Novel iloperidone pharmaceutical cocrystal and preparation method thereof
A technology of iloperidone and drugs, applied in the field of new iloperidone drug co-crystal and its preparation, to achieve the effect of improving bioavailability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
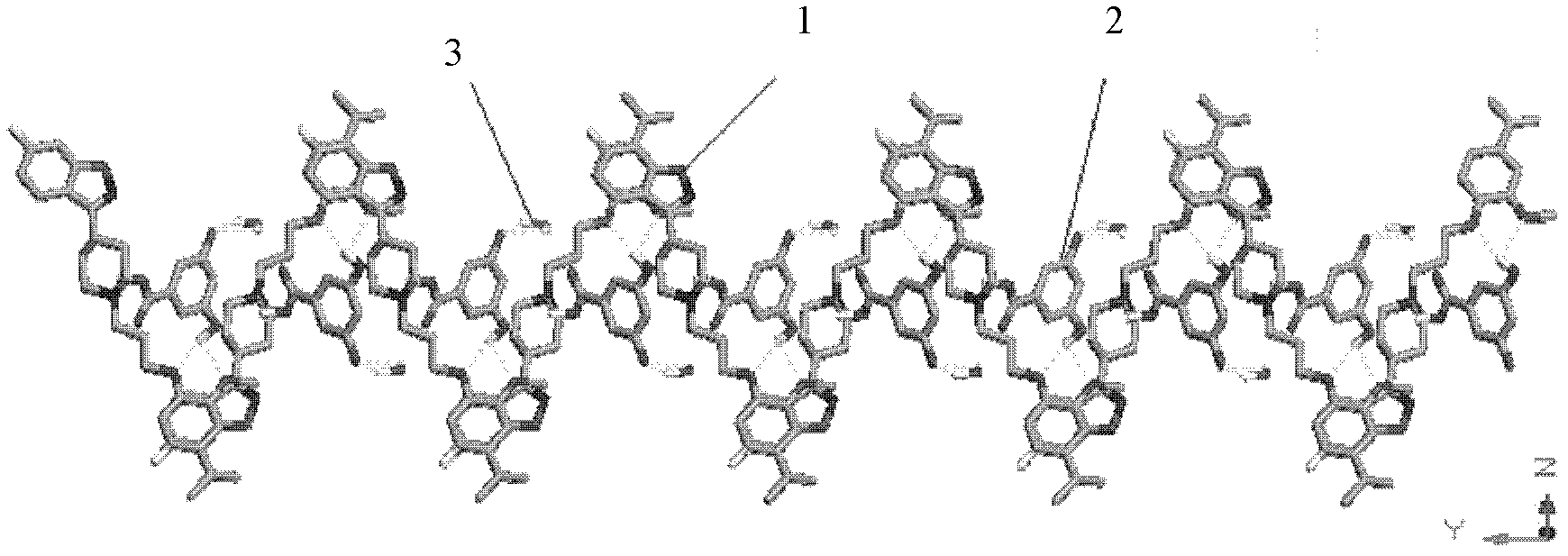
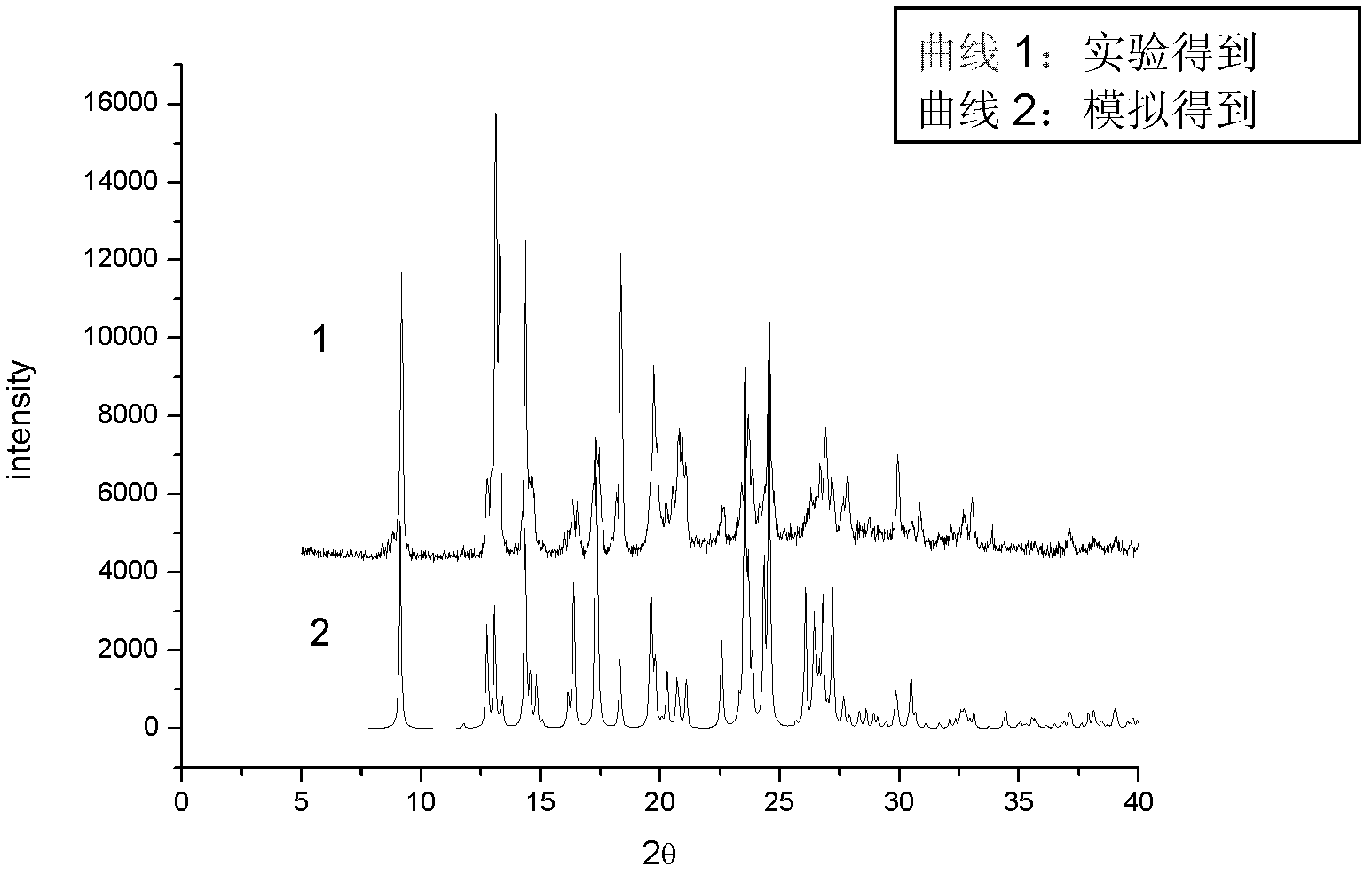
Image
Examples
Embodiment 1
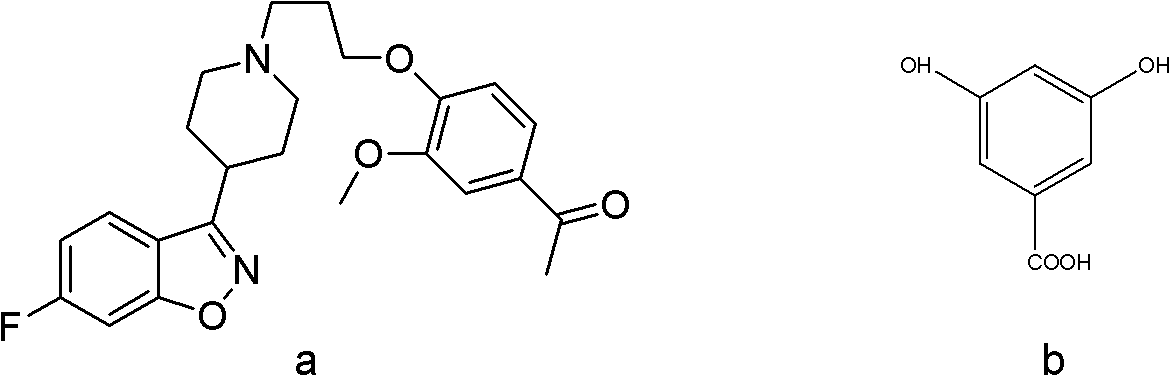
[0029] Synthesis of co-crystals using iloperidone and 3,5-dihydroxybenzoic acid:
[0030] Weighing:
[0031] The reactant is fed according to iloperidone:3,5-dihydroxybenzoic acid=1:1 mass ratio. Accurately weigh 20.00 mg of iloperidone and 20 mg of 3,5-dihydroxybenzoic acid in a 25 ml single-necked round bottom flask with an analytical balance.
[0032] Dissolution of API:
[0033] Use a 5ml pipette to accurately measure 5ml of ethanol into 25ml single-necked round bottom flasks.
[0034] Reflux-volatilization heat method:
[0035] Put the stirring bar in the round-bottomed flask of iloperidone and 3,5-dihydroxybenzoic acid dissolved uniformly above, set up the reflux device, the reflux time is 2h, the reflux temperature is 90°C, turn on the magnetic stirrer and condense water.
[0036] After reflux, the reaction solution was filtered, and the filtrate was placed in a 25ml transparent glass vial and placed at room temperature. After 6 hours of slow volatilization of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com