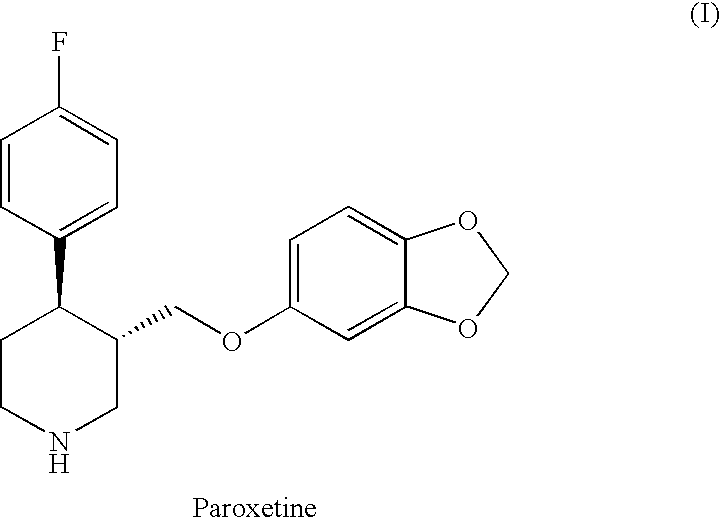
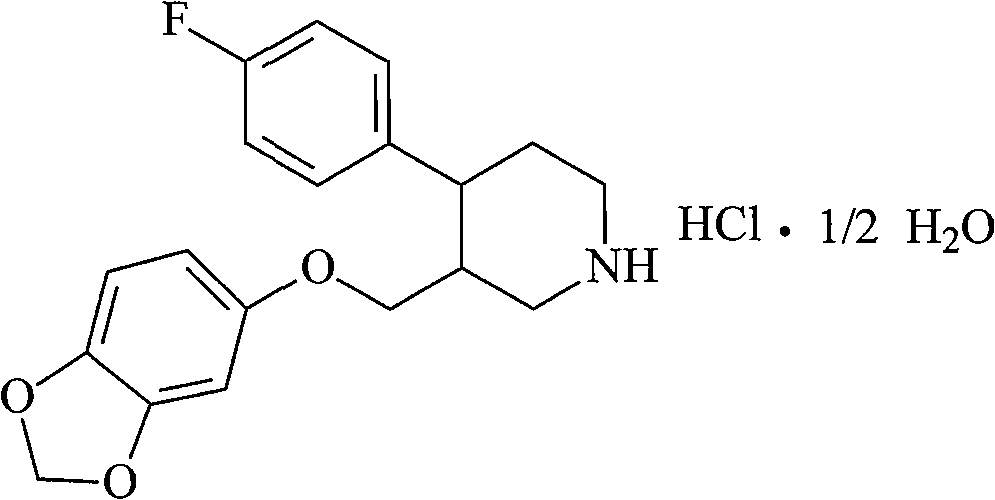
The hydrochloride salt form of paroxetine, a phenylpiperidine derivative and a selective serotonin reuptake inhibitor (SSRI) with antidepressant and anxiolytic properties. Paroxetine binds to the pre-synaptic serotonin transporter complex resulting in negative allosteric modulation of the complex thereby blocking reuptake of serotonin by the pre-synaptic transporter. Inhibition of serotonin recycling enhances serotonergic function through serotonin accumulation in the synaptic cleft, resulting in long-term desensitization and downregulation of 5HT1 (serotonin) receptors and leading to symptomatic relief of depressive illness.
Serotonin reuptake inhibitor formulations
A process for preparing amorphous paroxetine hydrochloride or sertraline hydrochloride is provided, which comprises preparing a solution in which paroxetine hydrochloride or sertraline hydrochloride and a water-soluble polymer are dissolved in a co-solvent of a volatile organic solvent and water. Said solution is dried to obtain a composition comprising amorphous paroxetine hydrochloride or sertraline hydrochloride and the water-soluble matrix.
Owner:ANDRX PHARMA INC
Serotonin reuptake inhibitor formulations
InactiveUS20020156066A1Easy to operateBiocidePowder deliveryOrganic solventSerotonin receptor inhibitors
A process for preparing amorphous paroxetine hydrochloride or sertraline hydrochloride is provided, which comprises preparing a solution in which paroxetine hydrochloride or sertraline hydrochloride and a water-soluble polymer are dissolved in a co-solvent of a volatile organic solvent and water. Said solution is dried to obtain a composition comprising amorphous paroxetine hydrochloride or sertraline hydrochloride and the water-soluble matrix.
Owner:ANDRX PHARMA INC
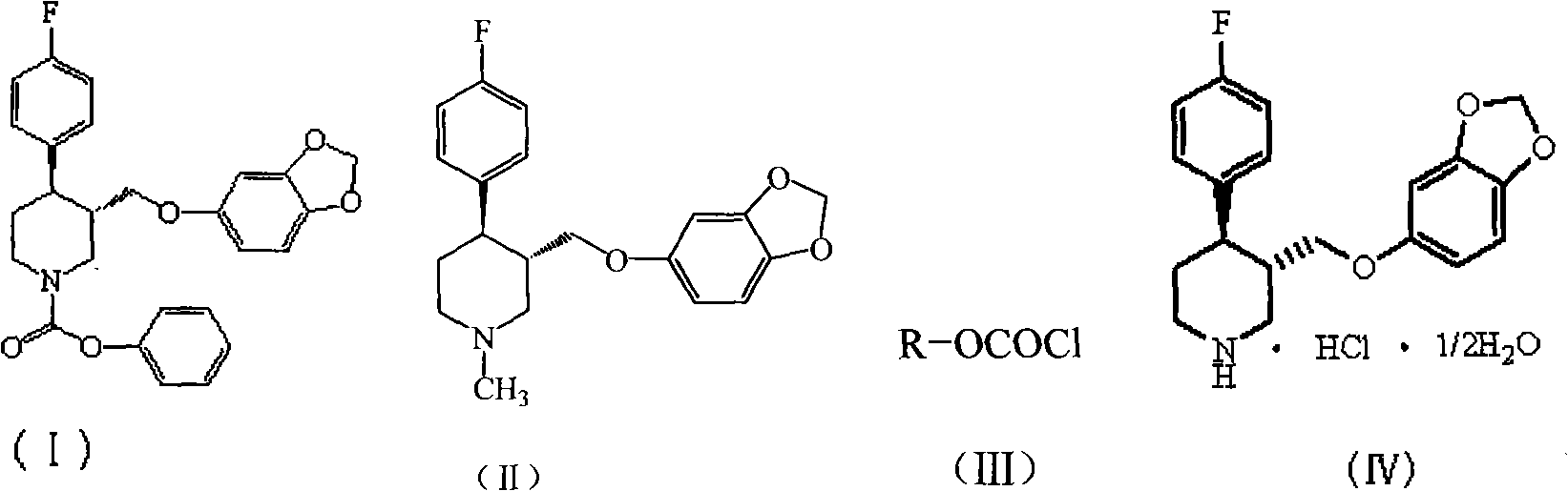
Preparation of paroxetine involving novel intermediates
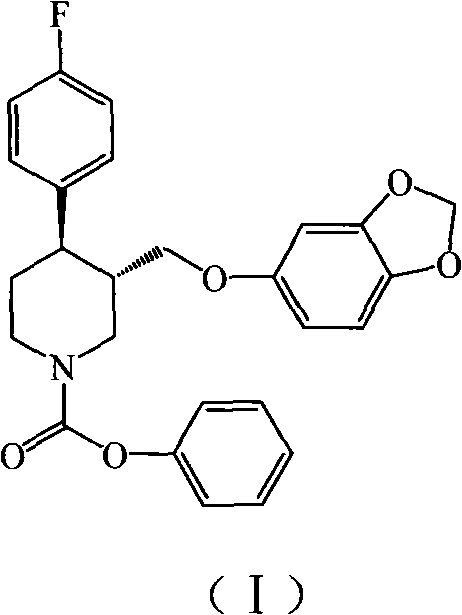
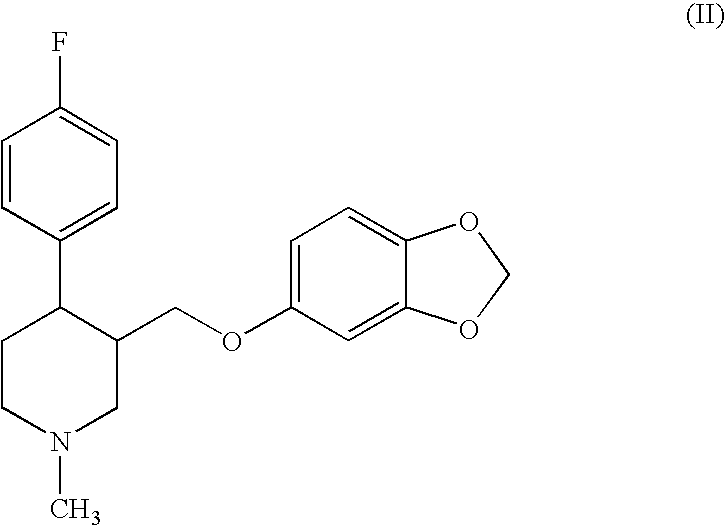
Disclosed are processes for preparing novel carbamate intermediates of paroxetine comprising dealkylating N-alkylparoxetine by reaction thereof with a haloalkyl ester of a haloformic acid, in a suitable organic solvent. Also disclosed are processes for preparing paroxetine comprising hydrolyzing the novel carbamate intermediates in a suitable solvent. Paroxetine prepared by the above processes can be neutralized with hydrogen chloride and crystallized as paroxetine hydrochloride anhydrous, hemihydrate or as a solvate of isopropanol. The invention is further directed to the novel carbamate intermediates formed by the disclosed processes.
Owner:TEVE PHARMA USA INC
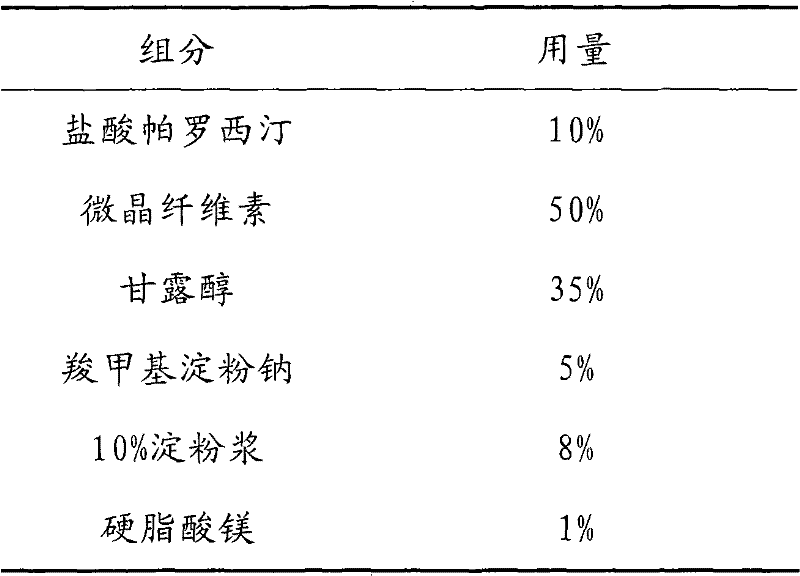
Tablet containing paroxetine and preparation method thereof
ActiveCN102525966AQuality assuranceGuaranteed stabilityOrganic active ingredientsNervous disorderParoxetine HydrochlorideChemistry
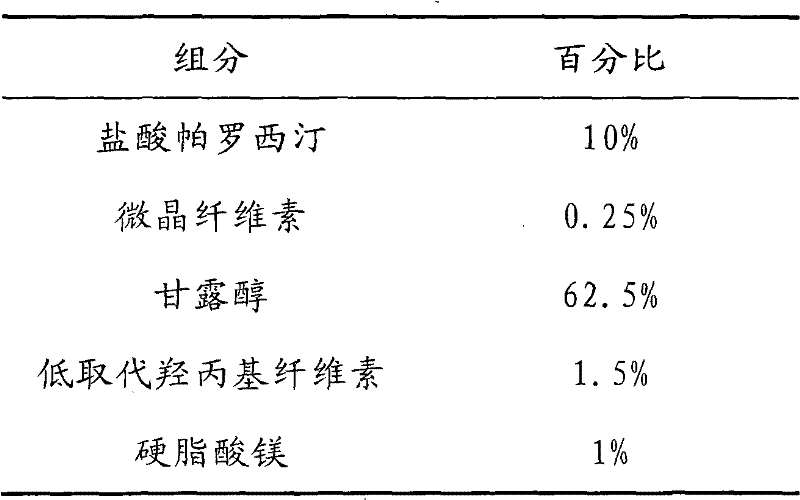
The invention discloses a tablet containing paroxetine and a preparation method thereof. The tablet is composed of paroxetine hydrochloride and auxiliary materials accepted in pharmacy and prepared in a wet granulation process, thereby not affecting tablet manufacturing modes and guaranteeing quality and stability of tablets. The tablet is simple in preparation method, wide in application scope, convenient to transport and store and suitable for scale production. The preparation method is convenient to operate.
Owner:江苏万全特创医药生物技术有限公司
Method for drying anhydrous paroxetine hydrochloride
InactiveUS6541637B1Efficient executionReduce pressureBiocideNervous disorderHydrogen chlorideIsopropyl alcohol
A process for drying paroxetine hydrochloride anhydrate comprising (A) reacting a paroxetine compound with hydrogen chloride in the presence of isopropyl alcohol and crystallizing the resulting product, to obtain paroxetine hydrochloride anhydrate, and drying the resulting paroxetine hydrochloride anhydrate at a temperature of not more than 60° C. and under normal pressure or lower in an atmosphere which does not substantially contain moisture until the content of isopropyl alcohol attains to not more than 15% by weight; and (B) further drying the paroxetine hydrochloride anhydrate at a temperature of 80° to 110°C. in an atmosphere reduced to not more than 20 mm Hg until the content of isopropyl alcohol attains to not more than 5% by weight. According to the present invention, the amount of remaining isopropyl alcohol contained in the paroxetine hydrochloride anhydrate, crystallized in the presence of isopropyl alcohol, can be efficiently reduced in a short period of time without necessitating a large-scaled apparatus.
Owner:SUMITOMO CHEM CO LTD
Methods of crystal precipitation
InactiveUS20060048696A1High yieldImprove solubilityPolycrystalline material growthNervous disorderOrganic solventHydrogen chloride
Crystals of paroxetine hydrochloride ½-hydrate are allowed to separate out by adding water to a solution or suspension comprising paroxetine hydrochloride and a polar organic solvent which contains no water or at most 60% by weight of water to adjust the water content to at least 70% by weight when crystals of paroxetine hydrochloride ½-hydrate are allowed to separate out in a water-containing polar organic solvent. Crystals of paroxetine hydrochloride ½-hydrate being not colored in pink can be allowed to separate out in the presence of hydrogen chloride when crystals of paroxetine hydrochloride ½-hydrate are allowed to separate out in water or a water-containing polar organic solvent.
Owner:SUMITOMO CHEM CO LTD
Dripping pills of paroxetine hydrochloride and its preparation method
InactiveCN1568987ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsNervous disorderMedicineDysphagia
The invention relates to an Paroxetine Hydrochloride drop pill prepared by utilizing ultramicro disintegration and drop pill manufacturing process, which has the advantages of improving collapse and dissolving speed, dissolving out speed and degree, quick effect, increased medicament stability, reduced adjuvant consumption, lowered production costs, and easiness in carrying and use. oral or swallow administration, It has good compliance, thus is especially suitable for children, the elderly, bedridden patients and dysphagia patients.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Preparation method of paroxetine hydrochloride and intermediate thereof
InactiveCN101560207AEasy to operateHigh yieldNervous disorderOrganic chemistryPhenyl groupStereochemistry
Owner:万全万特制药江苏有限公司
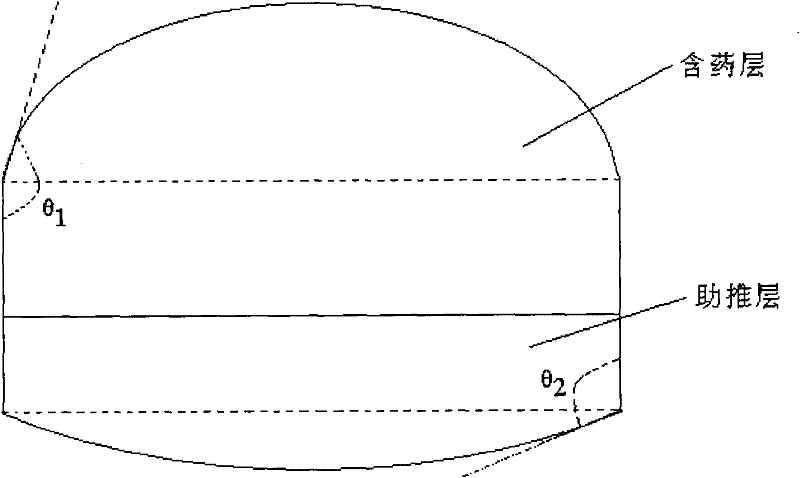
Paroxetine hydrochloride osmotic pump type enteric controlled release tablet
The invention provides a novel paroxetine hydrochloride osmotic pump type enteric controlled release tablet. In an osmotic pump type enteric controlled release tablet in an enteric coating, ethyl cellulose and polyvinylpyrrolidone are used as semipermeable membrane formation material, an asymmetric tablet type is preferable, the ageing phenomenon of the semipermeable membrane can be overcome, and the medicine residue is lowered. The invention also provides a method for improving the anti-ageing performance of the paroxetine hydrochloride osmotic pump type enteric controlled release tablet. The method is characterized in that the ethyl cellulose-polyvinylpyrrolidone is adopted to serve as the semipermeable membrane material. In addition, the invention also provides an application of the ethyl cellulose-polyvinylpyrrolidone composite to prepare the paroxetine hydrochloride osmotic pump type enteric controlled release tablet with the anti-ageing performance.
Owner:内蒙古天衡医院管理有限公司
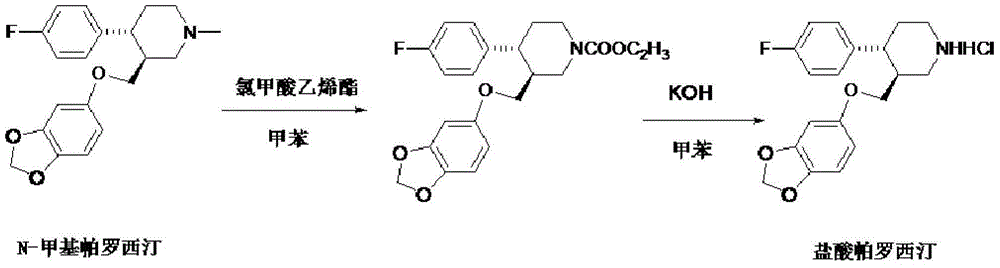
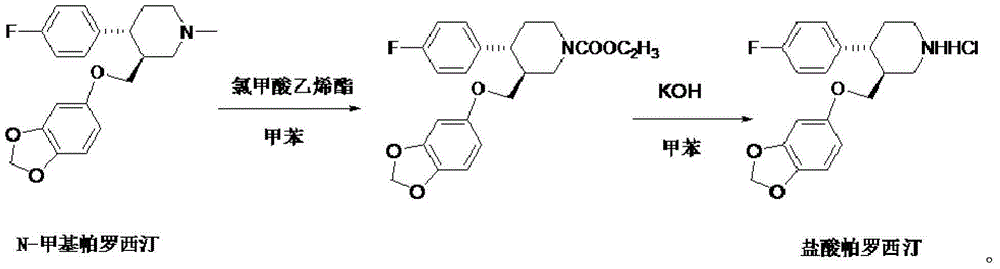
Production process of paroxetine hydrochloride
The invention discloses a production process of paroxetine hydrochloride and belongs to the technical field of medicines. The compound paroxetine hydrochloride has the following structure as shown in the specification. According to the invention, the demethylation reaction of N-methyl paroxetine is mainly studied in detail and the refining process of paroxetine hydrochloride is relevantly inspected. Industrially available vinyl chloroformate is adopted as a demethylation reagent, reaction conditions are mild and the total yield is above 90%. By adopting acetone of which the use amount is 10 times amount of a paroxetine hydrochloride crude product as a refining solvent, a majority of impurities can be well removed to obtain pure white crystalline powder of which the content is above 99.8%.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Paroxetine hydrochloride oral suspension and preparation process thereof
The present disclosure relates to the pharmaceutical field, and in particular, relates to a paroxetine hydrochloride oral suspension and a preparation process thereof. The paroxetine hydrochloride oral suspension is prepared by the steps: firstly, screening and pre-mixing raw material drugs with a suspending aid silicon dioxide, and then dispersing in propylene glycol; then adding a prescription amount of double distilled water, glycerin, a preservative, a taste bud paralysant, a sweetener, a colorant and a pH regulator, and adjusting the pH of the solution to 3-7; after uniform magnetic stirring, carrying out high pressure homogenization by a micro-jet high-pressure homogenizer; and finally, adding a foaming agent and an essence. The oral suspension masks the bitterness of drugs and is more acceptable to patients, wherein high pressure homogenization is performed by the micro-jet high-pressure homogenizer, and the prepared suspension has large sedimentation volume and is stable. The paroxetine hydrochloride oral suspension improves the compliance of patients, facilitates the use of children and adults with difficulty in swallowing oral solid preparations, ensures the accuracy of dosage of administration, and improves the safety and effectiveness of drugs.
Owner:AVENTIS PHARMA HAINAN
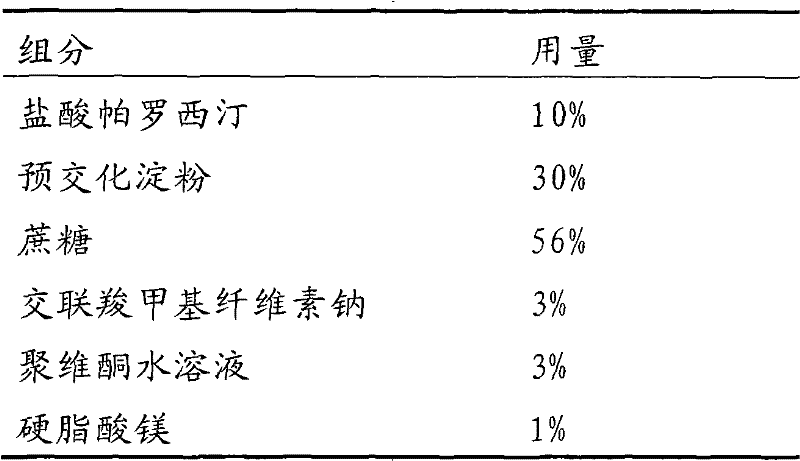
Stable paroxetine hydrochloride tablet and preparation method thereof
ActiveCN110037995AInhibition of reddeningHave an unexpected effectOrganic active ingredientsNervous disorderSucroseFiller Excipient
The invention belongs to the technical field of medicines, and provides a stable paroxetine hydrochloride tablet and a preparation method thereof. The paroxetine hydrochloride tablet comprises the following components in parts by weight: 7.6 parts of paroxetine hydrochloride, 67.4-81.9 parts of a filler, 2-5 parts of an adhesive, 3-10 parts of a stabilizer, 5-7 parts of a disintegrant and 0.5-3 parts of a lubricant, wherein the stabilizer is sucrose, the filler is calcium hydrogen phosphate dihydrate, the disintegrant is sodium carboxymethyl starch, the adhesive is povidone K30, and the lubricant is magnesium stearate. The preparation method of the paroxetine hydrochloride tablet adopts a wet granulation process. The method solves the problems that paroxetine hydrochloride products turn red in the wet granulation process and the hardness of paroxetine hydrochloride tablets is poor at high temperature when calcium phosphate dihydrate is used as a main filler.
Owner:山东启荣科技有限公司 +1
Controlled Release Compositions of an Antidepressant Agent
The present invention relates to controlled release compositions comprising an anti-depressant compound. More particularly, the present invention relates to controlled release compositions comprising paroxetine hydrochloride.
Owner:AUROBINDO PHARMA LTD
Paroxetine hydrochloride compound and synthetic method thereof
The invention relates to the field of medicine and particularly relates to a paroxetine hydrochloride compound and a synthetic method thereof. The paroxetine hydrochloride compound has the following structural formula. According to the paroxetine hydrochloride compound, ethyl chloroformate for industrial production is used as a demethylation reagent, the reagent is stable, the production condition of demethylation is moderate, the total yield is high and about 75%, and the paroxetine hydrochloride compound accords with the requirement of a 2010 edition of a pharmacopeia and applicable to industrial production.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Paroxetine hydrochloride enteric-coated and sustained-release tablet and its preparation method
InactiveCN108143722AConsistent in vitro releaseImprove solubilityOrganic active ingredientsNervous disorderDrug release rateSustained Release Tablet
The invention discloses a paroxetine hydrochloride enteric-coated and sustained-release tablet, which comprises a tablet core, an isolating layer, a semipermeable membrane, an enteric coating and a moistureproof coat; the isolating layer is arranged between the tablet core and the semipermeable membrane, the enteric coating is arranged between the semipermeable membrane and the moistureproof coat,wherein the tablet core is prepared from paroxetine hydrochloride, penetrating agent, filling agent, binding agent and lubricant; the penetrating agent comprises one or more drug release hole. The invention further provides a preparation method of the paroxetine hydrochloride enteric-coated and sustained-release tablet, which includes steps of preparing tablet core grains and performing compression moulding to obtain the tablet core; making the tablet core pass through the isolating layer, the emipermeable membrane, the enteric coating and moistureproof coat, drying to obtain the paroxetine hydrochloride enteric-coated and sustained-release tablet. The paroxetine hydrochloride enteric-coated and sustained-release tablet can effectively control the drug release rate, and keep stable blooddrug concentration and durable drug effect, increase the safety, effectiveness and compliance of patients after taking drugs; the production preparation technique is simple, and the cost is low.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Preparation of paroxetine involving novel intermediates
Disclosed are processes for preparing novel carbamate intermediates of paroxetine comprising dealkylating N-alkylparoxetine by reaction thereof with a haloalkyl ester of a haloformic acid, in a suitable organic solvent. Also disclosed are processes for preparing paroxetine comprising hydrolyzing the novel carbamate intermediates in a suitable solvent. Paroxetine prepared by the above processes can be neutralized with hydrogen chloride and crystallized as paroxetine hydrochloride anhydrous, hemihydrate or as a solvate of isopropanol. The invention is further directed to the novel carbamate intermediates formed by the disclosed processes.
Owner:TEVA PHARMA IND LTD +1
Compound preparation for treating premature ejaculation and erectile dysfunction
PendingCN111297869AGood treatment effectRelieve anxiety and depressionOrganic active ingredientsSexual disorderSexual impotencePharmaceutical drug
The invention discloses a compound preparation for treating premature ejaculation and erectile dysfunction. The compound preparation is composed of the following medicines by weight: 40-120 mg of sildenafil citrate, and 10-50 mg of paroxetine hydrochloride. The compound preparation has the following beneficial effects: the sildenafil citrate treats the erectile dysfunction, the paroxetine hydrochloride relieves anxiety and depression in patients, so that the treatment effect on the erectile dysfunction is improved; and the paroxetine hydrochloride treats the premature ejaculation, the sildenafil citrate treats side effects of the erectile dysfunction due to the paroxetine hydrochloride, so that the effective rate of the premature ejaculation treatment is improved.
Owner:白文智
Paroxetine hydrochloride time-controlled release pharmaceutical composition and preparation method thereof
InactiveCN104434851AOptimizing Prescription VolumeFix timed release issuesOrganic active ingredientsNervous disorderControlled releaseIsolation layer
The invention relates to the technical field of drug dosage form structure, and in particular relates to a paroxetine hydrochloride time-controlled release pharmaceutical composition and a preparation method thereof. The paroxetine hydrochloride time-controlled release medicinal composition comprises a paroxetine hydrochloride drug release layer, a coated isolation layer and a coated paroxetine hydrochloride drug release layer. Through a large number of tests and prescription screening, the invention provides the paroxetine hydrochloride time-controlled release pharmaceutical composition for the majority of patients, and fills the domestic blank to meet the needs of clinical medication.
Owner:TIANJIN SONGRUI MEDICAL TECH
Paroxetine hydrochloride oral disintegrating tablet
The invention provides a PEG6000-DSPE-paroxetine hydrochloride composite material, and a preparation method thereof. According to the preparation method, paroxetine hydrochloride and amphiphilic polymeric material PEG6000-DSPE are subjected to self assembling solvent evaporation so as to obtain a polymer micelle, freeze drying technology is adopted for freeze drying of the polymer micelle so as toobtain free dried powder, wet granulation is adopted to prepare the oral disintegrating tablet. The preparation method is capable of improving tablet mouthfeel, increasing main drug stability and dissolution rate, and increasing in vivo bioavailability.
Owner:万全万特制药江苏有限公司
Pharmaceutical composition containing paroxetine hydrochloride and application of pharmaceutical composition
InactiveCN105816457AImprove convenienceGood synergyOrganic active ingredientsNervous disorderTreatment effectSide effect
The invention relates to a pharmaceutical composition containing paroxetine hydrochloride and its application, belonging to the technical field of medicine. In order to overcome the technical deficiencies of the existing antidepressants that have relatively large side effects and unsustainable drug effects, the present invention provides a pharmaceutical composition containing paroxetine hydrochloride, which consists of paroxetine hydrochloride and silybin. The pharmaceutical composition can significantly improve the behavioral score of depression rats when treating depression, and has a significant improvement effect on the cerebral cortex monoamine neurotransmitters of depression rats, thus playing a role in depression. Very good therapeutic effect.
Owner:杨强
Process for producing paroxetine hydrochloride hydrate
InactiveUS20060041138A1Efficiently obtainedNervous disorderOrganic chemistryHydrogen chlorideParoxetine Hydrochloride
A process for preparing a paroxetine hydrochloride hydrate, comprising reacting (3S, 4R)-1-tert-butoxycarbonyl-4-(4-fluorophenyl)-3-[(3,4-methylene-dioxy)phenoxymethyl]piperidine with hydrogen chloride in the presence of water, and allowing crystals to separate out from the resulting reaction mixture in the presence of water.
Owner:SUMITOMO CHEM CO LTD
Paroxetine hydrochloride sustained release tablet and preparation method of same
InactiveCN107412178ARealize the function of shading protectionQuality improvementOrganic active ingredientsNervous disorderSustained Release TabletPharmacy
The invention relates to a paroxetine hydrochloride sustained release tablet and belongs to the technical field of pharmacy. According to a technical scheme, the paroxetine hydrochloride sustained release tablet is composed of a sustained release micro-pellet and common excipients. The sustained release micro-pellet is composed of a medicine-carrier layer, a sustained release layer and an enteric layer. The use amount of talcum powder in the sustained release layer is 20-100% of a high-molecular sustained release material in the layer. The use amount of talcum powder in the enteric layer is 50-200% of a high-molecular enteric material in the layer. Through regulation on the use amount of talcum powder, an effect of shading protection on the active component is achieved.
Owner:北京满格医药科技有限公司
Stable pharmaceutical formulation of paroxetine hydrochloride anhydrous and a process for preparation thereof
InactiveUS20050191350A1Organic active ingredientsBiocidePharmaceutical formulationParoxetine Hydrochloride
Owner:FOX MICHAEL +3
Paroxetine hydrochloride orally disintegrating tablets and preparation technology thereof
PendingCN112137970AGreat tasteDisintegrates quicklyOrganic active ingredientsNervous disorderOrally disintegrating tabletPharmaceutical Substances
The invention discloses paroxetine hydrochloride orally disintegrating tablets and preparation method thereof. The invention is characterized in that the paroxetine hydrochloride orally disintegratingtablets are prepared by adopting a particle coating process after wet granulation. Through the process, the taste of the medicine can be remarkably improved.
Owner:BEIJING VENTUREPHARM BIOTECH
New compound
Owner:SMITHKLINE BECKMAN CORP
Preparation method of key intermediate of paroxetine hydrochloride
ActiveCN109020872AImprove benefitsLow input costOrganic chemistryCombinatorial chemistryHydroxymethyl
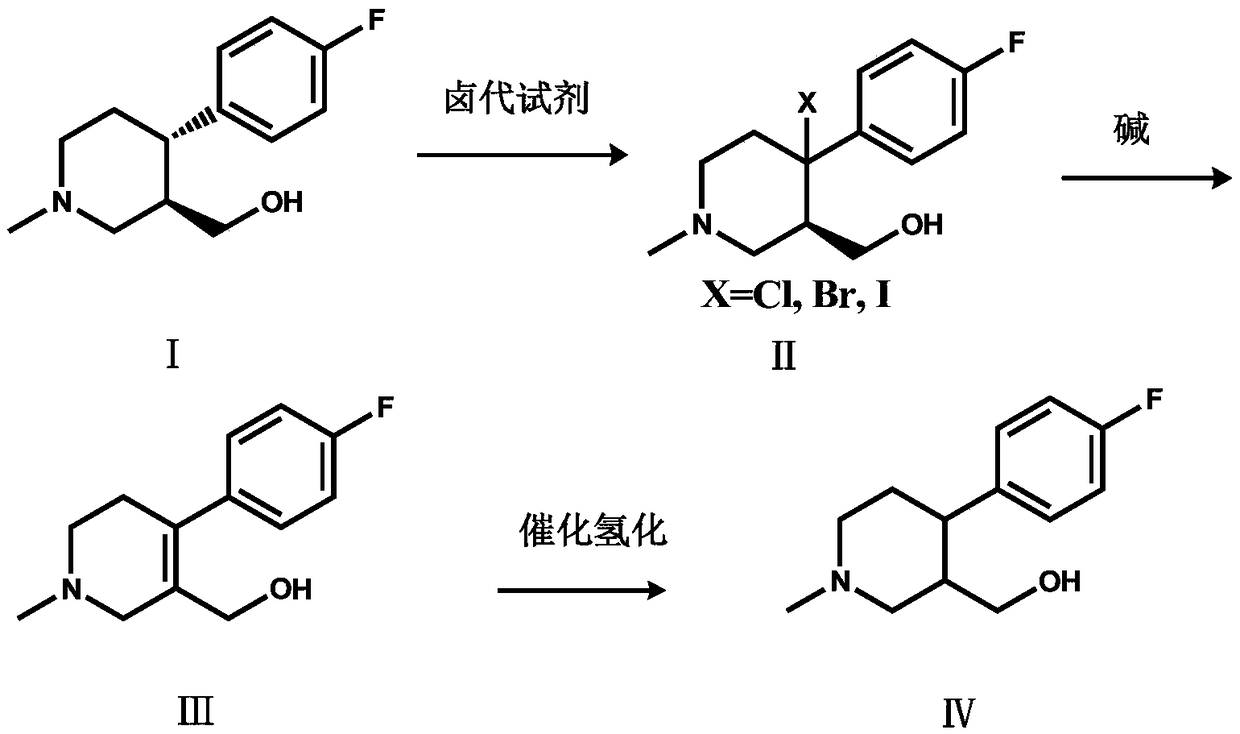
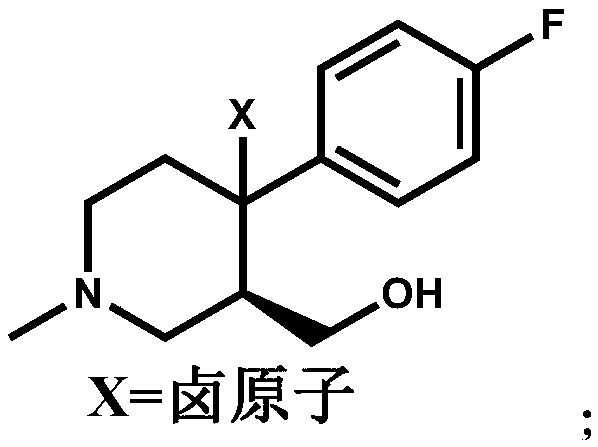
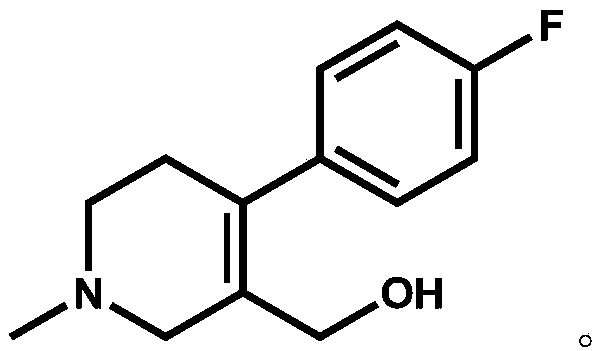
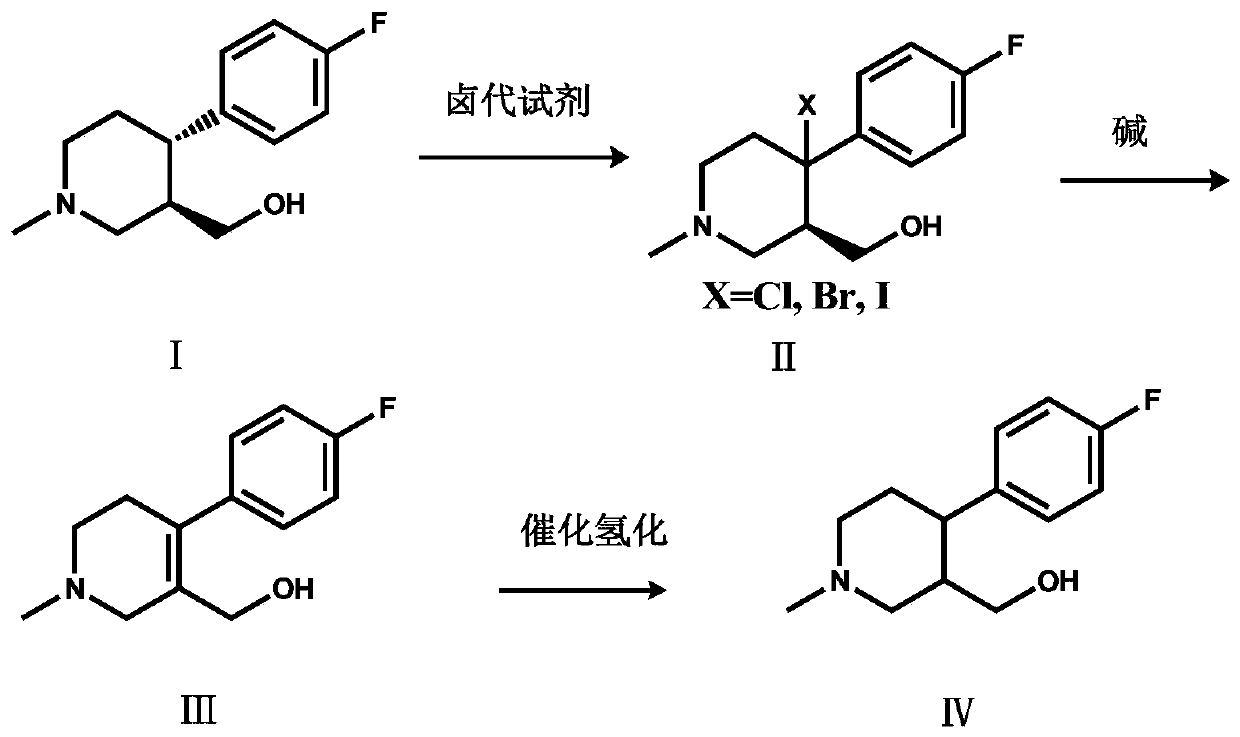
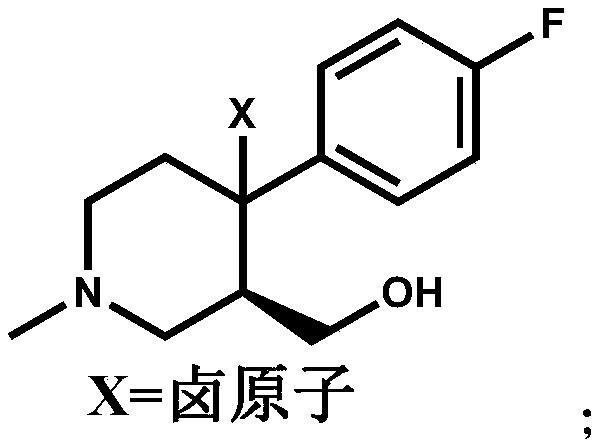
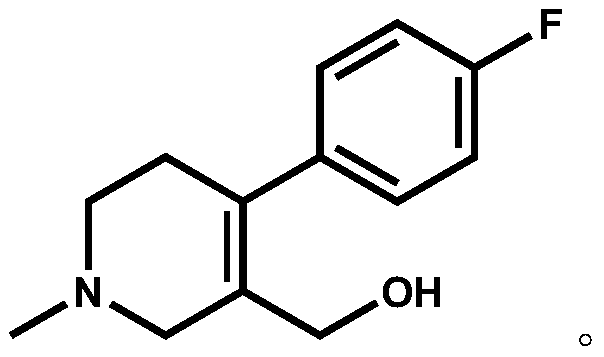
The invention discloses a preparation method of a key intermediate of paroxetine hydrochloride. The preparation method comprises: carrying out benzyl site halogenation on (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine as a starting material through a halogenation reagent to obtain a compound II, carrying out an elimination reaction process on the compound II to obtain a compound III, and carrying out catalytic hydrogenation reduction on the compound III to obtain (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine. The preparation method can convert a by-product (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine, which cannot be directly utilized, into (+ / -)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine. The product can be directly used as an intermediate for synthesizing paroxetine hydrochloride, change wastes into valuables, improve the benefits of enterprises and reduce the input cost of the enterprise.
Owner:ZHEJIANG HUABANG MEDICAL & CHEM
A kind of preparation method of paroxetine hydrochloride key intermediate
The invention discloses a preparation method of a key intermediate of paroxetine hydrochloride. The preparation method comprises: carrying out benzyl site halogenation on (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine as a starting material through a halogenation reagent to obtain a compound II, carrying out an elimination reaction process on the compound II to obtain a compound III, and carrying out catalytic hydrogenation reduction on the compound III to obtain (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine. The preparation method can convert a by-product (+)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine, which cannot be directly utilized, into (+ / -)-trans-4-(4-fluorophenyl)-3-hydroxymethyl-1-methylpiperidine. The product can be directly used as an intermediate for synthesizing paroxetine hydrochloride, change wastes into valuables, improve the benefits of enterprises and reduce the input cost of the enterprise.
Owner:ZHEJIANG HUABANG MEDICAL & CHEM
One step preparation method of paluxitin hydrochloride A crystal type crystal molecule assembly
A process for preparing paroxetine A hydrochloride includes such steps as adding semi-hydrous paroxetine hydrochloride and solvent (oxylipohydrocarbon derivative) to the crystallizer with jacket, heating, stirring, natural cooling while educting out crystal, and stirring for 30-120 min.
Owner:TIANJIN UNIV +1
Methods of crystal precipitation
Owner:SUMITOMO CHEM CO LTD
Paroxetine hydrochloride compound and preparation method thereof
InactiveCN102285973BChange the status quo of low purityImprove product qualityOrganic chemistryElutionStructural formula
The invention relates to a paroxetine hydrochloride compound with the purity of larger 99.5% and the following structural formula, and also relates to a preparation of the paroxetine hydrochloride compound. The preparation method comprises the following steps of: (1) dissolving the raw material paroxetine hydrochloride in the proper amount of methanol, extrating and filtering, abandoning solid impurities, and obtaining a methanol solution containing the paroxetine hydrochloride; (2) carrying out column chromatography on the methanol solution containing the paroxetine hydrochloride to load samples, collecting paroxetine hydrochloride elution parts, decompressing, concentrating, and obtaining a paroxetine hydrochloride methanol solution; and (3) adding an aqueous solution of diluted hydrochloric acid of 0.001-0.1M to the paroxetine hydrochloride methanol solution at the temperature not higher than 80 DEG C, regulating a pH value to be 4-6, reducing temperature in a gradient manner, re-crystallizing, and obtaining the purified paroxetine hydrochloride compound. The paroxetine hydrochloride compound disclosed by the invention has higher purity, the production quality of preparation is improved, the toxic and side effects are reduced, and the preparation method is suitable for industrial large-scale production.
Owner:HAINAN MEIDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com