Stable paroxetine hydrochloride tablet and preparation method thereof
A technology of paroxetine hydrochloride tablets and paroxetine hydrochloride, which is applied in the field of medicine, can solve the problems of reddening of paroxetine hydrochloride products and deterioration of the hardness of paroxetine hydrochloride tablets, etc., and achieve the goal of solving changes in hardness, inhibiting reddening of products, and complete dissolution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
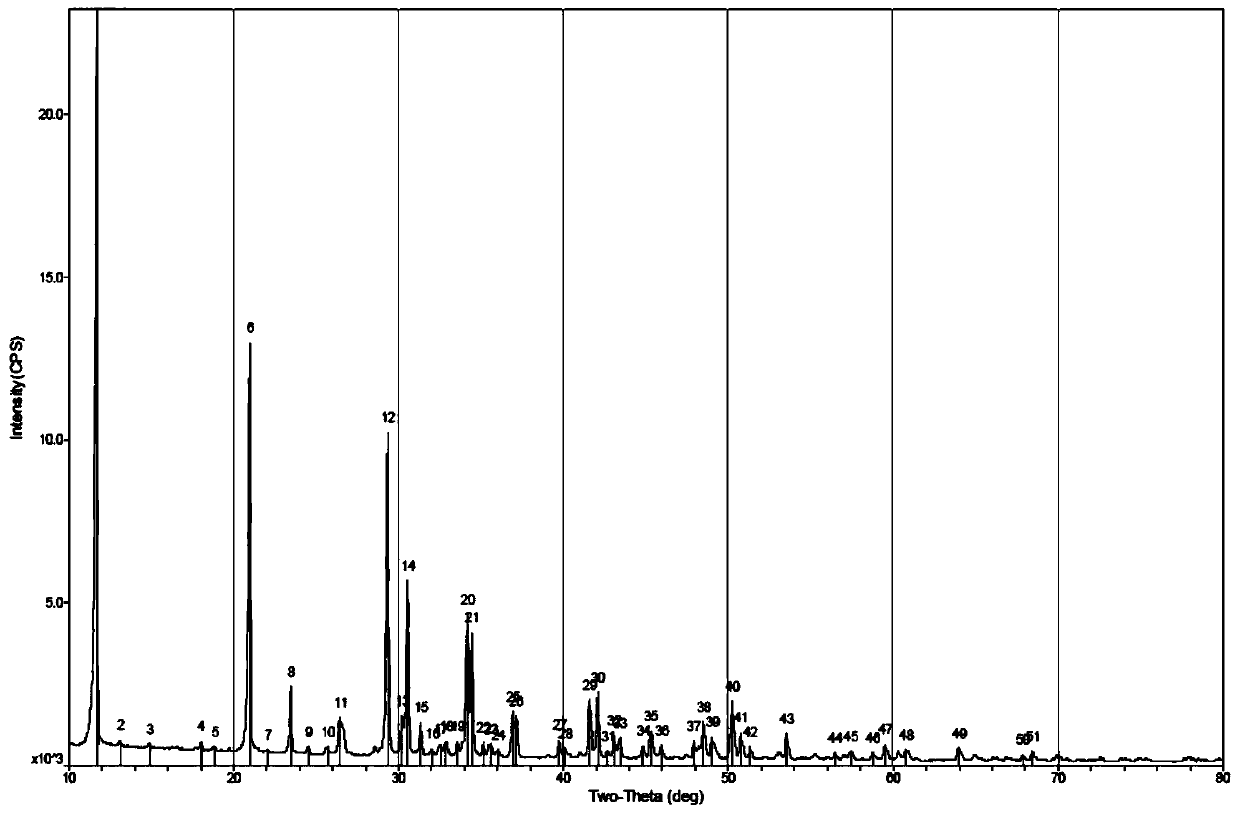
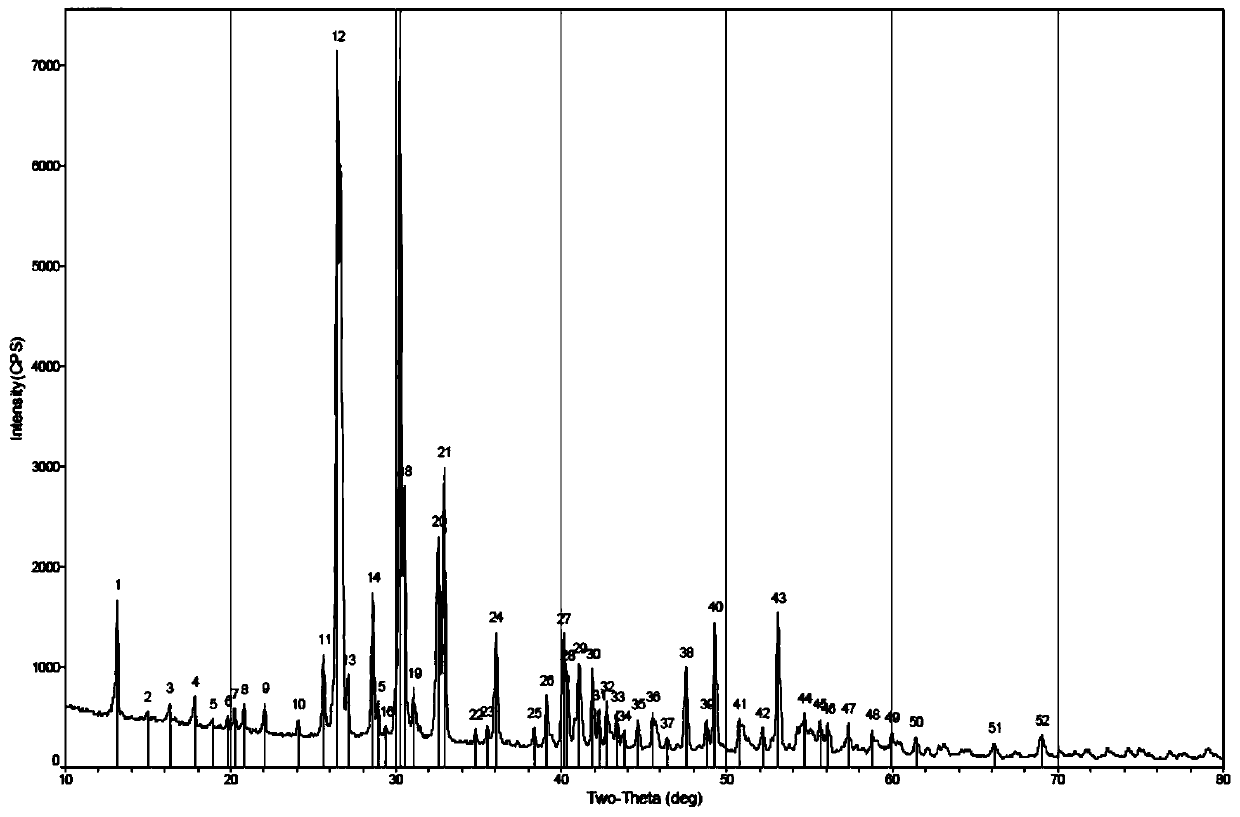
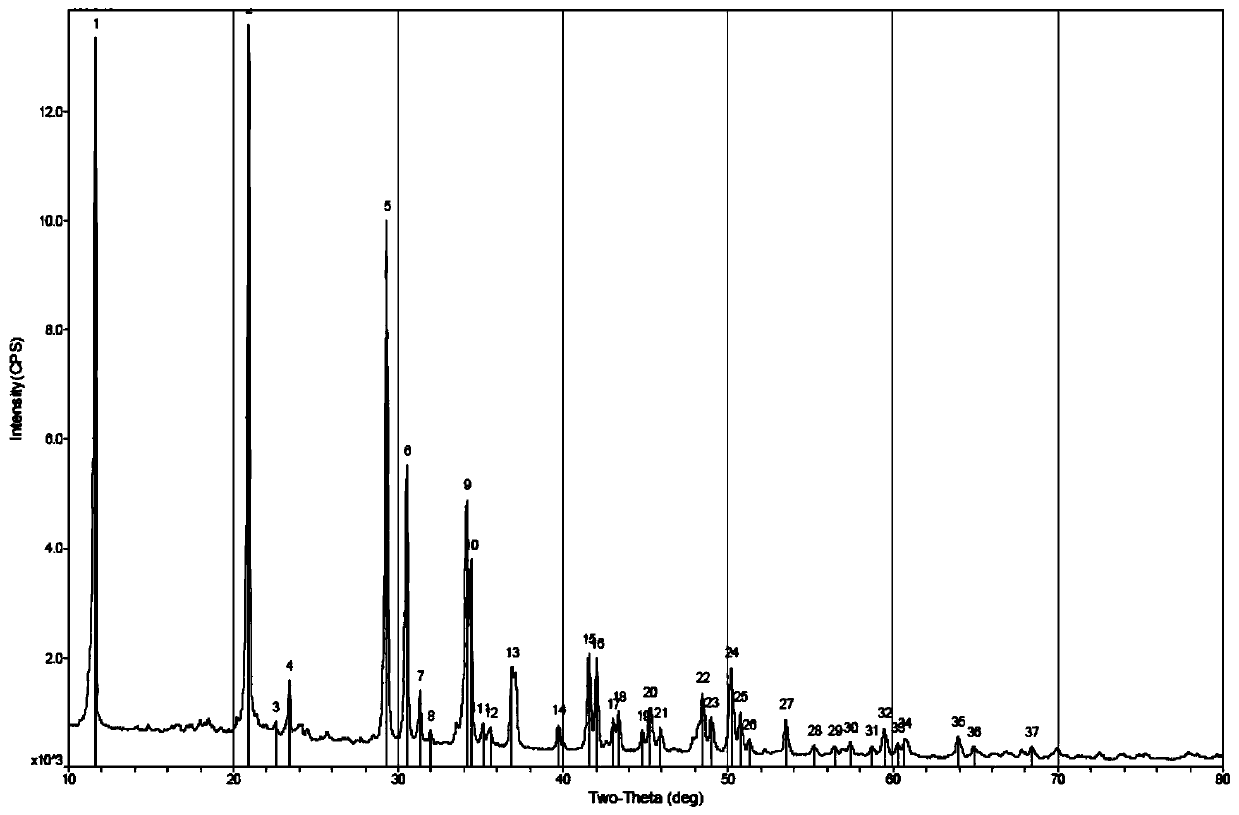
Image
Examples
Embodiment 1
[0039] A stabilized tablet of paroxetine hydrochloride containing the following components by weight per 1000 tablets:
[0040] Paroxetine Hydrochloride 22.8g, Calcium Hydrogen Phosphate Dihydrate 245.7g, Povidone K30 15g, Sucrose 9g, Carboxymethyl Starch Sodium 18g, Magnesium Stearate 1.5g, Opadry 9g,
[0041] Its preparation method adopts a wet granulation process, and the wetting agent is purified water, which specifically includes the following steps:
[0042] S1. According to the above formula, weigh each component for later use;
[0043] S2, mixing paroxetine hydrochloride evenly with other components in the formula;
[0044] S3, add wetting agent, continue granulation;
[0045] S4, drying the prepared wet granules, and pressing into tablets;
[0046] S5, coating the tablet core to obtain a stable paroxetine hydrochloride tablet.
Embodiment 2
[0048] A stabilized tablet of paroxetine hydrochloride containing the following components by weight per 1000 tablets:
[0049] Paroxetine Hydrochloride 22.8g, Calcium Hydrogen Phosphate Dihydrate 241.2g, Povidone K30 15g, Sucrose 15g, Carboxymethyl Starch Sodium 18g, Magnesium Stearate 3g, Opadry 9g,
[0050] Its preparation method adopts the wet granulation process, and the wetting agent is an aqueous solution of povidone K30, and the specific steps are the same as in Example 1.
Embodiment 3
[0052] A stabilized tablet of paroxetine hydrochloride containing the following components by weight per 1000 tablets:
[0053] Paroxetine Hydrochloride 22.8g, Calcium Hydrogen Phosphate Dihydrate 229.2g, Povidone K30 9g, Sucrose 18g, Carboxymethyl Starch Sodium 18g, Magnesium Stearate 3g, Opadry 9g,
[0054] Its preparation method adopts the wet granulation process, the wetting agent is purified water, and the specific steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com