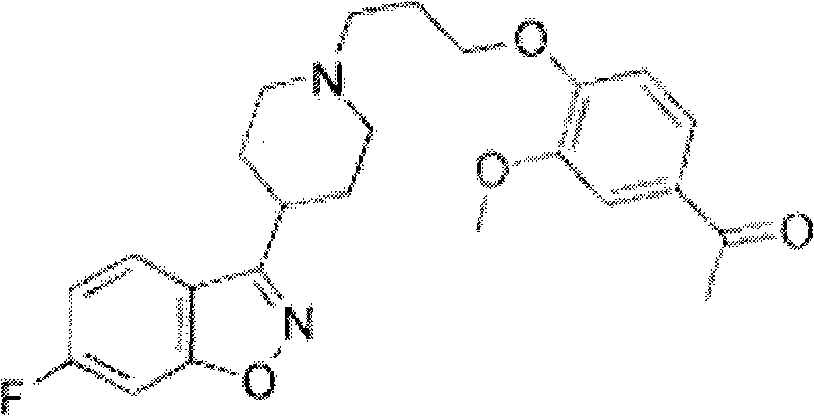
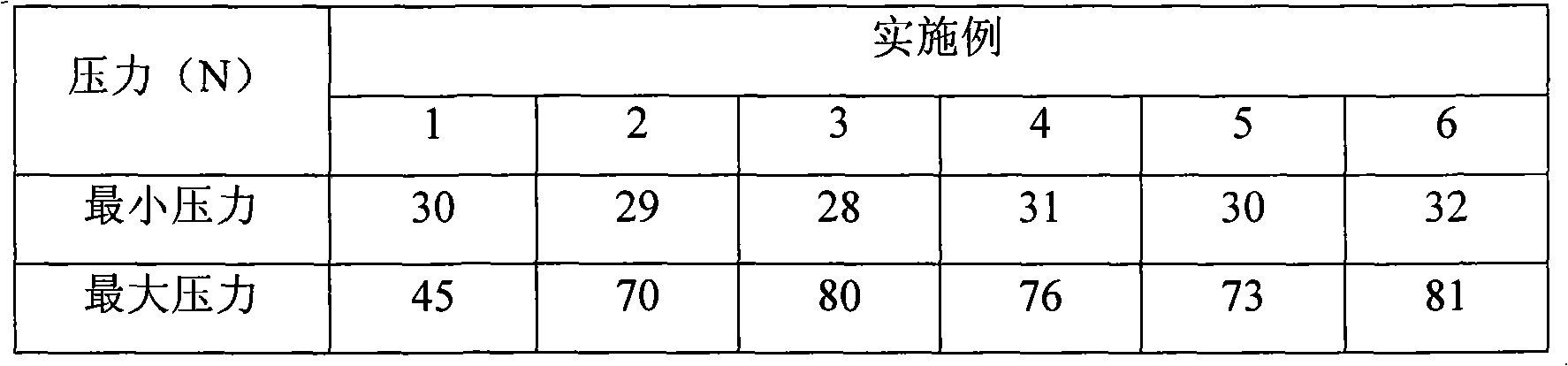
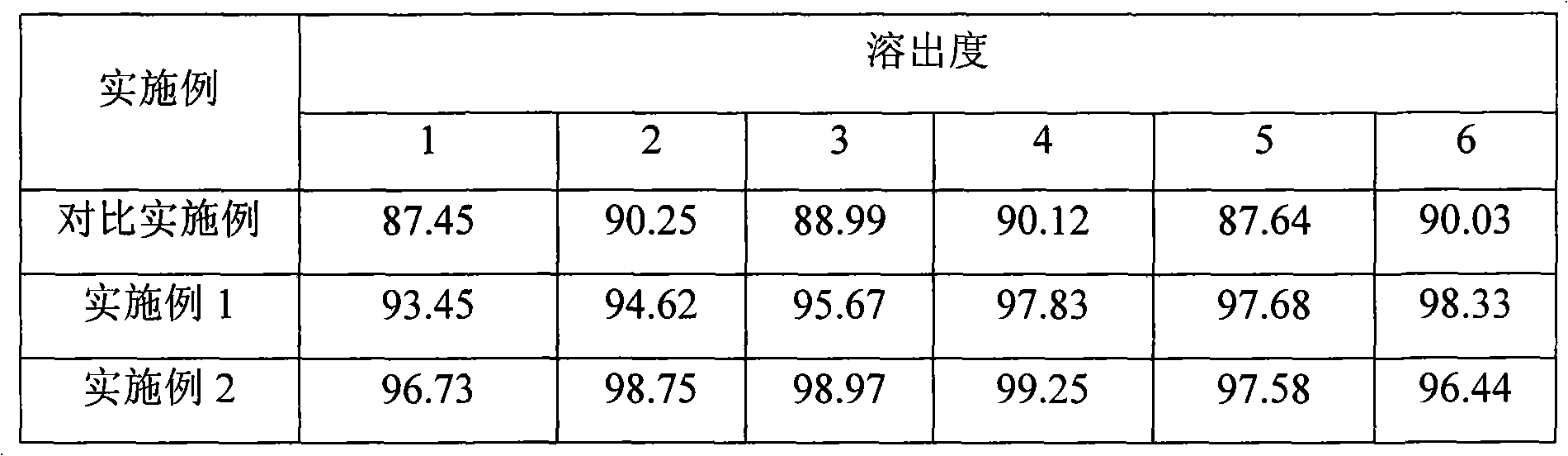
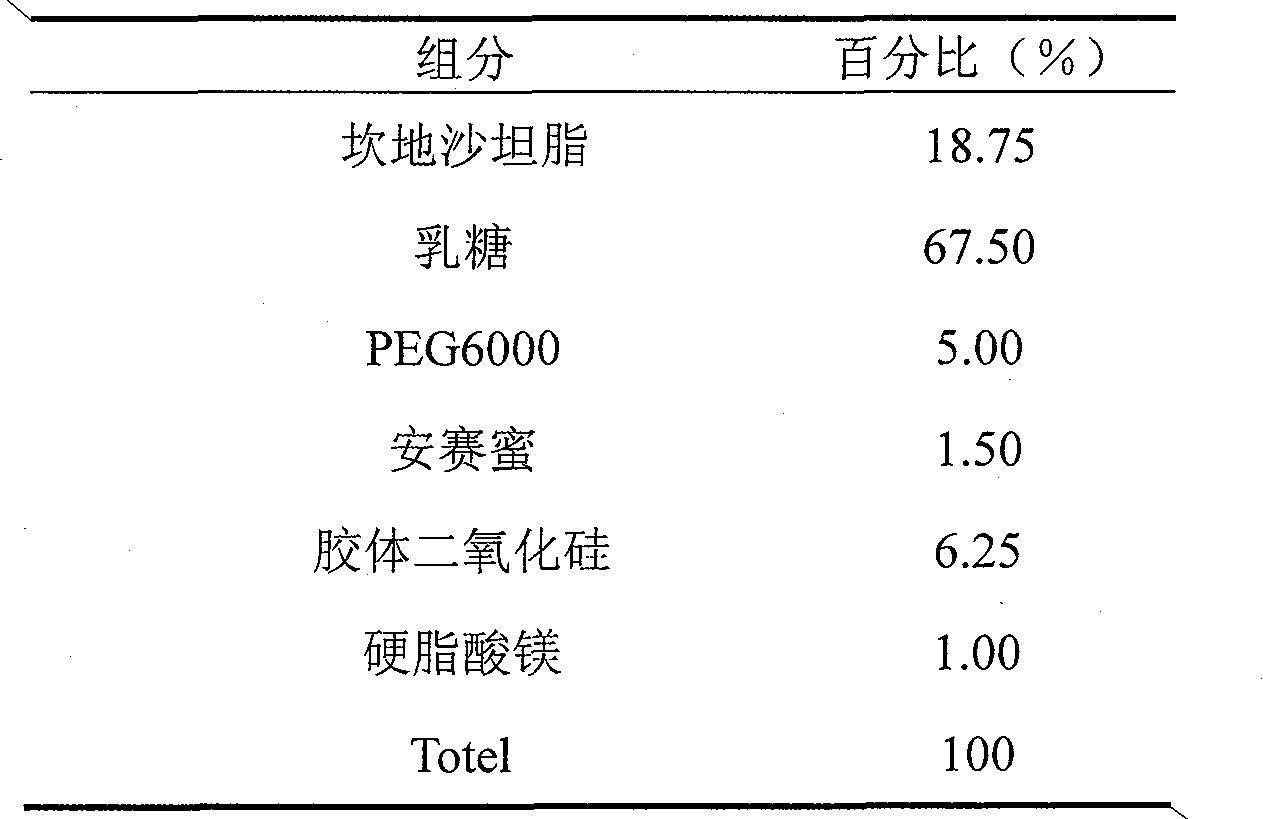
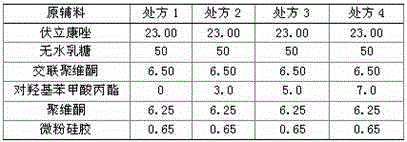
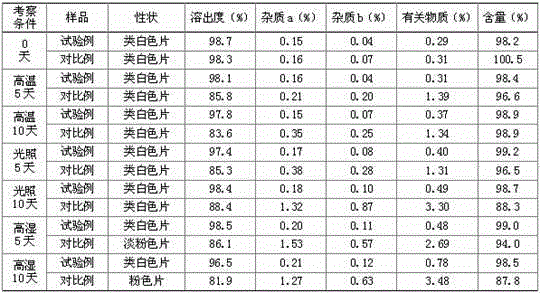
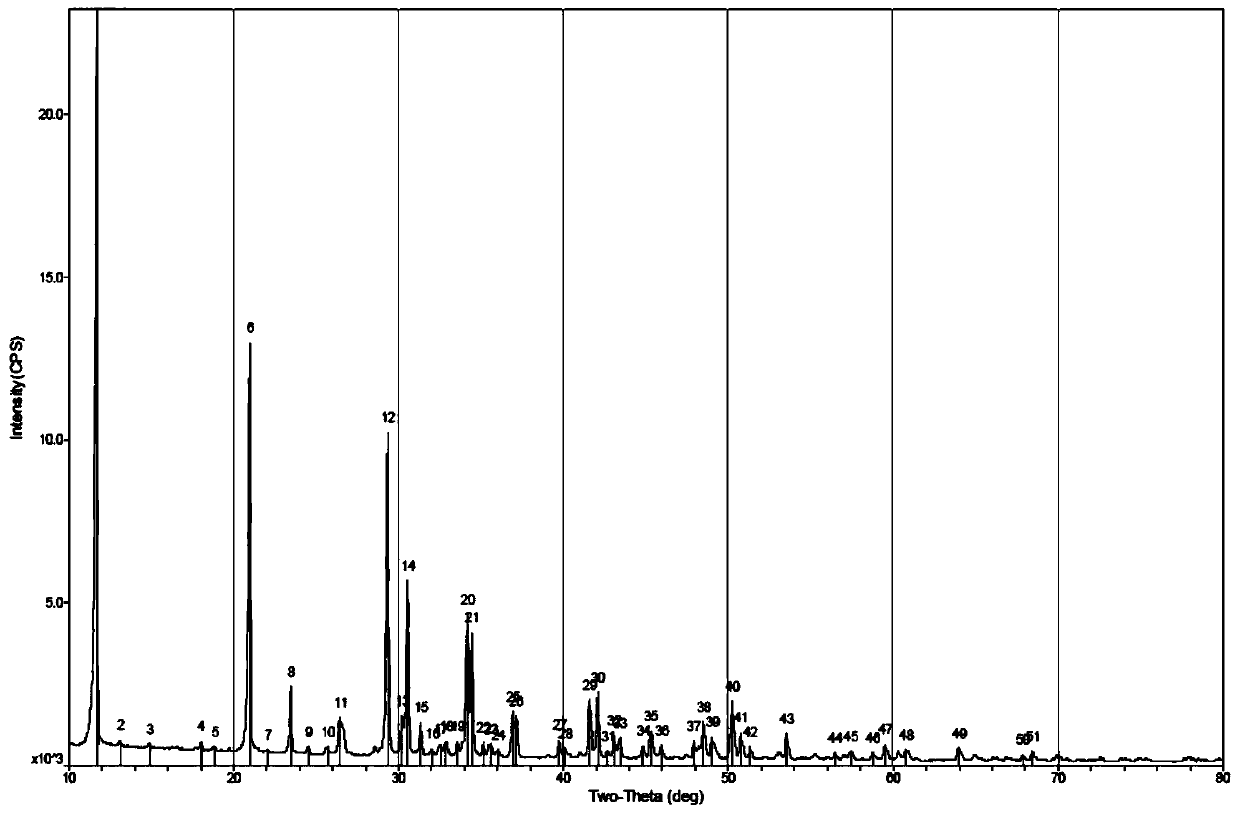
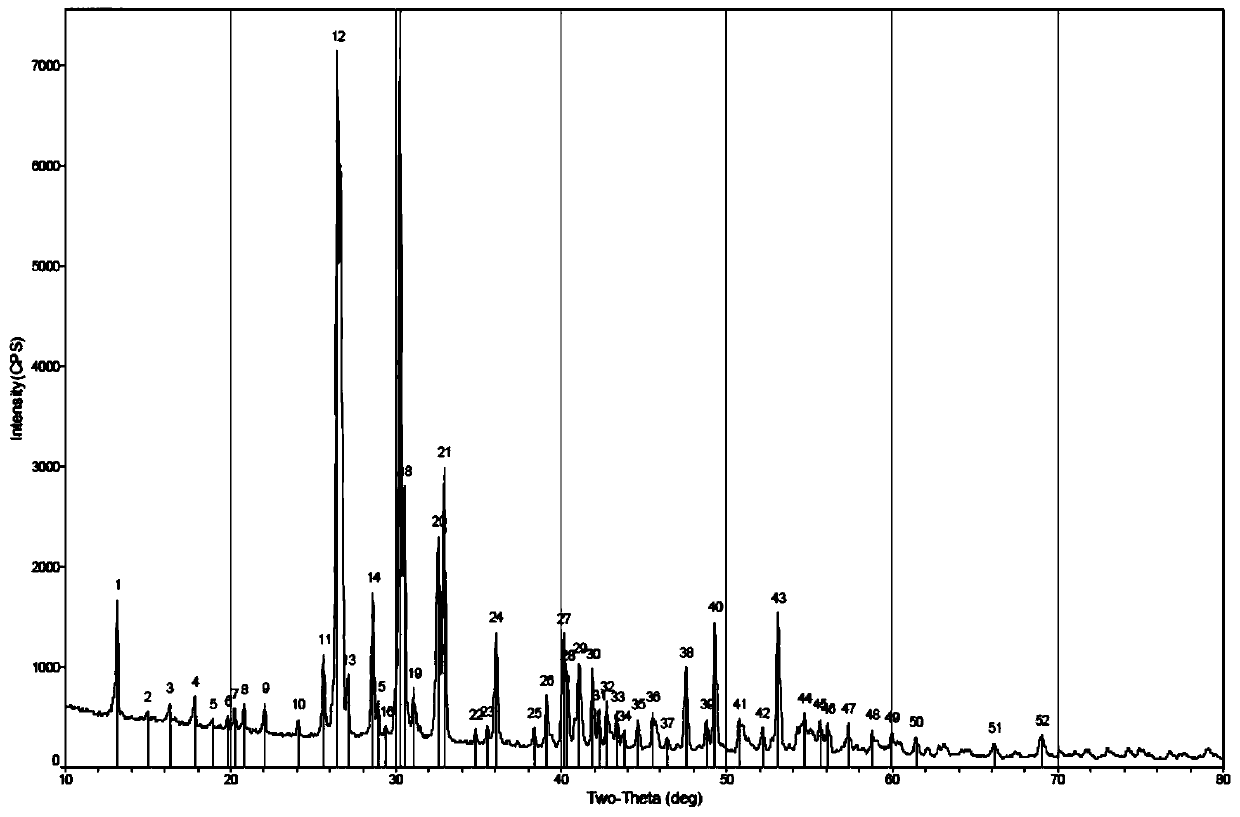
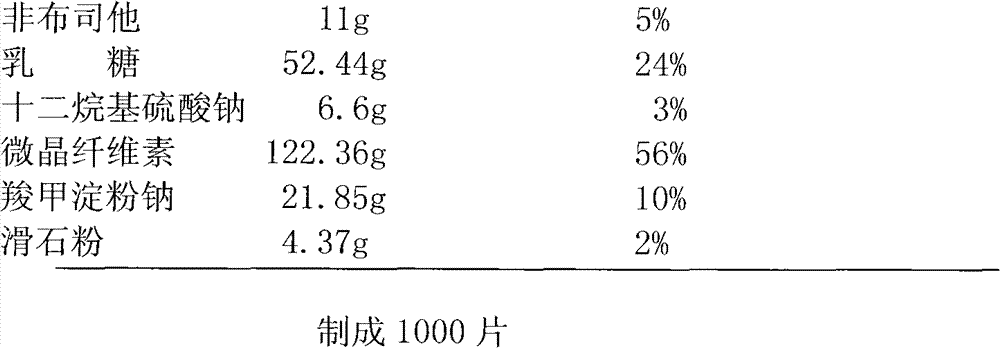
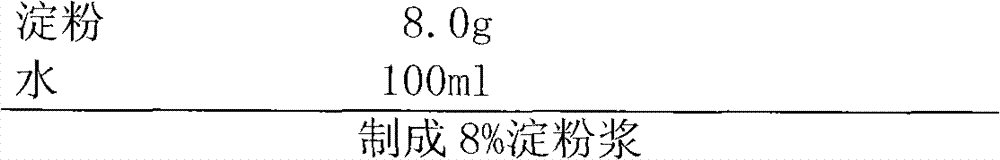
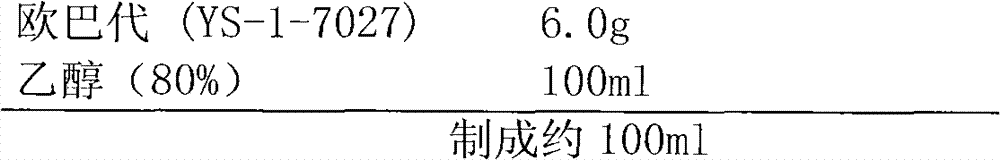
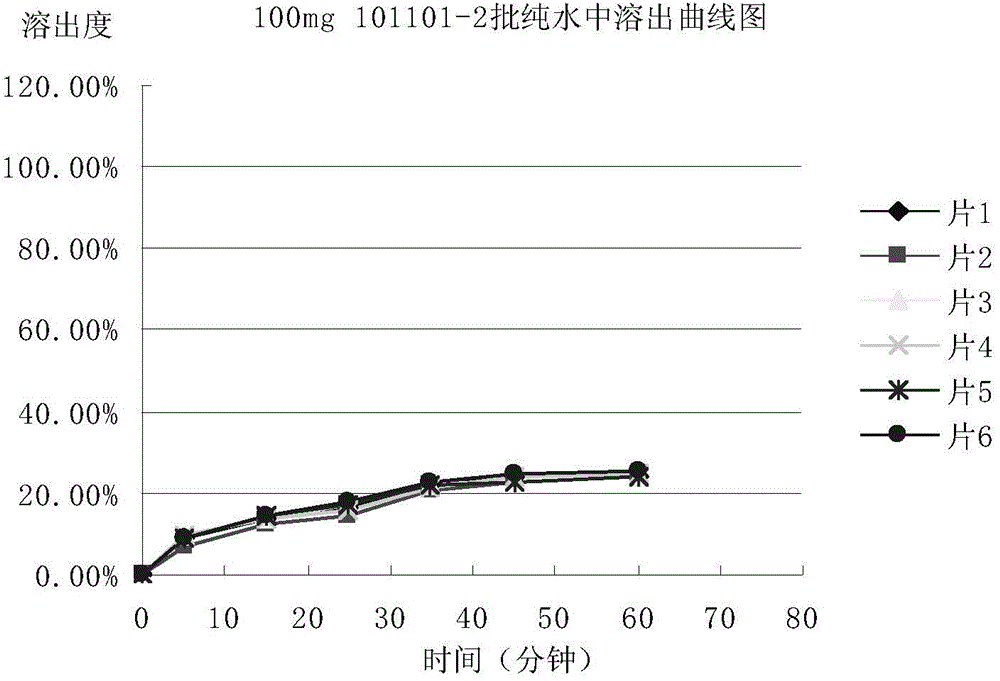
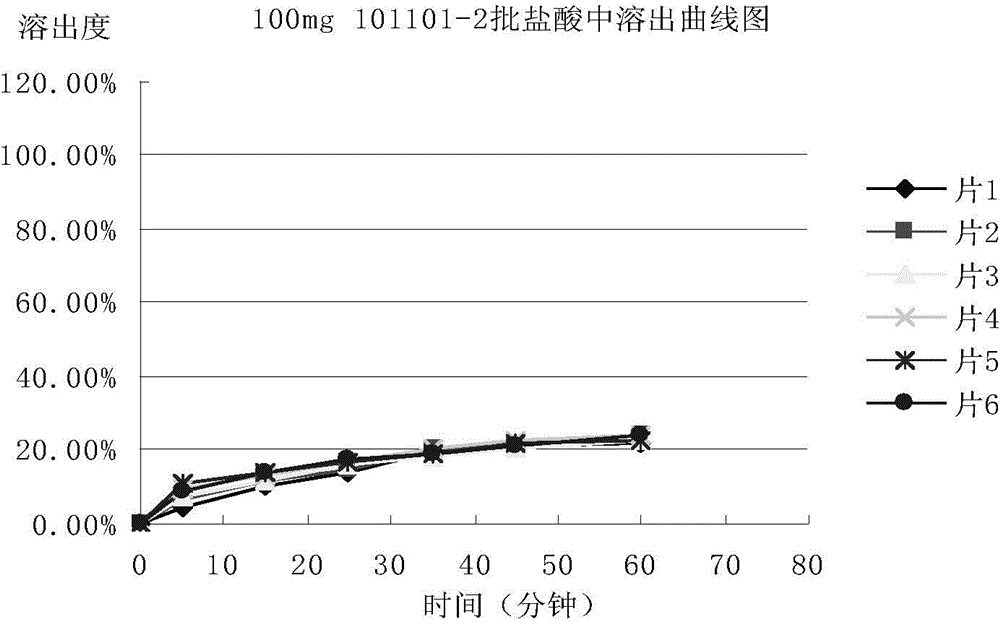
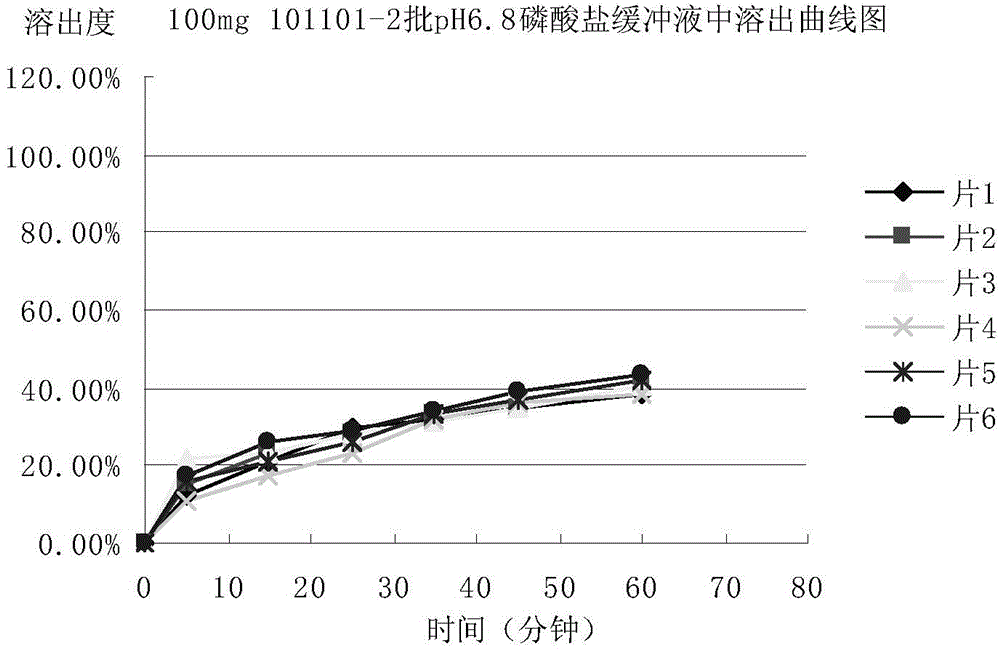
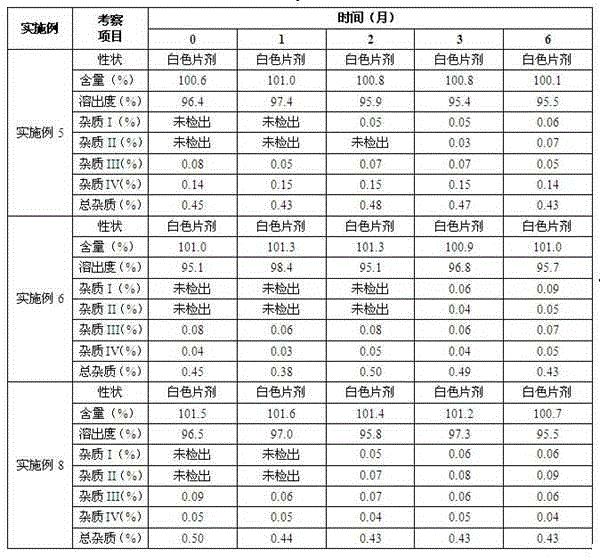
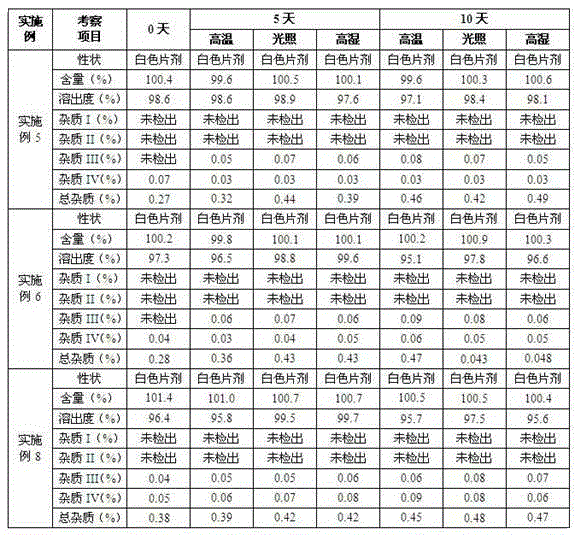
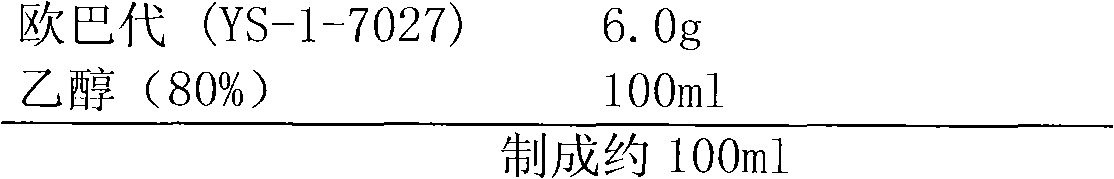Patents
Literature
68results about How to "Dissolution complete" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
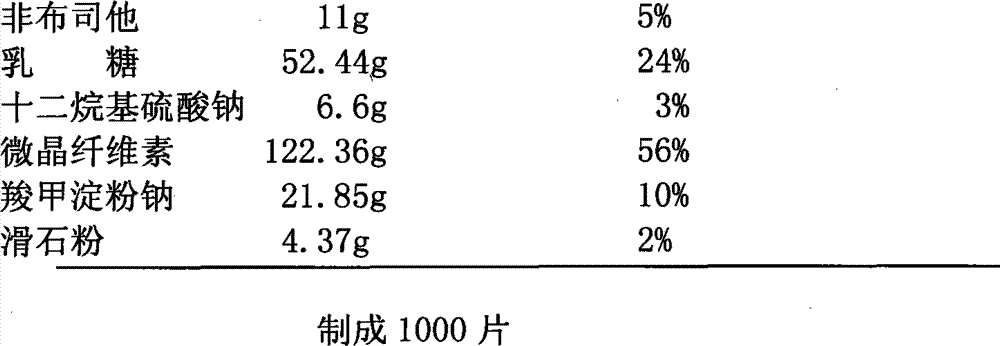
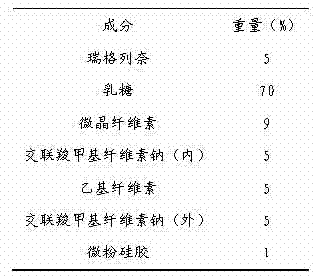
Iloperidone drug composition and preparation method thereof
ActiveCN101822674AGood compressibilityIncrease forceOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTIloperidone
The invention discloses a drug composition containing an Iloperidone active ingredient, which contains micronization Iloperidone or Iloperidone smashed with other accessories, can effectively enhances dissolution effect of Iloperidone.
Owner:AVENTIS PHARMA HAINAN
Febuxostat tablet and preparation method thereof
ActiveCN102488665ALess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderActive agentCombinatorial chemistry
The invention discloses a febuxostat tablet and a preparation method thereof. The febuxostat tablet comprises a tablet core and a coating, the tablet core comprises the following compositions: by weight percentage, from 5% to 30% of febuxostat, from 15% to 60% of filler, from 1% to 20% of disintegrating agent, from 0.1% to 5% of surfactant, from 0.1% to 8% of lubricating agent and a defined amount of adhesive. The febuxostat tablet adopts the high-efficient disintegrating agent within a reasonable proportion range, simultaneously, the febuxostat which is difficultly soluble medicine is dissolved by the aid of the surfactant and the high-efficient disintegrating agent, so that solubility of the febuxostat is improved, and bioavailability of the febuxostat is increased. In addition, the preparation method for the febuxostat tablet is simple, and is controllable in quality and fine in stability.
Owner:KANGYA OF NINGXIA PHARMA
Microbubble oxidization and acid dissolution method for copper-cobalt alloy
InactiveCN101812585ADissolution completeNo noise pollutionProcess efficiency improvementAcid dissolutionOxygen
The invention discloses a microbubble oxidization and acid dissolution method for copper-cobalt alloy. The current commonly used copper-cobalt alloy wet-process treatment method has the defects of low reaction speed, low efficiency, high cost, large equipment investment and the like. The technical scheme adopted by the invention is that dilute sulfuric acid is used as solvent and air is used as oxidant under normal temperature and normal pressure, a reaction device is formed by a centrifugal pump, a liquid-air injection pump and an acid dissolution reactor, the dilute sulfuric acid enters the acid dissolution reactor from the centrifugal pump through the injection pump, air is sucked in by using negative pressure generated when the injection pump injects high-speed liquid flow, the air is dispersed in the dilute sulfuric acid to form emulsion and to generate a large amount of oxygen-enriched microbubbles, and the microbubbles participate in the oxidization and acid dissolution reaction of the copper-cobalt alloy and the dilute sulfuric acid. The microbubble oxidization and acid dissolution method has the advantages that the dissolution speed is high, the dissolution rate is high, the fine grinding is not required for the alloy water quenching particles, the energy consumption is low, the dissolution cost is low, the air utilization ratio is high, no environmental pollution is caused and the like.
Owner:ZHEJIANG HUAYOU COBALT
Active tea cream high in extraction rate and complete in beneficial component preservation and preparation method thereof
The invention discloses active tea cream high in extraction rate and complete in beneficial component preservation and a preparation method thereof, and belongs to the technical field of tea leaf deep processing. The active tea cream comprises the following components by weight: 30 to 50 % of tea polyphenol, 20 to 30 % of tea polysaccharide, 6 to 10 % of free amino acid and 3 to 4 % of caffeine, and the sum of the weight percentage of the components is not larger than 100 %. The preparation method comprises processes of stocking, extracting, dirt-removing, condensing and forming: placing pulverized tea leaves in an extracting jar, adding an extractive at the temperature of 40 to 60 DEG C according to the feed-liquid ratio of 1: (10 to 15), extracting once or twice, extracting for 10 to 20 min every time, and collecting low temperature extract liquid; adding an extractive at the temperature of 60 to 80 DEG C according to the feed-liquid ratio of 1: (5 to 10), extracting once or twice, extracting for 10 to 20 min every time, and collecting high temperature extract liquid, mixing the extract liquid gained after double extraction according to certain proportion to gain total extract liquid, and gaining the active tea cream after dirt-removing, condensing and drying. The preparation method is high in extraction rate, complete in beneficial component preservation and simple in manufacturing technology; the prepared active tea cream is rich in nutrition, strong in fragrance and mellow in taste, and has a good market popularizing value.
Owner:YUNNAN DEFENG TEA IND CO LTD
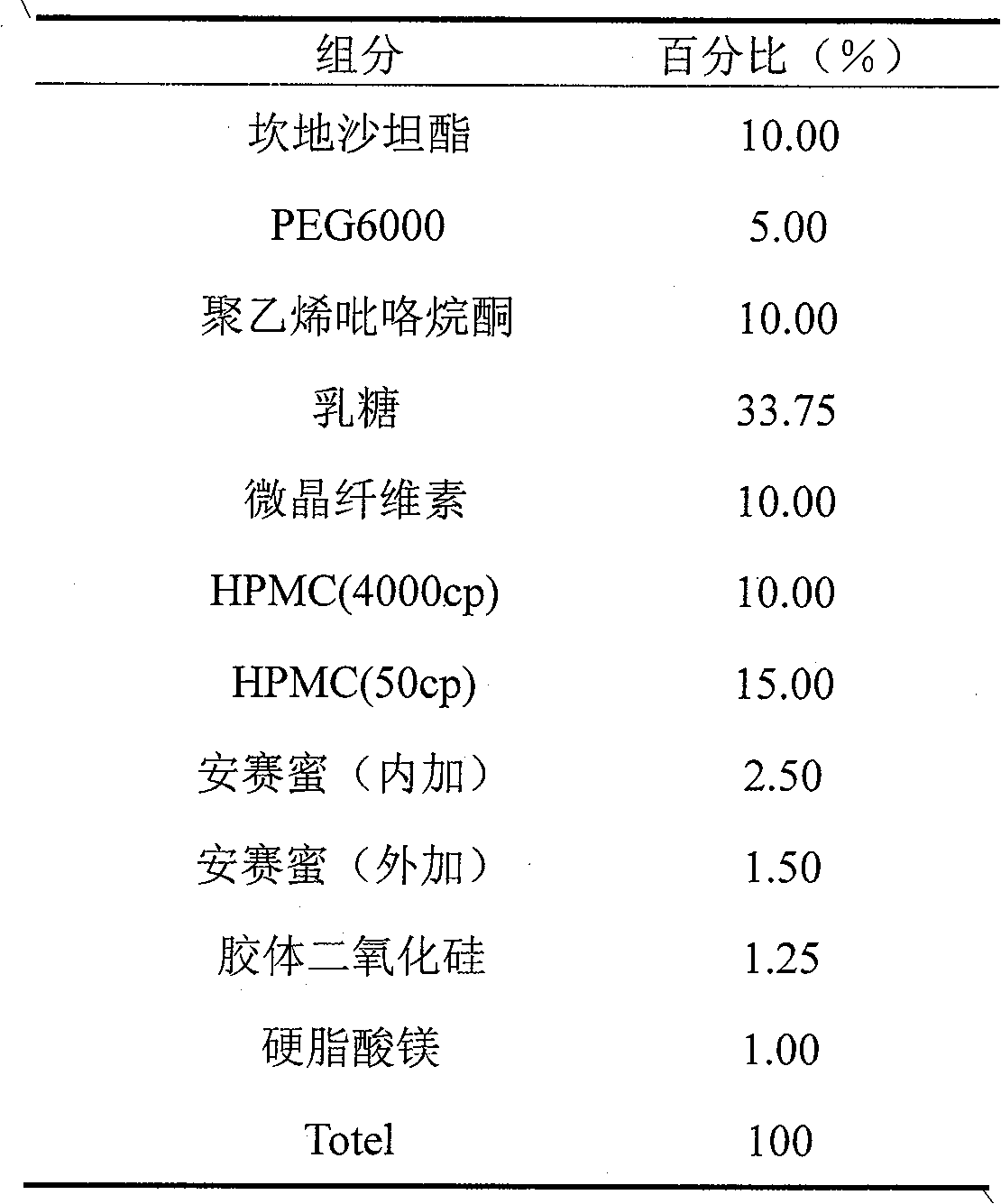
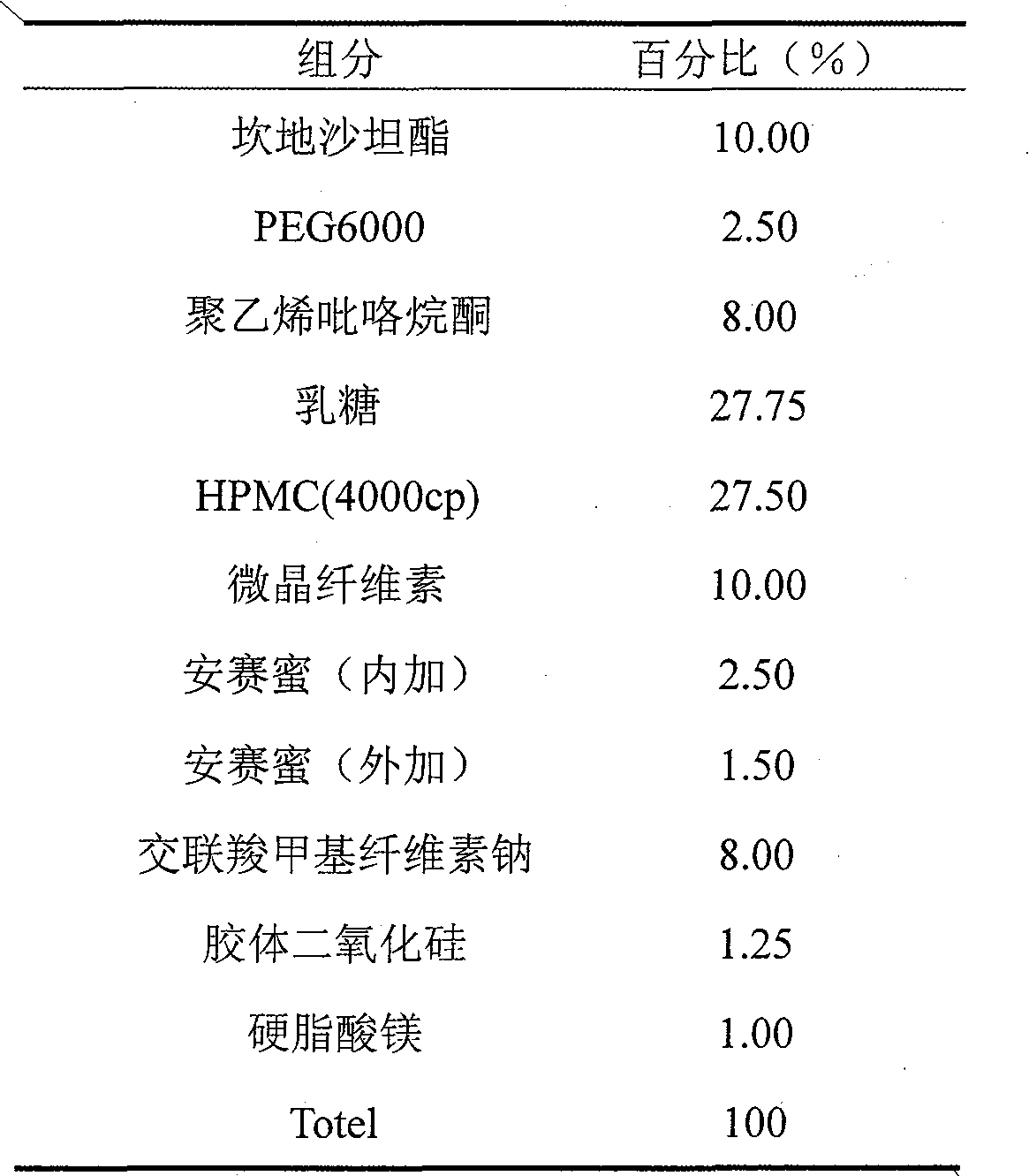
Medicine compound containing candesartan cilexetil
ActiveCN101862325AAvoid hydrolysisImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanEssential hypertension
The invention discloses a medicine compound containing candesartan cilexetil, and a preparation method thereof; and the medicine compound improves the stability of the base medicine and improves the dissolution rate of the medicine by preparing solid dispersion. The medicine compound is used for curing primary hypertension.
Owner:AVENTIS PHARMA HAINAN
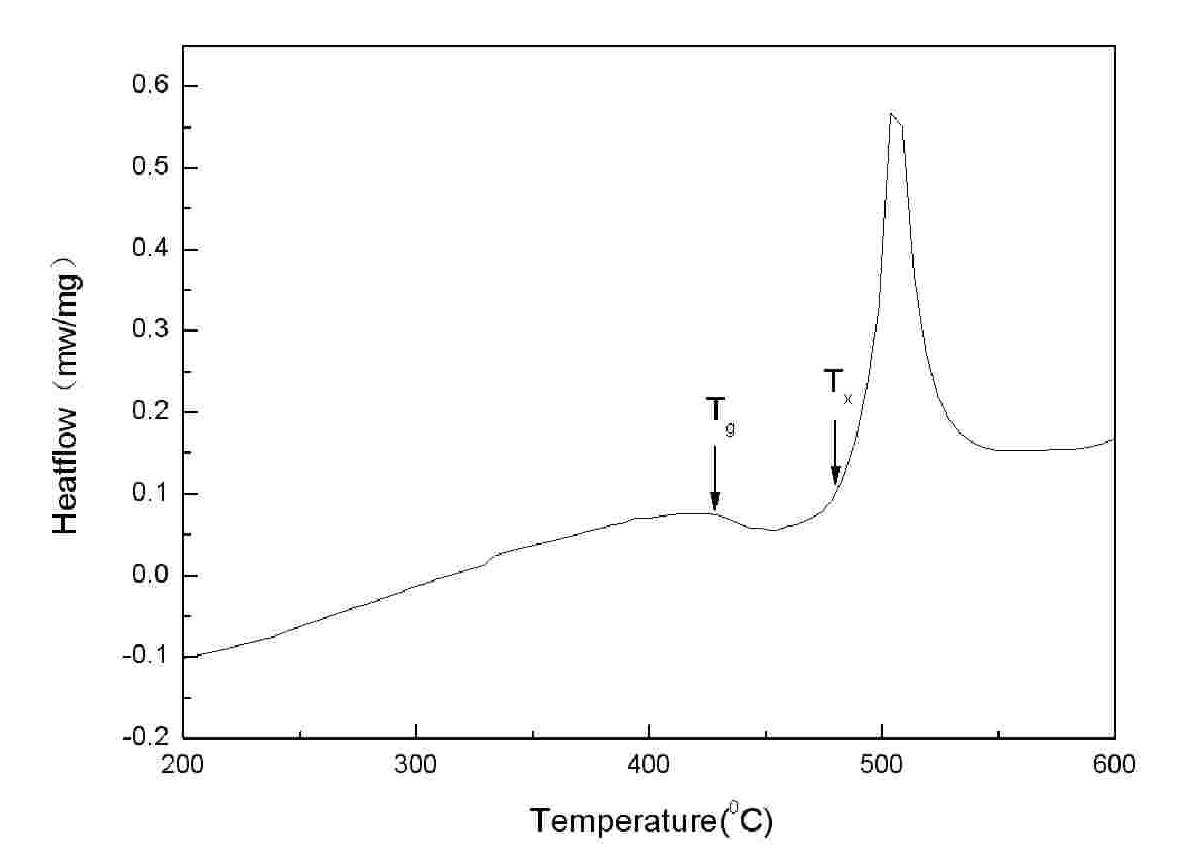
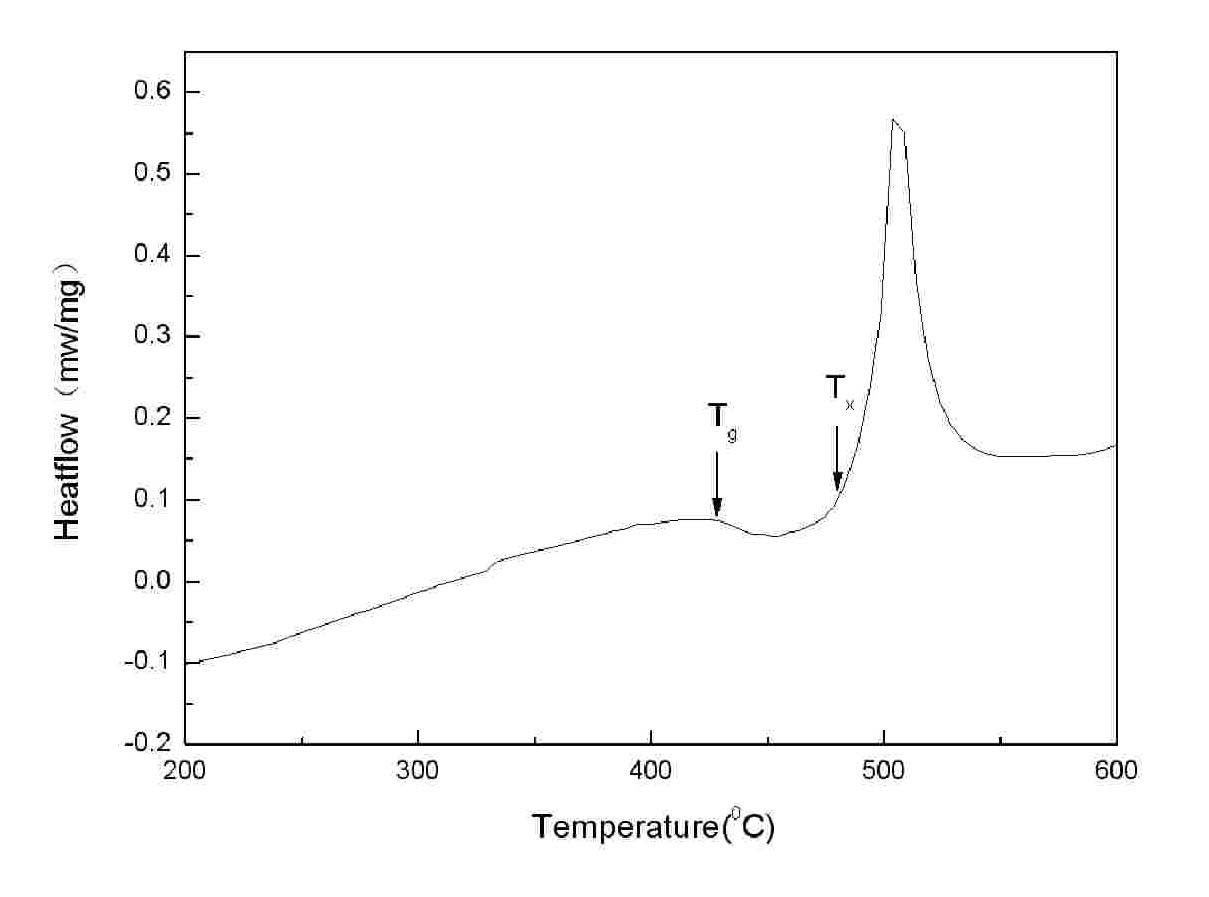
Powder forging and molding method for preparing porous amorphous alloy block material
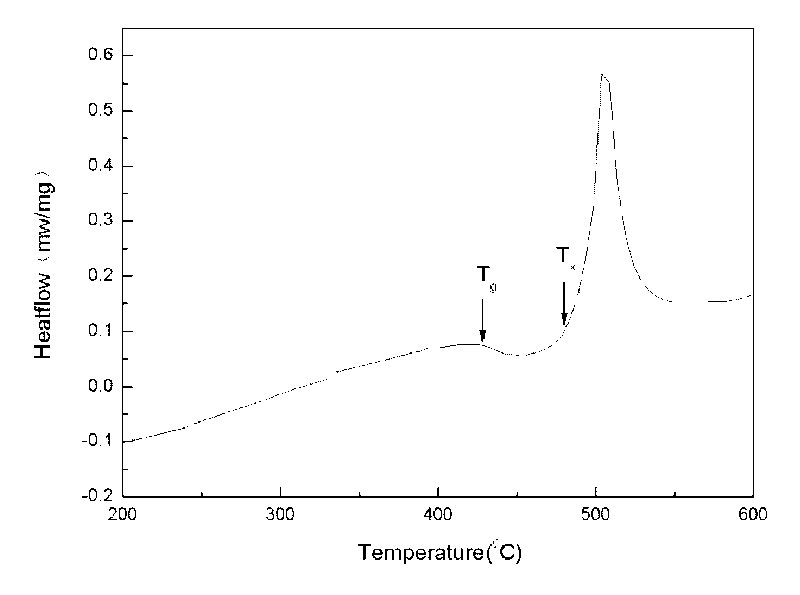
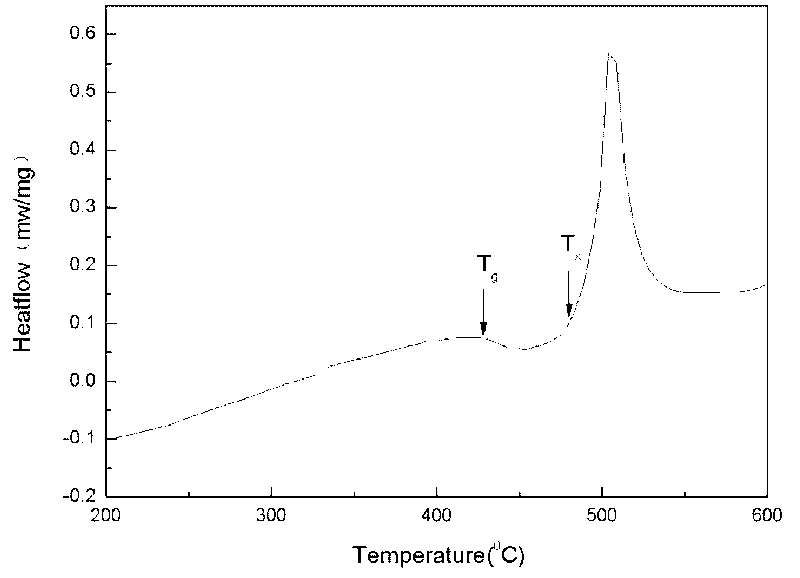
The invention discloses a powder forging and molding method for preparing a porous amorphous alloy block material. The method comprises the following steps of: after respectively sieving amorphous powder and a powdered NaCl pore-forming agent, proportionally weighing the two components according to a porosity requirement; after uniformly mixing the components by a material mixer, carrying out sheathing, degassing and enveloping; carrying out heating and heat preservation on the sheathed and enveloped powder in a cold liquid phase area (Tg-Tx) for 5-15 minutes; forging and molding according to a set forging ratio, maintaining pressure for 30-90s and then forging; removing sheaths by adopting a machining method; and removing the pore forming agent by a water dissolving method to obtain the porous amorphous alloy block material. The method is simple and easy to apply, the prepared porous amorphous alloy material has the characteristics of controlled porosity, opening structure, uniform pore size distribution, and the like; meanwhile, the structural behavior of the powder can also be maintained. The dissolution of the pore forming agent is environmentally friendly, and the pore forming agent can be repeatedly used after treated.
Owner:CENT SOUTH UNIV
Febuxostat tablet
ActiveCN102895209AImprove solubilityImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveMedicine
The invention discloses a Febuxostat tablet. The Febuxostat tablet comprises a tablet core and a coating, wherein the tablet core comprises the following components in weight percentage: 5-30 percent of Febuxostat, 15-60 percent of filler, 1-20 percent of disintegrating agent, 0.1-5 percent of surface active agent, 0.1-8 percent of lubricant and a right amount of adhesive. According to the Febuxostat tablet, trough adopting the powerful disintegrating agent within a reasonable proportional region, and meanwhile, through jointly using the surface active agent, the poorly water-soluble drug Febuxostat dissolves out, and further, the dissolvability of the Febuxostat is increased, and the bioavailability of the Febuxostat is improved. Moreover, the Febuxostat tablet is simple in preparation method, controllable in quality and high in stability.
Owner:KANGYA OF NINGXIA PHARMA
Imatinib mesylate tablet and preparation method thereof
InactiveCN103222965APromote dissolutionDissolution completeOrganic active ingredientsPharmaceutical non-active ingredientsImatinib mesylateCrospovidones
The invention discloses an imatinib mesylate tablet and a preparation method thereof. The imatinib mesylate tablet comprises 8-30% of crospovidone and 8 to 40% of silica. The preparation method comprises the following steps of carrying out granulation of imatinib mesylate and a waterless organic solvent, drying the granules, uniformly mixing the granules, crospovidone, silica, a filler and a lubricant, and carrying out tabletting. The preparation method solves the problem of a slow dissolution rate of a preparation obtained by the prior art.
Owner:QINGDAO UNIV
Imatinib mesylate tablet cores, coated tablets, and preparation method thereof
InactiveCN103083273AGood drug stabilityEasy to prepareOrganic active ingredientsAntineoplastic agentsUrologyPlasticizer
The invention discloses imatinib mesylate tablet cores comprising, by weight, 20-70% of imatinib mesylate alpha crystalline form, 10-50% of a filling agent, 0-10% of an adhesive, 10-30% of a disintegrant, 0.1-5% of a flow aid, and 0.1-2% of a lubricant. According to the tablet core preparation method, granulation and tabletting are carried out with the formula. The invention also discloses imatinib mesylate coated tablets and a preparation method thereof. The coated tablets comprise, by weight, 95-97% of the imatinib mesylate tablet cores, 0.5-4% of a film forming agent, 0.1-1% of a plasticizer, 0.1-1% of a coloring agent, and 0.1-1% of a lubricant. The preparation method of the coated tablets comprises the steps that the tablet cores are coated, such that the imatinib mesylate coated tablets are prepared. The tablets have the advantages of high dissolution rate and fast release.
Owner:SHANGHAI YINGLI PHARM CO LTD
Dutasteride self-microemulsion composition and preparation method thereof
InactiveCN103655470AImprove the dissolution rate of dutasterideSimple preparation processOrganic active ingredientsUrinary disorderSolubilityEmulsion
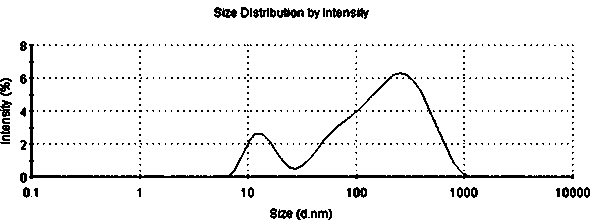
The invention relates to a self-microemulsion dutasteride orally-taken preparation and a preparation method thereof. The self-microemulsion composition comprises the following components in percent by weight: 0.05%-0.3% of dutasteride, 5%-75% of oil, 20%-60% of an emulsifier and 4%-50% of a co-emulsifier. After being cured, the self-microemulsion auxiliary materials can be prepared into preparations such as tablets or capsules; after being quickly disintegrated in a stomach, the preparation can be form emulsion drops with average grain size of 10 nm-100 nm in a gastrointestinal tract after being self-microemulsified, so that solubility and dissolution rate of the dutasteride can be improved, and absorption of the dutasteride in the gastrointestinal tract can be promoted.
Owner:CHONGQING PHARMA RES INST
Febuxostat tablet with improved dissolution rate
ActiveCN102895210ALess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderAdhesiveMedicine
The invention discloses a Febuxostat tablet and a preparation method thereof. The Febuxostat tablet comprises a tablet core and a coating. The tablet core comprises the following components in weight percentage: 5-30 percent of Febuxostat, 15-60 percent of filler, 1-20 percent of disintegrating agent, 0.1-5 percent of surface active agent, 0.1-8 percent of lubricant and a right amount of adhesive. According to the Febuxostat tablet, trough adopting the powerful disintegrating agent within a reasonable proportional region, and meanwhile, through jointly using the surface active agent, the poorly water-soluble drug Febuxostat dissolves out, and further, the dissolvability of the Febuxostat is increased, and the bioavailability of the Febuxostat is improved. Moreover, the Febuxostat tablet is simple in preparation method, controllable in quality and high in stability.
Owner:KANGYA OF NINGXIA PHARMA
Powder extrusion forming method for preparing porous amorphous alloy block material
The invention discloses a powder extrusion forming method for preparing a porous amorphous alloy block material, which comprises: sieving amorphous powder and powder NaCl pore-forming agent respectively, weighing the amorphous powder and powder NaCl pore-forming agent according to porosity requirements, mixing the powder uniformly in a mixer, and sheathing the mixed powder, exhausting air and sealing the sheath; and heating the powder packaged by the sheath in a cold liquid phase area (Tg-Tx), keeping the temperature for 5 to 15 minutes, performing extrusion forming according to a design extrusion ratio, removing the sheath by machining, and removing the pore-forming agent by using a water solution process to obtain the blocky porous amorphous alloy material. The method is simple and easy to implement. The prepared porous amorphous alloy material has the characteristics of controllable porosity, open structure, uniform aperture distribution and the like. Meanwhile, the structural state of the powder can be retained, the dissolution of the pore-forming agent is environmentally-friendly, and the pore-forming can be recycled after processing.
Owner:CENT SOUTH UNIV
Indissolvable drug oral sustained-release composition and preparation method thereof
ActiveCN111728949AGrow fastAchieve dissolutionOrganic active ingredientsPill deliveryPharmaceutical drugOrganic chemistry
The invention discloses an indissolvable drug oral sustained-release composition, which is especially suitable for low-dose indissolvable drugs. The indissolvable drug oral sustained-release composition comprises sustained-release particles and a gel skeleton, wherein the sustained-release particles comprise an indissolvable drug, an enteric material and a liquid strong adsorption carrier; the gelskeleton comprises a hydrophilic gel skeleton material; and the sustained-release particles are obtained by preparing a suspension from the indissolvable drug and the enteric material and then spraying the suspension onto the liquid strong adsorption carrier. The sustained-release particles are partially wrapped by the gel skeleton to form a multi-sustained-release system technology, the releasetime is prolonged, the preparation process is simple, the efficiency is high, the drug mixing is uniform, and the content loss is less.
Owner:AC PHARMA CO LTD
Papaya anti-oxidant serum and preparation method thereof
InactiveCN101444562AEasy to carry and storeImprove extraction efficiencyAntinoxious agentsPharmaceutical non-active ingredientsGramHealth food
The invention discloses a papaya anti-oxidant serum, which comprises cyclodextrin inclusion compound of pawpaw natural antioxidant and carriers accepted in pharmacy. The papaya anti-oxidant serum consists of enzymolysis water extract and alcohol extract of the papaya, in which vitamin C and polyphenols are contained. Vitamin C contained in papaya anti-oxidant serum is no less than 2 milligram per gram, and the total polyphenols calculated in gallic acid is no less than 22 milligram. Therefore, the papaya anti-oxidant serum has the characteristics of high efficiency, durability and stable inoxidizability and easy storage and carrying, can be served as health food to eat, can used as cosmetics, animal food, food or medical mediate, and further can used for preparing antioxidation products; the invention also discloses a preparation method for the papaya anti-oxidant serum, which comprises seven steps of pulping, enzyme digestion, filtering, alcohol extraction, concentration, inclusion and pelletization, so as to be a system for efficiently, sufficiently and completely extracting papaya anti-oxidant, and the antioxidative activity can be effectively preserved.
Owner:ARMY MEDICAL UNIV
Aripiprazole orally dissolving film and preparation method thereof
ActiveCN103784426BEasy to takePromote dissolutionOrganic active ingredientsNervous disorderHigh absorptionCurative effect
The invention discloses an aripiprazole oral membrane and a preparation method thereof. The aripiprazole oral membrane contains cyclodextrin encapsulated aripiprazole, wherein the cyclodextrin is more than one of hydroxypropyl-beta-cyclodextrin and glucosyl-beta-cyclodextrin, and the weight ratio of the cyclodextrin to the aripiprazole is 1: 1 to 3: 1. The membrane preparation has the beneficial effects of even, smooth and clean membrane obtained, easy administration and direct administration without water, quick and safe dissolving-out, high absorption speed, high bioavailability, good curative effect, simple production process, low production cost, and high added value of products. The aripiprazole membrane is quick to dissolve out, good in stability and high in bioavailability, and excellent in bioavailability, and also good in compliance and popular with patients; the aripiprazole membrane is good in formability, and capable of directly adhering to the part to which the medicine is applied after administration, and thus cannot be spitted, and therefore, psychopaths can be prevented from spitting out and hiding the drug; and in the production process, almost no dust flies, and the problems of labor protection and environmental pollution can be solved.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Isatis root pre-treatment method for fermenting extraction of isatis root polysaccharide
The invention relates to an extraction method in the technical field of traditional Chinese medicines, in particular to an isatis root pre-treatment method for fermenting extraction of isatis root polysaccharide. The isatis roots are crushed by means of fluid energy milling, so that the isatis root structure can be more fined and extraction of polysaccharide is not affected; by damaging the mechanical supporting wall membrane structures of the isatis roots, polysaccharide can be more fully dissolved in later stage. Steam explosion is carried out for pre-treatment. High-temperature saturated steam quickly permeates into the cell tissues of the isatis roots and component separation and structure change of raw materials are achieved by moment decompression, so that the cell tissues of the isatis roots are damaged; interconnection among fiber bundles is damaged, so that cell walls are broken to form smooth porous structures; the fiber bundles are separated, and the cell walls are broken, so that the functional components wrapped in the fiber bundles or cells are released, the mass transfer ability is obviously reduced, and therefore, a purpose of increasing the polysaccharide extraction ratio is achieved.
Owner:芮志行
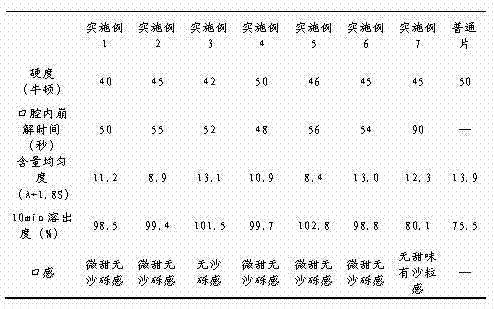
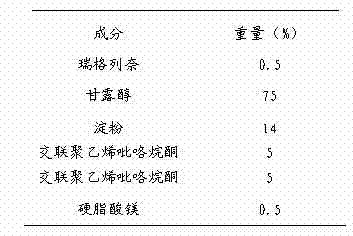
Repaglinide orally disintegrating tablet and preparation method thereof
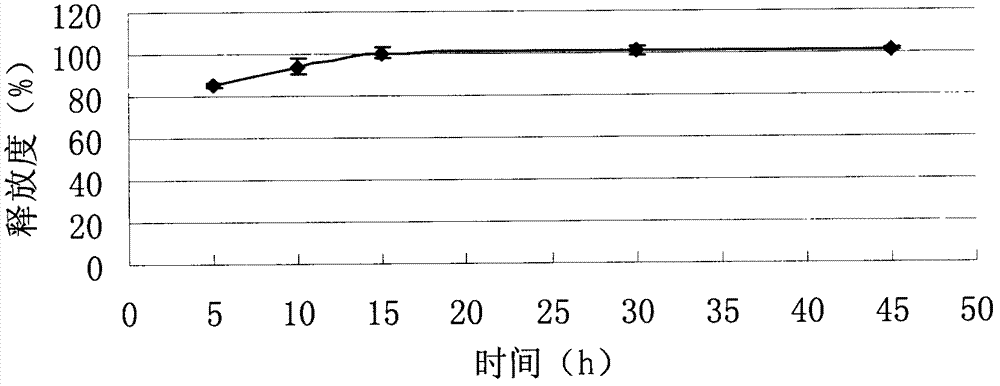
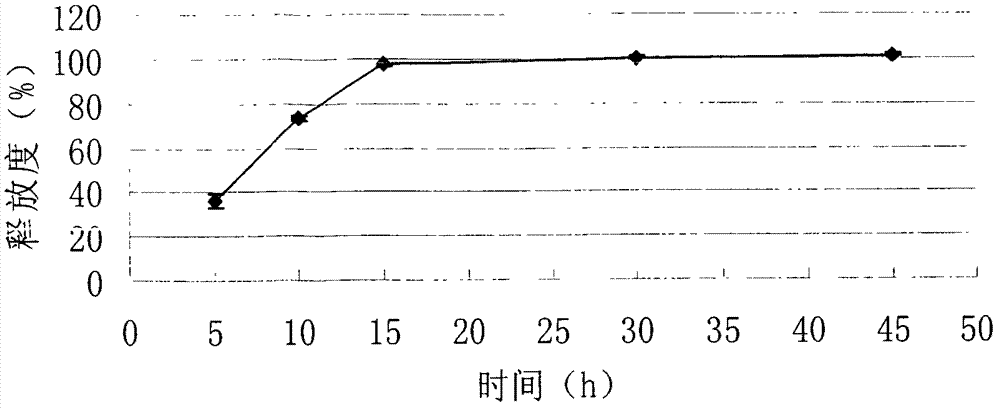
InactiveCN102525968ADissolution completeEvenly dispersedOrganic active ingredientsMetabolism disorderOrally disintegrating tabletBioavailability
The invention discloses a repaglinide orally disintegrating tablet and preparation method of the tablet. The repaglinide orally disintegrating tablet has a good taste, and disintegrates rapidly in water so that the repaglinide can be dissolved rapidly, and thus the dissolution and bioavailability of the drug can be improved greatly. The tablet is suitable for administration by senile patients and patients with difficulty in swallowing.
Owner:BEIJING D VENTUREPHARM TECH DEV
Pharmaceutical composition containing voriconazole
ActiveCN104688695AImprove stabilityDissolution completeOrganic active ingredientsAntimycoticsPropyl p-hydroxybenzoateDissolution
The invention specifically relates to a pharmaceutical composition containing voriconazole, belonging to the field of medicine. The pharmaceutical composition contains voriconazole, polyvinylpyrrolidone, a filler, a disintegrating agent, a lubricant and propyl p-hydroxybenzoate. The pharmaceutical composition has the advantages of good stability, complete dissolution and ,pre excellent quality; and a production method for the pharmaceutical composition is easy and practical to operate and applicable to industrial production.
Owner:长春海悦药业股份有限公司
Anoectochilus roxburghii healthcare baijiu and production technology thereof
InactiveCN107083314ADissolution completeGood for healthDigestive systemAlcoholic beverage preparationNutrientLycium chinense
The invention discloses anoectochilus roxburghii healthcare baijiu. The anoectochilus roxburghii healthcare baijiu is prepared from, by mass, 800-1500 parts of 40-60 %vol of brewed baijiu, 0.7-1.4 parts of anoectochilus roxburghii, 0.5-1.0 parts of wolfberries, 0.8-1.5 parts of radix glycyrrhizae and 15-30 parts of honey. The invention further discloses a production technology of the anoectochilus roxburghii healthcare baijiu, anoectochilus roxburghii, the wolfberries and radix glycyrrhizae are extracted by adopting the brewed baijiu, and are then subjected to solid-liquid separation to obtain filter liquid, the honey is added into the filter liquid to be homogenized, and then microfiltration is conducted to obtain the anoectochilus roxburghii healthcare baijiu. The anoectochilus roxburghii healthcare baijiu is rich in nutrient substances, remarkable in healthcare effect, the extraction efficiency is high, and the impurity separation is thorough.
Owner:JIAYING UNIV
Stable paroxetine hydrochloride tablet and preparation method thereof
ActiveCN110037995AInhibition of reddeningHave an unexpected effectOrganic active ingredientsNervous disorderSucroseFiller Excipient
The invention belongs to the technical field of medicines, and provides a stable paroxetine hydrochloride tablet and a preparation method thereof. The paroxetine hydrochloride tablet comprises the following components in parts by weight: 7.6 parts of paroxetine hydrochloride, 67.4-81.9 parts of a filler, 2-5 parts of an adhesive, 3-10 parts of a stabilizer, 5-7 parts of a disintegrant and 0.5-3 parts of a lubricant, wherein the stabilizer is sucrose, the filler is calcium hydrogen phosphate dihydrate, the disintegrant is sodium carboxymethyl starch, the adhesive is povidone K30, and the lubricant is magnesium stearate. The preparation method of the paroxetine hydrochloride tablet adopts a wet granulation process. The method solves the problems that paroxetine hydrochloride products turn red in the wet granulation process and the hardness of paroxetine hydrochloride tablets is poor at high temperature when calcium phosphate dihydrate is used as a main filler.
Owner:山东启荣科技有限公司 +1
Method for preparing carbon paper of gas diffusion layer of fuel cell by calendaring and carbon paper
ActiveCN111169030AHigh porosityLarge hole volumeFinal product manufactureCell electrodesFiberFuel cells
The invention provides a method for preparing carbon paper of a gas diffusion layer of a fuel cell by calendaring and the carbon paper. The carbon paper of the gas diffusion layer is prepared by the following steps of uniformly dispersing micropore conductive carbon fully infiltrated with a saturated salt solution, soluble salt particles, a hot-melt polymer and a fiber material, mixing, calendaring and stretching, and processing by a needle roller to obtain a thin sheet; and continuously passing the thin sheet through a guide roller in a solvent pool, leading out and drying the thin sheet, then leading the thin sheet into a clean water pool, and finally leading out and drying, trimming and coiling the thin sheet. According to the method, the conductive carbon material pre-occupying the soluble salt in the gaps and the soluble salt particles are dispersed in the polymer and then are subjected to hot calendering molding, small gaps of the carbon particles and macropores of the soluble salt particles after dissolution exist synergistically after washing desalination, needling treatment is further carried out, and finally infiltration and cold stretching are carried out in the solventpool, so that the carbon paper with high porosity is obtained. In addition, the preparation process is simple, the cost is low, and large-scale production is easy.
Owner:上海中海龙高新技术研究院
Iloperidone drug composition and preparation method thereof
ActiveCN101822674BGood compressibilityIncrease forceOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTActive ingredient
The invention discloses a drug composition containing an Iloperidone active ingredient, which contains micronization Iloperidone or Iloperidone smashed with other accessories, can effectively enhances dissolution effect of Iloperidone.
Owner:AVENTIS PHARMA HAINAN
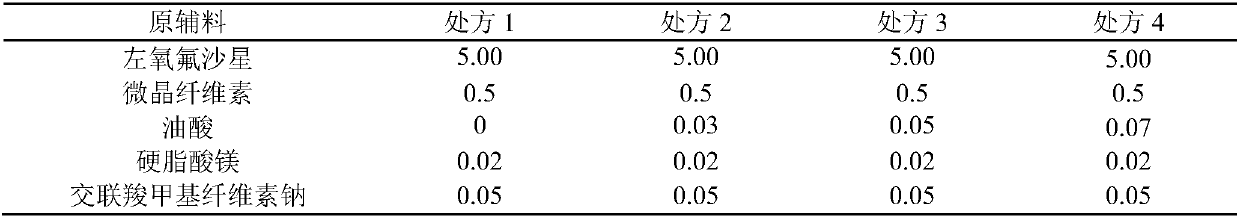
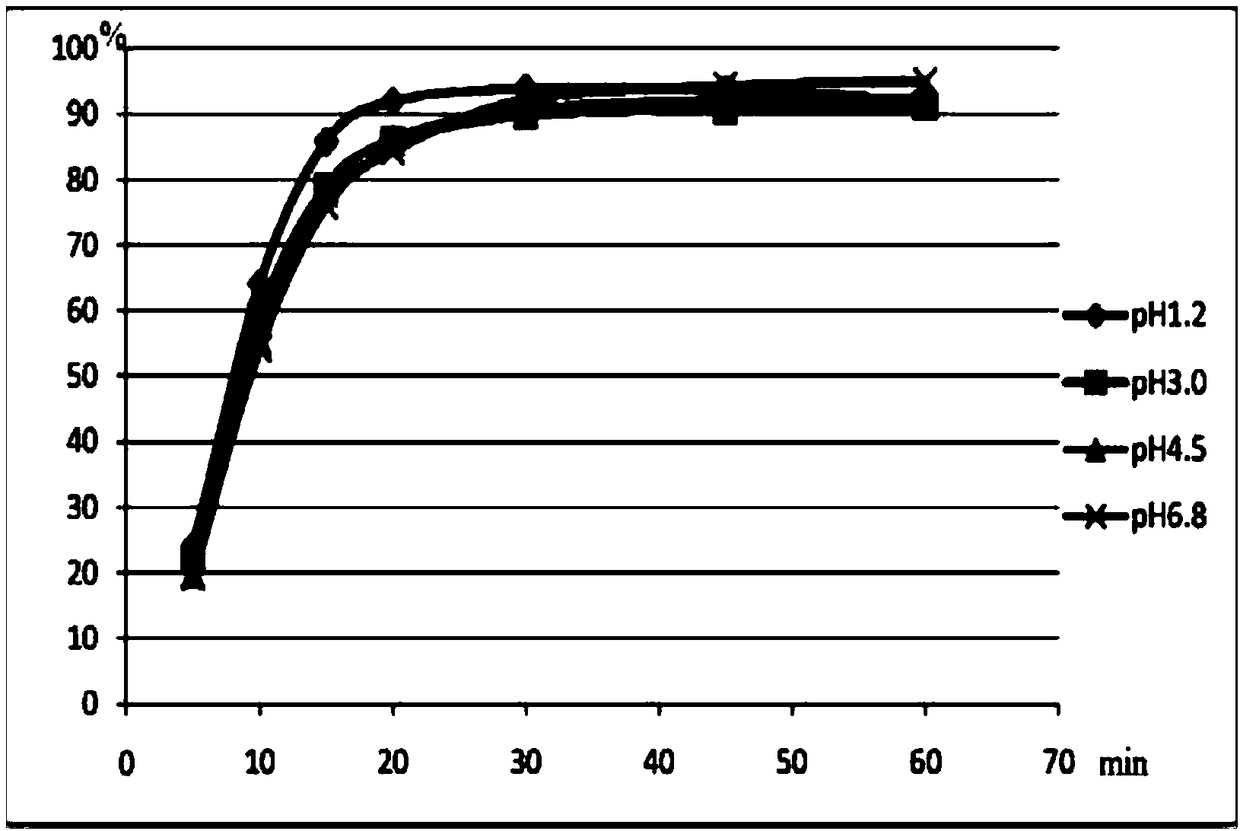
Levofloxacin tablet composition and preparation method thereof
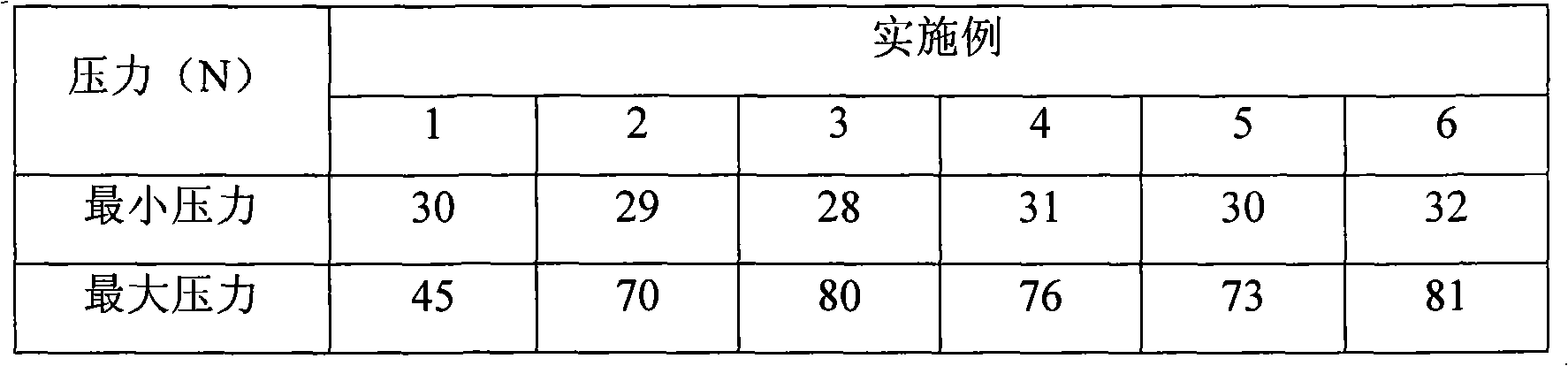
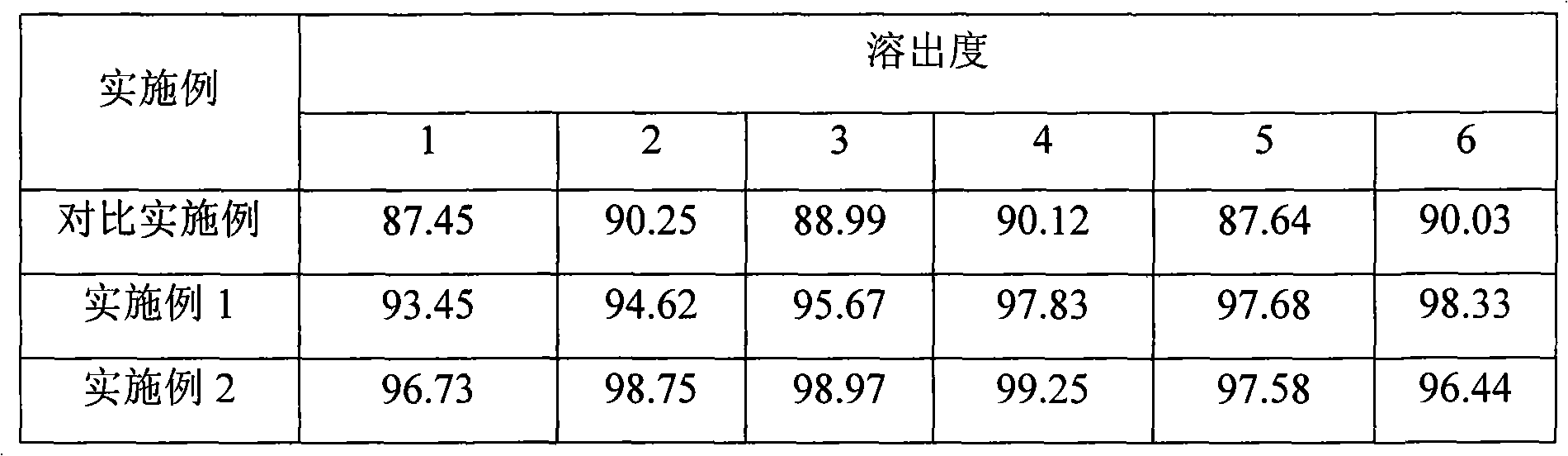
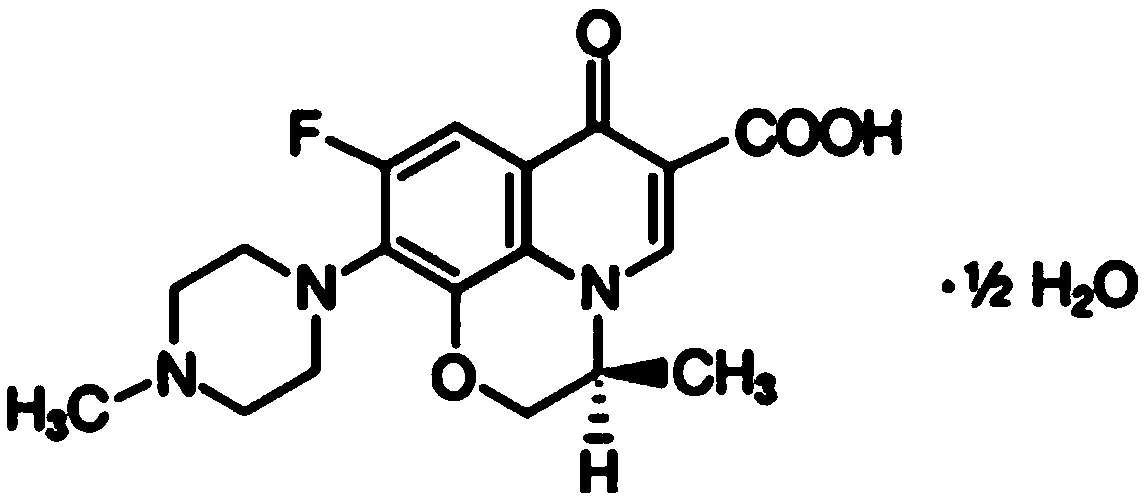
ActiveCN111110641AGood stabilityDissolution completeAntibacterial agentsOrganic active ingredientsOleic Acid TriglycerideBiomedical engineering
The invention belongs to the technical field of medicines, and particularly relates to a levofloxacin tablet composition and a preparation method thereof. The pharmaceutical composition contains levofloxacin, a filling agent, a lubricating agent, a disintegrating agent, an adhesive and oleic acid. The product is high in stability, is dissolved completely, and has higher product quality. The product is easy and feasible to produce and operate, and is suitable for industrial production.
Owner:长春海悦药业股份有限公司
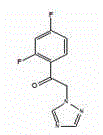
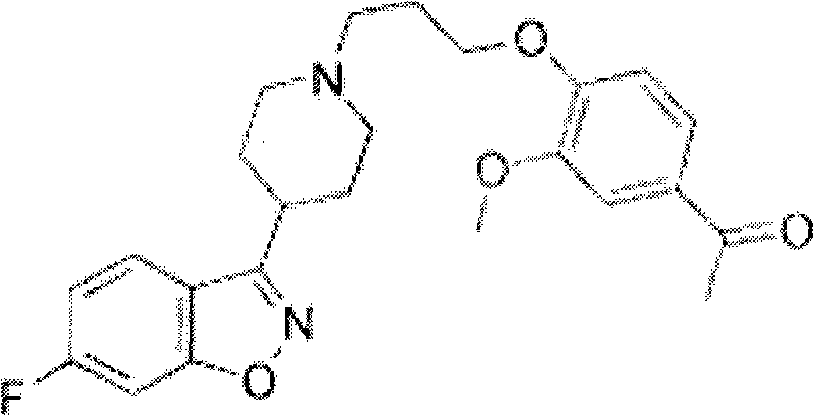
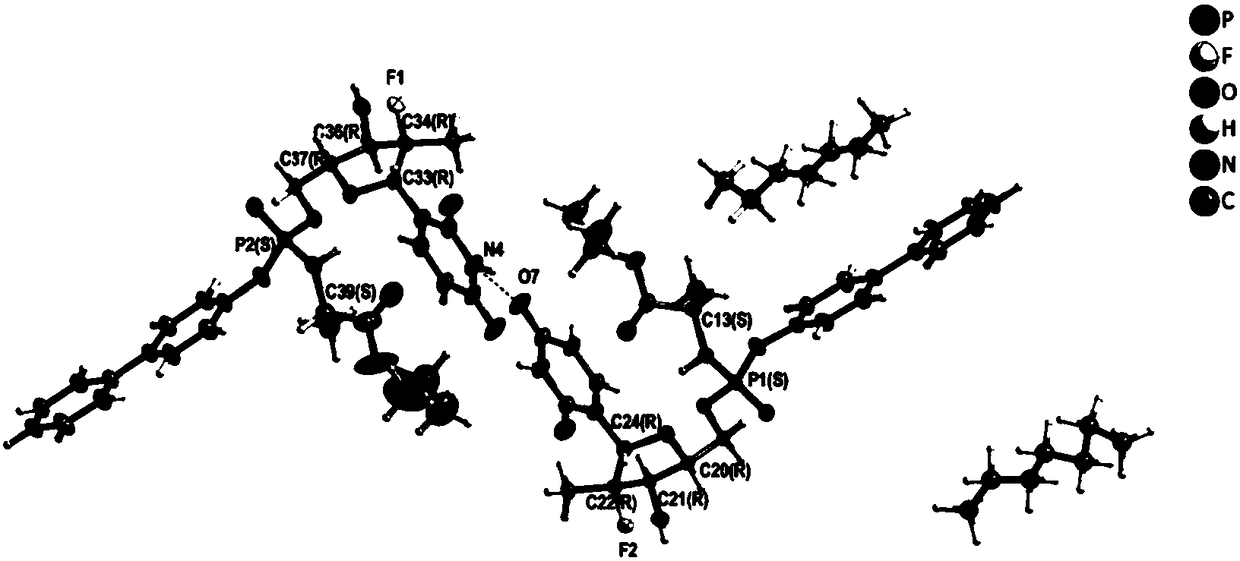
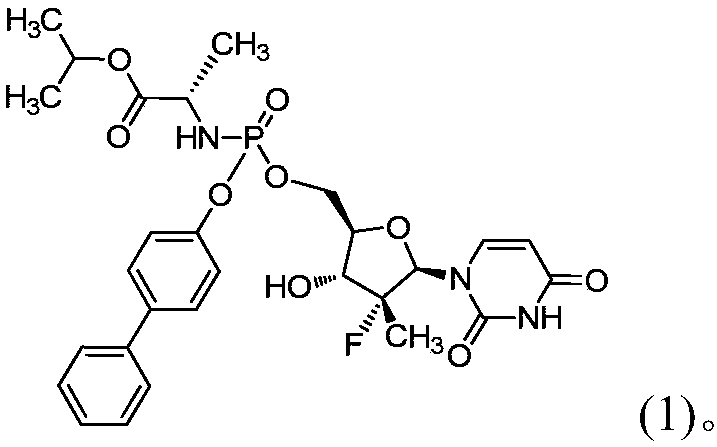
Novel composition of nucleoside amino phospholipid compound and preparation method thereof
PendingCN108210509AImprove stabilityFor long-term storageOrganic active ingredientsAntiviralsSolventIn vivo
The invention belongs to the field of pharmaceutical preparations, and relates to an oral preparation. The oral preparation is prepared from: (a) a compound shown as a formula (1) or a pharmaceutically-acceptable salt, isomer, solvent compound, hydrate or crystal thereof; and (b) an adsorbent. The invention further relates to a preparation method of the oral preparation and application of the oralpreparation to a medicament for preventing and / or treating hepatitis C. The oral preparation disclosed by the invention can be completely dissolved, is absorbed well in vivo, and has few impurities and high stability. The formula (1) is shown in the description.
Owner:南京汇诚制药有限公司
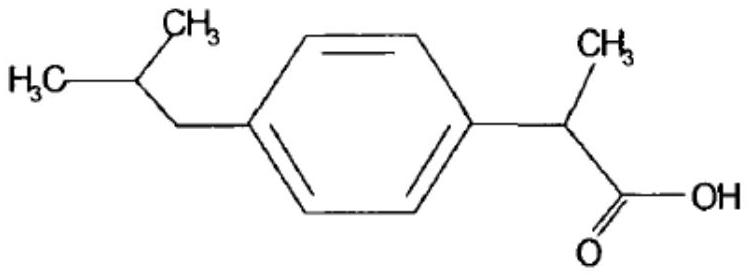
Ibuprofen sustained-release capsule and preparation method thereof
ActiveCN112263567AGood sustained release effectGood slow releaseOrganic active ingredientsAntipyreticSustained Release CapsulePolyethylene glycol
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to an ibuprofen sustained-release capsule and a preparation method thereof. The capsule is prepared by the following steps: enabling ibuprofen and a sustained-release carrier to be prepared into a solid dispersion by a spray drying method, adding a lubricant, performing mixing and performing filling, wherein preferably, the sustained-release carrier is polyacrylic resin polyethylene glycol. The capsule is good in slow release effect, good in process reproducibility and suitable for large-scaleproduction.
Owner:南京易亨制药有限公司
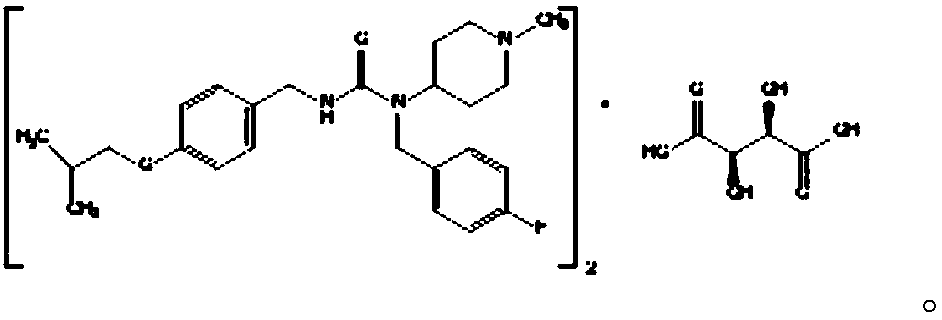
Pharmaceutical composition containing pimavanserin tartrate
InactiveCN109953965AImprove stabilityGreat tasteOrganic active ingredientsNervous disorderPimavanserin tartrateBeta-Cyclodextrins
The invention belongs to the pharmaceutical technical field, and in particular, relates to a pharmaceutical composition containing pimavanserin tartrate; the pharmaceutical composition comprises pimavanserin tartrate, a filling agent, a disintegrator and a lubricant, and is characterized by also comprising povidone and beta-cyclodextrin. The product has good stability, bitterness covering, good taste, fast absorption, and better product quality; the product is simple and feasible to produce and operate, and is suitable for industrial production.
Owner:DANYANG ZHENGYUAN BIOTECH
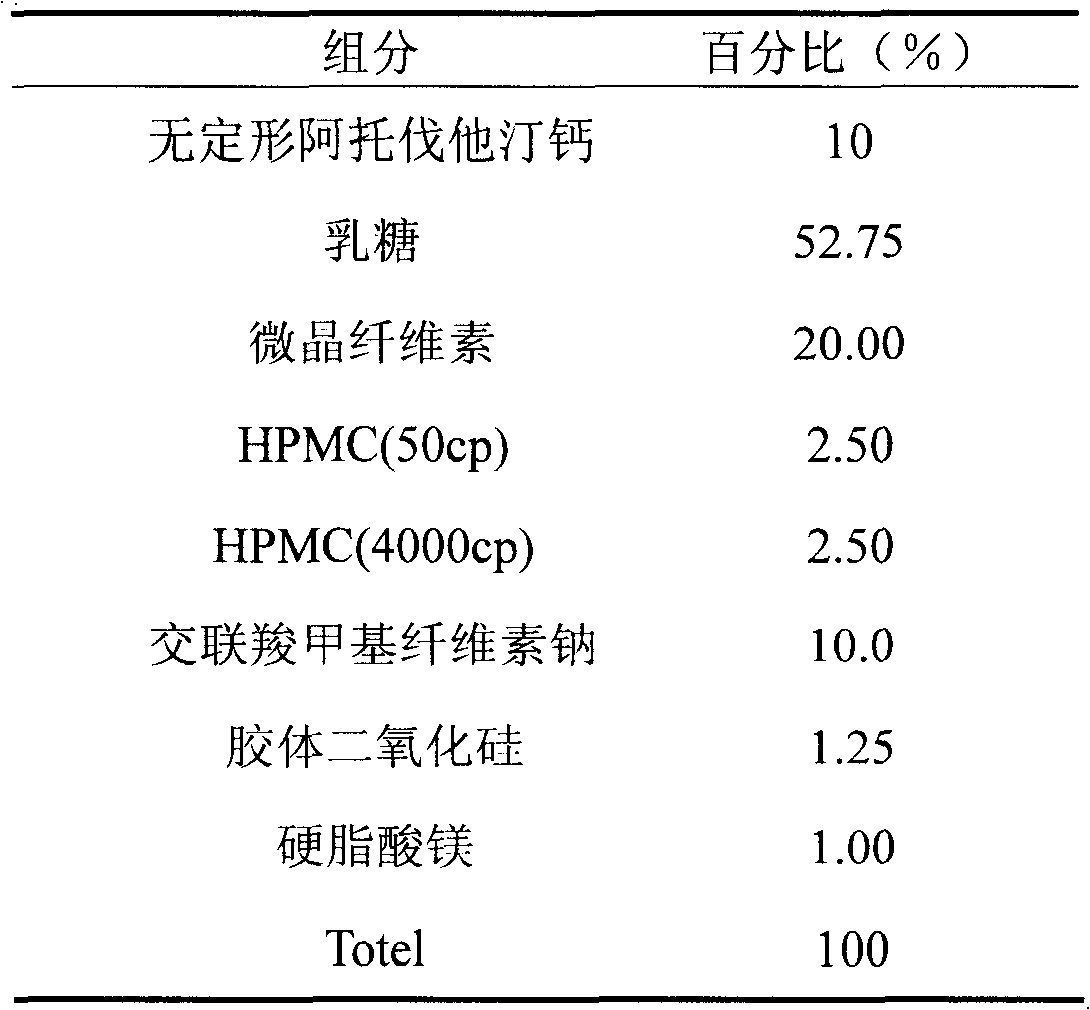
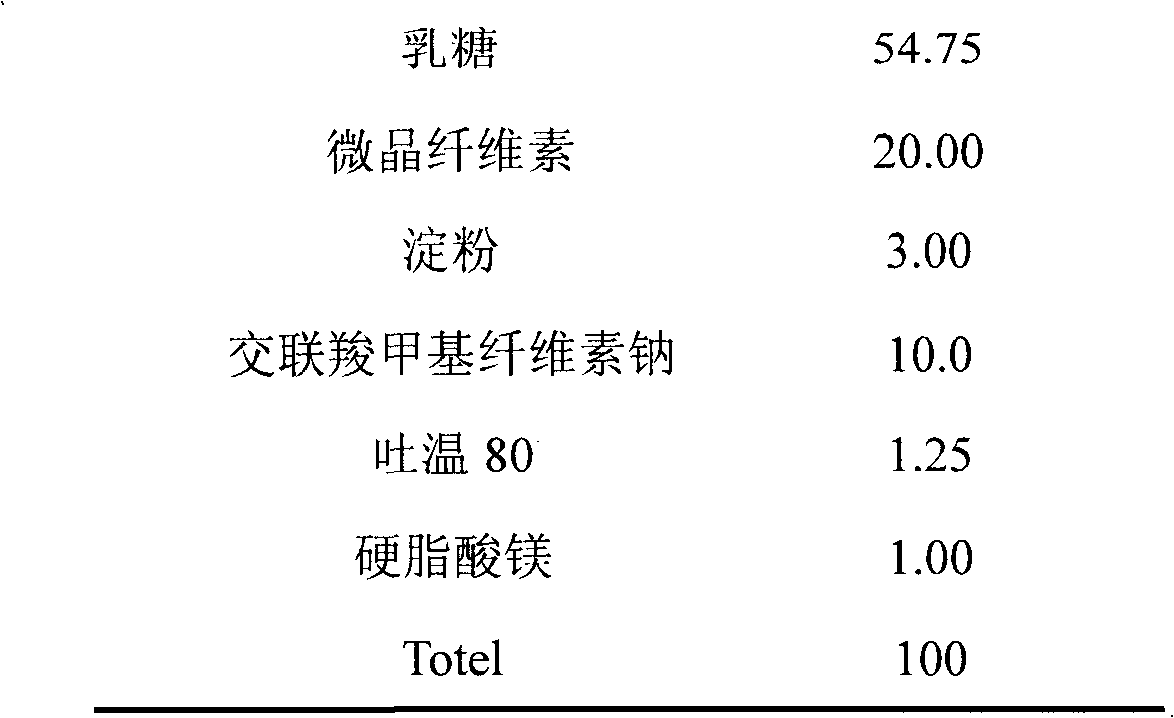
Medical composition containing amorphous atorvastatin calcium
InactiveCN101766593AImprove stabilityGood water solubilityMetabolism disorderHeterocyclic compound active ingredientsChemical compositionDissolution
The invention discloses a medical composition containing amorphous atorvastatin calcium and a preparation method thereof. By adopting the medical composition, the stability of the medicines is obviously improved, the disintegration speed fastened and the dissolution improved. The medical composition is used for treating high cholesterin lipidemia, can reduce administration dosage, avoids great quantity of medication, enables the blood drug concentration of patients to be smooth and simultaneously increases compliance.
Owner:BEIJING D VENTUREPHARM TECH DEV
Febuxostat tablet and preparation method thereof
ActiveCN102488665BLess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderSolubilityMedicine
The invention discloses a febuxostat tablet and a preparation method thereof. The febuxostat tablet comprises a tablet core and a coating, the tablet core comprises the following compositions: by weight percentage, from 5% to 30% of febuxostat, from 15% to 60% of filler, from 1% to 20% of disintegrating agent, from 0.1% to 5% of surfactant, from 0.1% to 8% of lubricating agent and a defined amount of adhesive. The febuxostat tablet adopts the high-efficient disintegrating agent within a reasonable proportion range, simultaneously, the febuxostat which is difficultly soluble medicine is dissolved by the aid of the surfactant and the high-efficient disintegrating agent, so that solubility of the febuxostat is improved, and bioavailability of the febuxostat is increased. In addition, the preparation method for the febuxostat tablet is simple, and is controllable in quality and fine in stability.
Owner:KANGYA OF NINGXIA PHARMA
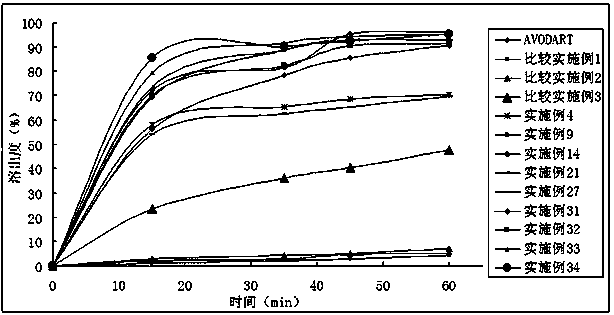
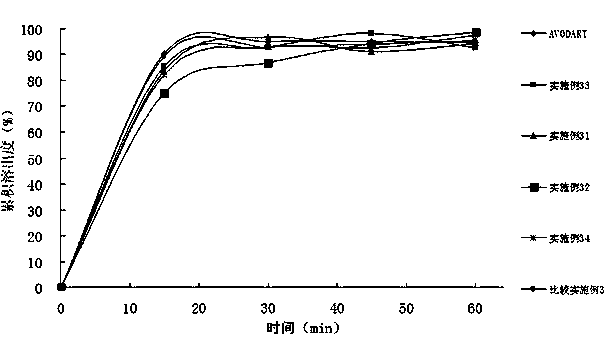
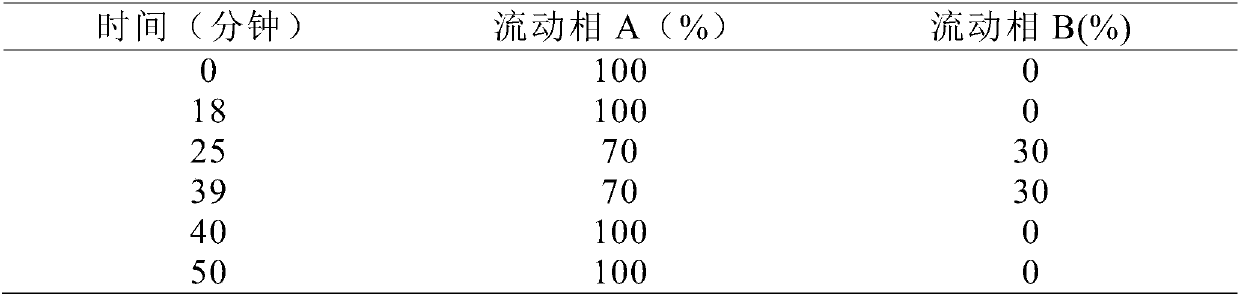
Method for determining dissolution rate of meisuoshuli tablets
InactiveCN105372187AShort analysis timeGood precisionColor/spectral properties measurementsMedicineDissolution
The invention discloses a method for determining the dissolution rate of meisuoshuli tablets. The method comprises the steps that the meisuoshuli tablets are dissolved by taking a phosphate buffer solution with the pH valve of 8.5-8.9 as a dissolution medium to obtain a test solution; absorbancy detection is performed on the test solution, and the dissolution rate of the meisuoshuli tablets is determined on the basis of a result of the absorbancy detection. According to the method, the dissolution rate of the meisuoshuli tablets can be effectively determined, the specificity is high, the needed analysis time is short, the precision and the repeatability are good, and the accuracy is high.
Owner:WUHAN OPTICS VALLEY HUMANWELL BIO PHARMA +2
Medicinal composition containing fenofibric acid
ActiveCN105581989AImprove stabilityDissolution completeOrganic active ingredientsMetabolism disorderFENOFIBRIC ACIDMedicine
The invention belongs to the technical field of medicines, and in particular relates to a medicinal composition containing fenofibric acid. The medicinal composition contains the fenofibric acid, a filling agent, a disintegrating agent, a lubricating agent, methylcellulose and mannitol granules. The medicinal composition is good in stability, dissolves out completely, and has excellent quality; the production of the medicinal composition is simple and feasible to operate, and the medicinal composition is suitable for industrial production.
Owner:长春海悦药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com