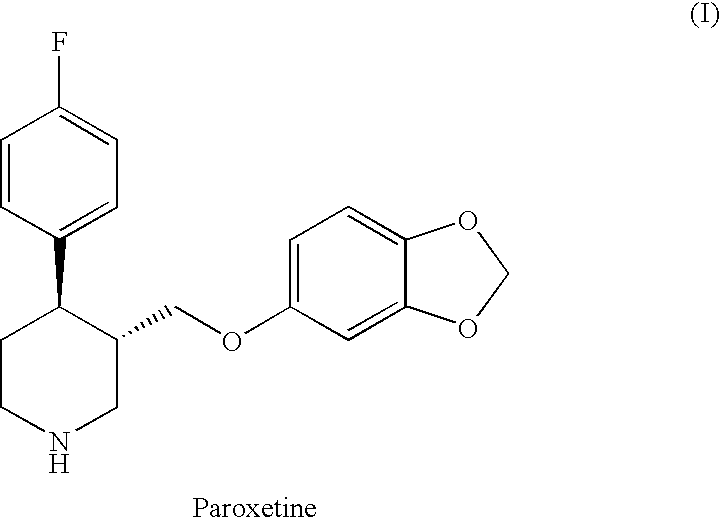
Stable pharmaceutical formulation of paroxetine hydrochloride anhydrous and a process for preparation thereof
a technology of paroxetine hydrochloride and pharmaceutical formulation, which is applied in the field of stable pharmaceutical formulation of paroxetine hydrochloride anhydrous and its preparation, can solve the problems of microcrystalline cellulose not being such a perfect excipient, and the hygroscopic nature of the anhydrous form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
Example 1
[0048] Composition of Stable Tablets of Paroxetine Hydrochloride:
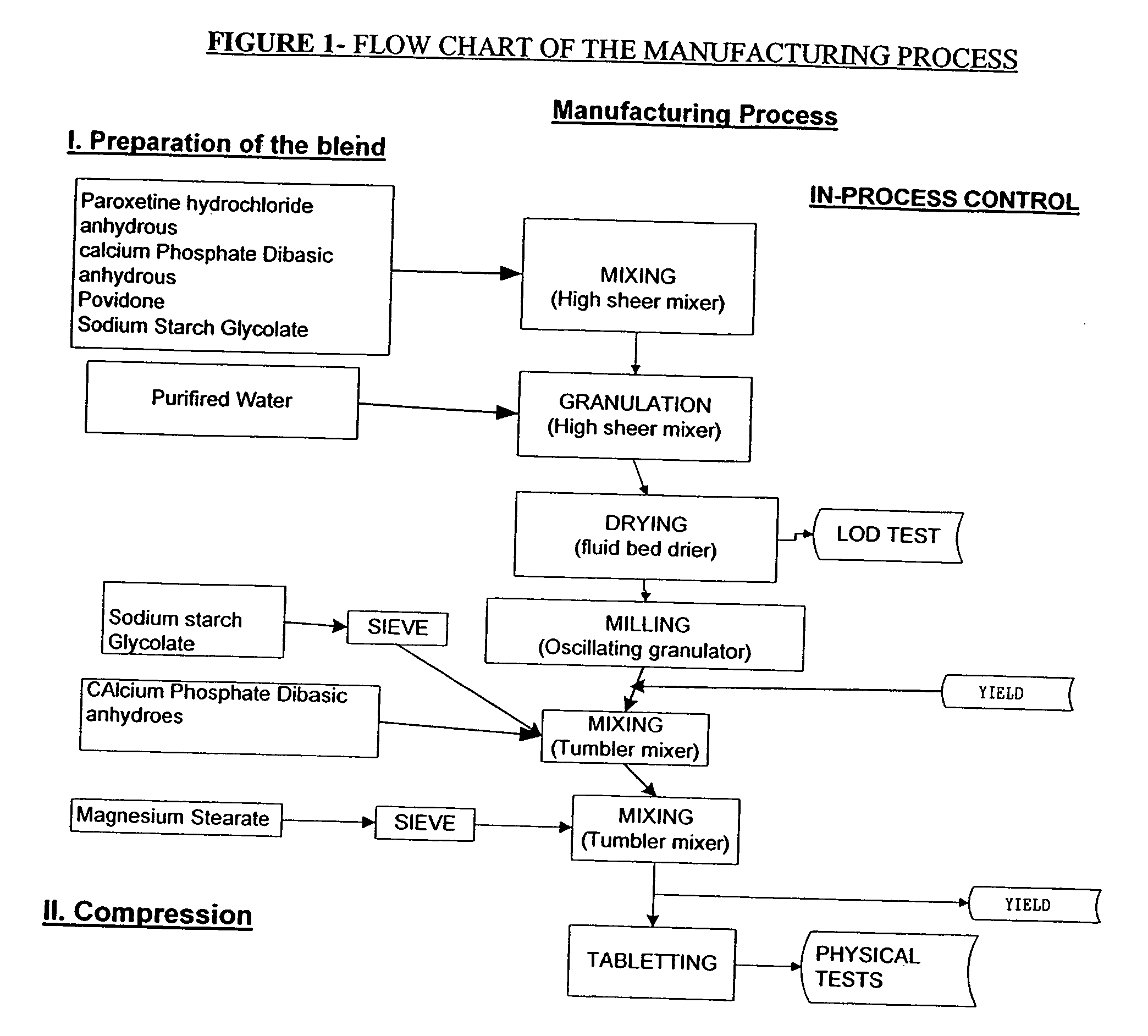
[0049] A paroxetine hydrochloride tablet was made which was of anhydrous form despite use of wet granulation. A tablet composition of paroxetine hydrochloride anhydrous, as made, is provided in Table 1. The manufacturing process is provided after the table.
TABLE 1Tablet Composition of Paroxetine Hydrochloride Anhydrous [Includesextra-granular excipients]Mg perINGREDIENTStabletFUNCTIONCoresParoxetine Hydrochloride22.21Active IngredientAnhydrousPovidone K-308binderDibasic Calcium Phosphate160.79FillerAnhydrousSodium starch Glycolate6.0DisintegrantMagnesium Stearate3.0LubricantPurified waterQ.S.Processing solvent (wetgranulation)Coating Suspension:*Opadry ®6.0*Composition of the Opadry ®% W / WTitanium Dioxide31.250Hydroxypropylmethylcellulose29.875(Methocel E3 Premium)Hydroxypropylmethylcellulose29.875(Methocel E5 Premium)Polyethylene Glycol 4008.000Polysorbate 80 (Tween)1.000
[0050] Manufacturing Process of St...
example 2
Paroxetine Anhydrous Tablet with Hydroxypropyl Methylcellulose as a Binder (Prepared Generally by the Same Process Disclosed in Example 1):
[0068] Paroxetine HCl Anhydrous (app. 11% w / w). [0069] Dibasic Calcium Phosphate Anhydrous (app. 82% w / w). [0070] Hydroxypropyl Methylcellulose, Grade E-5 (app. 2% w / w). [0071] Sodium Starch Glycolate (app. 3% w / w). [0072] Magnesium Stearate (app. 1.5% w / w). [0073] Purified Water (processing solvent only).
example 3
Prophetic Example
[0074] The paroxetine hydrochloride tablet prepared in Example 1 is subjected to a mechanical test-(Kraemer® Tablets Test System)
HARDNESS OF THE TABLETS, SCUHARDNESS OF THEAFTER 24 HOURS, 80° C., 75%TABLETS, SCU INITIALRELATIVE HUMIDITY1616
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com