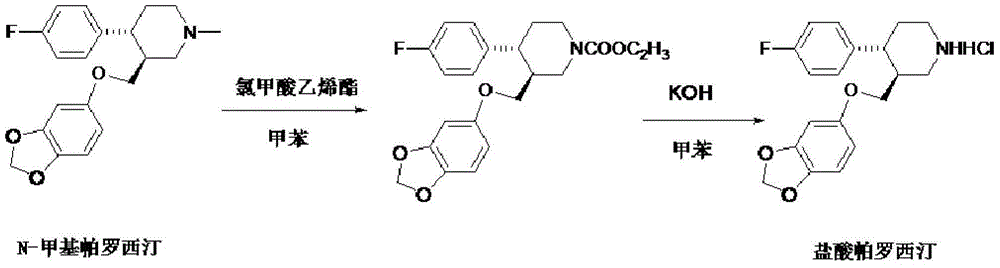
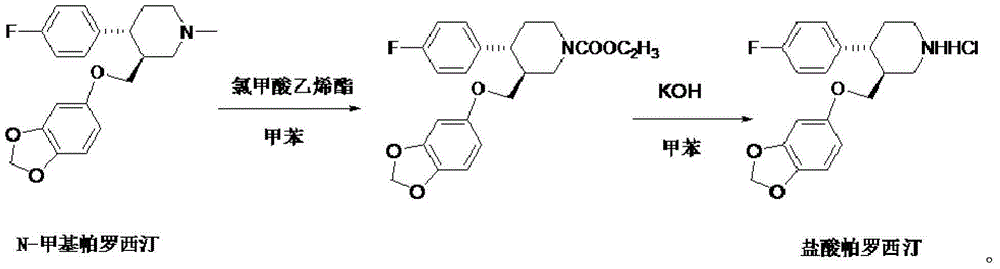
Production process of paroxetine hydrochloride
A paroxetine hydrochloride and production process technology, applied in the field of paroxetine hydrochloride production process, can solve the problems of low yield of paroxetine hydrochloride, incomplete reaction of N-methyl paroxetine, etc., to ensure stable quality and safety control, Improvement of yield and purity, effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 20 g of N-methylparoxetine and 200 ml of toluene into the three-necked flask, slowly add a mixture of 9.4 g of vinyl chloroformate and 20 ml of toluene dropwise at room temperature, and react at 80° C. for 6 hours after dropping by TLC (dichloromethane:methanol= 5:1) Monitor until the reaction is complete, add 200ml of water to the reaction solution, stir, separate layers, keep the organic layer, extract the water layer once with 100ml toluene, combine the organic layers, and wash three times with 200ml water respectively. The organic layer was concentrated under reduced pressure to obtain 22.2 g of oily substance, with a yield of 95%.
Embodiment 2
[0023] Add 20 g of the oil obtained in Example 1, 200 ml of toluene, and 6 g of potassium hydroxide to a three-neck flask, and react at 80° C. for 24 hours. TLC monitors until the reaction is complete (dichloromethane: ethyl acetate = 5:1), add water to separate layers , collect the organic layer, extract the water layer once with 100ml toluene, combine the organic layers, wash three times with 200ml respectively, take the organic layer and add concentrated hydrochloric acid under stirring, a large amount of light yellow crystals are precipitated, suction filtered and dried to obtain the crude product of light yellow paroxetine hydrochloride 18g, yield 95%.
Embodiment 3
[0025] Add 18 g of the crude product of paroxetine hydrochloride into the three-necked flask, and add 180 ml of acetone. Raise the temperature to 66°C, add 2% activated carbon, and reflux for 30 minutes to decolorize. Suction filtration, stirring the filtrate to cool down to 0°C, suction filtration and drying to obtain 16.5 g of finished paroxetine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com