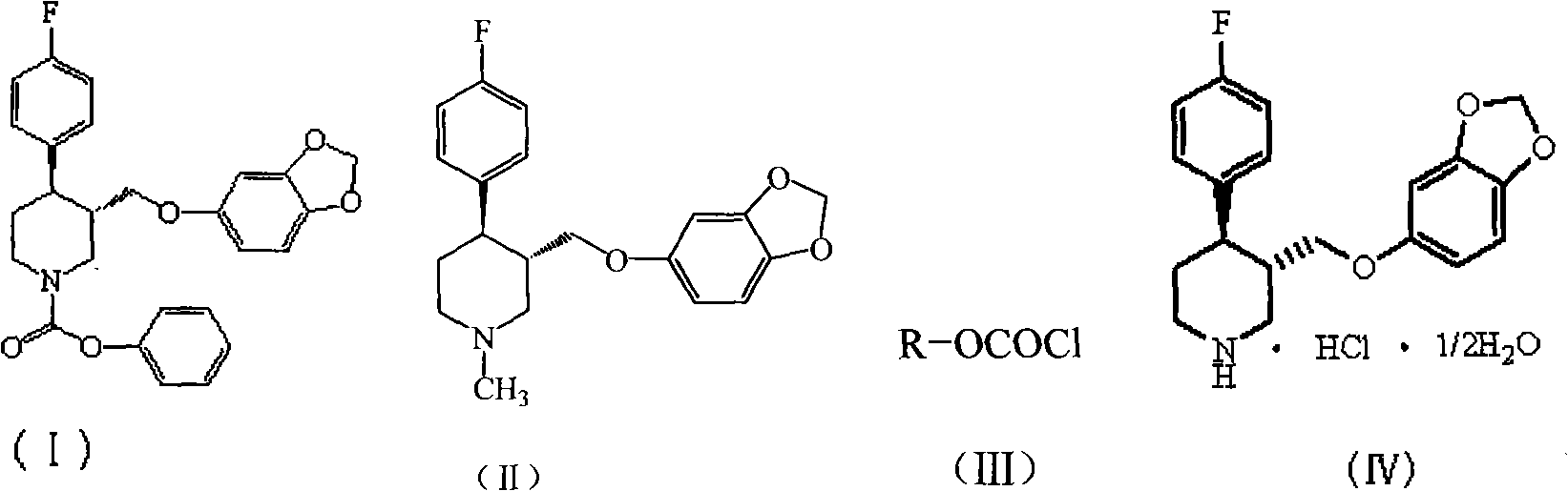
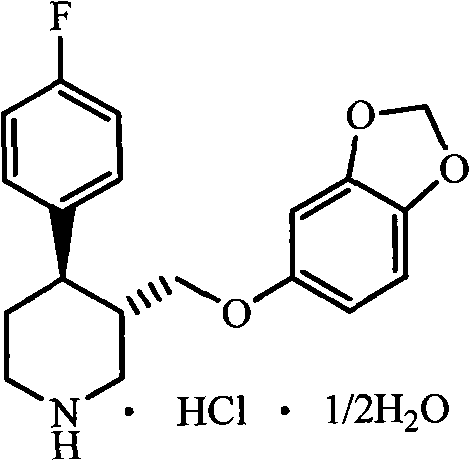
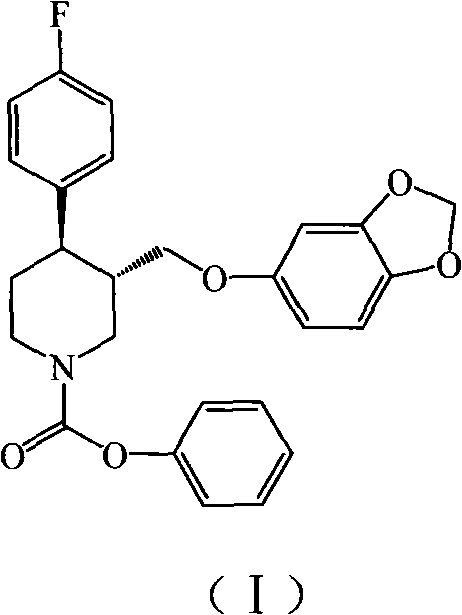
Preparation method of paroxetine hydrochloride and intermediate thereof
A technology of paroxetine hydrochloride and ethyl acetate, which is applied in the refining field of paroxetine hydrochloride, can solve the problems of small amount of recrystallization solvent, fast crystallization, loss and the like, and achieves a product with good product purity, strong controllability and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under nitrogen protection, 1500 g of N-methylparoxetine, 12.99 kg of toluene (C.P.), and 525 g of triethylamine (C.P.) were added to a 20 L reactor. The temperature was controlled at about 10°C, and 2055 g of phenyl chloroformate was slowly added dropwise to the system. After the dropwise addition, the reaction was refluxed for 2h. Remove from heat and let cool. Suction filtration, the filter cake was rinsed with 0.25kg toluene. The solvent was distilled off under reduced pressure. Add 6.54kg of petroleum ether and stir for 1h. Suction filtration, the filter cake was dried in a blast drying oven at 50°C for 6 hours to obtain 1904g (-)-trans-4R-(4-fluorophenyl)-3S-{[3′,4′-(methylenebis Oxy)phenoxy]methyl}-1-phenoxycarbonylpiperidine, yield 97%, TLC showed a single point.
Embodiment 2
[0022] Under nitrogen protection, 1500 g of N-methylparoxetine, 19.485 kg of toluene (C.P.), and 525 g of triethylamine (C.P.) were added to a 20 L reactor. The temperature was controlled at about 10°C, and 2055 g of phenyl chloroformate was slowly added dropwise to the system. After the dropwise addition, the reaction was refluxed for 2h. Remove from heat and let cool. Suction filtration, the filter cake was rinsed with 0.25kg toluene. The solvent was distilled off under reduced pressure. Add 6.54kg of methanol and stir for 1h. Suction filtration, the filter cake was dried in a blast drying oven at 50°C for 6 hours to obtain 1879g (-)-trans-4R-(4-fluorophenyl)-3S-{[3′,4′-(methylenebis Oxy)phenoxy]methyl}-1-phenoxycarbonylpiperidine, yield 96%, TLC showed a single point.
Embodiment 3
[0024] Under nitrogen protection, 1500 g of N-methylparoxetine, 15 L of dichloromethane (C.P.), and 525 g of triethylamine (C.P.) were added to a 20 L reactor. The temperature was controlled at about 10°C, and 2055 g of phenyl chloroformate was slowly added dropwise to the system. After the dropwise addition, the reaction was refluxed for 2h. Remove from heat and let cool. Suction filtration, filter cake rinse with 250ml toluene. The solvent was distilled off under reduced pressure. Add 6.54kg of methanol and stir for 1h. Suction filtration, the filter cake was dried in a blast drying oven at 50°C for 6h to obtain 1845g (-)-trans-4R-(4-fluorophenyl)-3S-{[3′,4′-(methylenebis Oxy)phenoxy]methyl}-1-phenoxycarbonylpiperidine, yield 94%, TLC showed a single point.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com