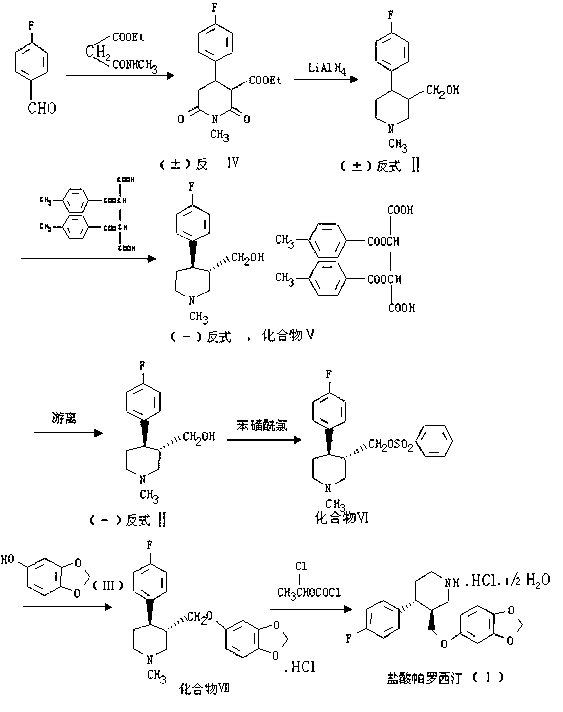
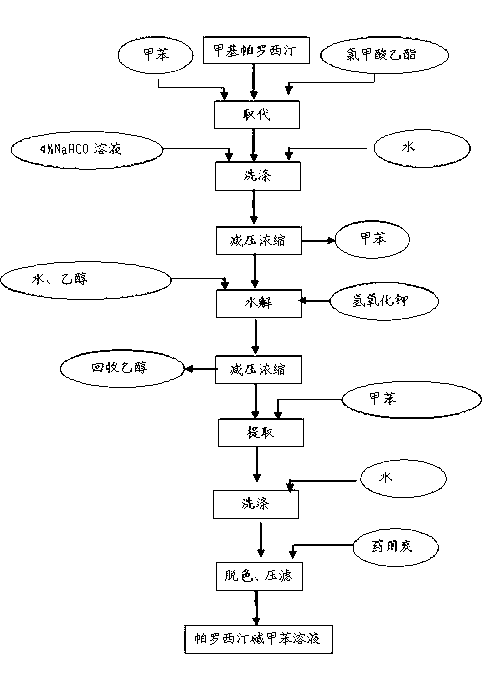
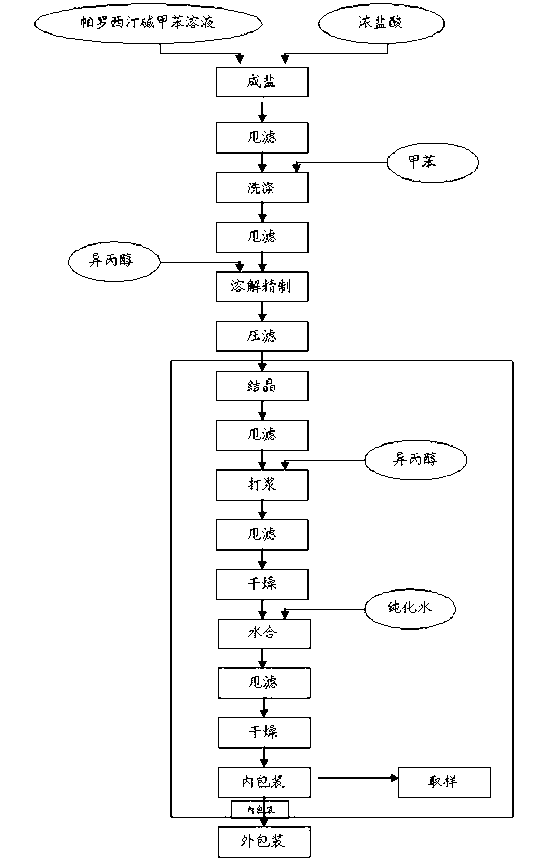
Paroxetine hydrochloride compound and synthetic method thereof
A technology of paroxetine hydrochloride and paroxetine methyl, which is applied in the field of medicine, can solve problems such as unfavorable removal of organic solvents, poor stability of chloroformic acid-α-chloroethyl ester, complicated operation, etc., and achieve mild production conditions and good stability , the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthetic process and refining method of paroxetine hydrochloride
[0031] 1) Preparation of crude paroxetine hydrochloride:
[0032] Such as figure 2 As shown, add 20 kg of N-methylparoxetine into 290 kg of toluene, stir, add ethyl chloroformate toluene mixture dropwise at room temperature, after the addition, stir for 30 minutes, heat up to 60-70 ° C, keep stirring for 4- After 6 hours (TLC detected that the reaction was complete, petroleum ether: ethyl acetate = 3:1), the organic layer was washed with water and aqueous sodium bicarbonate solution respectively, the water layer was separated, and the toluene was concentrated under reduced pressure to obtain a viscous substance.
[0033] N-methylparoxetine, source: Linhai Jinqiao Chemical Factory
[0034]
[0035] Add 700kg of ethanol to the residue, add 70.0Kg of potassium hydroxide, and heat up and reflux for 20-25 hours (about 80°C) (TLC test shows that the reaction is complete, if the reaction is too slow, add...
Embodiment 1 3
[0043] The stability situation of embodiment 1 three batches of samples is as follows:
[0044] Stability inspection items: selected according to the key inspection items stipulated in the above guiding principles and the properties of this variety, mainly including appearance, pH, moisture, related substances, and content.
[0045] Inspection basis: According to the 2010 edition of the Pharmacopoeia standard of paroxetine hydrochloride
[0046] Sample: three batches of 100401, 100402, and 100403 produced in Example 1.
[0047] accelerated test
[0048] Test method: Take this product to simulate the packaging of the marketed drug, and place it at 40 ° C ± 2 o C Place it under the condition of relative humidity RH75%±5% for 6 months. Samples were taken at 0, 1, 2, 3 and 6 months, and the indicators measured were compared with the samples at 0. The test results are shown in Table 1.
[0049] The accelerated test was carried out for 6 months, and the test results showed tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com