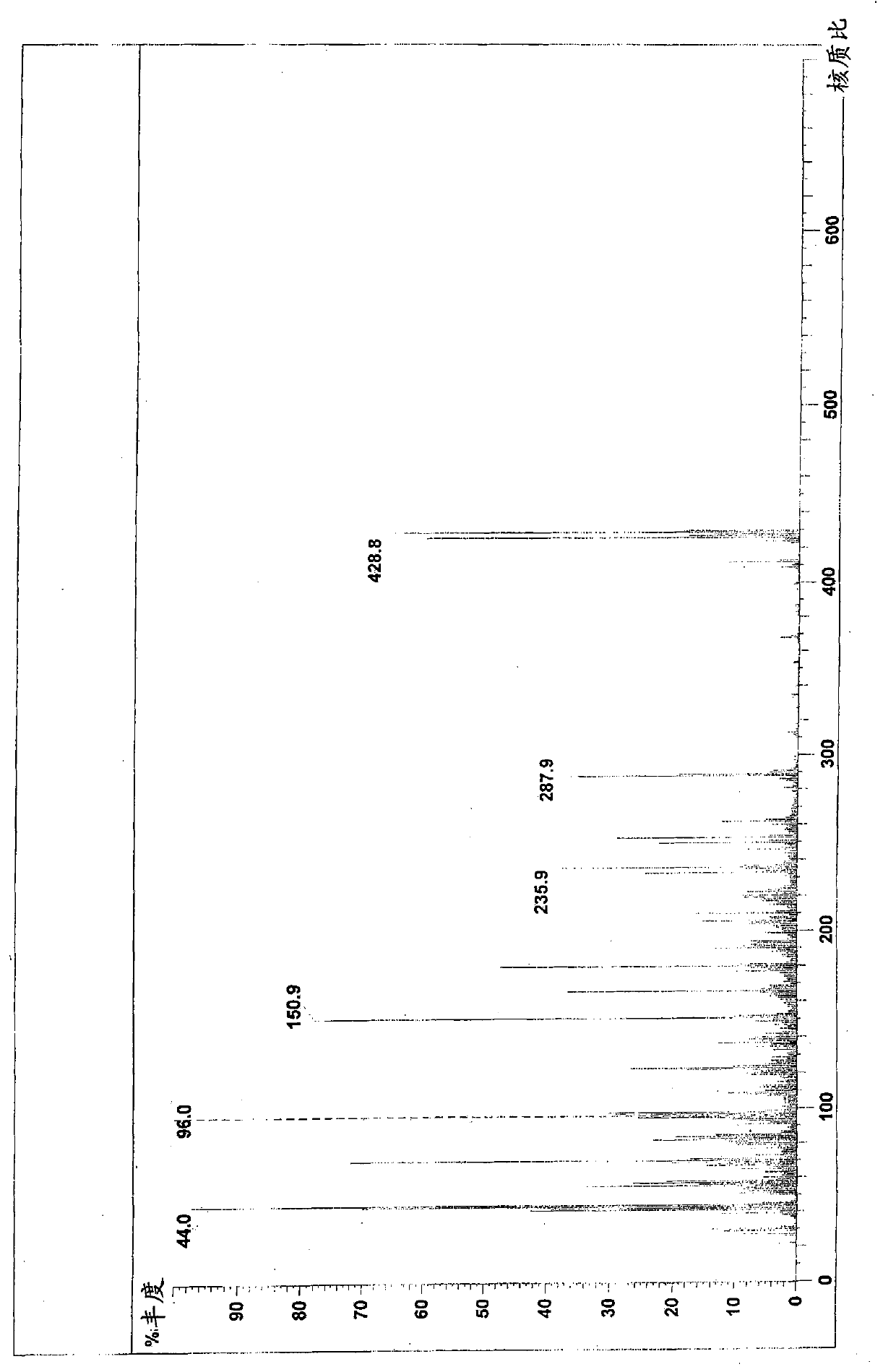
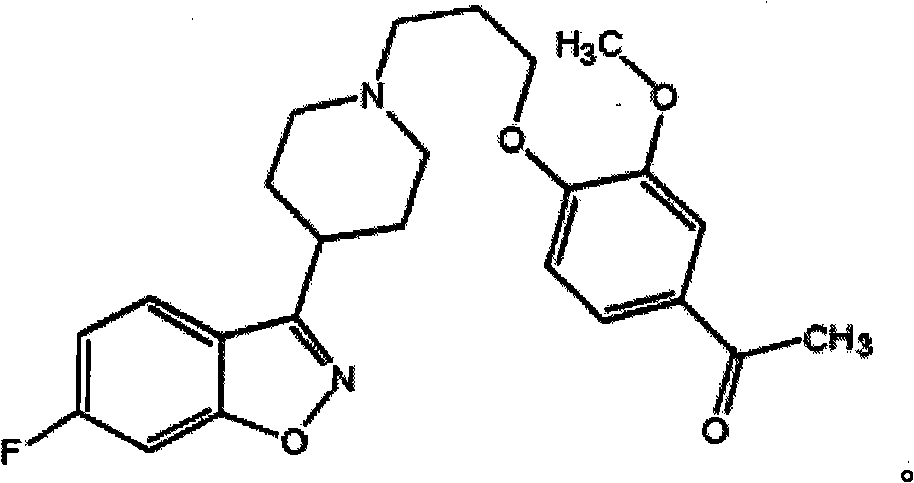
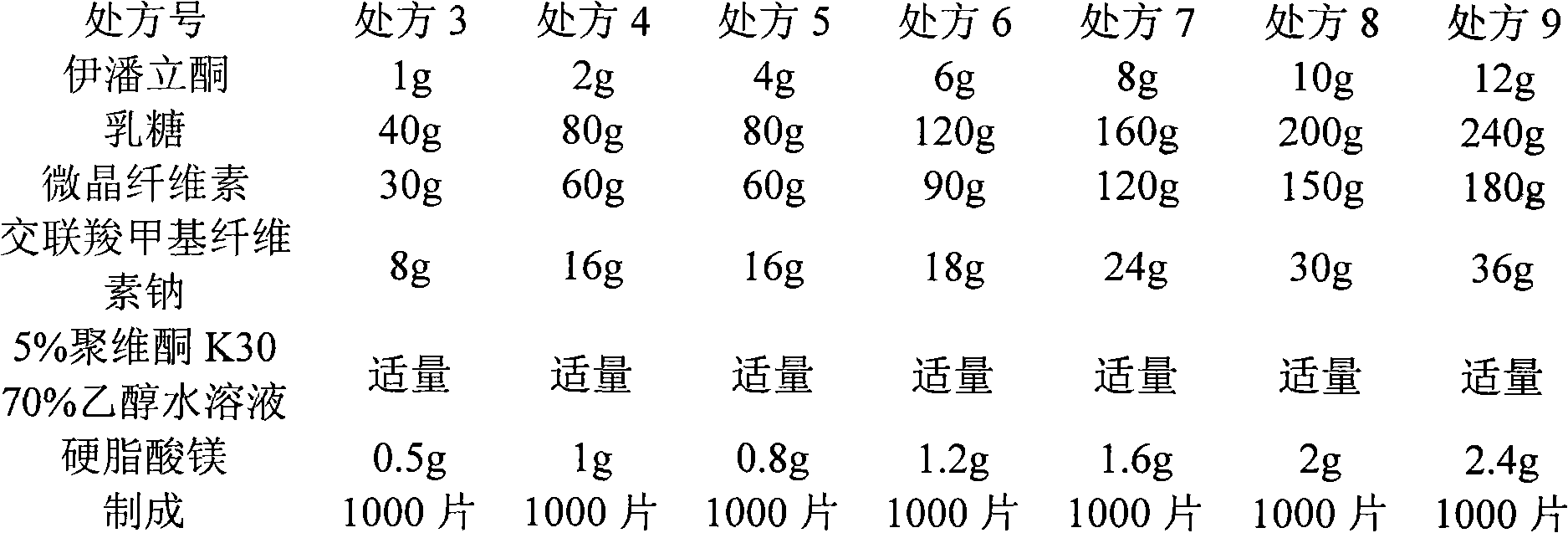
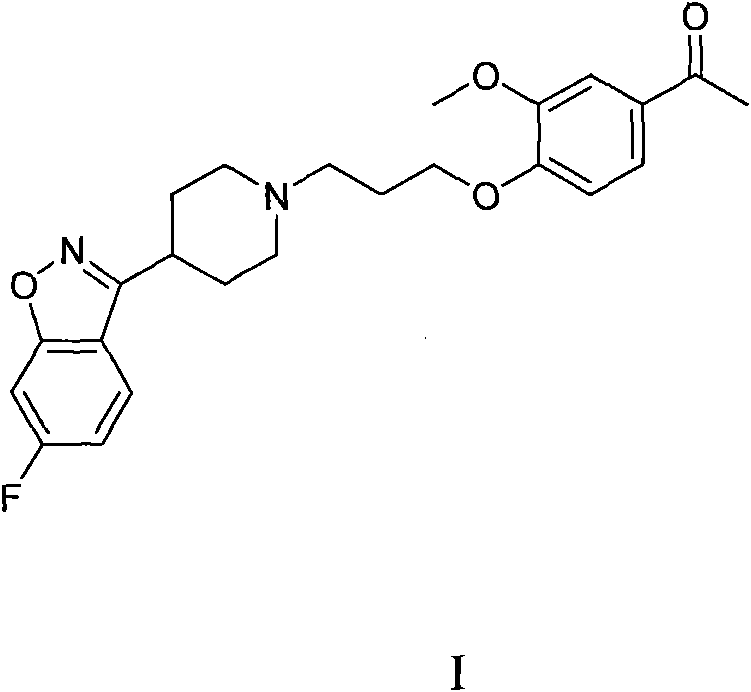
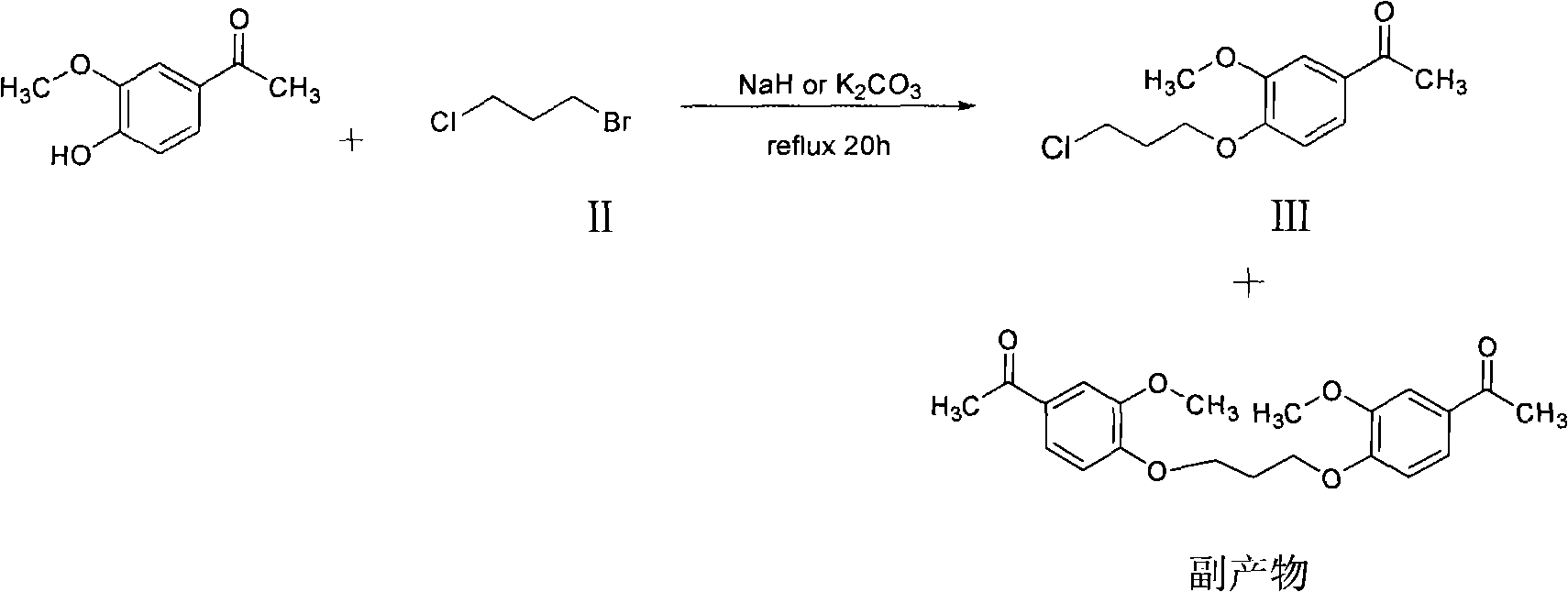
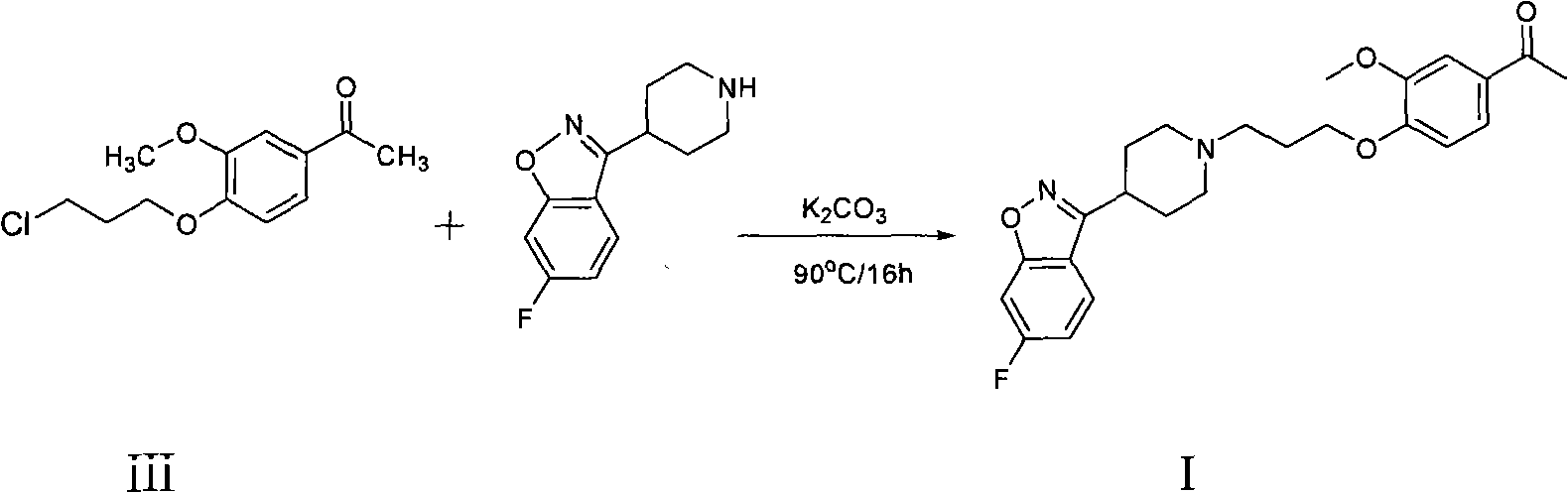
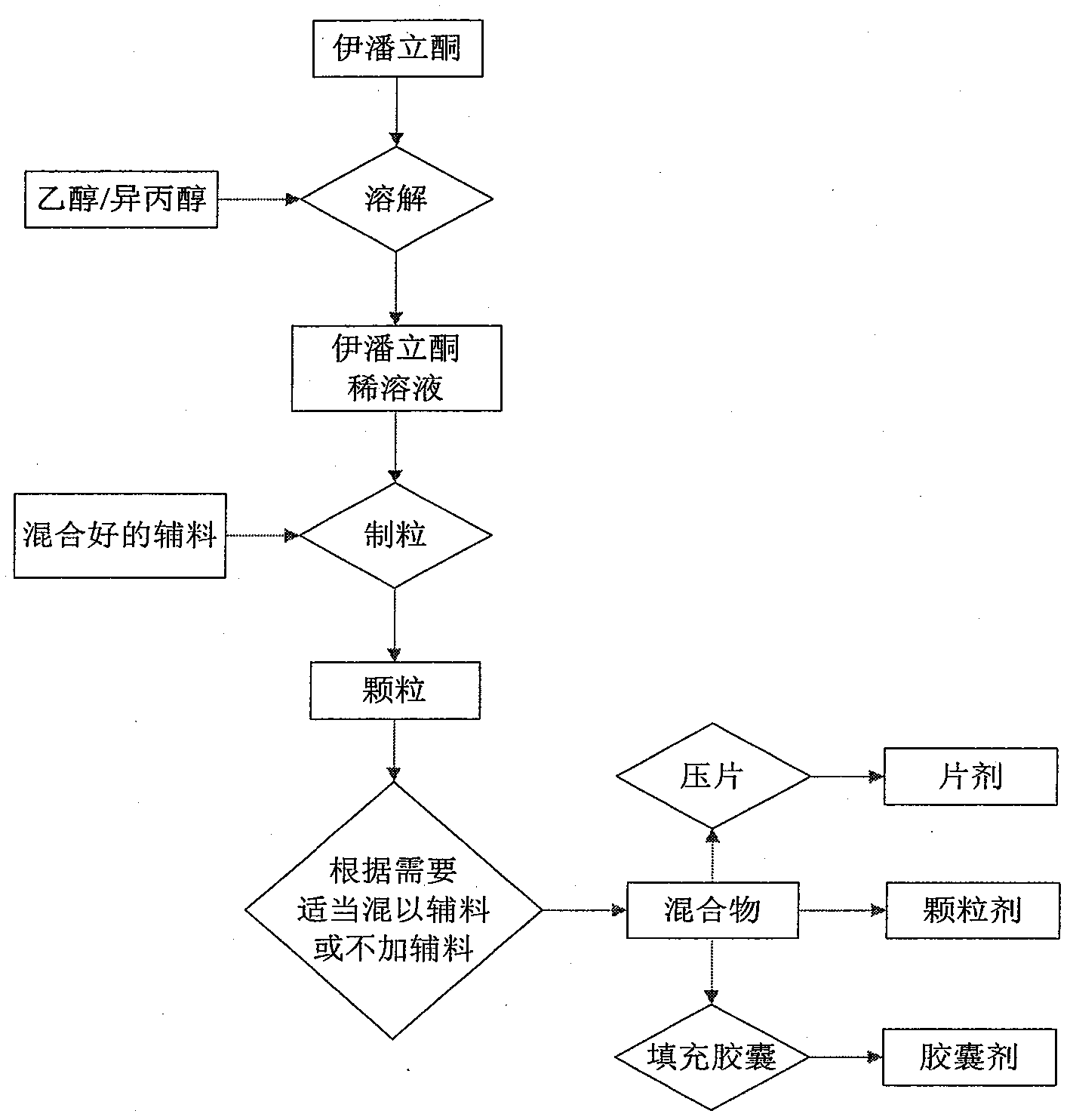
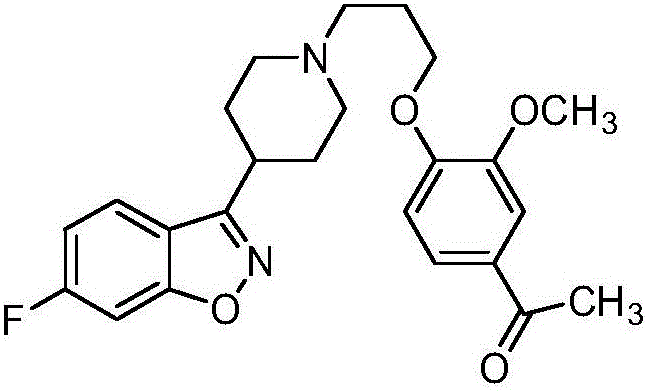
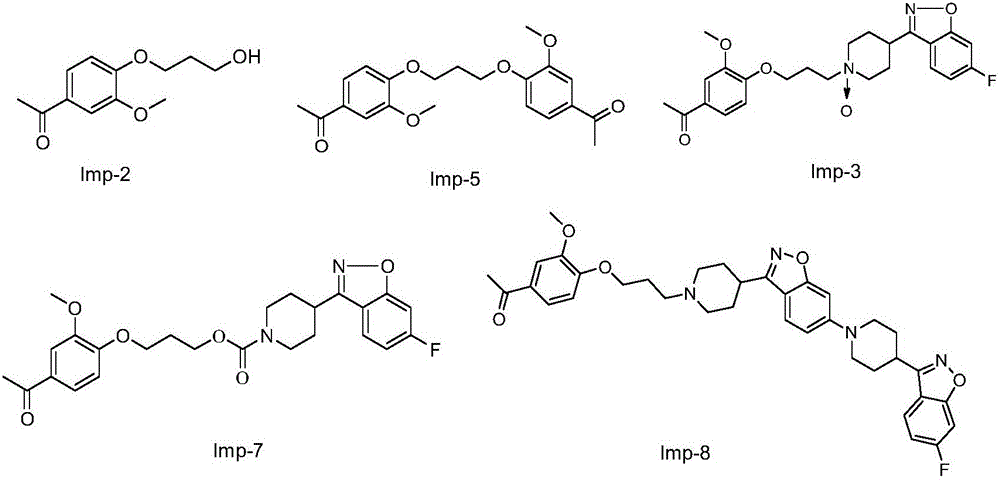
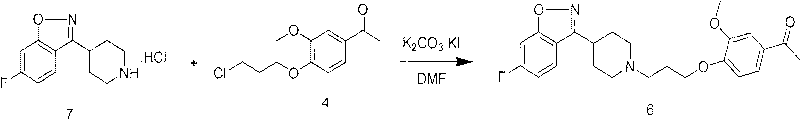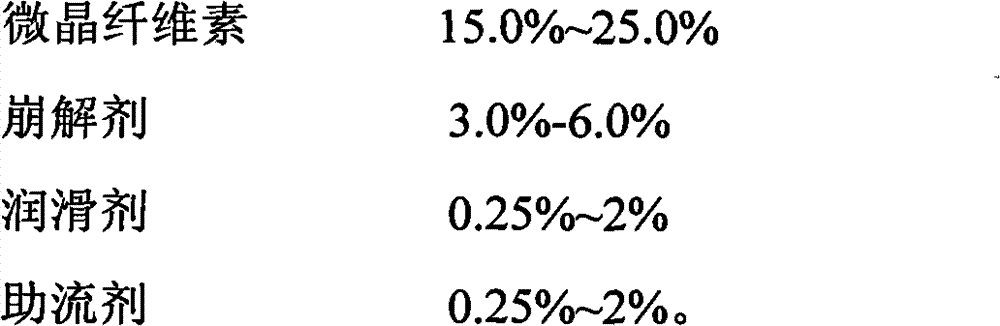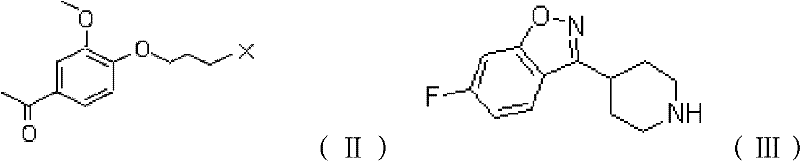Patents
Literature
83 results about "Iloperidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
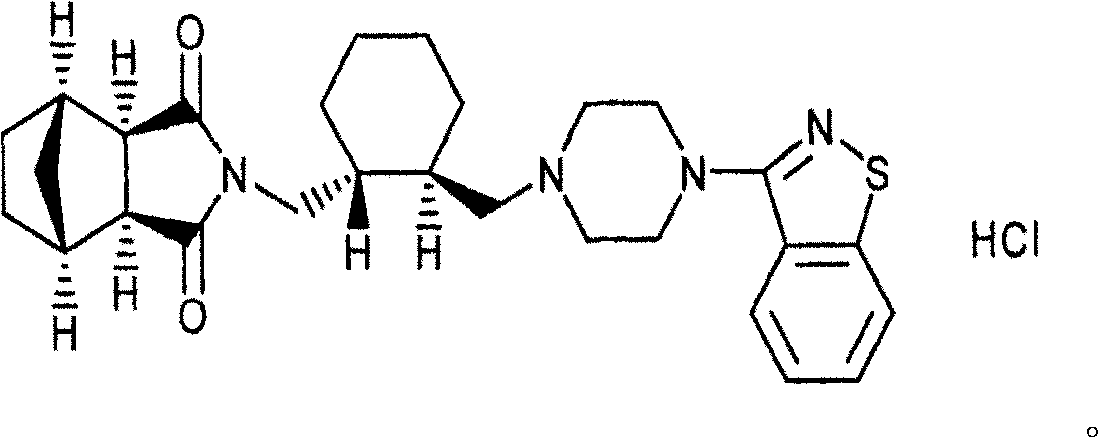
This medication is used to treat certain mental/mood disorders (such as schizophrenia).
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
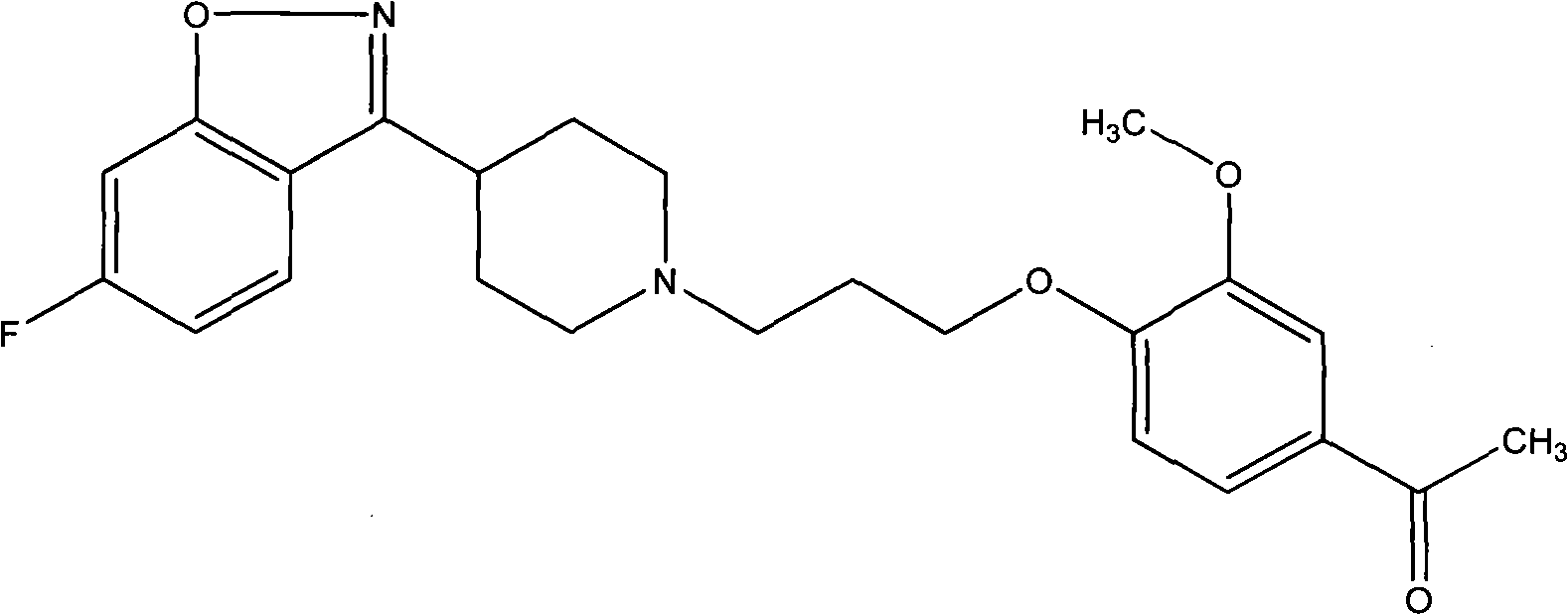
Iloperidone drug composition and preparation method thereof
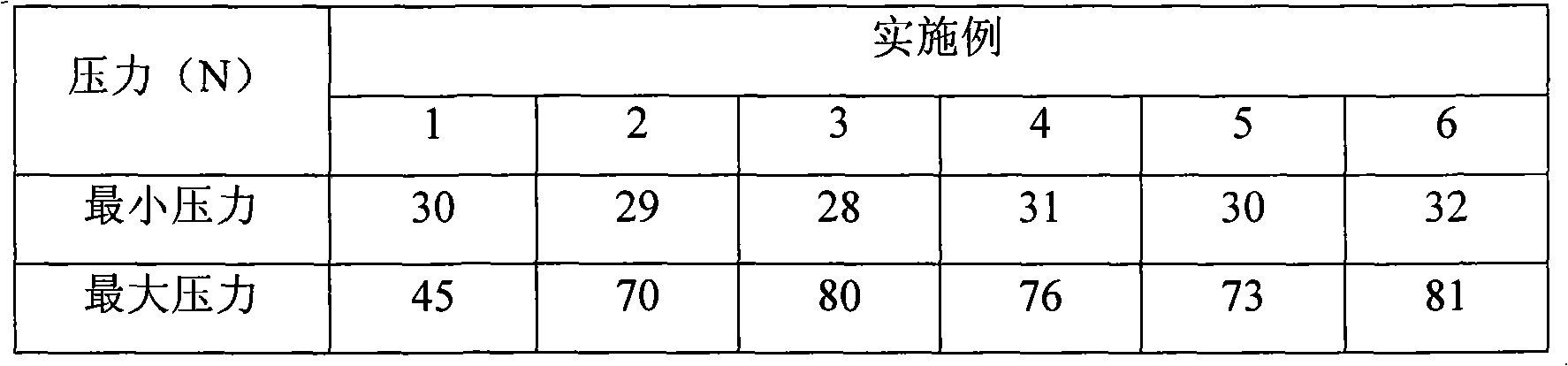
ActiveCN101822674AGood compressibilityIncrease forceOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTIloperidone
The invention discloses a drug composition containing an Iloperidone active ingredient, which contains micronization Iloperidone or Iloperidone smashed with other accessories, can effectively enhances dissolution effect of Iloperidone.
Owner:AVENTIS PHARMA HAINAN
Methods for the administration of iloperidone
Owner:VANDA PHARMA INC
A kind of novel iloperidone medicine co-crystal and preparation method thereof
ActiveCN102276594ASolubility changeImprove stabilityNervous disorderOrganic chemistrySolubilityCarboxylic acid
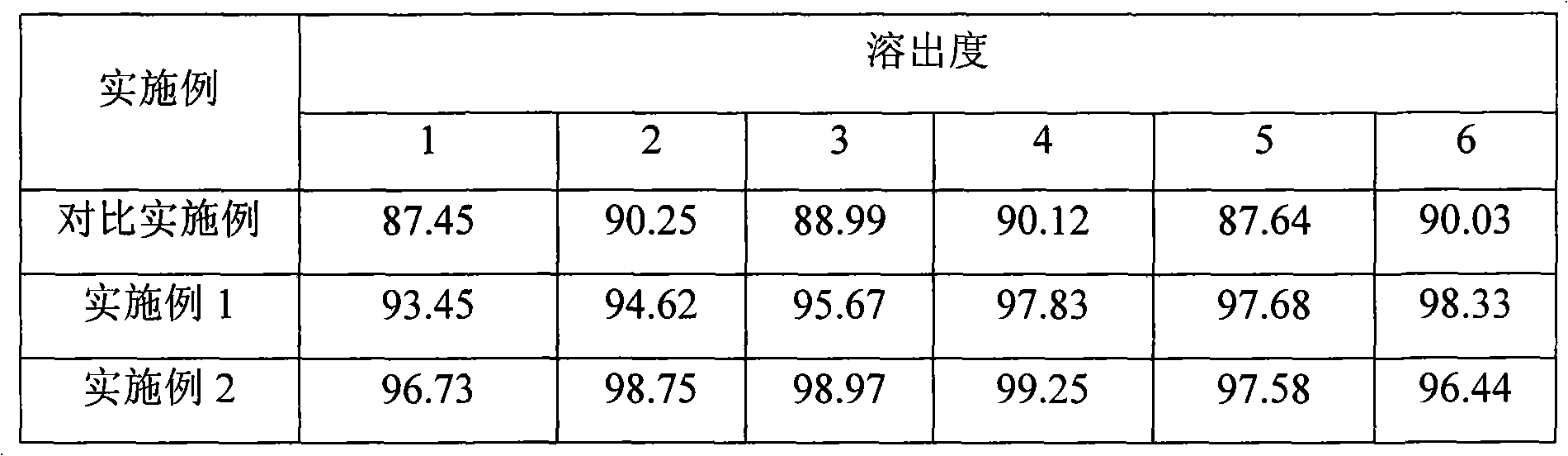
The invention belongs to the technical field of medicinal cocrystal, and particularly relates to a novel iloperidone medicinal cocrystal and a preparation method thereof. An N atom in a piperidine ring of iloperidone is used as a hydrogen bond donor, and an H atom on a carboxyl group in 3,5-pyridine dicarboxylic acid is used as a hydrogen bond receptor to form a hydrogen bond; an iloperidone molecule is combined with a 3,5-pyridine dicarboxylic acid molecule through the hydrogen bond to form a basic structural unit of the iloperidone medicinal cocrystal; and a space group of the medicinal cocrystal is a triclinic system. The preparation method of the iloperidone medicinal cocrystal is a reflux-room temperature volatilization method. The medicinal cocrystal prepared by using the preparation method disclosed by the invention has the characteristics of inheriting the conventional raw material medicament on treatment of schizophrenia; and the dissolubility, the stability and the bioavailability of the medicinal cocrystal are obviously improved.
Owner:JILIN UNIV
Method for synthesizing Iloperidone
Owner:EAST CHINA NORMAL UNIV
New preparation method of iloperidone
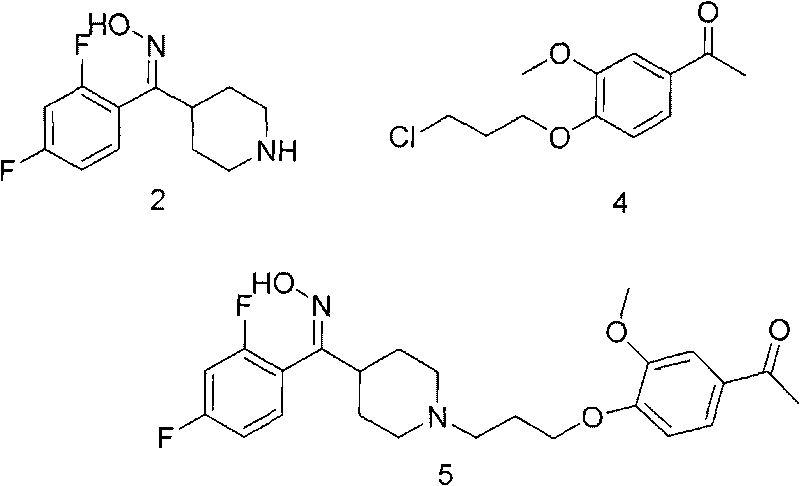
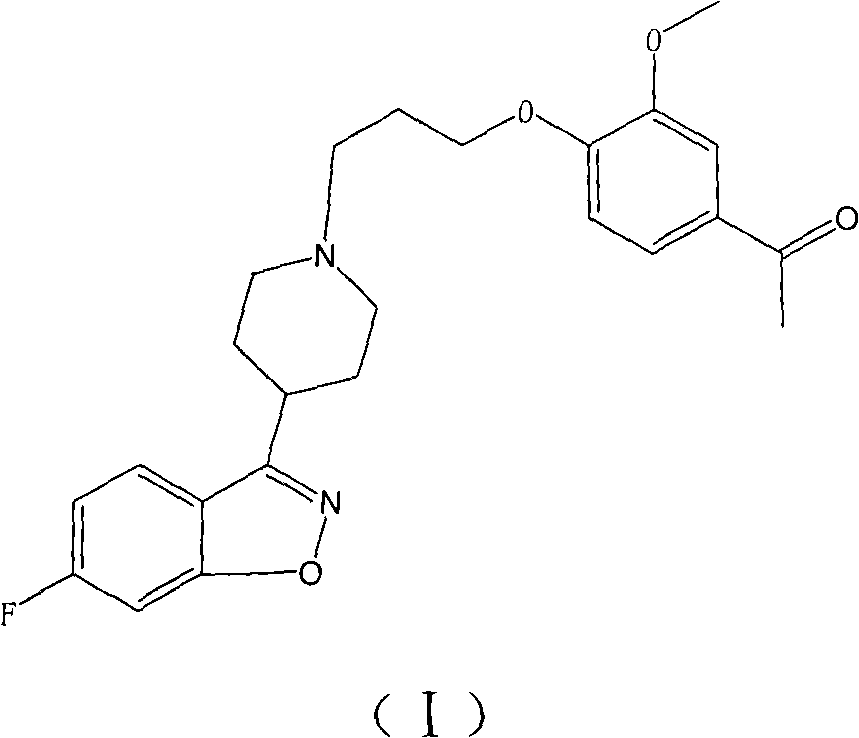
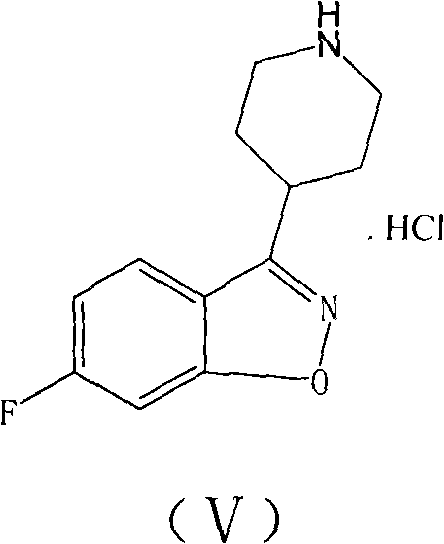
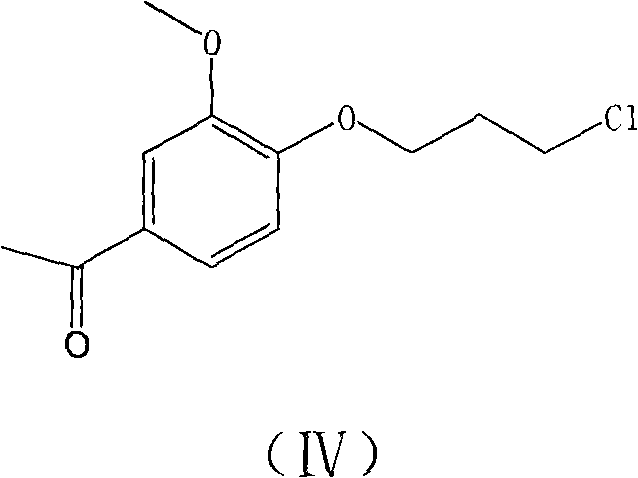
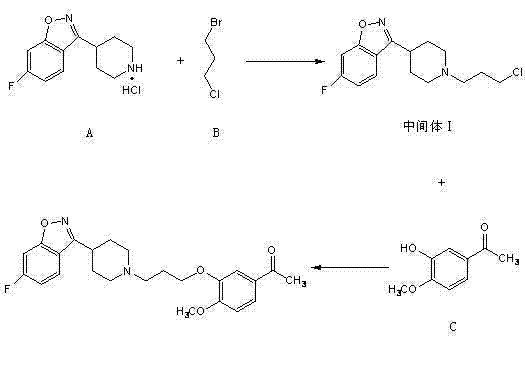
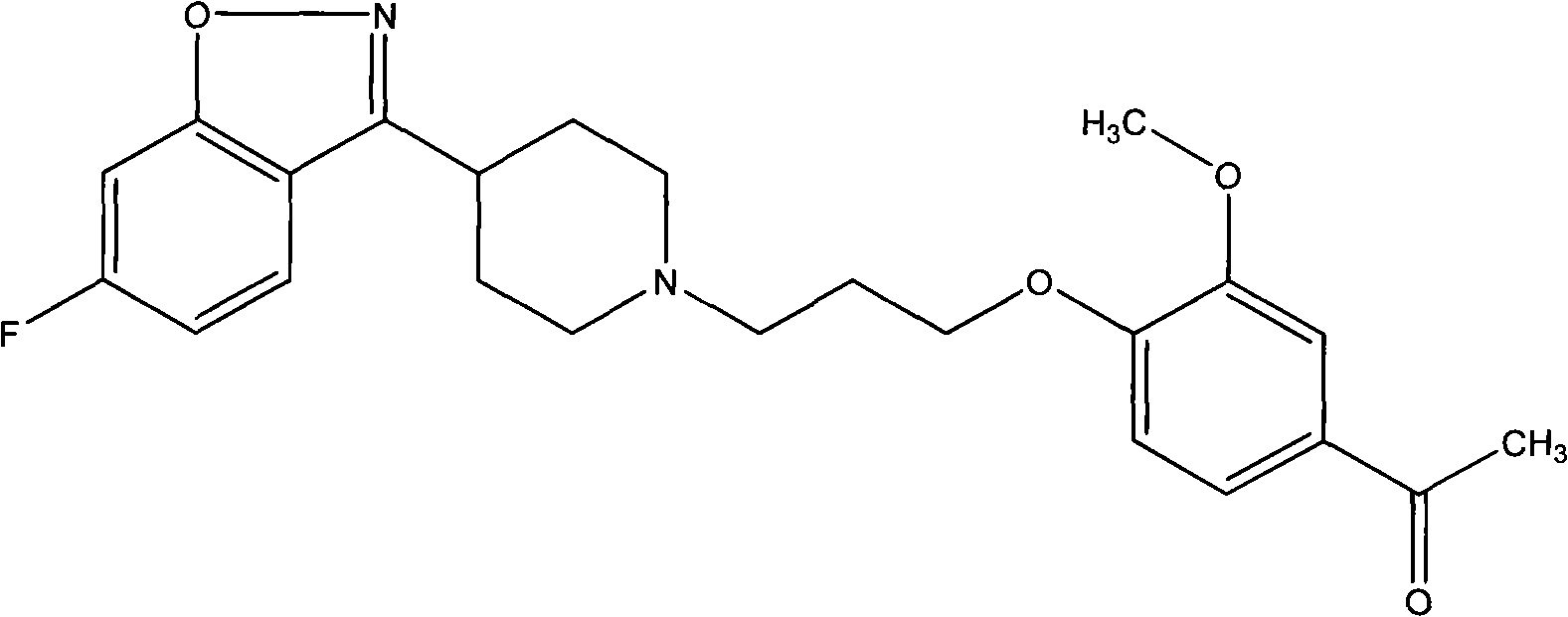
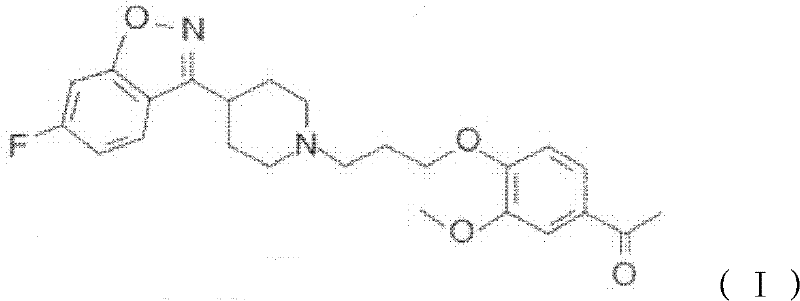
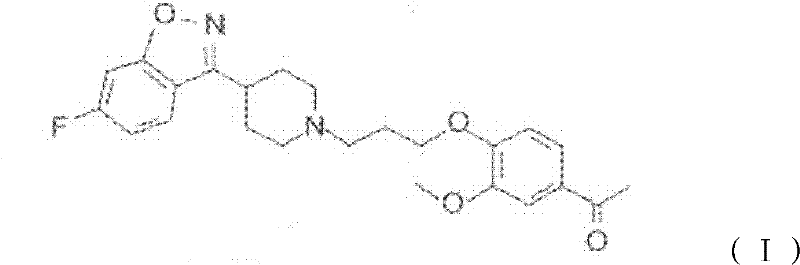
The invention provides a new preparation method of iloperidone, comprising leading 6 - F -3 - (4 - piperidinyl) -1,2 - benzisoxazole hydrochloride and 1 - [4 - (3 - chloropropoxy) -3 - methoxyphenyl] ethyl ketone to react in the inorganic alkaline water solution to obtain the iloperidone. The advantages of the invention are as follows: the method has high yield, low cost, no adverse effects for operators and environment, and is very suitable for industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Iloperidone sustained release microsphere and preparation method thereof
InactiveCN103599074AHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsNervous disorderMicrosphereGlycolic acid
The invention relates to an iloperidone sustained release microsphere and a preparation method thereof. The sustained release microsphere mainly comprises iloperidone and a biodegradable pharmaceutical polymer material PLGA (poly lactic-co-glycolic acid), wherein the molar ratio of lactic acid of PLGA to glycolic acid is (75-50):(25-50), the dosing weight ratio of the iloperidone to the PLGA is 1:(1-10), and the iloperidone is 3.5-15.5% of the total weight of the microsphere. According to the prepared iloperidone sustained release microsphere, the medicine encapsulation efficiency is high, the drug loading capacity is high, and the surface of the microsphere is smooth and round. The sustained release microsphere is used for curing psychosis, can prolong the action time of the medicine, reduces the dosing times, and greatly improves the obedience of patients in taking medicine.
Owner:CHONGQING PHARMA RES INST
Method for preparing iloperidone
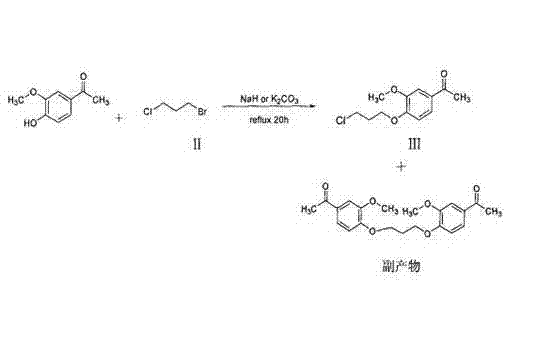
The invention belongs to the technical field of medicines. The invention discloses a method for preparing iloperidone. The method comprises the following steps of: reacting 6-fluoro-3-(4-piperidinyl)-1,2 benzisoxazole monohydrochloride and 1-bromine-3-chloropropane, which are both used as raw materials, to obtain 3-[1-(3-chloropropyl)piperidine-4-radical]-6-fluoro-1,2 benzoisoxazole, and reacting the obtained product and 4-hydroxyl-3-methoxyacetophenone serving as a raw material to obtain the iloperidone. Research shows that the byproducts generated in the method can be easily removed in the purifying process, so that the method has the advantages of high yield, high product purity and the like.
Owner:北京美迪康信医药科技有限公司
Novel iloperidone pharmaceutical cocrystal and preparation method thereof
The invention belongs to the technical field of pharmaceutical cocrystals and in particular relates to a novel iloperidone pharmaceutical cocrystal and a preparation method thereof. The pharmaceutical cocrystal is characterized in that iloperidone is taken as the active pharmaceutical ingredient, 3,5-dihydroxy-benzoic acid is taken as the former, and an iloperidone molecule, a 3,5-dihydroxy-benzoic acid molecule and a water molecule jointly form a basic structural unit of the iloperidone pharmaceutical cocrystal through hydrogen bonds and deposition. The pharmaceutical cocrystal is prepared by taking ethanol as the solvent and adopting the method of reflux-room temperature diffusion and volatilization. As the selected organic solvent has lower boiling point, crystals are prepared in the process of solvent volatilization after reflux and filtration. The prepared pharmaceutical cocrystal carries forward the characteristic of the traditional active pharmaceutical ingredients in treating schizophrenia and the dissolubility, stability and bioavailability of the pharmaceutical cocrystal are also obviously improved.
Owner:CHANGCHUN LICHENG BICHENG NEW MEDICINE TECHDEV
Methods for the administration of iloperidone
The present invention relates to methods for the identification of genetic polymorphisms that may be associated with a risk for QT prolongation after treatment with iloperidone and related methods of administering iloperidone to patients with such polymorphisms.
Owner:VANDA PHARMA INC
Iloperidone for the treatment of schizophrenia
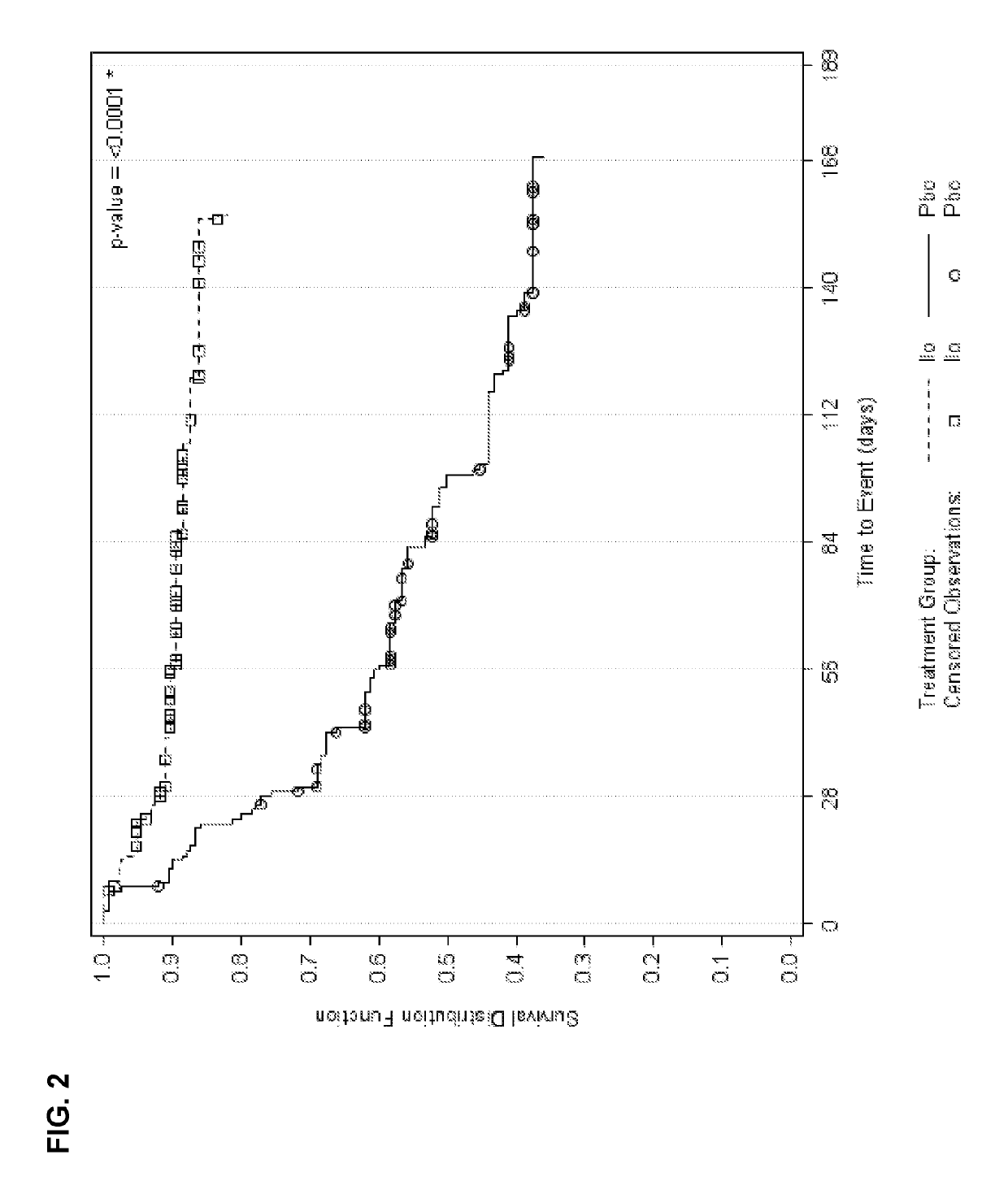
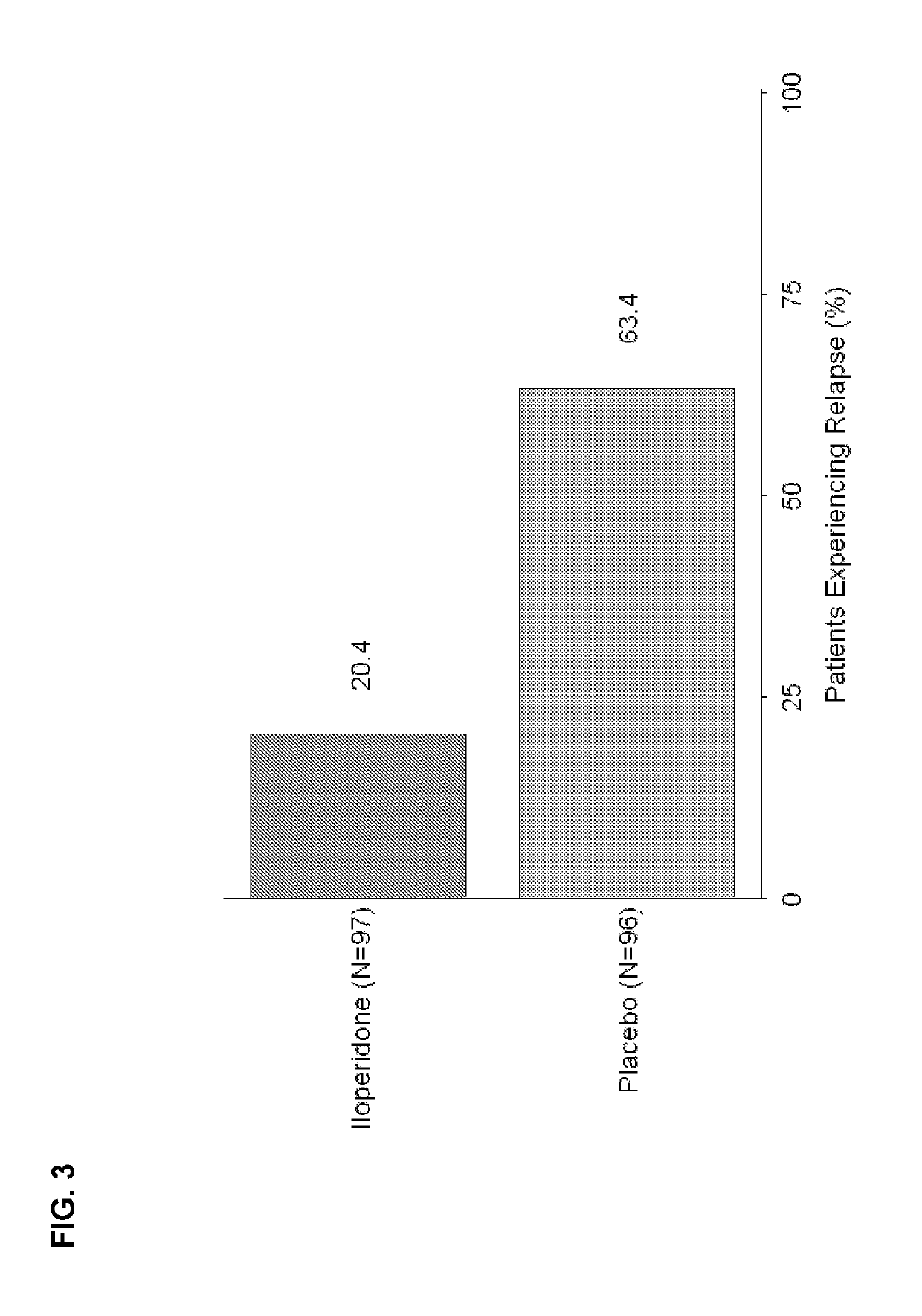
Aspects of the invention relate generally to the treatment of schizophrenia in an individual and, more specifically, to the treatment of an individual with iloperidone, an iloperidone metabolite, or a pharmaceutically-acceptable salt thereof. In one embodiment, the invention provides a method of preventing schizophrenic relapse in an individual diagnosed with schizophrenia, the method comprising: administering to the individual iloperidone, an iloperidone metabolite, or a pharmaceutically-acceptable salt thereof at a daily dose between about 12 mg and about 16 mg following a period in which the individual's schizophrenia was stabilized.
Owner:VANDA PHARMA INC
Method for preparing iloperidone
ActiveCN102796090AHigh yieldHigh purityOrganic chemistryPotassium hydroxideHydroxylamine Hydrochloride
The invention relates to a method for preparing iloperidone. The method comprises the following steps of: dissolving 4-(2,4-difluorobenzoyl)-piperidine hydrochloride and hydroxylamine hydrochloride serving as raw materials into 95 percent ethanol serving as a cheap solvent by taking excessive triethylamine as an alkali to obtain an intermediate, i.e., 2,4-difluorophenyl-4-piperidylketoxime; dissolving potassium hydroxide powder with absolute ethyl alcohol, reacting with the intermediate, purifying and salting to obtain a second intermediate, i.e., 6-fluoro-3-(4-piperidyl)-1,2-benzisoxazolehydrochloride; preparing and recrystallizing a third intermediate, i.e., 4-(3-chloropropyloxy)-3-methoxyacetophenone by taking 3-methoxyl-4-hydroxyacetophenone and 1-bromine-3-chloropropane serving raw materials; reacting the second intermediate, i.e., 6-fluoro-3-(4-piperidyl)-1,2-benzisoxazolehydrochloride with the third intermediate, i.e., 4-(3-chloropropyloxy)-3-methoxyacetophenone in a mixed solution of water and acetone under the action of potassium carbonate to obtain crude iloperidone; and refining the crude iloperidone with ethanol to obtain fine iloperidone.
Owner:TIANJIN HUAJIN PHARMA +1
Pharmaceutical composition containing Iloperidone and preparation method thereof
InactiveCN102327266ADissolution rate is fastChange in dissolution behaviorOrganic active ingredientsNervous disorderLactoseMedical prescription
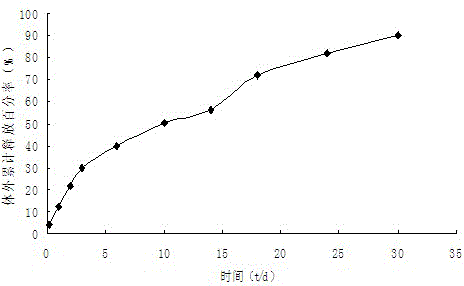
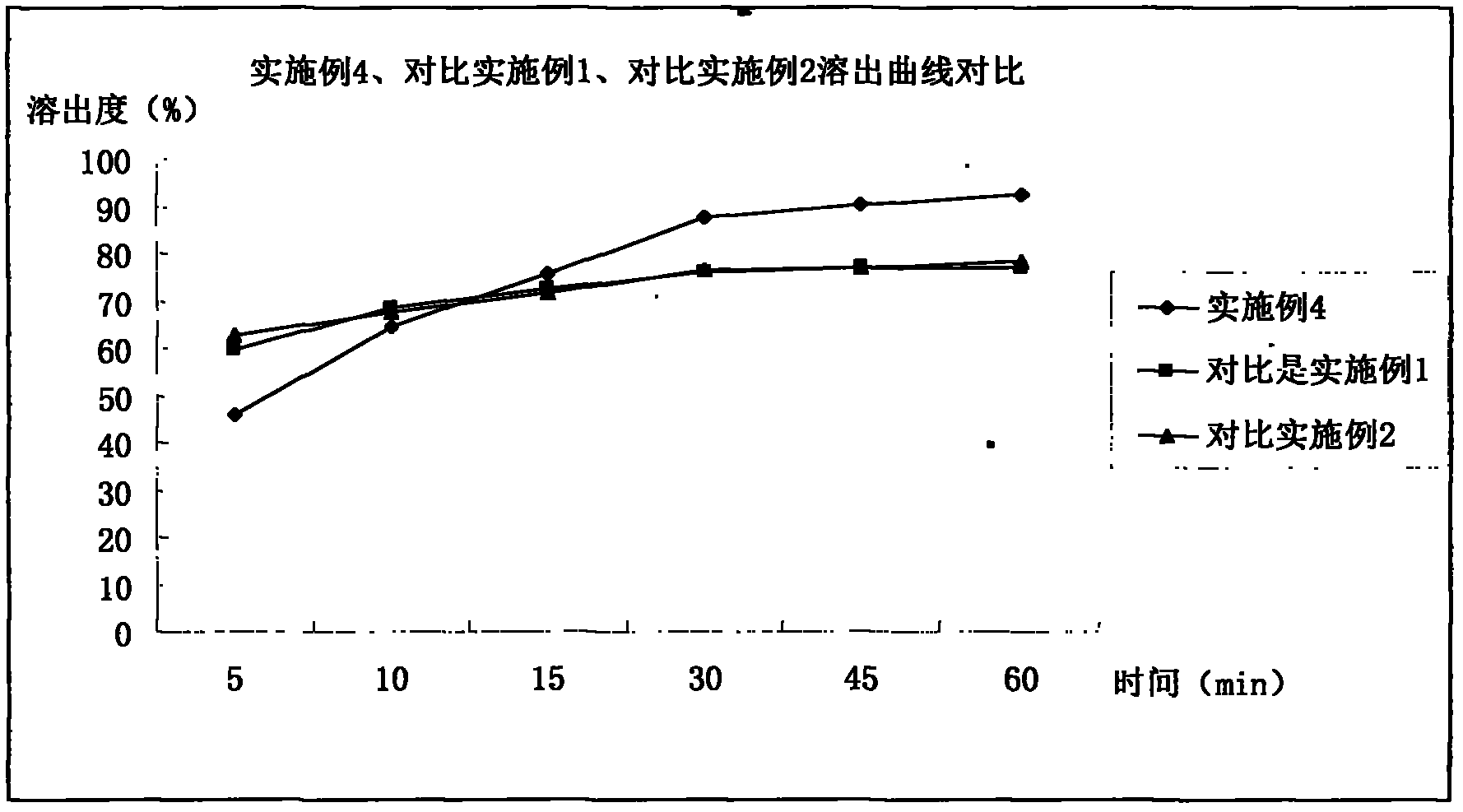
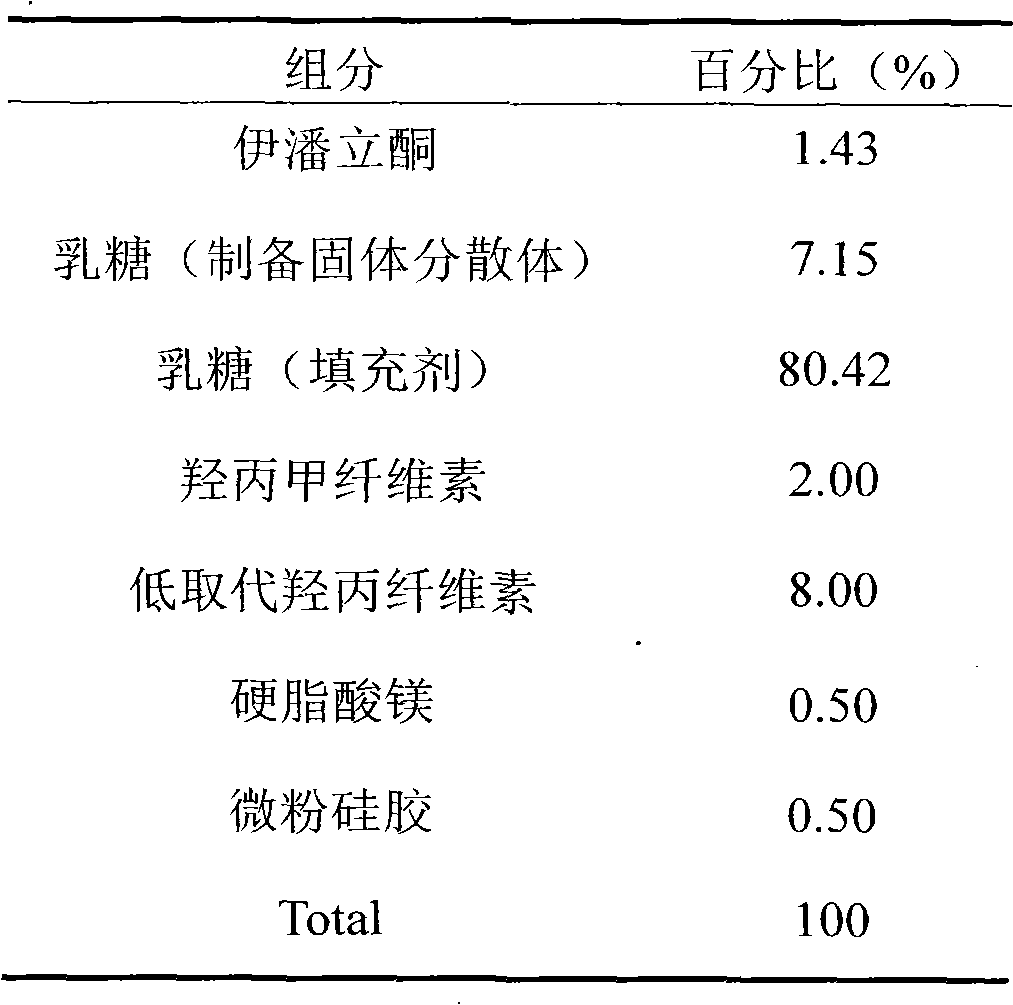
The invention belongs to the technical field of medicines, and particularly relates to a pharmaceutical composition containing Iloperidone and a preparation method thereof. In the invention, Iloperidone and lactose are prepared into solid dispersion, and a hydrophilic gel material is added to the prescription at the same time, thus improving the water insolubility of Iloperidone. The pharmaceutical composition containing Iloperidone provided by the invention has the characteristics that the water insolubility of Iloperidone is improved, the in-vitro dissolution is different from the characteristic that the traditional common preparation rapidly releases at the early stage, and the in-vitro dissolution is mild at the early stage, but is completed within 30 minutes.
Owner:北京德众万全医药科技有限公司
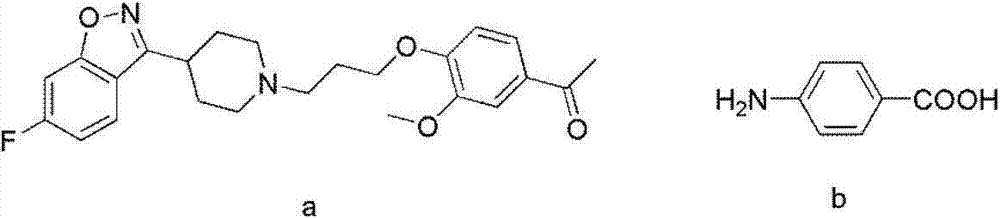
Pharmaceutical intermediate, preparation method thereof and method for preparing iloperidone by pharmaceutical intermediate
The invention provides a novel pharmaceutical intermediate compound in a formula (1), medicinal salt and a preparation method thereof. In the presence or absence of catalyst, the pharmaceutical intermediate compound of the invention is obtained by mixing, heating and stirring the compounds in formulas (2) and (3) with organic solvent in the presence of alkali. The medicinal salt of the pharmaceutical intermediate compound is obtained by mixing the compound in the formula (1) with another organic solvent, adding corresponding acid into the mixture and adjusting the pH value of the system. The preparation method is simple, has higher yield, and is very applicable to industrial production. The invention further provides a method for preparing iloperidone by the pharmaceutical intermediate compound or the medicinal salt thereof, which comprises the steps of mixing the compound in the formula (1) or the medicinal salt thereof with the solvent, cyclization reacting in the presence of alkali, and then obtaining the iloperidone in a formula (4). The invention overcomes the defect of the existing iloperidone synthesizing method, has higher yield compared with the existing method, and is applicable to large-scale industrial production.
Owner:天津泰普制药有限公司
Methods for the administration of iloperidone
The present invention relates to methods for the identification of genetic polymorphisms that may be associated with a risk for QT prolongation after treatment with iloperidone and related methods of administering iloperidone to patients with such polymorphisms.
Owner:VANDA PHARMA INC
Iloperidone orally disintegrating tablet
InactiveCN102440971AOrganic active ingredientsNervous disorderSolubilityOrally disintegrating tablet
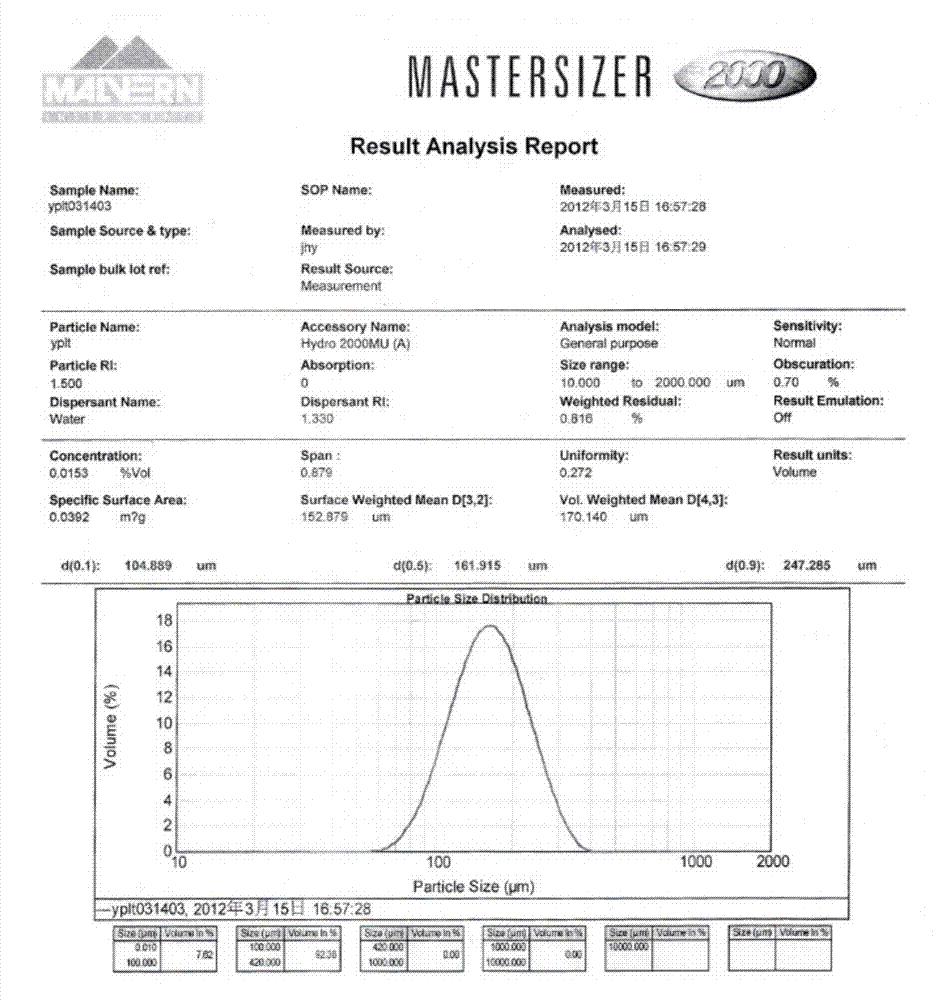
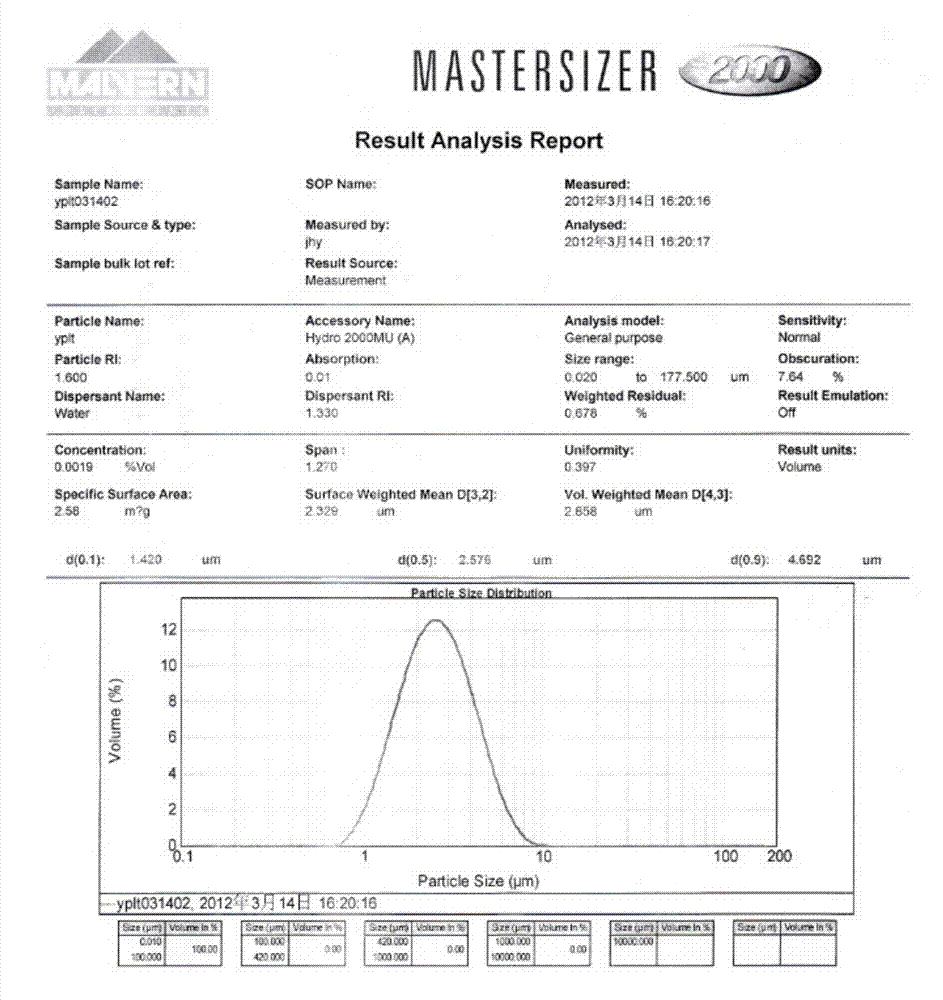
The invention relates to an iloperidone orally disintegrating tablet, which is characterized in that: 1) the grain size D of the iloperidone is less than or equal to 90 and more than or equal to 30 mum; 2) the proportion of water-solubility components in the tablet is more than or equal to 50%; and 3) the orally disintegrating tablet can be disintegrated when meeting spittle in an oral cavity.
Owner:重庆市力扬医药开发有限公司
Oral tablet containing iloperidone, and its preparation method
The invention discloses an oral tablet containing iloperidone, and its preparation method. The oral tablet is characterized in that the oral tablet contains iloperidone, hydroxypropyl methylcellulose, lactose, microcrystalline cellulose, a disintegrating agent, a lubricant, and a flow aid, wherein the application amount of the hydroxypropyl methylcellulose is 4.0-6.0%. The iloperidone tablet prepared according to the preparation method in the invention has good in-vitro dissolution to guarantee the iloperidone tablet has a good in-vivo biological utilization degree. Simultaneously the invention also provides the preparation method of the oral tablet, and the preparation method has the advantages of simple technology, low cost, and suitableness for commercial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Iloperidone pharmaceutical composition and preparation method thereof
InactiveCN102078320AReasonable prescriptionWorkmanship is feasibleOrganic active ingredientsNervous disorderTreatment effectLactose
The invention discloses an iloperidone pharmaceutical composition and a preparation method thereof. The invention is characterized in that 1000 iloperidone pharmaceutical composition tablets are prepared from the following components: 1-24g of iloperidone, lactose, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, 5% povidone K30 and a proper amount of 70% ethanol solution. The invention also relates to the preparation method of the iloperidone pharmaceutical composition. The iloperidone tablet prepared by the prescription and preparation method provided by the invention has the advantages of good liquidity, good dissolution rate, small tablet weight variation, high bioavailability and good treatment effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of Iloperidone
ActiveCN101824030AAvoid generatingAvoid vacuum distillation operationsNervous disorderOrganic chemistryKetoneIloperidone
Owner:AVENTIS PHARMA HAINAN
Iloperidone crystal and preparation method and medicinal composition thereof
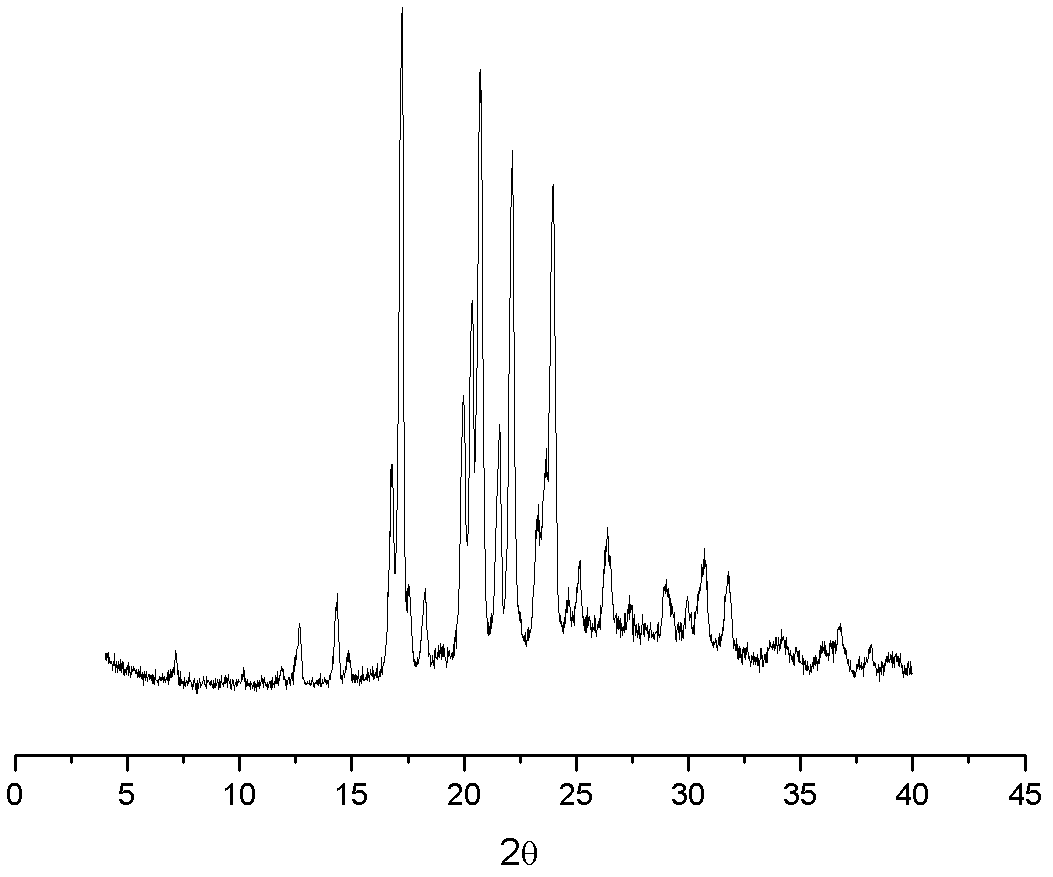
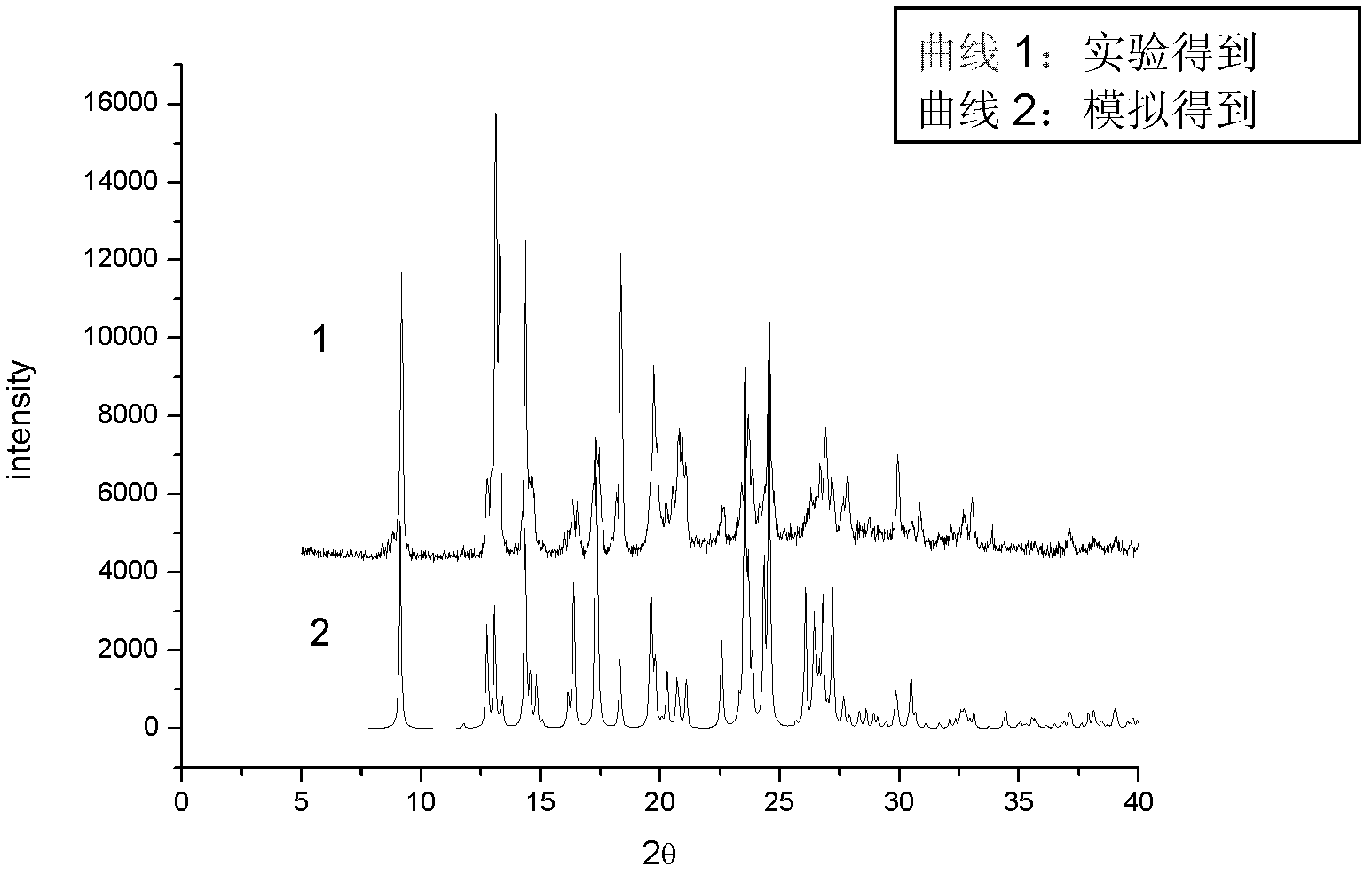
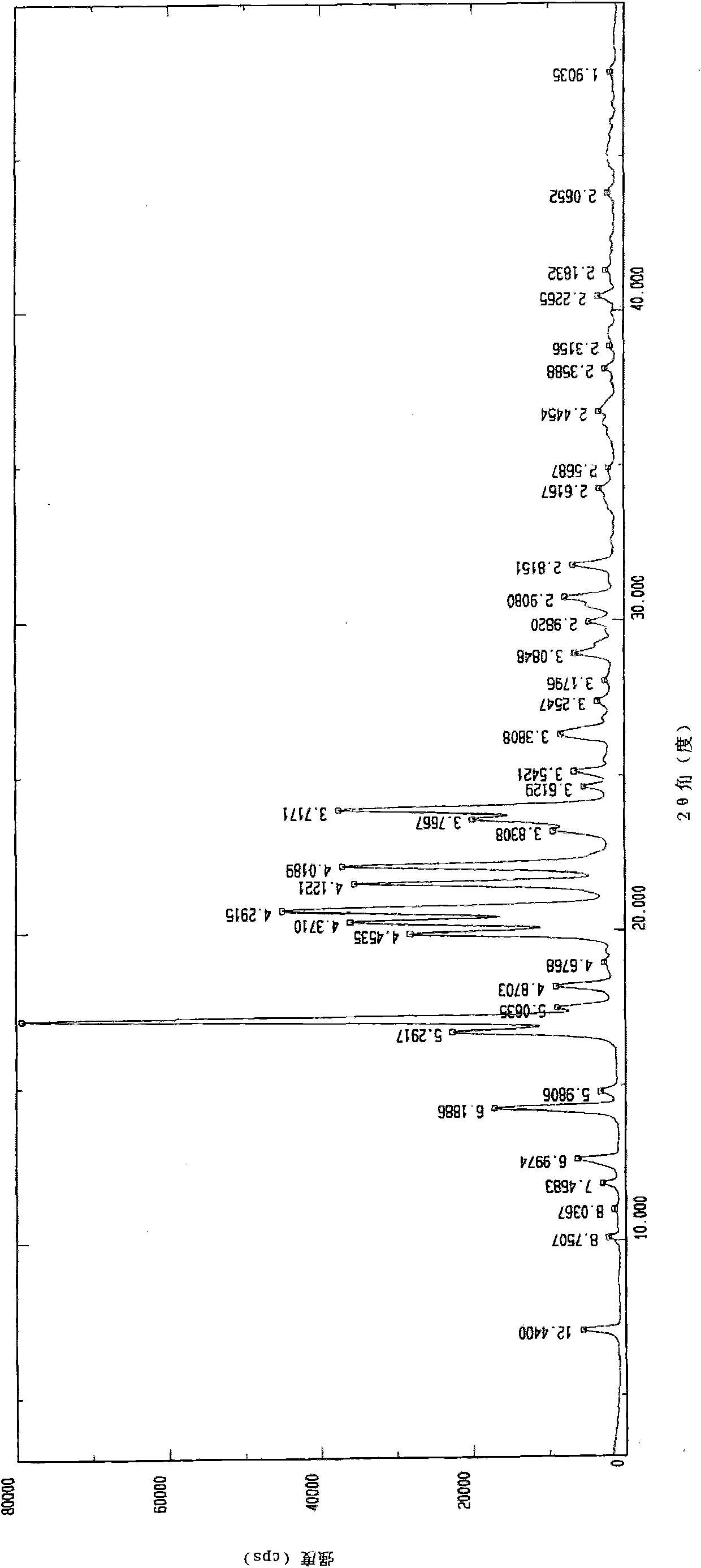
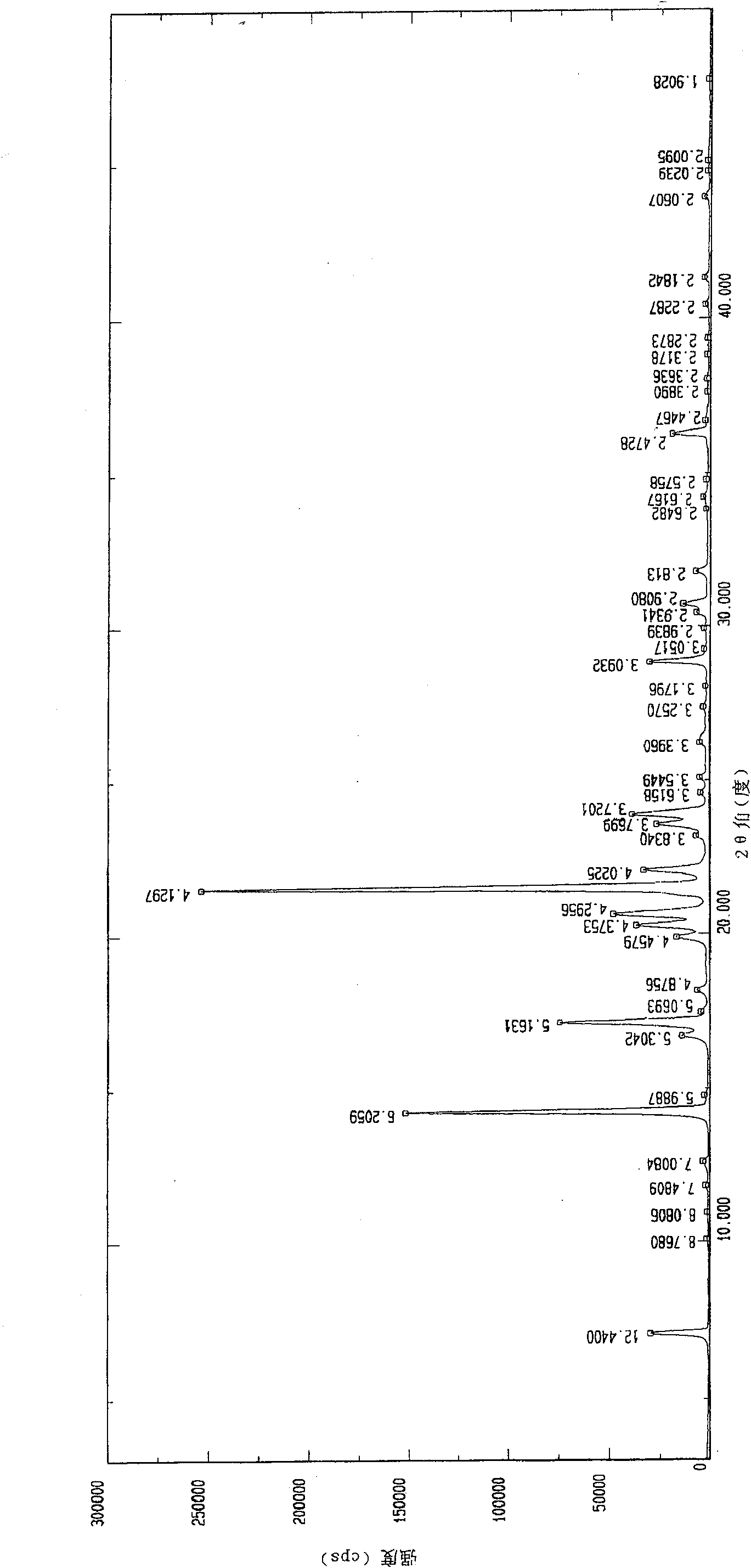
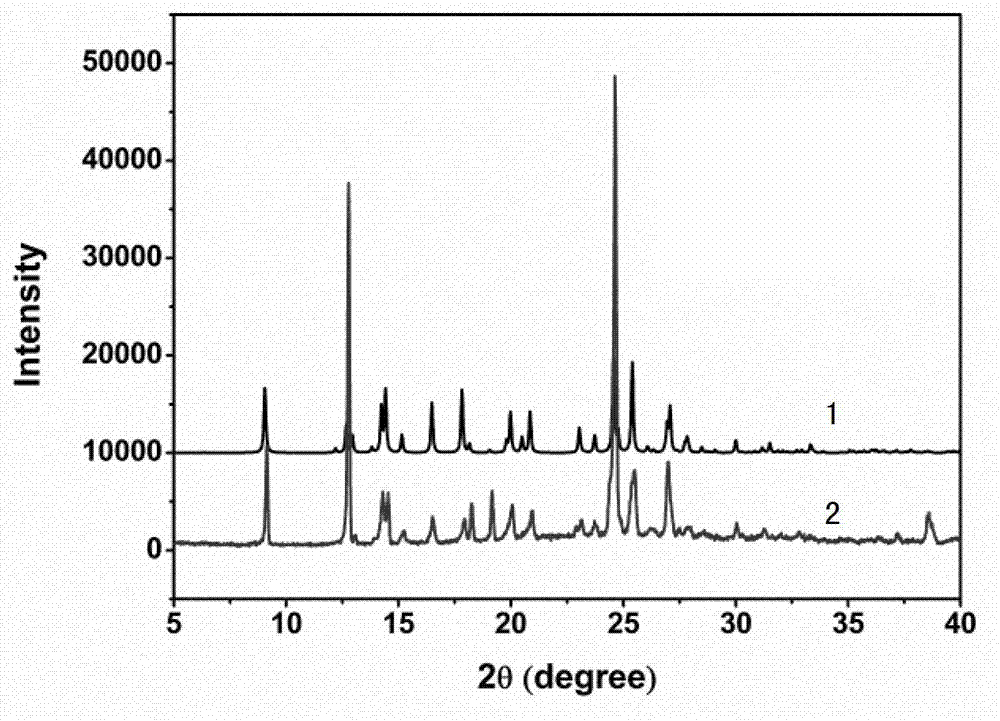
The invention provides an iloperidone crystal, which has X-ray diffraction peaks at 17.16 degrees, 20.68 degrees, 14.30 degrees, 16.74 degrees, 19.92 degrees, 20.30 degrees, 21.54 degrees, 22.10 degrees, 7.10 degrees, 12.64 degrees, 17.50 degrees, 18.20 degrees, 24.62 degrees, 25.12 degrees, 26.34 degrees, 28.92 degrees, 29.94 degrees, 30.72 degrees and 31.76 degrees represented by a 2theta anglein an X-ray diffraction pattern. The invention also provides a method for preparing the iloperidone crystal. The iloperidone crystal has a stable structure, can be stored for a long time, does not have any special requirement on temperature and humidity, and can be used for preparing a medicinal composition for treating schizophrenia.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Industrial preparation method of micronized iloperidone
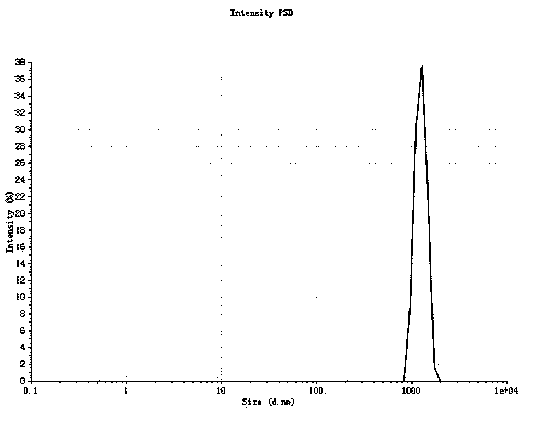
InactiveCN104324008AHigh puritySimple processPowder deliveryOrganic active ingredientsOrganic solventAnti solvent
An industrial preparation method of micronized iloperidone belongs to the field of medical ultrafine powder preparation, especially preparation of an iloperidone ultrafine powder. Specifically, iloperidone is dissolved in an organic solvent to obtain an iloperidone solution; at a certain temperature, the iloperidone solution is added into an anti-solvent according to a certain volume ratio of the solution to the anti-solvent; after full stirring and mixing, iloperidone crystals are precipitated; and after aging at a certain temperature, filtration and drying are carried out successively to obtain the iloperidone ultrafine powder product. The method is simple, and all operations are safe. In addition, the method is low-cost and is easy for industrial large-scale production. The product obtained has higher purity and faster dissolution rate.
Owner:山东省生物医药科学院有限公司
Preparation method of antipsychotic drug iloperidone
The invention provides a preparation method of a novel antipsychotic drug iloperidone, which comprises the following steps that: 6-fluoro-3-(4-piperidinyl)-1,2-benzo isoxazole hydrochloride is reacted with 1-[4-(3- chlorophenoxy)-3-methoxyphenyl]ethanone in an aqueous solution of a non-ionic surfactant by using an inorganic base as an acid binding agent and potassium iodide as a catalyst to obtain the iloperidone. The process has the advantages of high yield, high purity, good product appearance, simpleness and convenience in operation, and low cost, has no adverse impact on the environment, and is very suitable for industrial mass production.
Owner:HARBIN SANLIAN PHARMA CO LTD
Preparation method of iloperidone
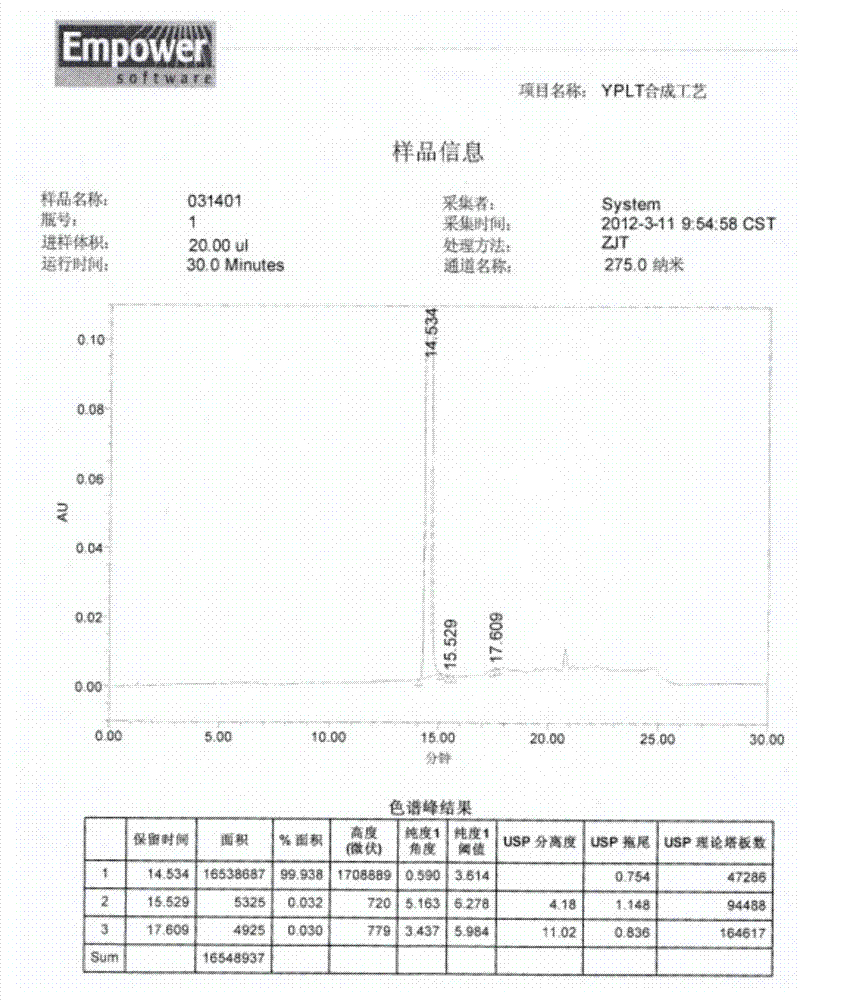
The invention provides a method for preparing iloperidone by a one-step method. Iloperidone is obtained directly by a one-step base catalytic reaction of a compound in formula 3 and a compound in formula 4. The operation is simplified greatly, and the product quality is not compromised; the obtained iloperidone has purity of more than 99% and a yield of more than 90%.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Iloperidone crystal, and preparation method and medicinal composition thereof
The invention provides iloperidone crystal. X-diffracted ray diffraction peaks are formed at angles 2theta of 14.26+ / -0.2 and 21.50+ / -0.2 degrees in an X-ray diffraction pattern of the iloperidone crystal. The invention also provides a preparation method of the iloperidone crystal; and the method comprises the following steps of: 1) mixing the iloperidone and an organic solvent, heating and dissolving; 2) naturally cooling the solution to 45 to 55 DEG C, and separating the crystal out; and 3) continuing to naturally cool the obtained crystal to room temperature, cooling to 5 to 10 DEG C in anice water bath, filtering, and drying under vacuum at the temperature of less than 60 DEG C to obtain the iloperidone crystal. The iloperidone crystal has a stable structure, can be stored for a longtime, does not have special requirements on temperature, luminosity and humidity, and can be used for preparing a medicinal composition and medicaments for treating schizophrenia.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Iloperidone composition and preparation method thereof
ActiveCN102805745AReduce manufacturing costEvenly dispersedPowder deliveryOrganic active ingredientsMedicineBULK ACTIVE INGREDIENT
The invention relates to an iloperidone composition and a preparation method thereof. According to the iloperidone composition, iloperidone which is taken as an active ingredient is pretreated, so that the particle size of the iloperidone is minimized; and therefore, the dissolution rate of the iloperidone is effectively increased.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Methods for the administration of iloperidone
The present invention relates to methods for the identification of genetic polymorphisms that may be associated with a risk for QT prolongation after treatment with iloperidone and related methods of administering iloperidone to patients with such polymorphisms.
Owner:VANDA PHARMA INC
Preparation method of iloperidone intermediate
The invention relates to a preparation method of an iloperidone intermediate. The preparation method comprises the following steps of (1) adding potassium hydroxide into methyl alcohol, and adding (2,4-difluorophenyl)-(4-piperidyl)ketoxime hydrochloride; (2) heating, and reacting for 2 to 3h at the controlled temperature of 50 to 60 DEG C; (3) cooling to room temperature, adding anhydrous MgSO4, stirring for 0.8 to 1.2h, sucking and filtering, and performing vacuum concentration on filtrate; (4) adding acetone, stirring for 0.4 to 0.6h at the room temperature, filtering, dripping a saturated HCl methyl alcohol solution while stirring the filtrate, so as to adjust a pH (potential of hydrogen) value to 2-3, sucking and filtering, and drying, so as to obtain a white solid, wherein the water content of methyl alcohol is less than 0.5%.
Owner:BEIJING PHARMA GRP CO LTD
Preparation method of iloperidone
InactiveCN103130785ASimple preparation processSimple and refined processOrganic chemistryHydroxylamine HydrochlorideIloperidone
The invention relates to the technical field of drug synthesis, in particular to a preparation method of iloperidone. 95-105 grams of 4-hydroxyl-3-methoxyacetophenone, 9.5-10.5 grams of hydroxylamine hydrochloride and 950-1050 millimeters of absolute ethyl alcohol are added into a reaction vessel in sequence and mixed with the temperate controlled between 35-45 DEG C, 124-126 millimeters of triethylamine are added dropwise, and heating of backflow are conducted for 15-16 hours after the droplet addition is finished, the temperature is reduced to room temperature, reaction products are subjected to suction filtration, a filter cake is washed and dried, and white powder is obtained. The preparation method of iloperidone is simple and direct in preparation process, suitable for industrial production, high in yield coefficient, and capable of effectively shortening reaction time. Meanwhile, purification processes are simple and direct, and product purity is high.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Iloperidone drug cocrystal and preparation method thereof
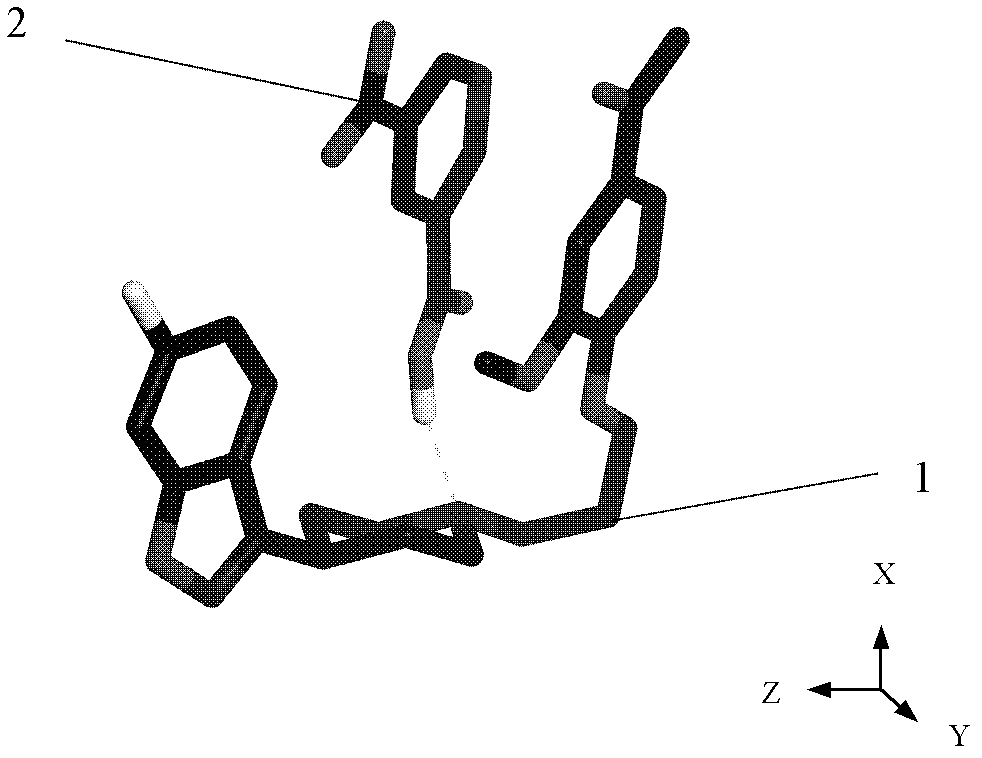
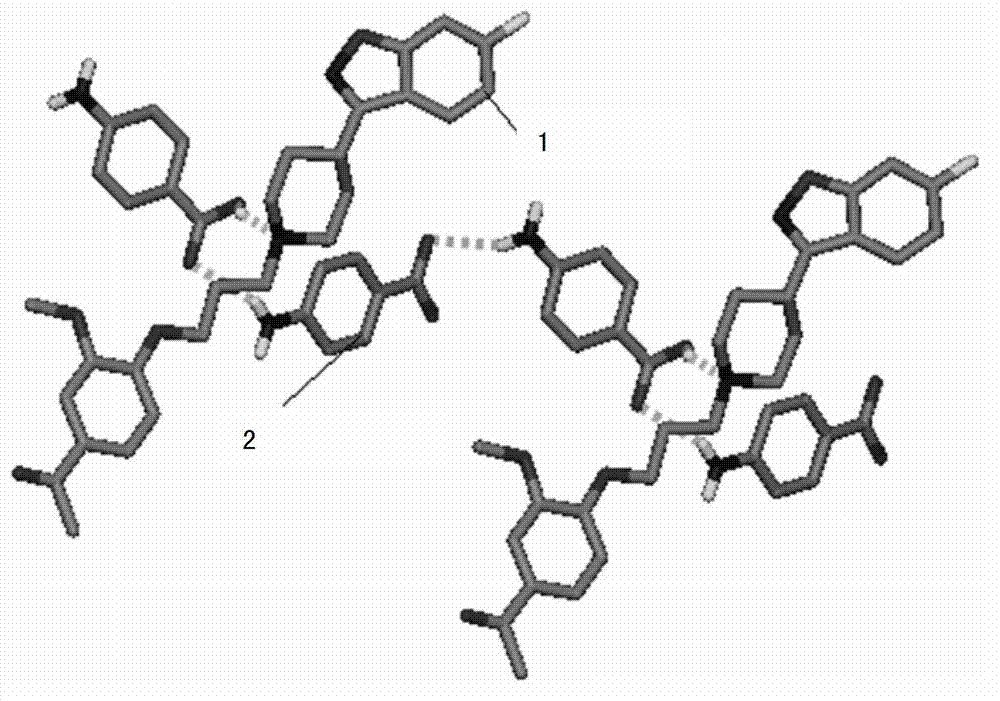
The invention belongs to the technical field of new crystal forms of organic drugs, and particularly relates to an iloperidone drug cocrystal and a preparation method of the iloperidone drug cocrystal. The method comprises the steps that iloperidone is taken as an active drug ingredient; 4-aminobenzoic acid is taken as a precursor; a three-dimensional net structure is formed by iloperidone and 4-aminobenzoic acid through hydrogen bonds and by the action of pi-pi accumulation; on a YZ plane, 4-aminobenzoic acid molecules stretch in a wave manner in a Y direction on the YZ plane through N-H...O hydrogen bonds; iloperidone molecules conduct the pi-pi accumulation on the YZ plane in an end-to-end accumulation manner and stretch in the Y direction; in an X direction, the iloperidone molecules and 4-aminobenzoic acid molecules form the hydrogen bonds through O-H...N, and the iloperidone molecules conduct the pi-pi accumulation in the X direction in an end-to-end accumulation manner. A crystal space group is a monoclinic system. The drug cocrystal carries on the characteristics of a traditional raw material drug in treating schizophrenia, and has obvious improvement in solubility, stability and bioavailability.
Owner:苏州纳埃净化科技有限公司
Iloperidone slow releasing microsphere and preparation method thereof
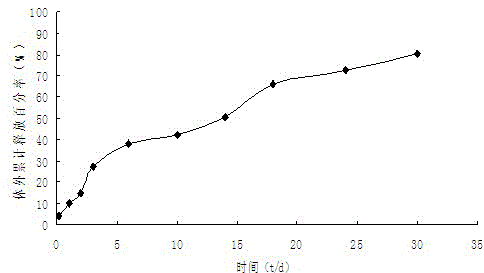
InactiveCN104352443AIncrease concentrationOrganic active ingredientsNervous disorderLactideMicrosphere
The invention provides an iloperidone slow releasing microsphere and a preparation method thereof. The microsphere contains an iloperidone and lactide-glycolide copolymer. The iloperidone slow releasing microsphere provided by the invention has the benefits that the drug loading capacity is higher, the slow releasing period is above 5 weeks, and an in vitro release curve is smooth and flat.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com