Construction method for compound thrombus clearing preparation bioactivity chromatography finger print
A compound Xueshuantong and biological activity technology, applied in the field of drug analysis, can solve the problems of single pharmacodynamic index, more mobile phase consumption, long analysis time, etc., to ensure safety and effectiveness, improve product quality, and prepare simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] The present invention will be further described below in conjunction with specific examples.
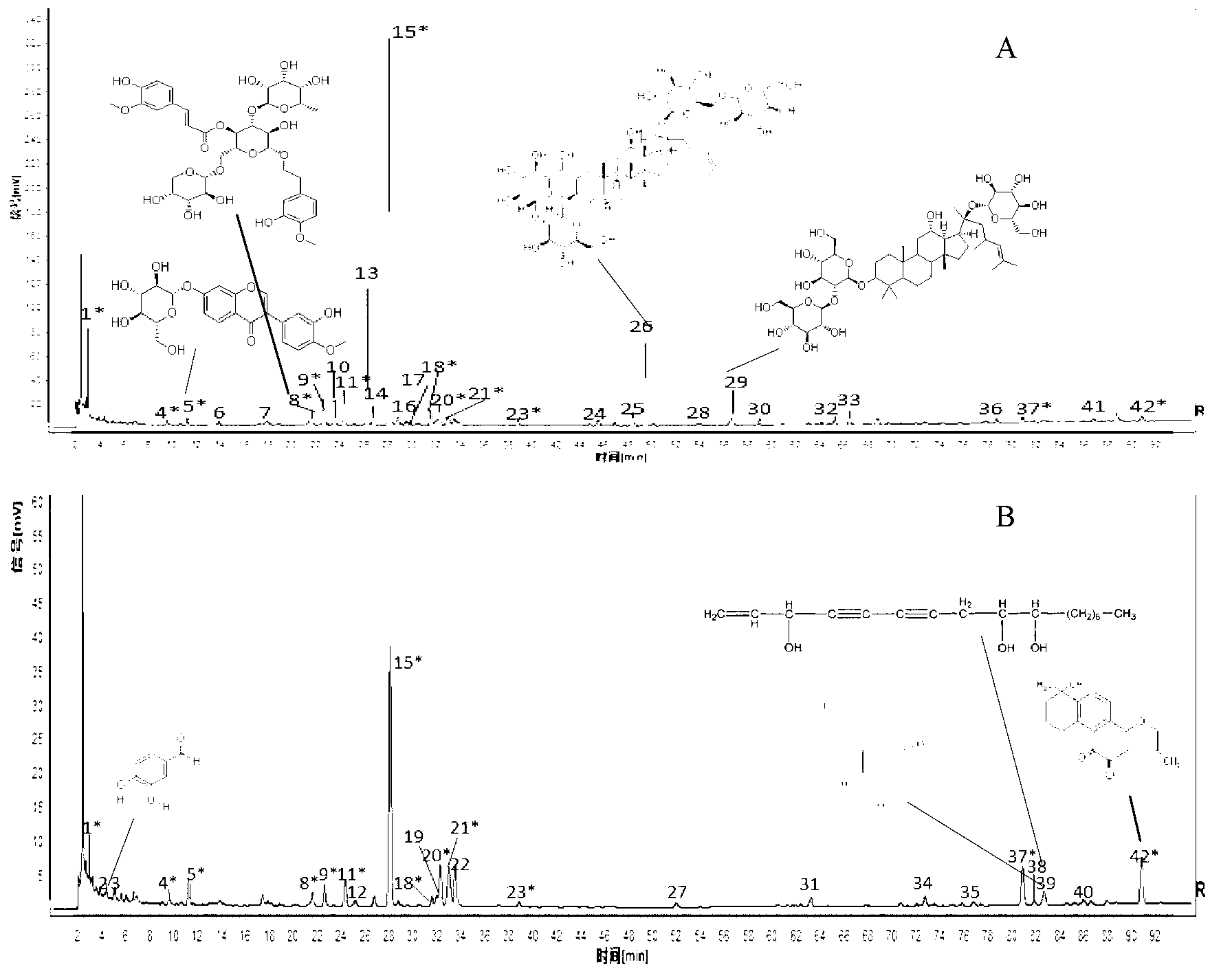
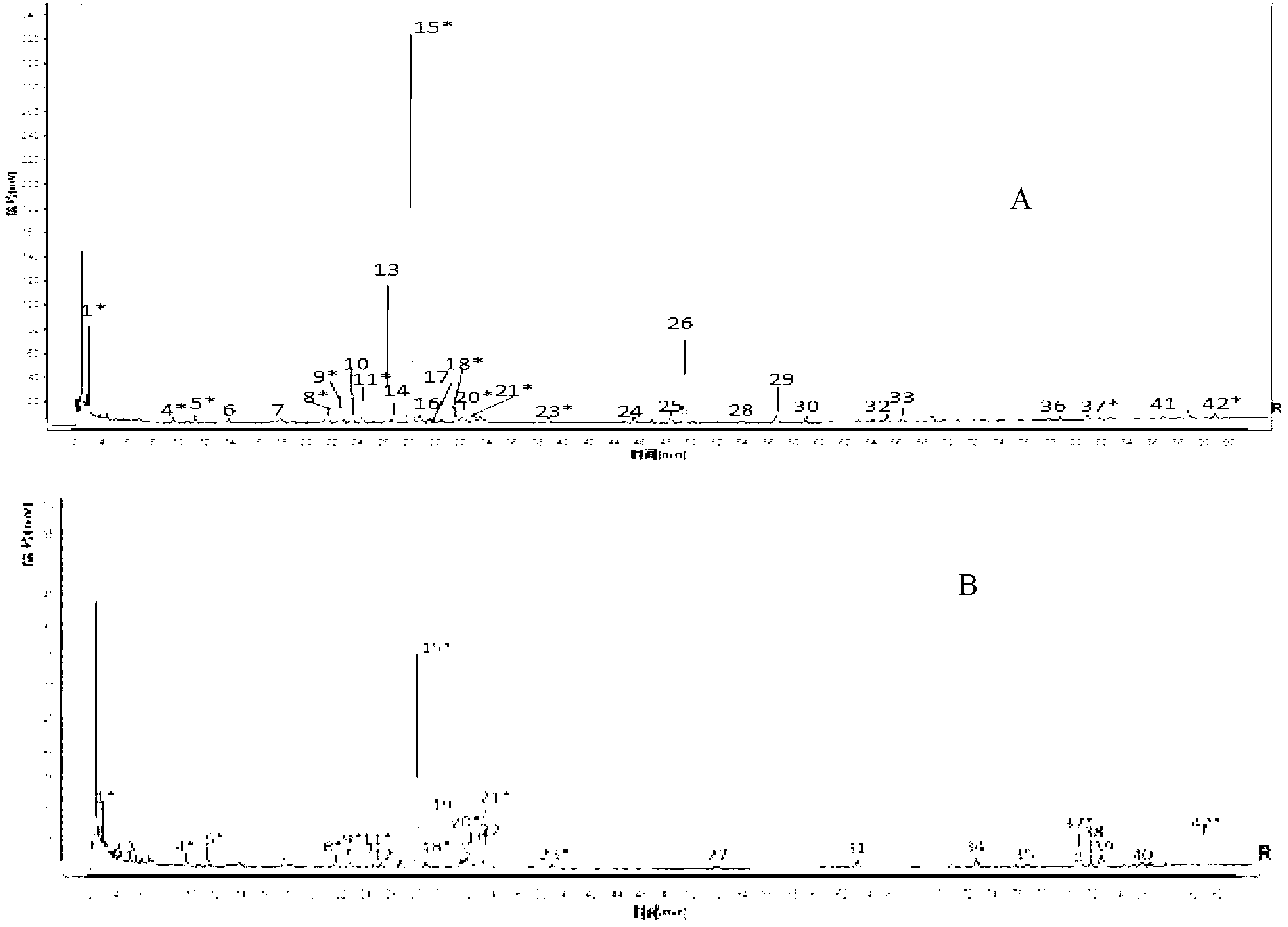
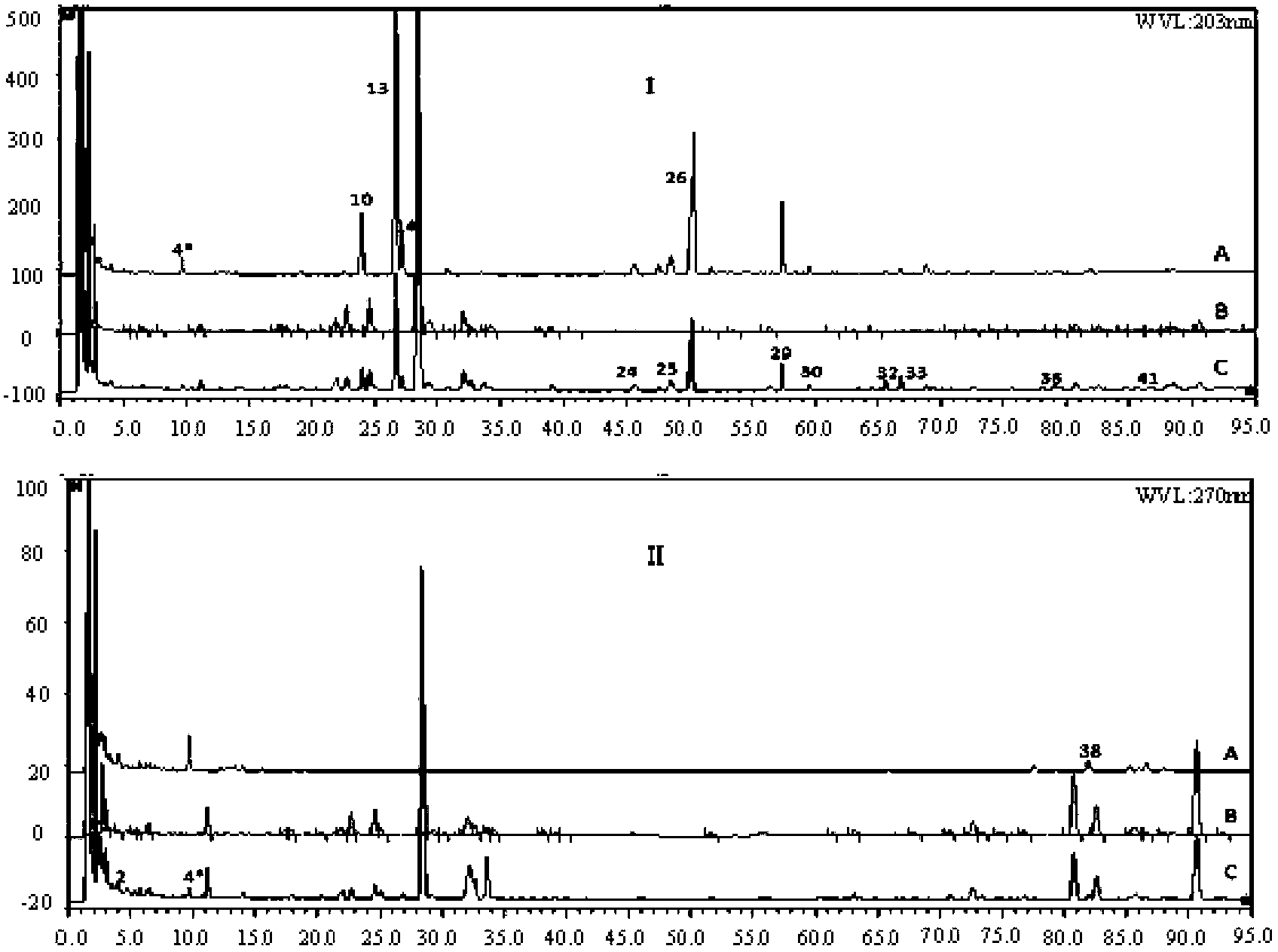
[0058] 1. Construction of HPLC fingerprint of Fufang Xueshuantong preparation
[0059] 1. Experimental materials
[0060] 1.1 Experimental samples
[0061] 10 batches of finished and semi-finished products of Fufang Xueshuantong Capsules Sanqi extract and three herbs (Danshen, Astragalus, Scrophulariae) extracts, Sanqi negative samples, Danshen negative samples, Astragalus negative samples, Scrophulariaceae negative samples, all produced by Guangdong Zhongsheng Provided by Pharmaceutical Co., Ltd.
[0062] 1.2 Experimental Instruments
[0063] One hundred thousandth electronic analytical balance (German Sartorius company, BP211D type); ultrapure water device (Millipore Millipore Company, Simplicity); rotary evaporator (Germany Laborota company, 4001 type); oven (Germany Memmert company, UFB400 type); CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., KQ-250DE);...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com