Method for determining dissolution rate of meisuoshuli tablets
A technology of mesosulide tablets and mesosulide tablets, applied in the field of medicine, can solve the problems of no mesosulide, etc., and achieve the effects of good precision and repeatability, short analysis time and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Mesosulide tablets with a specification of 50 mg are prepared according to the following method:
[0055] 1. Prescription composition:
[0056] Name of raw material
Dosage (g)
Mesosulide
50
180
Povidone K30 (10% aqueous solution (g / g))
8
Crospovidone (PVPP)
12
2.5
Co-made
1000 pieces
[0057] 2. Preparation method:
[0058] (1) material preparation: after the principal ingredient is pulverized, pass through a 60-mesh sieve, and the auxiliary materials pass through an 80-mesh sieve, and set aside;
[0059] (2) Preparation of adhesive: Weigh a certain amount of povidone K30 (PVPK30) in a beaker, add an appropriate amount of purified water, and stir until clear to obtain 10% PVPK30 aqueous solution I, which is set aside;
[0060] (3) Mixing: Weigh the main ingredient, lactose, microcrystalline cellulose, PVPP (internal addition) in the pres...
Embodiment 2
[0068] Mesosulide tablets with a specification of 100 mg are prepared according to the following method:
[0069] 1. Prescription composition:
[0070] Name of raw material
Dosage (g)
Mesosulide
100
80
40
Povidone K30 (10% aqueous solution (g / g))
7
Crospovidone (PVPP)
12
2
Co-made
1000 pieces
[0071] 2. Preparation method:
[0072] The preparation method is the same as in Example 1.
Embodiment 3
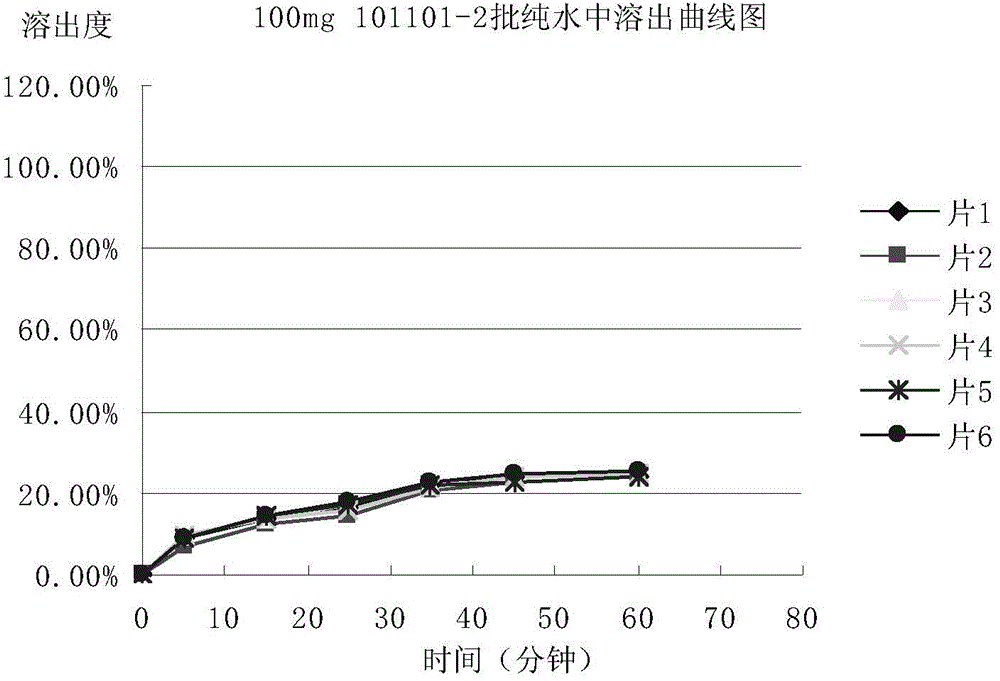
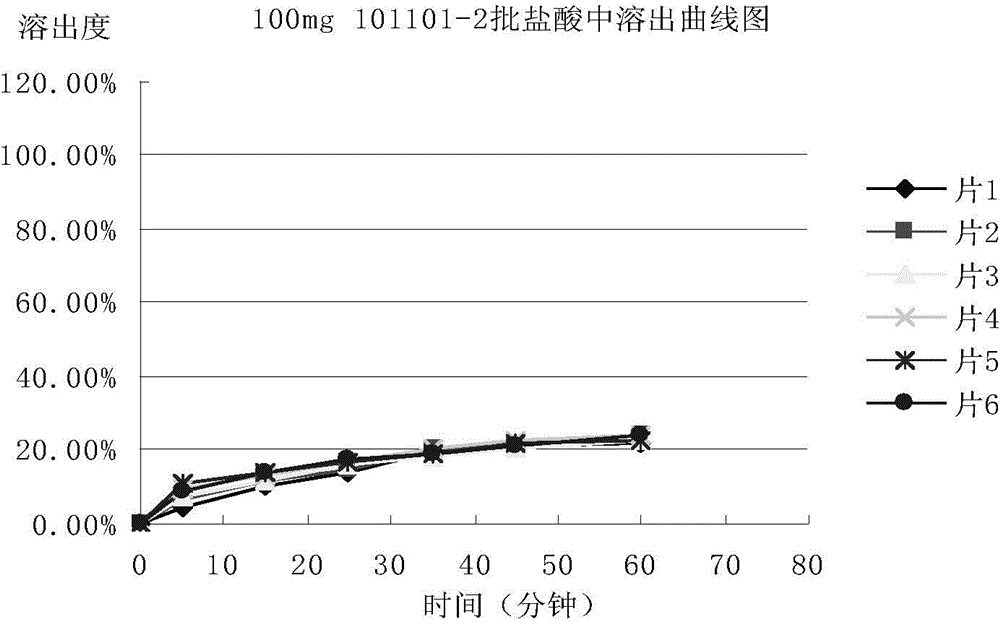
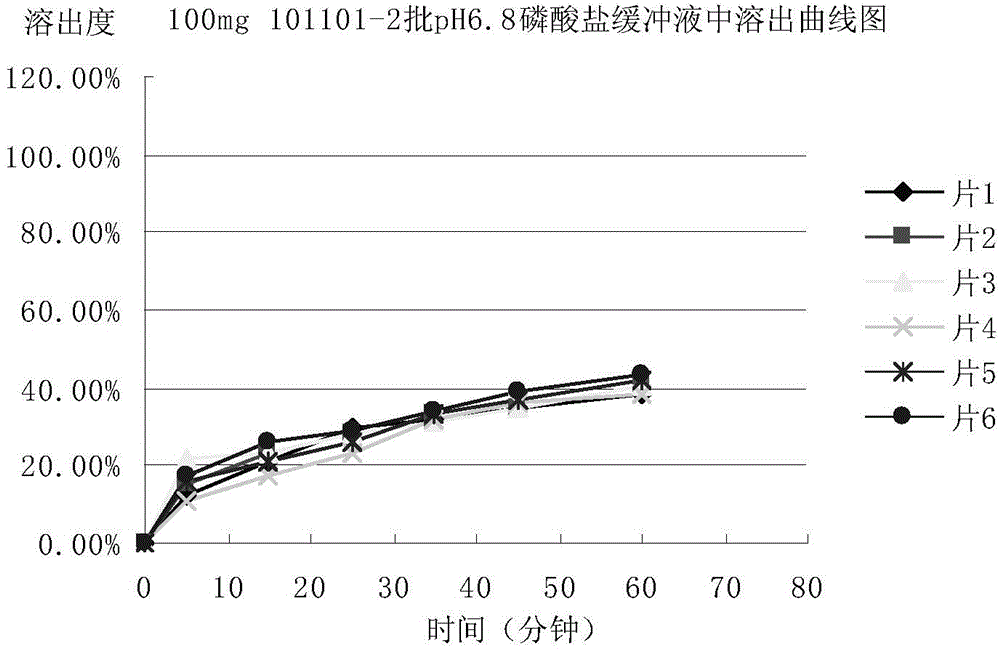
[0074] Adopt the mesosulide tablet prepared by embodiment 1 and 2, determine respectively the stripping condition of mesosulide tablet according to the following methods:
[0075] 1. Selection of dissolution medium
[0076] In order to select a suitable dissolution medium, the condition of the sink is set as follows: the dissolution medium is water, 0.1mol / L hydrochloric acid solution, acetate buffer solution of pH 4.5, phosphate buffer solution of pH 6.8 and pH 8.8 Phosphate buffer solution (preparation method: sodium hydroxide 2.30g, potassium dihydrogen phosphate 7.65g, add water to dissolve to 1000ml, adjust pH to 8.8 with phosphoric acid) put a piece of Messocid with a specification of 100mg / tablet Sharp sheet (prepared and obtained in Example 2), the dissolution medium volume is 330ml, the paddle method, the rotating speed is 75 revolutions per minute, the temperature is 37°C ± 0.5°C, sampling is carried out at 75min, and the absorbance is measured at a detection wavelen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com