Paroxetine hydrochloride orally disintegrating tablets and preparation technology thereof
A technology of paroxetine hydrochloride and orally disintegrating tablets, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as complicated preparation methods and difficulty in industrial scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
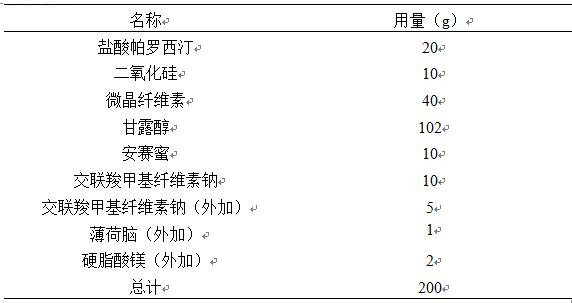
[0017]
[0018] Note: The raw and auxiliary materials in the table are the dosage per 1000 preparation units
[0019] Preparation Process:
[0020] Pass paroxetine hydrochloride and silicon dioxide through a 60-mesh sieve and mix, and then mix the rest of the auxiliary materials. Add 30% ethanol to the mixture to make soft materials, granulate with a 24-mesh sieve, control the drying temperature at 50°C, control the moisture content of the granules below 3%, sieve the granules with a 24-mesh sieve, and mix the obtained granules with the prescription amount of additional materials. Compressed into 1000 tablets on the tablet press, and packed after passing the inspection.
Embodiment 2
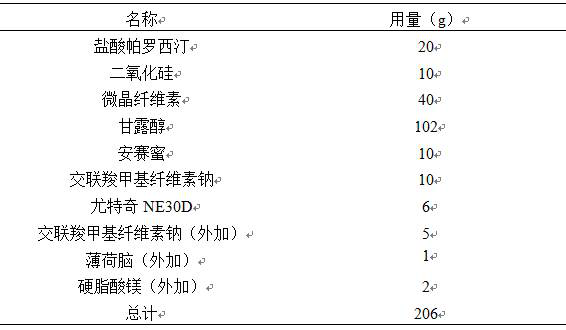
[0022]
[0023] Note: The raw and auxiliary materials in the table are the dosage per 1000 preparation units
[0024] Preparation Process:
[0025] Pass paroxetine hydrochloride and silicon dioxide through a 60-mesh sieve and mix, and then mix the rest of the auxiliary materials. Add 30% ethanol to the mixture to make soft materials, granulate with a 24 mesh sieve, control the drying temperature at 50°C, control the moisture content of the granules below 3%, sieve the granules with a 24 mesh sieve, and coat the obtained granules with Eudragit NE30D. Compressed into 1000 pieces on a tablet machine, and packed after passing the inspection.
Embodiment 3
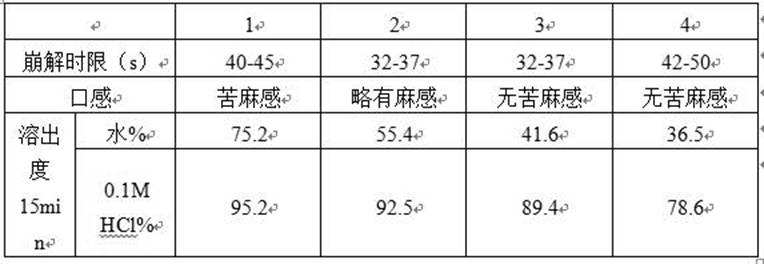
[0027]
[0028] Note: The raw and auxiliary materials in the table are the dosage per 1000 preparation units
[0029] Preparation Process:
[0030] Pass paroxetine hydrochloride and silicon dioxide through a 60-mesh sieve and mix, and then mix the rest of the auxiliary materials. Add 30% ethanol to the mixture to make soft materials, granulate with a 24 mesh sieve, control the drying temperature at 50°C, control the moisture content of the granules below 3%, sieve the granules with a 24 mesh sieve, and coat the obtained granules with Eudragit NE30D. Compressed into 1000 pieces on a tablet machine, and packed after passing the inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com