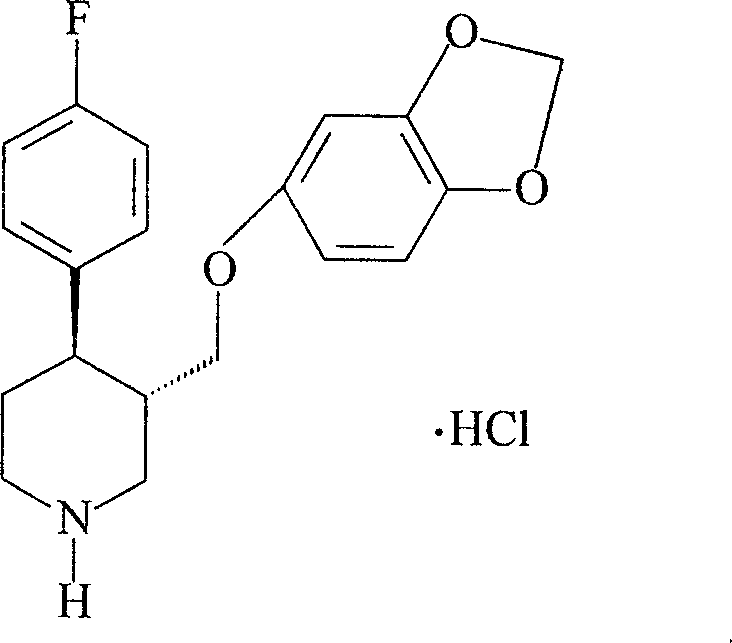
One step preparation method of paluxitin hydrochloride A crystal type crystal molecule assembly
A technology of paroxetine hydrochloride and crystals, which is applied in the field of one-step preparation of paroxetine hydrochloride A crystal crystal molecular assembly, which can solve the problems of increasing operation difficulty, affecting the quality of final products, and long operation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
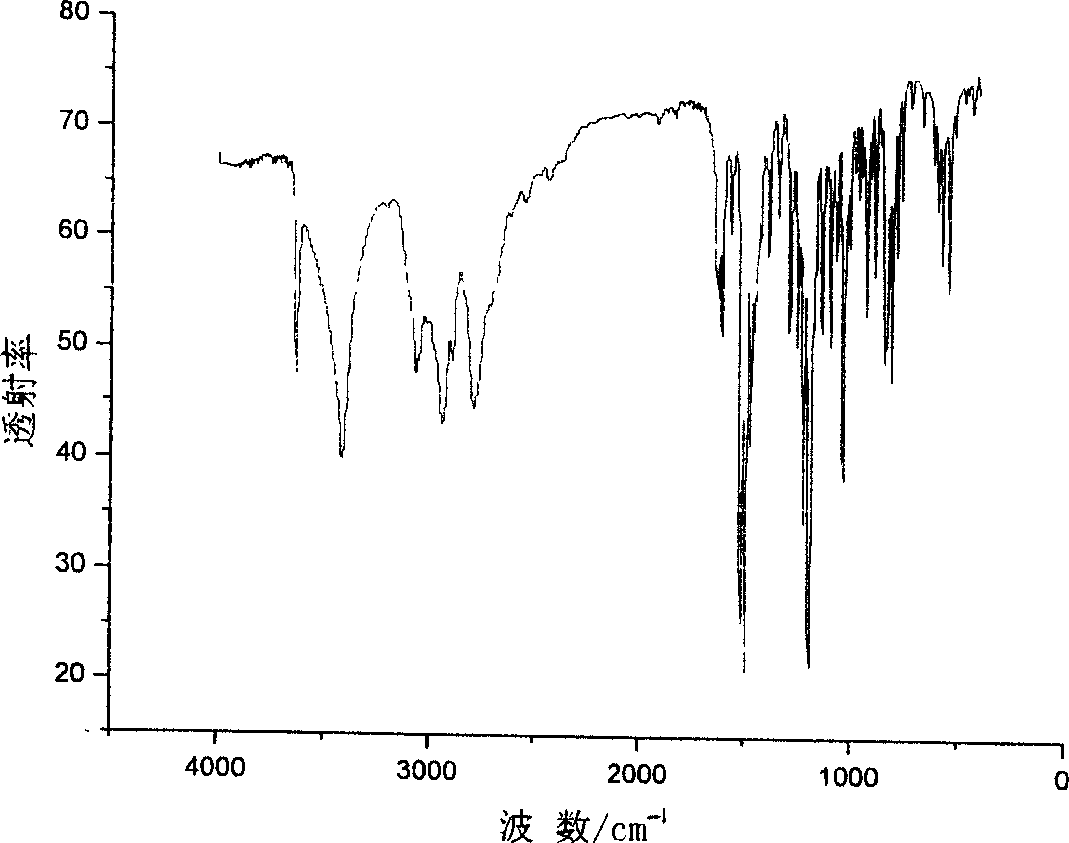
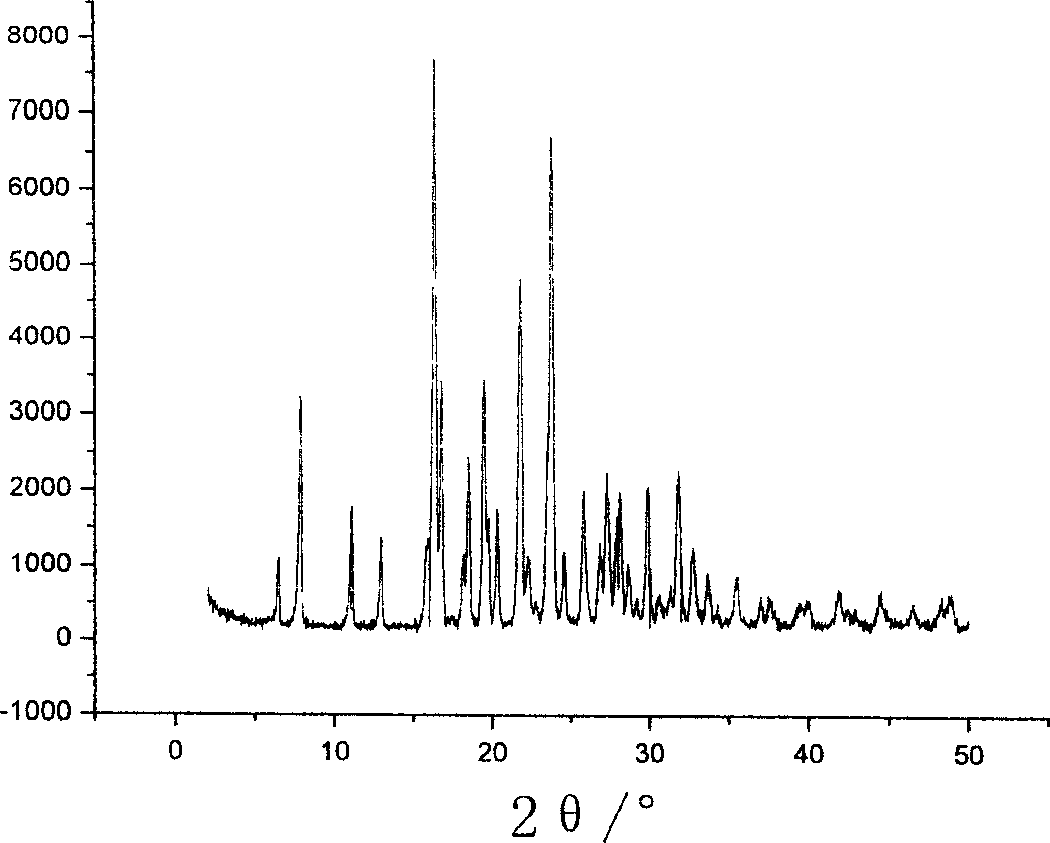
Image
Examples
example 1
[0026] A crystalline form of paroxetine hydrochloride was prepared by using a mixture of ketones and alcohols as a solvent.
[0027] Add 20.0012g of paroxetine hydrochloride hemihydrate into a jacketed 500ml glass crystallizer, add 300ml of acetone and 60ml of isopropanol at the same time, stir together, and heat to 55°C, stirring continuously for 40 minutes, paroxetine hydrochloride hemihydrate is completely dissolved .
[0028] The air is naturally cooled, and crystals are precipitated at about 30°C. Continue to stir, and the crystallizer is obviously turbid in about 3 minutes, and continue to stir for 2 hours.
[0029] After suction filtration for 15 minutes, the filter cake was dried under high vacuum with phosphorus pentoxide for 2 hours at a drying temperature of 50°C.
[0030] 14.6556 g of anhydrous paroxetine hydrochloride was obtained, with acetone content of 0.26% (NMR analysis), isopropanol content of 0.10% (NMR analysis), and water content of 0.32% (KF).
[0031]...
example 2
[0039] A crystalline form of paroxetine hydrochloride was prepared by using fatty alcohol as a solvent.
[0040] Add 10.0235g of paroxetine hydrochloride hemihydrate in a 700ml jacketed crystallizer, add 500ml of ethanol at the same time, stir together, and heat to 55°C, continue stirring for 60 minutes, paroxetine hydrochloride hemihydrate is completely dissolved.
[0041] The air is naturally cooled, and crystals are precipitated at about 35°C, and the crystallizer is obviously turbid in about 3 minutes, and the stirring is continued for 1 hour.
[0042] After suction filtration for 20 minutes, the filter cake was dried under high vacuum with phosphorus pentoxide for 1 hour at a drying temperature of 45°C.
[0043] 8.6532 g of paroxetine hydrochloride in crystal form A was obtained, with an ethanol content of 0.35% (NMR analysis) and a water content of 0.26% (KF).
[0044] The melting point is 122-124°C.
[0045] The DSC test results with a heating rate of 10K / min show tha...
example 3
[0052] Using ketones as solvents, the A crystal form of paroxetine hydrochloride was prepared.
[0053] Add 8.8235g of paroxetine hydrochloride hemihydrate in a 700ml jacketed crystallizer, add 500ml of acetone at the same time, stir together, and heat to 50°C, continue stirring for 60 minutes, paroxetine hydrochloride hemihydrate is completely dissolved.
[0054] The air is naturally cooled, and crystals are precipitated at about 30°C, and the crystallizer is obviously turbid in about 3 minutes, and the stirring is continued for 3 hours.
[0055] Suction filtration was performed for 30 minutes, and the filter cake was dried under high vacuum with phosphorus pentoxide for 3 hours at a drying temperature of 55°C.
[0056] 7.1532 g of anhydrous paroxetine hydrochloride was obtained, with an acetone content of 0.35% (NMR analysis) and a water content of 0.25% (KF).
[0057] The melting point is 122-124°C.
[0058] The DSC test results with a heating rate of 10K / min show that th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com