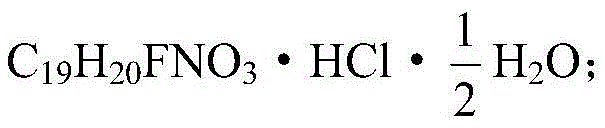Paroxetine hydrochloride time-controlled release pharmaceutical composition and preparation method thereof
A technology of paroxetine hydrochloride and its composition, which is applied in the field of paroxetine hydrochloride timed release pharmaceutical composition and its preparation, can solve the problems of paroxetine hydrochloride timed release, etc., and achieve the goal of solving the problem of timed release, stable product quality, and optimized prescription quantity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prepare 1000 tablets of paroxetine hydrochloride time-release pharmaceutical composition, the composition formula is as follows:
[0046] A particle
[0047]
[0048] B particle isolation layer
[0049]
[0050] When making:
[0051] 1) Take the prescribed amount of paroxetine hydrochloride and pregelatinized starch, mix well, use 3% methylcellulose solution, granulate with a 20-mesh sieve, dry at 50±5°C, granulate, and set aside;
[0052] 2) Add the remaining prescription amount of pregelatinized starch, sodium carboxymethyl starch, crospovidone, and magnesium stearate to 1), and mix to make Granule A;
[0053] 3) Mix the hydroxypropyl cellulose K15M, hydroxypropyl cellulose K4, mannitol, talcum powder, and blue lake in the prescribed amount evenly, use 8% povidone ethanol solution, granulate with a 20-mesh screen, 50± Drying at 5°C and sizing the granules to obtain granules B;
[0054] 4) Tablet compression: compressed into a cylindrical three-layer tablet w...
Embodiment 2
[0058] Simulate the large-scale production scale, with each batch of 30,000 pieces, scale up and produce 3 batches.
[0059] Sample trial batch number
[0060] It has been proved by experiments that the product prescription process is simple and feasible. More than three batches of products have been packaged in the accelerated test conditions of 40°C±2°C and relative humidity of 75%±5% for 6 months and long-term test conditions for 6 months. The results show that the quality is relatively stable and meets the production requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com