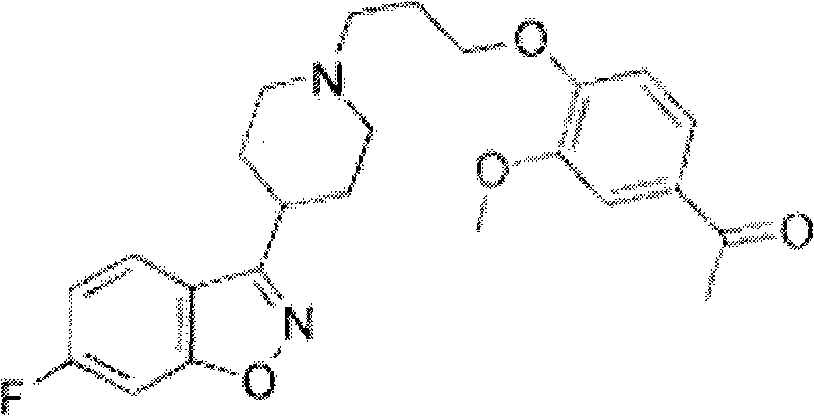
Iloperidone drug composition and preparation method thereof
A technology for iloperidone and a composition is applied in the field of pharmaceutical compositions containing iloperidone and the preparation thereof, and can solve the problems of poor tablet compressibility, poor action force, incomplete dissolution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
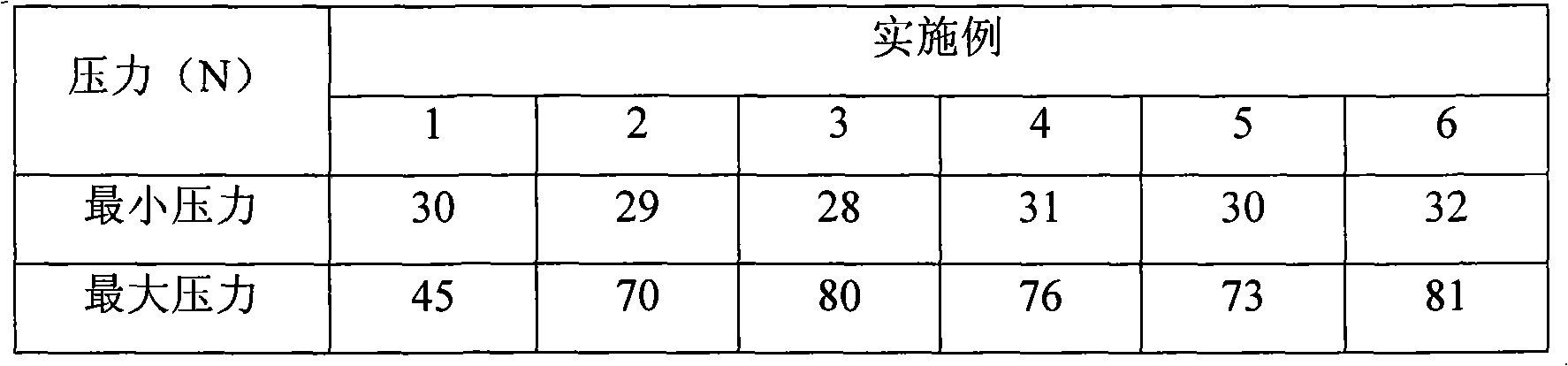
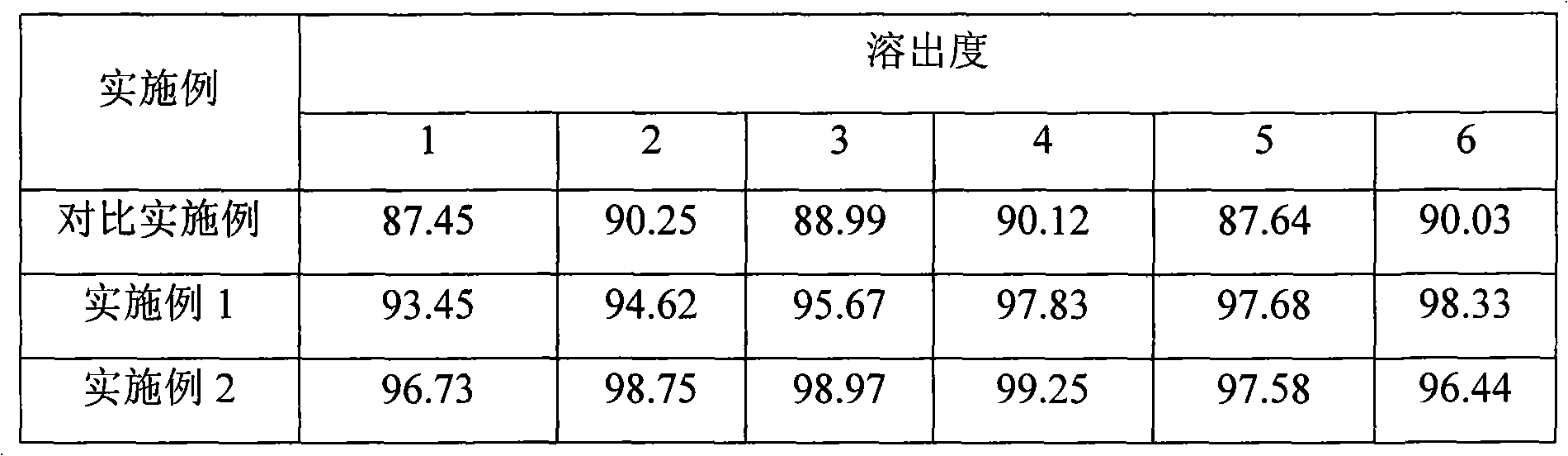
Examples
Embodiment 1
[0035] components
[0036] Preparation process: Weigh the micronized iloperidone according to the prescription quantity, the particle size of the active ingredient is more than 90% and less than 75 μm, weigh lactose and microcrystalline cellulose according to the prescription quantity, mix with 5% hydroxypropyl methylcellulose The plain aqueous solution is used as a binder to granulate, dried at 50°C, mixed with cross-linked povidone, micropowder silica gel and magnesium stearate, and compressed into tablets.
Embodiment 2
[0038] components
[0039] Preparation process: Weigh the co-pulverized iloperidone and lactose mixture according to the prescription amount, the particle size of the mixture is more than 90% and less than 75 μm, add the prescription amount of microcrystalline cellulose and mix, and use 5% hydroxypropyl methylcellulose The aqueous solution is used as a binder to granulate, dried at 50°C, mixed with cross-linked povidone, micropowder silica gel and magnesium stearate, and compressed into tablets.
Embodiment 3
[0041] components
[0042] Preparation process: Weigh the micronized iloperidone according to the prescription quantity, the particle size of the active ingredient is more than 90% and less than 75 μm, add the prescription quantity of lactose, microcrystalline cellulose and low-substituted hydroxypropyl cellulose and mix, 10% starch slurry Granulation. Dried at 50°C, mixed with low-substituted hydroxypropyl cellulose and magnesium stearate, and compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com